Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Chitosan Hemostatic Materials
- (1)
- Chitosan hemostatic sponge (ChSp) was prepared using 300 kDa molecular weight chitosan (Ch) with 95% deacetylation degree and 1% acetic acid purchased from YuDa Chemicals, Qingdao, PRC. Chitosan was dissolved in acetic acid and stirred for 24 h at room temperature until it becomes homogeneous. This solution was then placed in a reaction vessel with a maximum column height of 1.0 cm. The polymer solution was frozen at −25 °C for 24 h and then dried in the vacuum chamber (0.1 Pa, 24 h).
- (2)
- Chitosan electrospinning membranes (ChEsM) were prepared from chitosan powder, 300 kDa molecular weight (95% deacetylation degree) and polyethylene oxide (PEO) powder (400 g/mol) purchased from Glentham Life Sciences (Corsham, UK). Ch (2 g) and PEO (3 g) were dissolved in 100 mL of 50% acetic acid and stirred for 24 h at room temperature. After complete dissolution, solutions were mixed in a 3:1 PEO/Ch ratio. The conductivity of this solution was 1487 μS/cm. The electrospinning process was performed at room temperature and relative humidity of 15–20% in the safe cabinet with laminar airflow in the RT-Advanced machine (Linari Engineering, Pisa, Italy). Ch/PEO solution was poured into a 10 mL glass syringe with a needle diameter of 0.6 mm. The distance between the needle and the collector