Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease
Abstract
1. Introduction
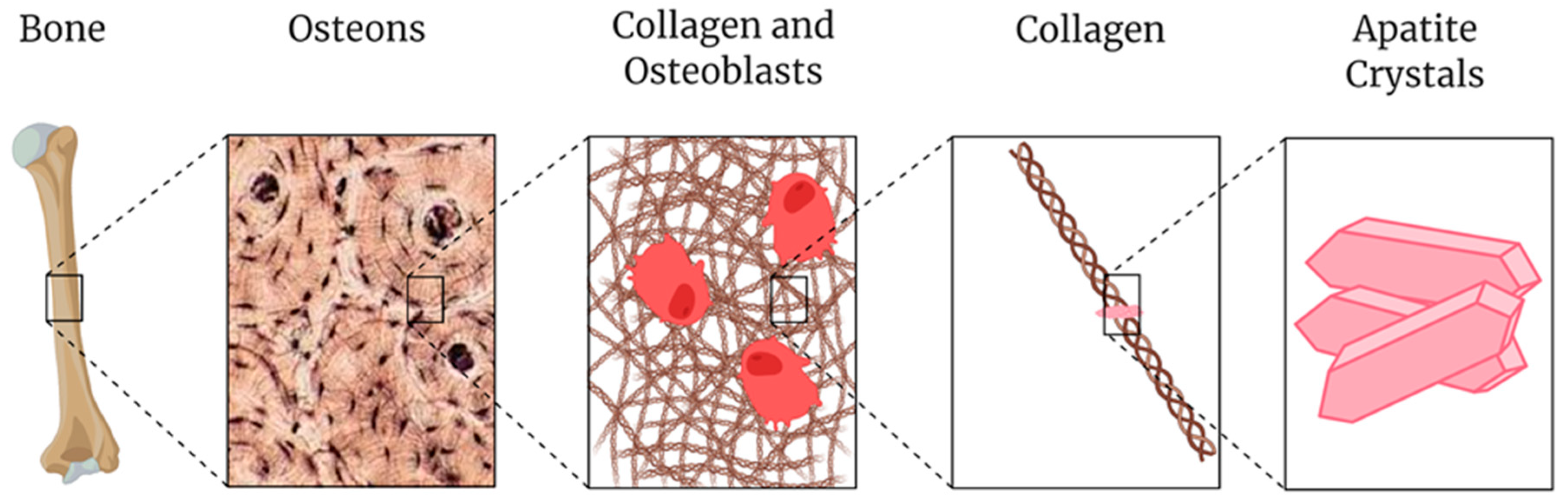
2. Process of Printing Bone Tissue
- bioceramic powders;
- hydrogels; and
- other polymers synthesized from raw materials.
3. Materials for Printing Bone Tissue
3.1. Polymers
3.2. Metals, Glasses, Plastic, and Ceramics
3.3. Apatite Derivatives
3.4. Cell-Material Interactions in Bone Tissue Bioprinting
4. Bone Diseases
4.1. Osteoporosis
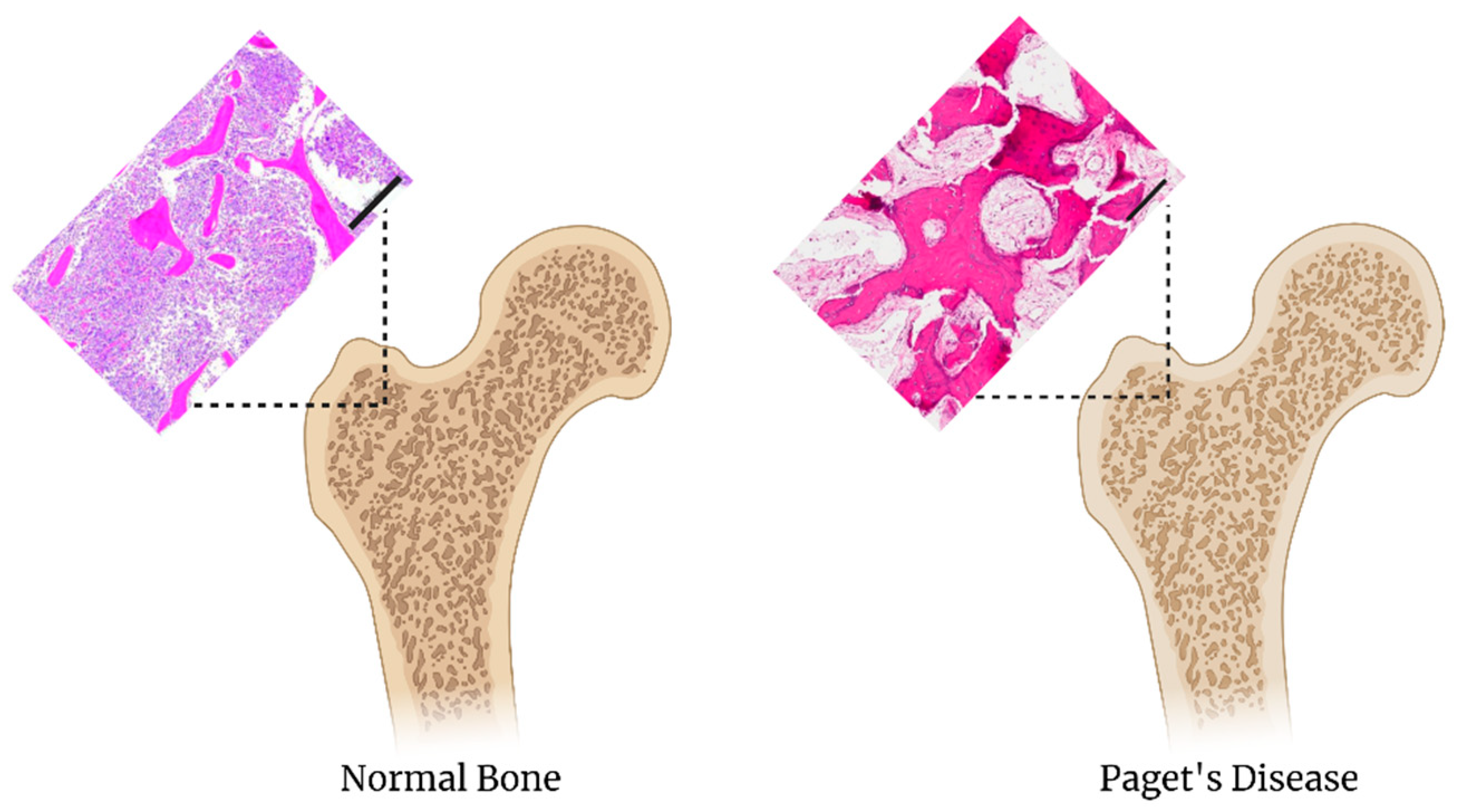
4.2. Paget’s Disease
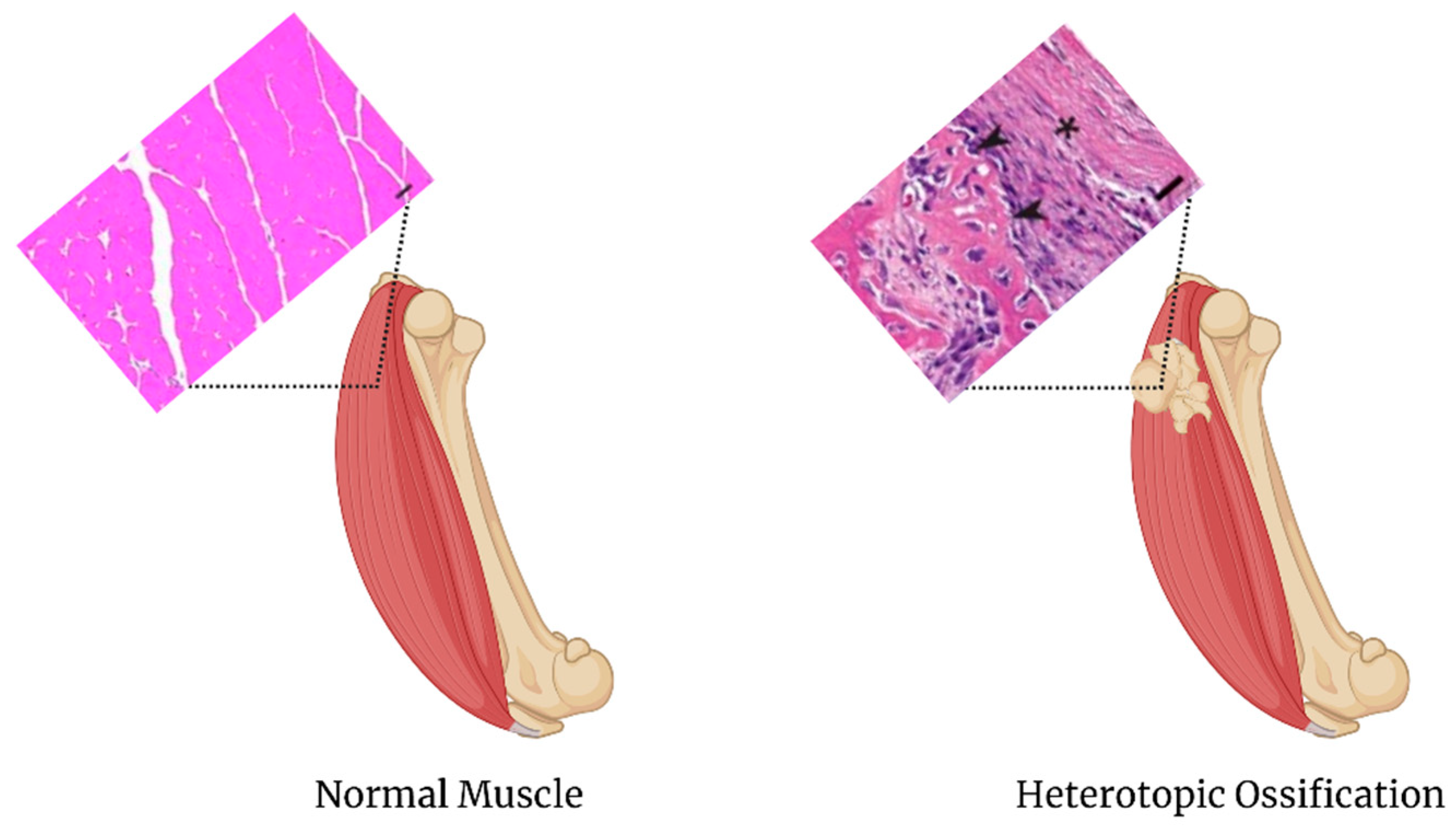
4.3. Heterotopic Ossification
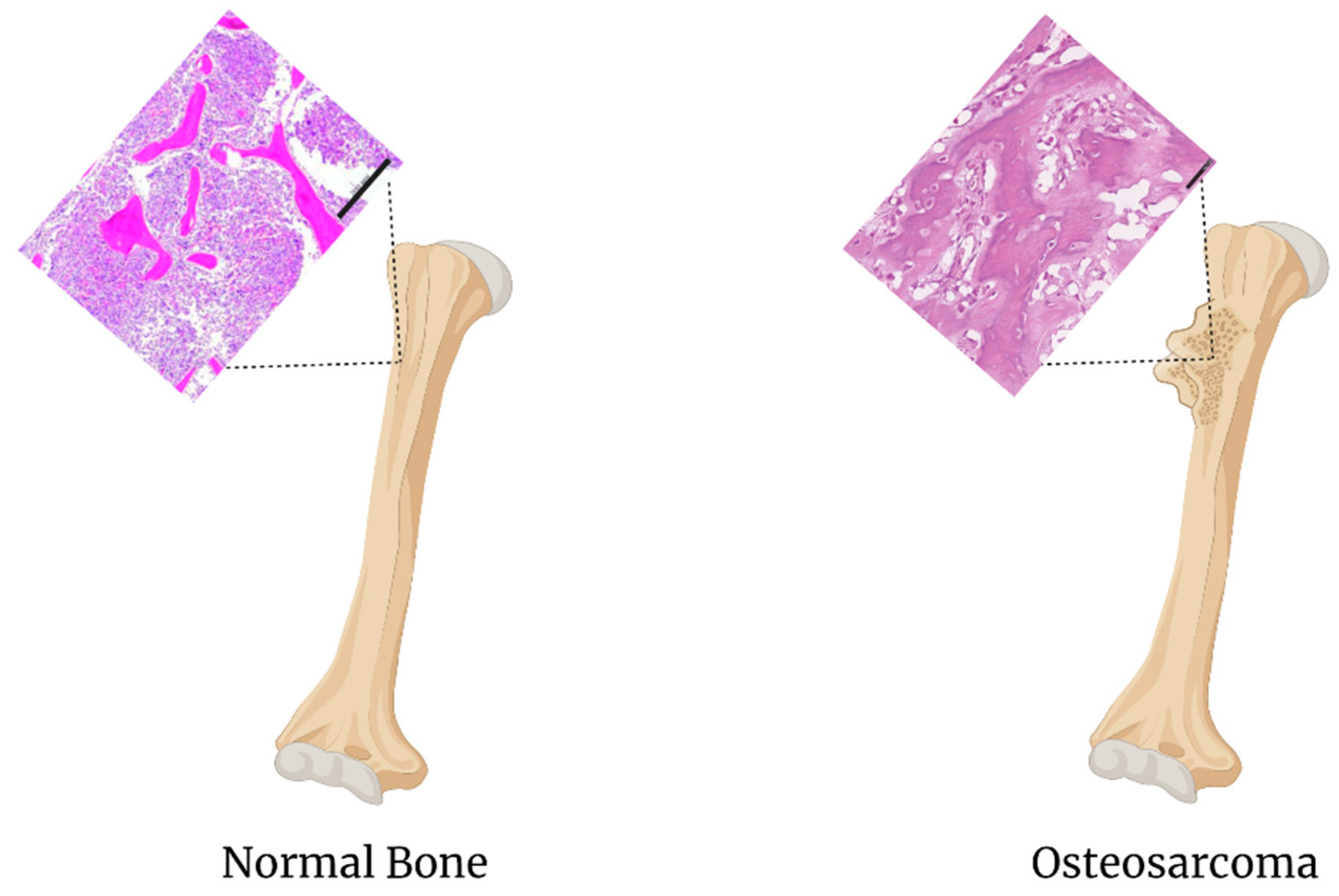
4.4. Osteosarcoma
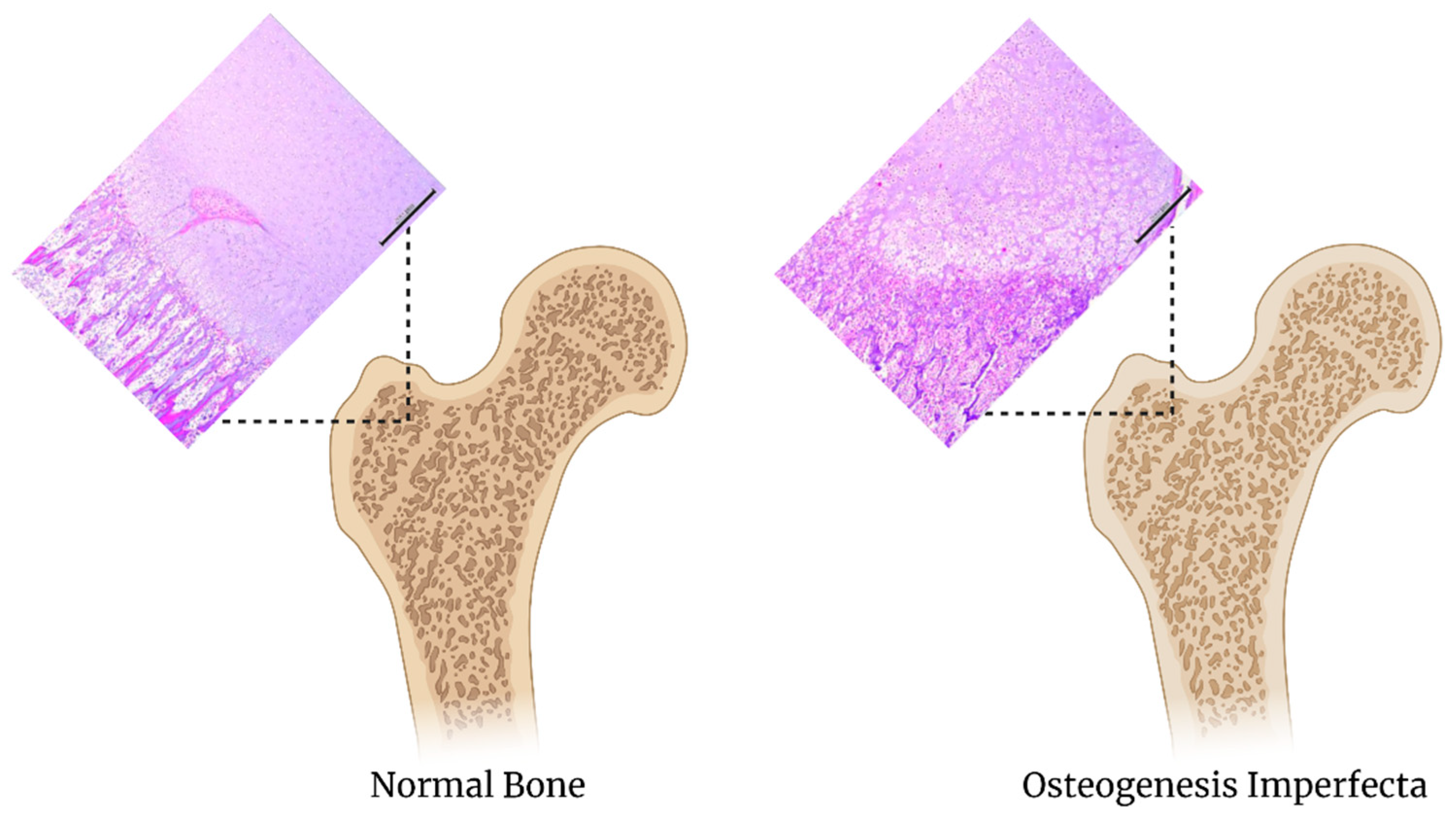
4.5. Osteogenesis Imperfecta
4.6. Rickets Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-TCP | α-tricalcium phosphate |
| ABS | acrylonitrile butadiene styrene |
| AMP | adenosine monophosphate |
| APEX1 | apurinic/apyrimidinic endodeoxyribonuclease 1 |
| AURKA | aurora kinase A |
| β –TCP | β-tricalcium phosphate |
| BG | bioglass |
| BP | black phosphorous |
| BMP | bone morphogenic protein |
| C-X-C | C-X-C chemokine receptor type 4 |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| CX3CL | C-X3-C motif Chemokine ligand 1 |
| CXCL5 | C-X-C motif chemokine ligand 5 |
| CX3CR1 | C-X3-C motif Chemokine Receptor 1 |
| CXCL14 | C-X-C motif chemokine ligand 14 |
| CCL5 | C-C motif chemokine 5 precursor |
| CRTAP | cartilage associated protein |
| Cyr61 | cysteine-rich angiogenic inducer 61 |
| c-Myc | cellular myeloctyomatosis |
| CD40L | cluster of differentiation 40 ligand |
| CD152 | cluster of differentiation 152 |
| COL1A1 | collagen type 1 alpha 1 chain |
| COL1A2 | collagen type 1 alpha 2 chain |
| CAD | computational aided design |
| DCPA | dicalcium phosphate anhydrous |
| DCPD | dicalcium phosphate dehydrate |
| ECM | extracellular matrix |
| FA | Fluorapatite |
| FOP | fibrodysplasia ossificans progressiva |
| CSF3/G-CSF | granulocyte colony stimulating factor 3 |
| CSF2/GM-CSF | granulocyte macrophage colony stimulating factor 2 |
| HNRNPA1 | heterogeneous nuclear ribonucleoprotein A1 |
| HNRNPA2B1 | heterogeneous nuclear ribonucleoproteins A2/B1 |
| HIF-1α | hypoxia inducible factor 1α |
| HA | hydroxyapatite |
| IL-1B | interleukin 1 beta |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IKB | international klein blue |