was 12 cm. The parameters of electrospinning were set as follows: the flow rates, 0.2 mL/h; the voltage applied to the needle, 17 kV; the rotational speed of the collector (10 cm in diameter), 800 rpm. The electrospun membrane was vacuum dried at room temperature within 12 h.
2.2. Scanning Electron Microscopy (SEM)
2.3. Porosity and Density
2.4. Degradation and Biodegradation Study
2.5. Antibacterial Test
2.6. Cell Viability Assay
2.7. Live/Dead Staining
2.8. Blood Interaction Test
2.9. Animals and Surgical Procedures
2.10. Histological Tissues Processing
2.11. Immunohistochemical Study
2.12. Statistical Analysis
3. Results and Discussion
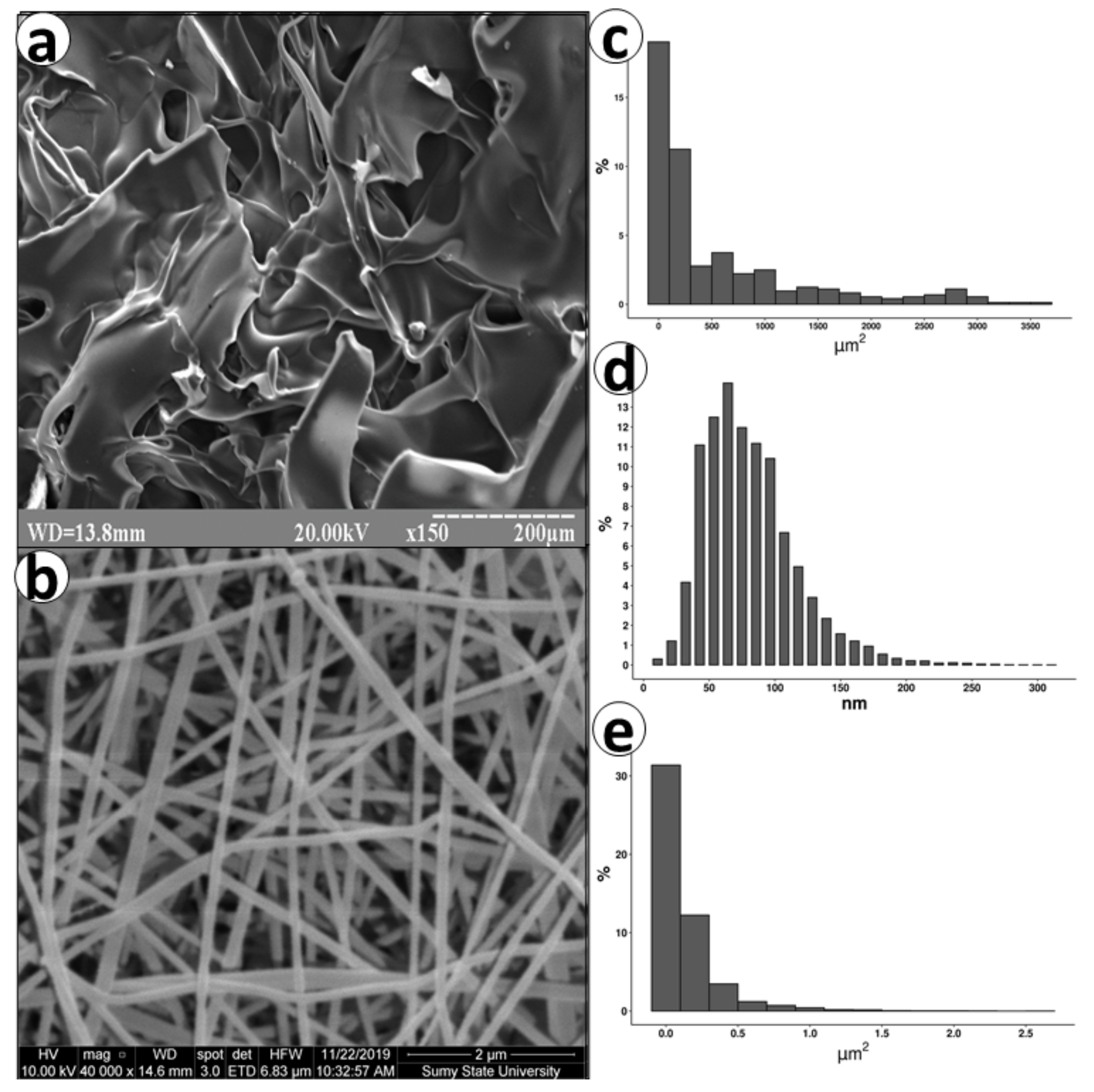
3.1. SEM
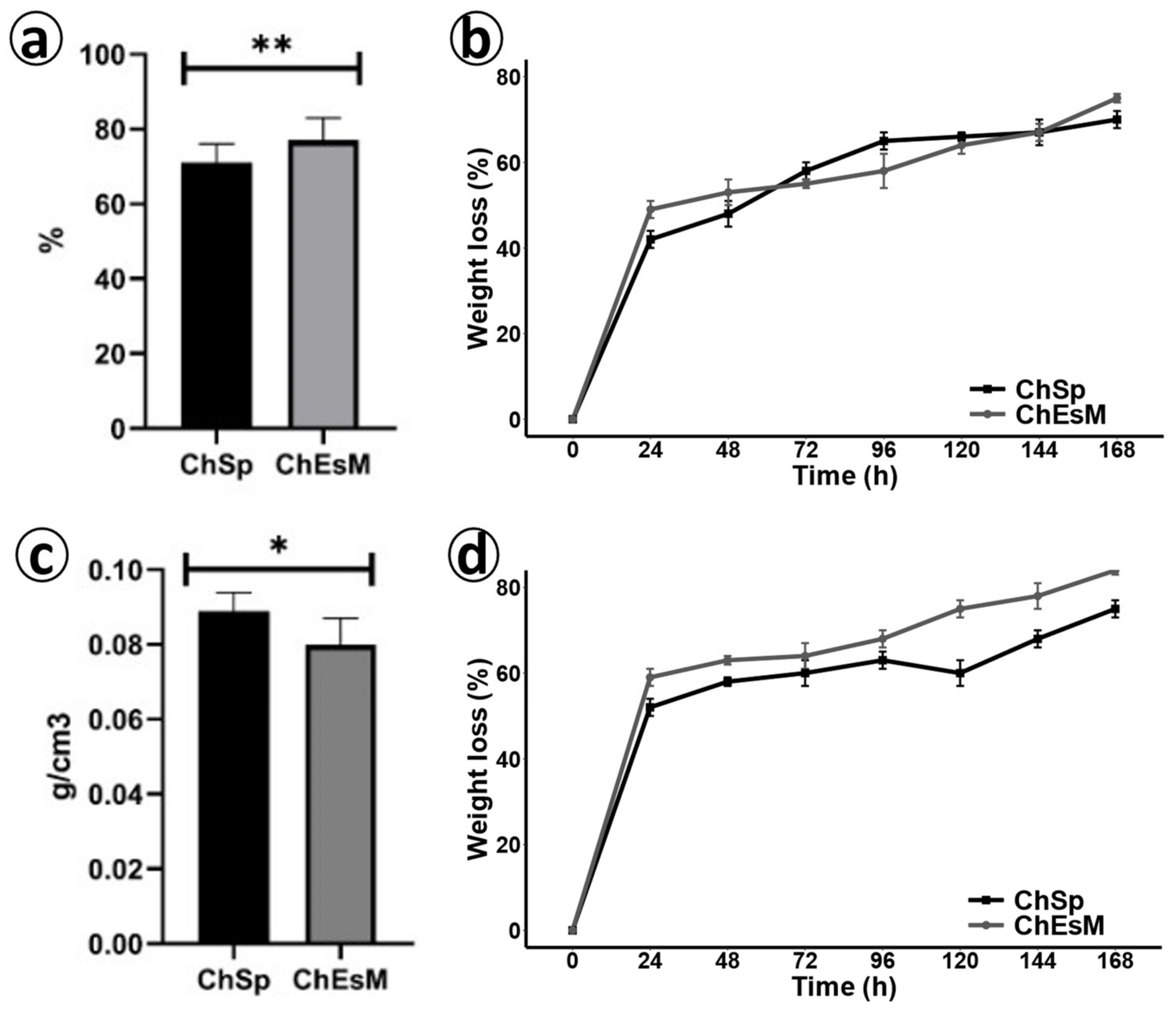
3.2. Porosity and Density
3.3. Degradation
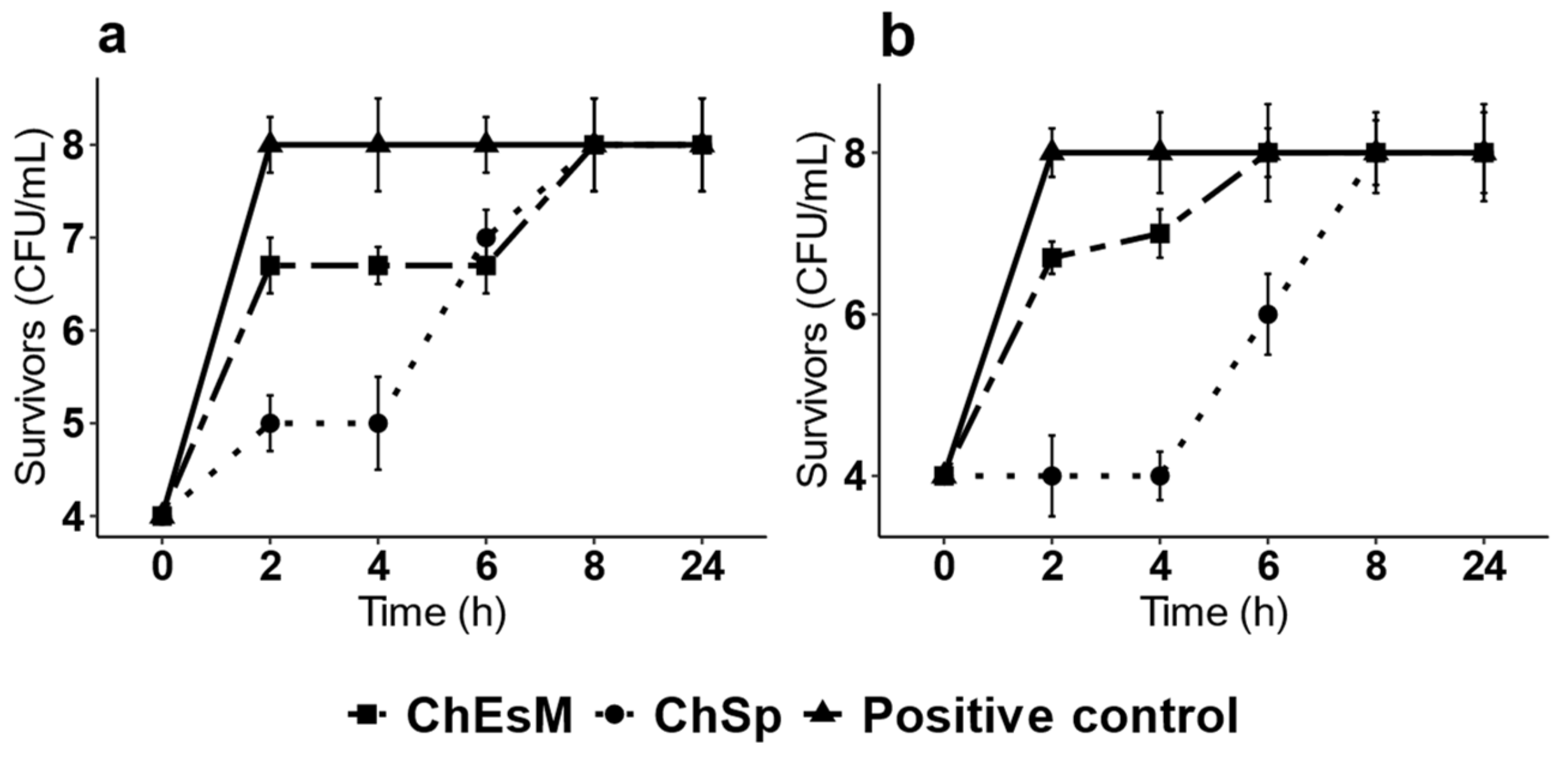
3.4. Antibacterial Properties
3.5. Cell Viability Assay
3.6. Blood Interaction Test
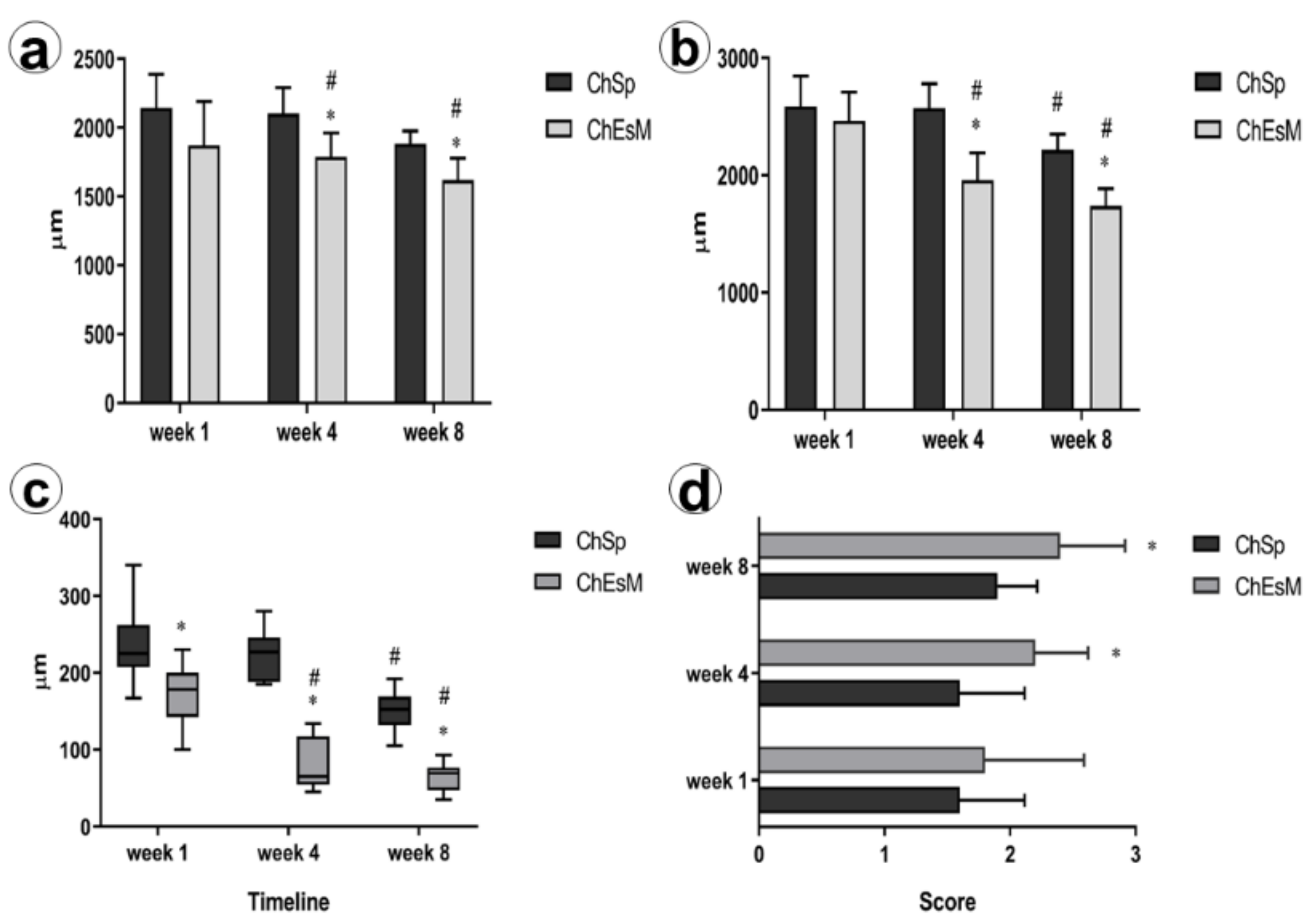
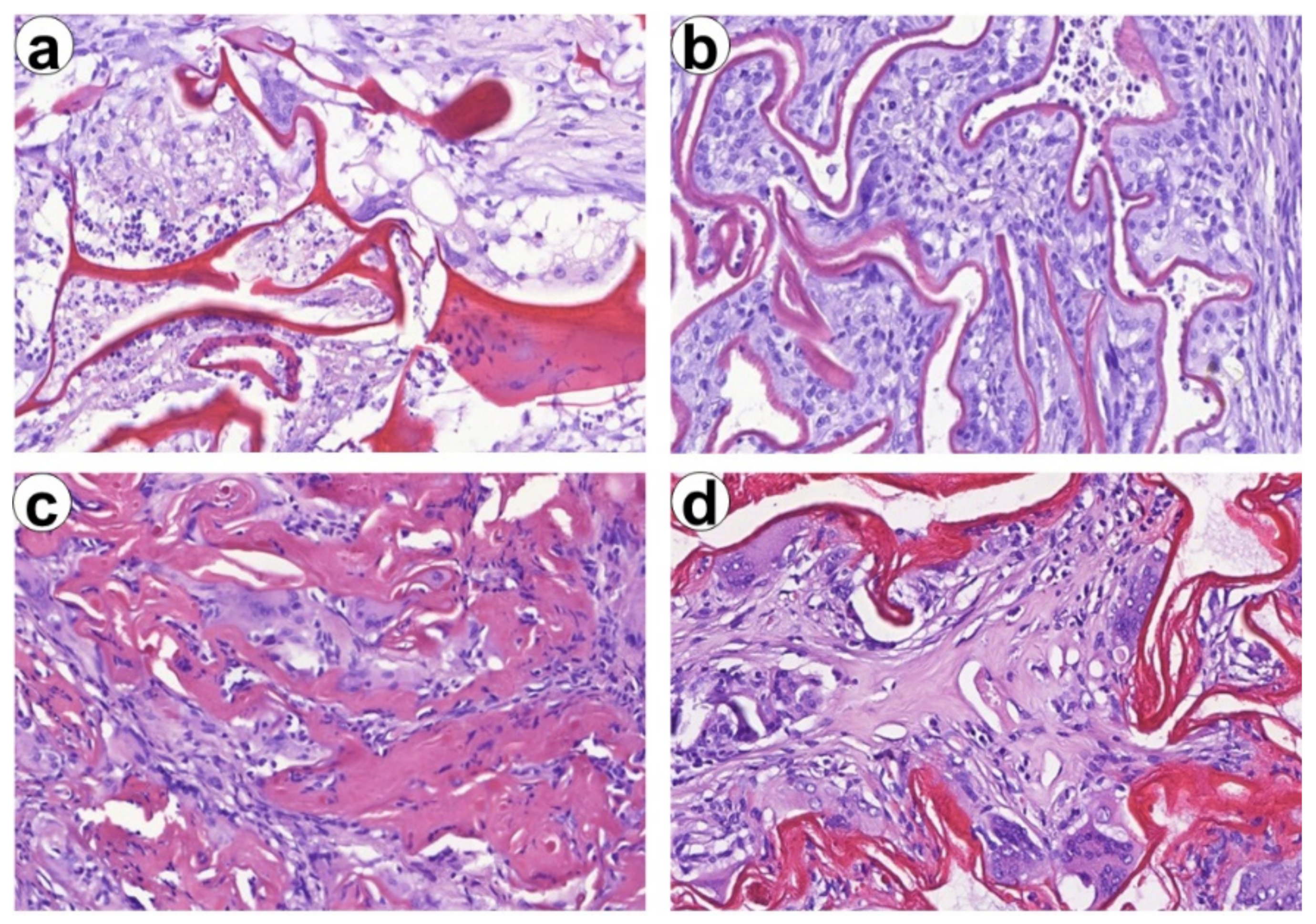
3.7. In Vivo Results
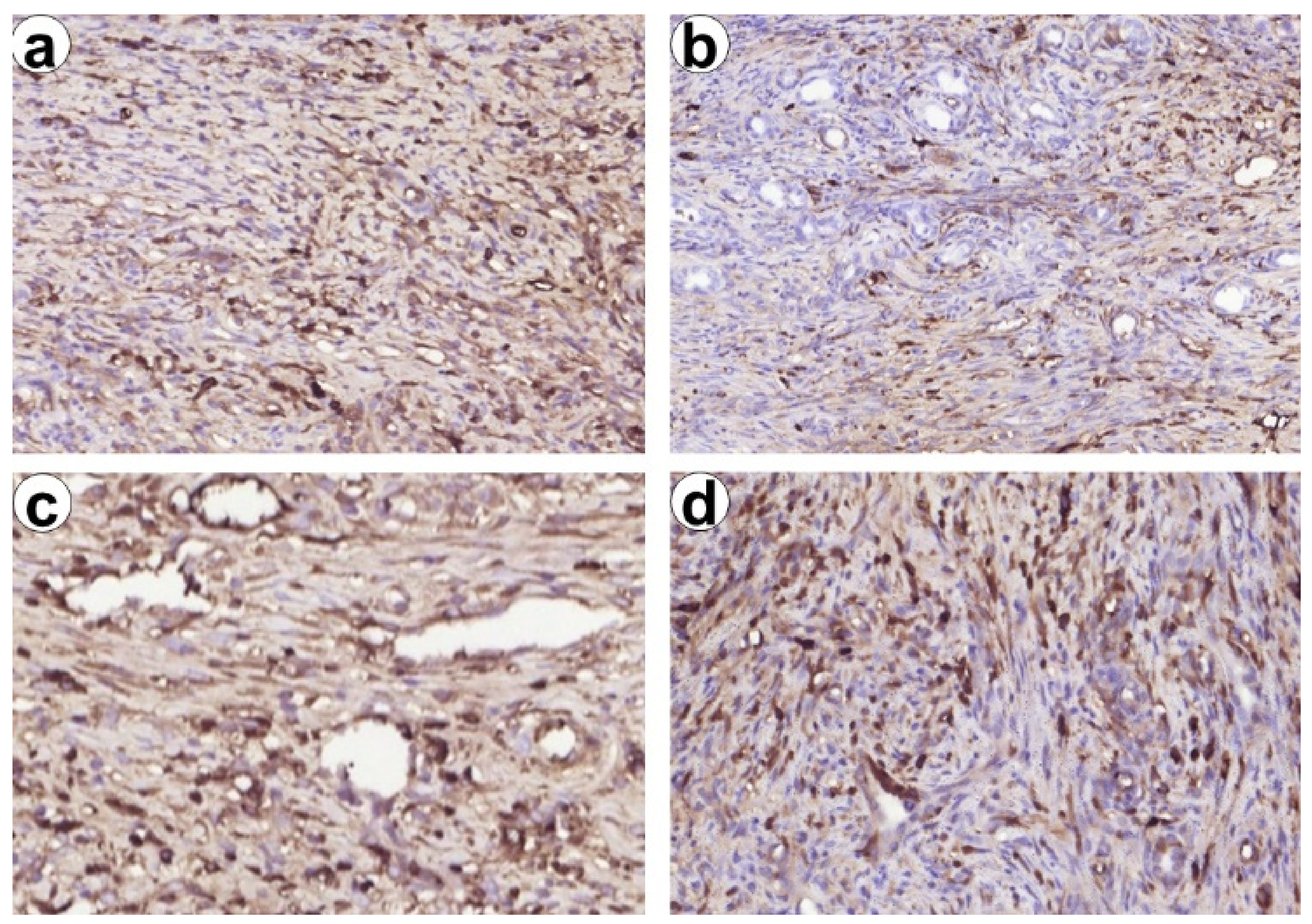
3.8. Histological and Immunohistochemical Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, N.; Toogood, G. Principles of liver resection. Surgery 2017, 35, 692–699. [Google Scholar] [CrossRef]
- Hickman, D.S.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv. Mater. 2018, 30, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, G.L.; Kaye, A.D.; Liu, H. Advances in topical hemostatic agent therapies: A comprehensive update. Adv. Ther. 2020, 37, 4132–4148. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Dai, C.; Tong, T.; Wu, J. Hemostatic nanotechnologies for external and internal hemorrhage management. Biomater. Sci. 2020, 8, 4396–4412. [Google Scholar] [CrossRef]
- Tompeck, A.J.; Gajdhar, A.U.R.; Dowling, M.; Johnson, S.B.; Barie, P.S.; Winchell, R.J.; King, D.; Scalea, T.M.; Britt, L.D.; Narayan, M. A comprehensive review of topical hemostatic agents. J. Trauma Acute Care Surg. 2020, 88, e1–e21. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Gu, S.; Tang, K. A systematic review and meta-analysis of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus traditional staged hepatectomy. Medicine 2019, 98, e15229. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef]
- Rad, A.T.; Ali, N.; Kotturi, H.S.R.; Yazdimamaghani, M.; Smay, J.; Vashaee, D.; Tayebi, L. Conducting scaffolds for liver tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4169–4181. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015, 10, 15014. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Sun, J.; Perry, S.L.; Schiffman, J.D. Electrospinning nanofibers from chitosan/hyaluronic acid complex coacervates. Biomacromolecules 2019, 20, 4191–4198. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; Mo, X. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Sergi, R.; Cannillo, V.; Boccaccini, A.R.; Liverani, L. A new generation of electrospun fibers containing bioactive glass particles for wound healing. Materials 2020, 13, 5651. [Google Scholar] [CrossRef]
- Garcia, C.E.G.; Bossard, F.; Rinaudo, M. Electrospun biomaterials from chitosan blends applied as scaffold for tissue regeneration. Polymers 2021, 13, 1037. [Google Scholar] [CrossRef]
- Zanchetta, F.C.; Trinca, R.B.; Gomes Silva, J.L.; Breder, J.D.; Cantarutti, T.A.; Consonni, S.R.; Moraes, Â.M.; Pereira de Araújo, E.; Saad, M.J.; Adams, G.G.; et al. Effects of electrospun fibrous membranes of poly-caprolactone and chitosan/poly(ethylene oxide) on mouse acute skin lesions. Polymers 2020, 12, 1518. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, J.; Hu, X.; Guo, H.; Wang, F.; Qiao, Y.; Wang, L. Collagen/polyethylene oxide nanofibrous membranes with improved hemostasis and cytocompatibility for wound dressing. Appl. Sci. 2018, 8, 1226. [Google Scholar] [CrossRef]
- Yuan, T.T.; Foushee, A.M.D.; Johnson, M.C.; Jockheck-Clark, A.R.; Stahl, J.M. Development of electrospun chitosan-polyethylene oxide/fibrinogen biocomposite for potential wound healing applications. Nanoscale Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Sasmal, P.; Datta, P. Tranexamic acid-loaded chitosan electrospun nanofibers as drug delivery system for hemorrhage control applications. J. Drug Deliv. Sci. Technol. 2019, 52, 559–567. [Google Scholar] [CrossRef]