| IRX1 | Iroquois homeobox 1 |
| KLF6 | krupple like factor 6 |
| LEPRE1 | leucine proline-enriched proteoglycan 1 |
| LOX | lysyl oxidase |
| mTOR | mechanistic target of rapamycin |
| MATR3 | matrin 3 |
| MMPs | matrix metalloproteinases |
| MK | midkine |
| MCPA | monocalcium phosphate anhydrous |
| MCPM | monocalcium phosphate monohydrate |
| MDM2 | mouse double minute 2 homolog |
| NTx | naltrexone |
| nHAp | nano-hydroxyapatite |
| NIR | near infrared light |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| NFkB | nuclear factor kappa-light-chain-enhancer |
| OCP | octocalcium phosphate |
| PPIB | peptidylprolyl isomerase B |
| PI3K | phosphoinositide 3-kinase |
| PAR | photoreactive acrylic resin |
| PBMCs | peripheral blood mononuclear cells |
| PCL | polycaprolactone |
| PDA | phenylenediamine |
| pDA | polydopamine |
| PLA | polylactic acid |
| PLGA | poly-lactide-co-glycolic acid |
| PVA | polyvinyl alcohol |
| PCL/HA | porous polycaprolactone/hydroxyapatite |
| PLGA | polylactic-co-glycolic acid |
| pTi | porous titanium |
| PD1 | programmed cell death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| P3H1 | prolyl 3-hydroxylase 1 |
| PEMF | pulse electromagnetic felds |
| RANKL | receptor activator of nuclear factor kappa-B ligand |
| RB1 | retinoblastoma 1 |
| ROCK1 | rho-associated coiled-coil-containing protein kinase 1 |
| Runx-2 | runt-related transcription factor |
| SQSTM1 | sequestosome 1 |
| SOX9 | SRY-Box Transcription Factor 9 |
| STAT3 | signal transducer and activator of transcription 3 |
| AgNPs | silver nanoparticles |
| TRACP-6b | tartrate-resistant acid phosphatase isoform 6b |
| TTCP | tetracalcium phosphate |
| TIA1 | T-cell restricted intracellular antigen 1 |
| RelA | transcription factor p65 A subunit |
| RelB | transcription factor p65 B subunit |
| TGF-B | transforming growth factor beta |
| TNF-α | tumor necrosis factor alpha |
| OX-40L | tumor necrosis factor receptor superfamily member 4 ligand |
| tP53 | tumor protein 53 |
| VCP | valosin-containing protein |
| VEGF | vascular endothelial growth factor |
| WWOX | WW domain containing oxidoreductase |
| YB-1 | y box binding protein 1 |
| ZNF 687 | zinc finger protein 687 |
References
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [CrossRef]