- Zlatska, A.; Gordiienko, I.; Vasyliev, R.; Zubov, D.; Gubar, O.; Rodnichenko, A.; Syroeshkin, A.; Zlatskiy, I. In vitro study of deuterium effect on biological properties of human cultured adipose-derived stem cells. Sci. World J. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Zhang, H.; Yu, Z.; Yuan, H.; Wang, X.; Zhang, Y. Fabrication of high-performance silk fibroin fibers: Via stable jet electrospinning for potential use in anisotropic tissue regeneration. J. Mater. Chem. B 2018, 6, 3934–3945. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.R.; Reis, R.L.; Martins, A.; Neves, N.M. The use of electrospinning technique on osteochondral tissue engineering. Adv. Exp. Med. Biol. 2018, 1058, 247–263. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, L.; Liu, N.Q.; Yao, Q.; van Handel, B.; Xu, Y.; Wang, C.; Evseenko, D.; Wang, L. In vitro behavior of tendon stem/progenitor cells on bioactive electrospun nanofiber membranes for tendon-bone tissue engineering applications. Int. J. Nanomed. 2019, 14, 5831–5848. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, J.; Deng, D.; Jin, S.; Li, J.; Man, Y. Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1505–1522. [Google Scholar] [CrossRef]
- Luo, J.; Meng, Y.; Zheng, L.; Xu, K.; Li, C. Fabrication and characterization of Chinese giant salamander skin composite collagen sponge as a high-strength rapid hemostatic material. J. Biomater. Sci. Polym. Ed. 2019, 30, 247–262. [Google Scholar] [CrossRef]
- Li, D.; Li, P.; Zang, J.; Liu, J. Enhanced hemostatic performance of tranexamic acid-loaded chitosan/alginate composite microparticles. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef][Green Version]
- Takagi, T.; Tsujimoto, H.; Torii, H.; Ozamoto, Y.; Hagiwara, A. Two-layer sheet of gelatin: A new topical hemostatic agent. Asian J. Surg. 2018, 41, 124–130. [Google Scholar] [CrossRef]
- Che, C.; Liu, L.; Wang, X.; Zhang, X.; Luan, S.; Yin, J.; Li, X.; Shi, H. Surface-adaptive and on-demand antibacterial sponge for synergistic rapid hemostasis and wound disinfection. ACS Biomater. Sci. Eng. 2020, 6, 1776–1786. [Google Scholar] [CrossRef]
- Atay, H.Y. Antibacterial activity of chitosan-based systems. In Functional Chitosan; Jana, S., Ed.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Tzur, I.; Barchel, D.; Izhakian, S.; Swarka, M.; Garach-Jehoshua, O.; Krutkina, E.; Plotnikov, G.; Gorelik, O. Platelet distribution width: A novel prognostic marker in an internal medicine ward. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 464–470. [Google Scholar] [CrossRef]