- Singer, F.R. Paget disease: When to treat and when not to treat. Nat. Rev. Rheumatol. 2009, 5, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Handforth, C.; D’Oronzo, S.; Coleman, R.; Brown, J. Cancer Treatment and Bone Health. Calcif. Tissue Int. 2018, 102, 251–264. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone Tissue Engineering: State of the Art and Future Trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Annabi, N.; Nikkhah, M.; Bae, H.; Binan, L.; Park, S.; Kang, Y.; Yang, Y.; Khademhosseini, A. Vascularized Bone Tissue Engineering: Approaches for Potential Improvement. Tissue Eng. Part B: Rev. 2012, 18, 363–382. [Google Scholar] [CrossRef]
- Byambaa, B.; Annabi, N.; Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Jia, W.; Kazemzadeh-Narbat, M.; Shin, S.R.; Tamayol, A.; Khademhosseini, A. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv. Healthc. Mater. 2017, 6, 1700015. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; De La Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, 2000095. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 2013, 5, 015014. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Iannace, S.; Sorrentino, L.; Di Maio, E. Biodegradable biomedical foam scaffolds. In Biomedical Foams for Tissue Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 163–187. [Google Scholar]
- Galeja, M.; Wypiór, K.; Wachowicz, J.; Kędzierski, P.; Hejna, A.; Marć, M.; Klewicz, K.; Gabor, J.; Okła, H.; Swinarew, A.S. POM/EVA Blends with Future Utility in Fused Deposition Modeling. Materials 2020, 13, 2912. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Q.; Sun, Y.; Wang, L.; Lu, J.; Nie, Q.; Ma, Y.; Jing, W. Fabrication of Ag Nanosheet@TiO2 Antibacterial Membranes for Inulin Purification. Ind. Eng. Chem. Res. 2020, 59, 7797–7804. [Google Scholar] [CrossRef]
- De Melo, L.P.; Salmoria, G.V.; Fancello, E.A.; Roesler, C.R.D.M. Effect of Injection Molding Melt Temperatures on PLGA Craniofacial Plate Properties during In Vitro Degradation. Int. J. Biomater. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Zou, F.; Jiang, J.; Lv, F.; Xia, X.; Ma, X. Preparation of antibacterial and osteoconductive 3D-printed PLGA/Cu(I)@ZIF-8 nanocomposite scaffolds for infected bone repair. J. Nanobiotech. 2020, 18, 1–14. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, D.; Feng, X.; Ma, L.; Deng, X.; Han, C.; Wei, Q.; Yang, C. 3D-printed porous titanium scaffolds incorporating niobium for high bone regeneration capacity. Mater. Des. 2020, 194, 108890. [Google Scholar] [CrossRef]
- Mitsouras, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B.; et al. Medical 3D Printing for the Radiologist. Radiographics 2015, 35, 1965–1988. [Google Scholar] [CrossRef]
- Fattahi, P.; Dover, J.T.; Brown, J.L. 3D Near-Field Electrospinning of Biomaterial Microfibers with Potential for Blended Microfiber-Cell-Loaded Gel Composite Structures. Adv. Health Mater. 2017, 6, 1700456. [Google Scholar] [CrossRef]
- Burg, K. Chapter 6—Poly(α-ester)s. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 115–121. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Ma, P.X. The engineering of patient-specific, anatomically shaped, digits. Biomaterials 2009, 30, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.S.R.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Grémillard, L. 3-D printing of chitosan-calcium phosphate inks: Rheology, interactions and characterization. J. Mater. Sci. Mater. Med. 2018, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M. Calcium phosphate/chitosan composite scaffolds for controlledin vitro antibiotic drug release. J. Biomed. Mater. Res. 2002, 62, 378–386. [Google Scholar] [CrossRef]
- Guzmán, R.; Nardecchia, S.; Gutierrez, M.C.; Ferrer, M.L.; Ramos, V.; Del Monte, F.; Abarrategi, A.; López-Lacomba, J.L. Chitosan Scaffolds Containing Calcium Phosphate Salts and rhBMP-2: In Vitro and In Vivo Testing for Bone Tissue Regeneration. PLoS ONE 2014, 9, e87149. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13, 1–13. [Google Scholar] [CrossRef]
- Malikova, T.V.; Golovanova, O.A.; Chikanova, E.S. Physicochemical Properties of Calcium Phosphate–Chitosan Composites and Scaffolds. Inorg. Mater. 2018, 54, 957–964. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Granular Honeycombs Composed of Carbonate Apatite, Hydroxyapatite, and β-Tricalcium Phosphate as Bone Graft Substitutes: Effects of Composition on Bone Formation and Maturation. ACS Appl. Bio Mater. 2020, 3, 1787–1795. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; A Park, S. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, Y.; Lee, H.; Kim, G. A new hybrid scaffold constructed of solid freeform-fabricated PCL struts and collagen struts for bone tissue regeneration: Fabrication, mechanical properties, and cellular activity. J. Mater. Chem. 2012, 22, 15901. [Google Scholar] [CrossRef]