- Mori, T.; Murakami, M.; Okumura, M.; Kadosawa, T.; Uede, T.; Fujinaga, T. Mechanism of macrophage activation by chitin derivatives. J. Vet. Med. Sci. 2005, 67, 51–56. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Fonseca, A.C.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Águas, A.P.; Barbosa, J.N. Macrophage polarization following chitosan implantation. Biomaterials 2013, 34, 9952–9959. [Google Scholar] [CrossRef]
- Moore, L.B.; Kyriakides, T.R. Molecular characterization of macrophage-biomaterial interactions. Adv. Exp. Med. Biol. 2015, 865, 109–122. [Google Scholar] [CrossRef]
- Farace, C.; Sánchez-Moreno, P.; Orecchioni, M.; Manetti, R.; Sgarrella, F.; Asara, Y.; Peula-García, J.M.; Marchal, J.A.; Madeddu, R.; Delogu, L.G. Immune cell impact of three differently coated lipid nanocapsules: Pluronic, chitosan and polyethylene glycol. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Lei, H.; Schmidt-Bleek, K.; Dienelt, A.; Reinke, P.; Volk, H.D. Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front. Pharmacol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Korn, T.; Muschaweckh, A. Stability and maintenance of Foxp3+ treg cells in non-lymphoid microenvironments. Front. Immunol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Khan, S.; Lele, S.S.; Prabhakar, B.S. Restoring self-tolerance in autoimmune diseases by enhancing regulatory T-cells. Cell. Immunol. 2019, 339, 41–49. [Google Scholar] [CrossRef]