- Ravi, P.; Shiakolas, P.S.; Welch, T.R. Poly-l-lactic acid: Pellets to fiber to fused filament fabricated scaffolds, and scaffold weight loss study. Addit. Manuf. 2017, 16, 167–176. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; Tan, K.H.; Wiria, F.E.; Cheah, C.M. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J. Mater. Sci. Mater. Electron. 2004, 15, 1113–1121. [Google Scholar] [CrossRef]
- Clinical Applications of Digital Dental Technology; Wiley-Blackwell: Ames, IA, USA, 2015.
- Ghai, S.; Sharma, Y.; Jain, N.; Satpathy, M.; Pillai, A.K. Use of 3-D printing technologies in craniomaxillofacial surgery: A review. Oral Maxillofac. Surg. 2018, 22, 249–259. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Szymor, P. Comparison of pre-bent titanium mesh versus polyethylene implants in patient specific orbital reconstructions. Head Face Med. 2013, 9, 32. [Google Scholar] [CrossRef]
- Cohen, A.; Laviv, A.; Berman, P.; Nashef, R.; Abu-Tair, J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 661–666. [Google Scholar] [CrossRef]
- Shi, X.; Nommeots-Nomm, A.; Todd, N.M.; Devlin-Mullin, A.; Geng, H.; Lee, P.D.; Mitchell, C.A.; Jones, J.R. Bioactive glass scaffold architectures regulate patterning of bone regeneration in vivo. Appl. Mater. Today 2020, 20, 100770. [Google Scholar] [CrossRef]
- Wong, T.M.; Jin, J.; Lau, T.W.; Fang, C.; Yan, C.H.; Yeung, K.; To, M.; Leung, F. The use of three-dimensional printing technology in orthopaedic surgery. J. Orthop. Surg. 2017, 25, 2309499016684077. [Google Scholar] [CrossRef]
- Vlad, M.D.; Aguado, E.F.; González, S.G.; Ivanov, I.C.; Şindilar, E.V.; Poeată, I.; Iencean, A. Ştefan; Butnaru, M.; Avădănei, E.R.; López, J.L. Novel titanium-apatite hybrid scaffolds with spongy bone-like micro architecture intended for spinal application: In vitro and in vivo study. Mater. Sci. Eng. C 2020, 110, 110658. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration—A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef]
- Which Component of Bone Tissue Makes Bone Hard? Available online: https://en.lifeder.com/component-bone-tissue-makes-bone-hard/ (accessed on 26 January 2021).
- Liu, H.; Du, Y.; Yang, G.; Hu, X.; Wang, L.; Liu, B.; Wang, J.; Zhang, S. Delivering Proangiogenic Factors from 3D-Printed Polycaprolactone Scaffolds for Vascularized Bone Regeneration. Adv. Health Mater. 2020, 9, e2000727. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Shi, X.; Chen, Y.; Yang, W.; Ma, Y.; Shi, X.-L.; Song, P.; Dargusch, M.S.; Chen, Z.-G. Bacteria-Triggered pH-Responsive Osteopotentiating Coating on 3D-Printed Polyetheretherketone Scaffolds for Infective Bone Defect Repair. Ind. Eng. Chem. Res. 2020, 59, 12123–12135. [Google Scholar] [CrossRef]
- Yeo, T.; Ko, Y.-G.; Kim, E.J.; Kwon, O.K.; Chung, H.Y. Promoting bone regeneration by 3D-printed poly(glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 2021, 94, 343–351. [Google Scholar] [CrossRef]
- Bhaumik, S.; Bandyopadhyay, R.; Rohit, T.A.; Banerjee, A.; Therese, H.A.; Pathak, R. Friction and Wear Performance of Nano Hydroxy Apatite (nHAp) Polyoxymethylene Composites on 316L Steel. In Machining and Machinability of Fiber Reinforced Polymer Composites; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2021; pp. 149–164. [Google Scholar]
- Staehlke, S.; Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, J.B. Enhanced calcium ion mobilization in osteoblasts on amino group containing plasma polymer nanolayer. Cell Biosci. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Zani, B.G.; Edelman, E.R. Cellular bridges. Commun. Integr. Biol. 2010, 3, 215–220. [Google Scholar] [CrossRef]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.C.; A Van Blitterswijk, C.; Barralet, J. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef]
- Stevens, N.M.; Konda, S.R. Pathophysiology and Epidemiology of Osteoporosis. In Vertebral Compression Fractures in Oste-oporotic and Pathologic Bone: A Clinical Guide to Diagnosis and Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 9–20. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Nabil, N.; Ghaleb, S. Pathophysiology of the risk factors associated with osteoporosis and their correlation to the T-score value in patients with osteopenia and osteoporosis in the United Arab Emirates. J. Pharm. Bioallied Sci. 2019, 11, 364–372. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Turczyn, P.; Dobies-Krzesniak, B.; Frasunska, J.; Tarnacka, B. Role of CX3CL1/CX3CR1 Signaling Axis Activity in Osteoporosis. Mediat. Inflamm. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Miura, M.; Ichimura, S.; Inaba, M.; Imanishi, Y.; Shiraki, M.; Takada, J.; Chaki, O.; Hagino, H.; Fukunaga, M.; et al. Executive summary of the Japan Osteoporosis Society Guide for the Use of Bone Turnover Markers in the Diagnosis and Treatment of Osteoporosis (2018 Edition). Clin. Chim. Acta 2019, 498, 101–107. [Google Scholar] [CrossRef]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-Based Treatment Strategies for Osteoporosis: A Literature Review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef]
- Barak, M.M.; Black, M.A. A novel use of 3D printing model demonstrates the effects of deteriorated trabecular bone structure on bone stiffness and strength. J. Mech. Behav. Biomed. Mater. 2018, 78, 455–464. [Google Scholar] [CrossRef]
- Boerckel, J.D.; E Mason, D.; McDermott, A.M.; Alsberg, E. Microcomputed tomography: Approaches and applications in bioengineering. Stem Cell Res. Ther. 2014, 5, 144. [Google Scholar] [CrossRef]
- Ye, M.; Liu, W.; Yan, L.; Cheng, S.; Li, X.; Qiao, S. 3D-printed Ti6Al4V scaffolds combined with pulse electromagnetic fields enhance osseointegration in osteoporosis. Mol. Med. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef]
- Qiao, S.; Sheng, Q.; Li, Z.; Wu, D.; Zhu, Y.; Lai, H.; Gu, Y. 3D-printed Ti6Al4V scaffolds coated with freeze-dried platelet-rich plasma as bioactive interface for enhancing osseointegration in osteoporosis. Mater. Des. 2020, 194, 108825. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, N.; Liu, P.; Sun, Y.; Wang, Y.; Fei, F.; Zhang, S.; Zheng, J.; Han, B. Poly(Dopamine) Coating on 3D-Printed Poly-Lactic-Co-Glycolic Acid/β-Tricalcium Phosphate Scaffolds for Bone Tissue Engineering. Molecules 2019, 24, 4397. [Google Scholar] [CrossRef]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar]
- Gennari, L.; Rendina, D.; Falchetti, A.; Merlotti, D. Paget’s Disease of Bone. Calcif. Tissue Int. 2019, 104, 483–500. [Google Scholar] [CrossRef]
- Ralston, S.H.; Taylor, J.P. Rare Inherited forms of Paget’s Disease and Related Syndromes. Calcif. Tissue Int. 2019, 104, 501–516. [Google Scholar] [CrossRef]
- Moul, A.; Alladin, A.; Navarrete, C.; Abdenour, G.; Rodriguez, M.M. Osteogenesis Imperfecta Due to Compound Heterozygosity for theLEPRE1Gene. Fetal Pediatr. Pathol. 2013, 32, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Labajian, V.; Landry, E.; Alfawwaz, F.; Lai, C.; Kilty, S.J. An Uncommon Osseous Frontal Sinus Tumor: Monostotic Paget’s Disease. Case Rep. Otolaryngol. 2013, 2013, 1–4. [Google Scholar] [CrossRef]