- Bal, S.M.; Stadhouders, R. Tregs in fibrosis: To know your enemy, you must become your enemy. Sci. Immunol. 2019, 4, 6–9. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deineka, V.; Sulaieva, O.; Pernakov, M.; Korniienko, V.; Husak, Y.; Yanovska, A.; Yusupova, A.; Tkachenko, Y.; Kalinkevich, O.; Zlatska, A.; et al. Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials. Biomedicines 2021, 9, 588. https://doi.org/10.3390/biomedicines9060588
Deineka V, Sulaieva O, Pernakov M, Korniienko V, Husak Y, Yanovska A, Yusupova A, Tkachenko Y, Kalinkevich O, Zlatska A, et al. Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials. Biomedicines. 2021; 9(6):588. https://doi.org/10.3390/biomedicines9060588
Chicago/Turabian StyleDeineka, Volodymyr, Oksana Sulaieva, Mykola Pernakov, Viktoriia Korniienko, Yevheniia Husak, Anna Yanovska, Aziza Yusupova, Yuliia Tkachenko, Oksana Kalinkevich, Alena Zlatska, and et al. 2021. "Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials" Biomedicines 9, no. 6: 588. https://doi.org/10.3390/biomedicines9060588
APA StyleDeineka, V., Sulaieva, O., Pernakov, M., Korniienko, V., Husak, Y., Yanovska, A., Yusupova, A., Tkachenko, Y., Kalinkevich, O., Zlatska, A., & Pogorielov, M. (2021). Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials. Biomedicines, 9(6), 588. https://doi.org/10.3390/biomedicines9060588