- Zheng, C.; Attarilar, S.; Li, K.; Wang, C.; Liu, J.; Wang, L.; Yang, J.; Tang, Y. 3D-printed HA15-loaded β-Tricalcium Phosphate/Poly (Lactic-co-glycolic acid) Bone Tissue Scaffold Promotes Bone Regeneration in Rabbit Radial Defects. Int. J. Bioprinting 2020, 7, 317. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Athirasala, A.; Twohig, C.; Boda, S.K.; Bertassoni, L.E. Biomaterials for Craniofacial Bone Regeneration. Dent. Clin. N. Am. 2017, 61, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Wheatley, B.M.; Cholok, D.; Agarwal, S.; Yu, P.B.; Levi, B.; Davis, T.A. The traumatic bone: Trauma-induced heterotopic ossification. Transl. Res. 2017, 186, 95–111. [Google Scholar] [CrossRef]
- Cholok, D.; Chung, M.T.; Ranganathan, K.; Ucer, S.; Day, D.; Davis, T.A.; Mishina, Y.; Levi, B. Heterotopic ossification and the elucidation of pathologic differentiation. Bone 2018, 109, 12–21. [Google Scholar] [CrossRef]
- Wu, J.; Ren, B.; Shi, F.; Hua, P.; Lin, H. BMP and mTOR signaling in heterotopic ossification: Does their crosstalk provide therapeutic opportunities? J. Cell. Biochem. 2019, 120, 12108–12122. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, Y.J.; Cho, H.; Park, D.; Jung, G.; Ku, S.K.; Choi, J. Extracellular polysaccharides purified from Aureobasidium pullulans SM-2001 (Polycan) inhibit dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2017, 41, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.; Lisiecki, J.; Miller, S.; Levin, A.; Fayad, L.; Ding, C.; Sono, T.; McCarthy, E.; Levi, B.; James, A.W. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019, 3, e10172. [Google Scholar] [CrossRef]
- Yu, D.; Xiao, H.; Xue, F.; Pan, M.; Ju, J.; Tang, G. [Expression And Significance Of Hypoxia Inducible Factor 1α In Rat Model Of Heterotopic Ossification After Achilles Tenotomy]. Chin. J. Reparative Reconstr. Surg. 2016, 30, 1098–1103. [Google Scholar] [CrossRef]
- Kirkham, M.; Kalivas, A.; Fatema, K.; Luelling, S.; Dubansky, B.H.; Dubansky, B.; Jones, K.B.; Barrott, J.J. Underlying Ossification Phenotype in a Murine Model of Metastatic Synovial Sarcoma. Int. J. Mol. Sci. 2020, 21, 2636. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, M.; Huber, A.K.; Hwang, C.; Iv, W.F.C.; Menon, R.; Li, J.; Vasquez, K.; Pagani, C.; Patel, N.; Li, S.; et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 2018, 162, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Sekar, D.; Mani, P.; Biruntha, M.; Sivagurunathan, P.; Karthigeyan, M. Dissecting the functional role of microRNA 21 in osteosarcoma. Cancer Gene Ther. 2019, 26, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hao, M.; Du, X.; Chen, K.; Wang, G.; Yang, J. Advances in targeted therapy for osteosarcoma. Discov. Med. 2014, 17, 301–307. [Google Scholar] [PubMed]
- Osteosarcoma of the Jaw: Classification, Diagnosis and Treatment. Available online: https://www.intechopen.com/books/osteosarcoma-biology-behavior-and-mechanisms/osteosarcoma-of-the-jaw-classification-diagnosis-and-treatment (accessed on 26 January 2021).
- Tang, N.; Song, W.-X.; Luo, J.; Haydon, R.C.; He, T.-C. Osteosarcoma Development and Stem Cell Differentiation. Clin. Orthop. Relat. Res. 2008, 466, 2114–2130. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Di Pompo, G.; Chano, T.; Errani, C.; Ibrahim-Hashim, A.; Gillies, R.J.; Donati, D.M.; Baldini, N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int. J. Cancer 2017, 140, 1331–1345. [Google Scholar] [CrossRef]
- Lu, J.; Song, G.; Tang, Q.; Zou, C.; Jinchang, L.; Zhao, Z.; Yong, B.; Yin, J.; Xu, H.; Xie, X.; et al. IRX1 hypomethylation promotes osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J. Clin. Investig. 2015, 125, 1839–1856. [Google Scholar] [CrossRef]
- Chaiyawat, P.; Sungngam, P.; Teeyakasem, P.; Sirikaew, N.; Klangjorhor, J.; Settakorn, J.; Diskul-Na-Ayudthaya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Svasti, J.; et al. Protein profiling of osteosarcoma tissue and soft callus unveils activation of the unfolded protein response pathway. Int. J. Oncol. 2019, 54, 1704–1718. [Google Scholar] [CrossRef]
- Gang, W.; Tanjun, W.; Yong, H.; Jiajun, Q.; Yi, Z.; Hao, H. Inhibition of miR-9 decreases osteosarcoma cell proliferation. Bosn. J. Basic Med Sci. 2019, 20, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Kendal, J. Studying the Effects of Bone Morphogenetic Protein-2 on Osteosarcoma Tumour Biology. Unpublished Master’s Thesis, University of Calgary, Calgary, AB, USA, 2019. Available online: http://hdl.handle.net/1880/109433 (accessed on 26 January 2021).
- Faisham, W.I.; Saad, A.Z.M.; Alsaigh, L.N.; Azman, M.Z.N.; Imran, M.K.; Biswal, B.M.; Bhavaraju, V.M.; Salzihan, S.; Hasnan, J.; Ezane, A.M.; et al. Prognostic factors and survival rate of osteosarcoma: A single-institution study. Asia-Pac. J. Clin. Oncol. 2015, 13, 104. [Google Scholar] [CrossRef]
- Letai, A. Functional precision cancer medicine—moving beyond pure genomics. Nat. Med. 2017, 23, 1028–1035. [Google Scholar] [CrossRef]
- Narasimhan, V.; Wright, J.A.; Churchill, M.; Wang, T.; Rosati, R.; Lannagan, T.R.; Vrbanac, L.; Richardson, A.B.; Kobayashi, H.; Price, T.; et al. Medium-throughput Drug Screening of Patient-derived Organoids from Colorectal Peritoneal Metastases to Direct Personalized Therapy. Clin. Cancer Res. 2020, 26, 3662–3670. [Google Scholar] [CrossRef]
- Mitsis, D.; Francescutti, V.; Skitzki, J. Current Immunotherapies for Sarcoma: Clinical Trials and Rationale. Sarcoma 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Pan, S.; Yin, J.; Yu, L.; Zhang, C.; Zhu, Y.; Gao, Y.; Chen, Y. 2D MXene-Integrated 3D-Printing Scaffolds for Augmented Osteosarcoma Phototherapy and Accelerated Tissue Reconstruction. Adv. Sci. 2019, 7, 1901511. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Martin, E.; Shapiro, J.R. Osteogenesis imperfecta: Epidemiology and pathophysiology. Curr. Osteoporos. Rep. 2007, 5, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Kuhn, H.; Netzer, C.; Semler, O. Osteogenesis imperfecta: Pathophysiology and treatment. Wien. Med. Wochenschr. 2015, 165, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.S.; Glorieux, F.H. Osteogenesis Imperfecta: Update on presentation and management. Rev. Endocr. Metab. Disord. 2008, 9, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hoyer-Kuhn, H.; Netzer, C.; Koerber, F.; Schoenau, E.; Semler, O. Two years’ experience with denosumab for children with Osteogenesis imperfecta type VI. Orphanet J. Rare Dis. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Akiva, A.; Melke, J.; Ansari, S.; Liv, N.; van der Meijden, R.; van Erp, M.; Zhao, F.; Stout, M.; Nijhuis, W.H.; de Heus, C.; et al. An Organoid for Woven Bone. Adv. Funct. Mater. 2021, 31, 2010524. [Google Scholar] [CrossRef]
- Shore, R.M.; Chesney, R.W. Rickets: Part I. Pediatr. Radiol. 2012, 43, 140–151. [Google Scholar] [CrossRef]
- Shore, R.M.; Chesney, R.W. Rickets: Part II. Pediatr. Radiol. 2013, 43, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Miyamoto, K.-I. Hereditary hypophosphatemic rickets with hypercalciuria: Pathophysiology, clinical presentation, diagnosis and therapy. Pflügers Archiv—Eur. J. Physiol. 2019, 471, 149–163. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Shaw, N.J.; Portale, A.A.; Ward, L.M.; Abrams, S.A.; Pettifor, J.M. Rickets. Nat. Rev. Dis. Prim. 2017, 3, 17101. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, N.C.W.; Zhu, D.; Milne, E.M.; Hof, R.V.T.; Martin, A.; Quarles, D.L.; Millán, J.L.; Farquharson, C.; Macrae, V.E. Altered Bone Development and an Increase in FGF-23 Expression in Enpp1−/− Mice. PLoS ONE 2012, 7, e32177. [Google Scholar] [CrossRef]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]


| Material | Biocompatibility | Resolution | Tm | Derivatives and Blends | Ref |
|---|---|---|---|---|---|
| PCL | +++ | 50–60 °C | HA, VEGF, TCP | [14] | |
| PVA | +++ | 50 µm | 220–240 °C | [15] | |
| PLA | + | 130–180 °C | [14,16] | ||
| PDA | 130–180 °C | AgNP | [17] | ||
| PEOT/PBT | +++ | 225 °C | [14] | ||
| POM | ++ | 10 µm | 232 °C | nHAp, EVA | [18] |
| PEEK | +/+++ | 350 °C | HA, AgNPs, pDA | [14,19] | |
| PLGA | +++ | ~300 µm | 240–280 °C | Cu(I) | [20,21] |
| Titanium | ++ | 40 µm | 1670 °C | [22] | |
| PMMA | + | 50 µm | 160 °C | [23,24] | |
| ABS plastic | - | 190 °C | [14,23] | ||
| PLLA | 50 µm | 170–180 °C | [25,26] | ||
| CCP | ++ | 30 µm | 1670 °C | TCP, BMP-2, nHAP, HA, DCPD | [27,28,29,30,31] |
| Carbonate Apatite | +++ | ~2 µm | 1000 °C | HA | [32] |
| HOA | ++ | 115 µm | 1100 °C | TCP, AgNPs | [32] |
| Bone Biomarker | Sample Location |
|---|---|
| Osteocalcin | Serum |
| Bone alkaline phosphatase | Serum |
| Type I procollagen-C-propeptide | Serum |
| Intact type I procollagen-N-propeptide | Serum |
| Total type I procollagen-N-propeptide | Serum |
| Bone resorption markers: | |
| Pyridinoline | Urine |
| Deoxypyridinoline | Urine |
| Type I collagen cross-linked N-telopeptide | Serum/Urine |
| Type I collagen cross-linked C-telopeptide | Serum/Urine |
| Type I collagen C-telopeptide | Serum |
| Tartrate-resistant acid phosphatase 5b | Serum |
| Bone matrix-related markers: | |
| Undercarboxylated osteocalcin | Serum |
| Pentosidine | Plasma/Urine |
| Homocysteine | Plasma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavek, A.; Nartker, C.; Saleh, M.; Kirkham, M.; Khajeh Pour, S.; Aghazadeh-Habashi, A.; Barrott, J.J. Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease. Biomedicines 2021, 9, 551. https://doi.org/10.3390/biomedicines9050551
Pavek A, Nartker C, Saleh M, Kirkham M, Khajeh Pour S, Aghazadeh-Habashi A, Barrott JJ. Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease. Biomedicines. 2021; 9(5):551. https://doi.org/10.3390/biomedicines9050551
Chicago/Turabian StylePavek, Adriene, Christopher Nartker, Maamoon Saleh, Matthew Kirkham, Sana Khajeh Pour, Ali Aghazadeh-Habashi, and Jared J. Barrott. 2021. "Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease" Biomedicines 9, no. 5: 551. https://doi.org/10.3390/biomedicines9050551
APA StylePavek, A., Nartker, C., Saleh, M., Kirkham, M., Khajeh Pour, S., Aghazadeh-Habashi, A., & Barrott, J. J. (2021). Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease. Biomedicines, 9(5), 551. https://doi.org/10.3390/biomedicines9050551








