Natural Autoimmunity to the Thyroid Hormone Monocarboxylate Transporters MCT8 and MCT10
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Commercial Antibodies
2.3. Construction of MCT8 and MCT10 Luciferase Fusion Proteins for aAb Detection
2.4. Quantification of MCT8 and MCT10 Autoantibodies
2.5. Characterization of MCT8-aAb by Immunoprecipitation and Thyroid Hormone Uptake
2.6. Statistical Analysis
3. Results
3.1. Test for Linearity of the MCT8-aAb and MCT10-aAb Assays with Commercial Antibodies
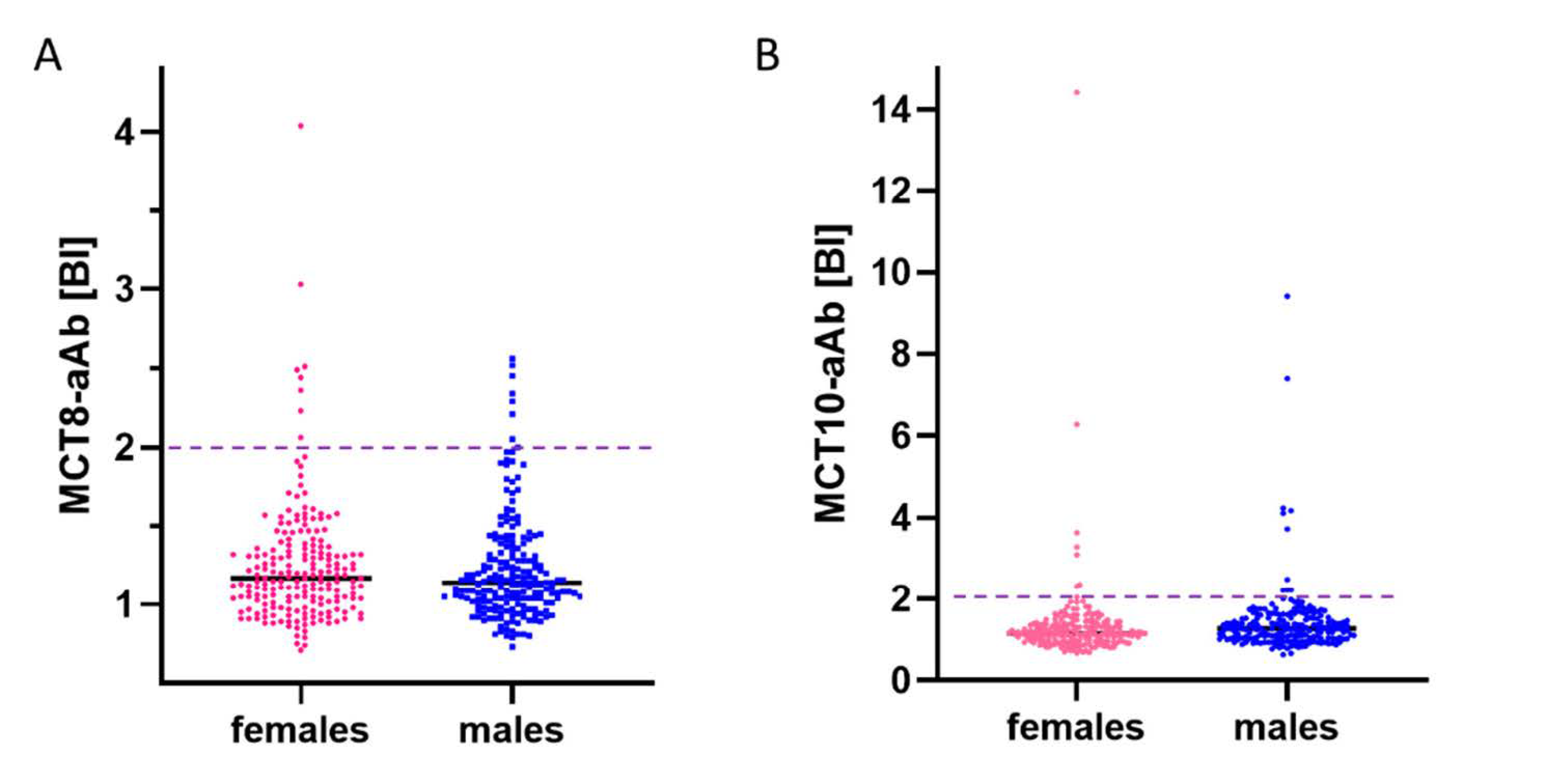
3.2. Prevalence of aAb to MCT8 and MCT10 in Healthy Subjects
3.3. Prevalence of MCT8-aAb and MCT10-aAb in Overweight Young Subjects
3.4. In Vitro Activity of MCT8-aAb Affecting TH Uptake into Cells In Vitro
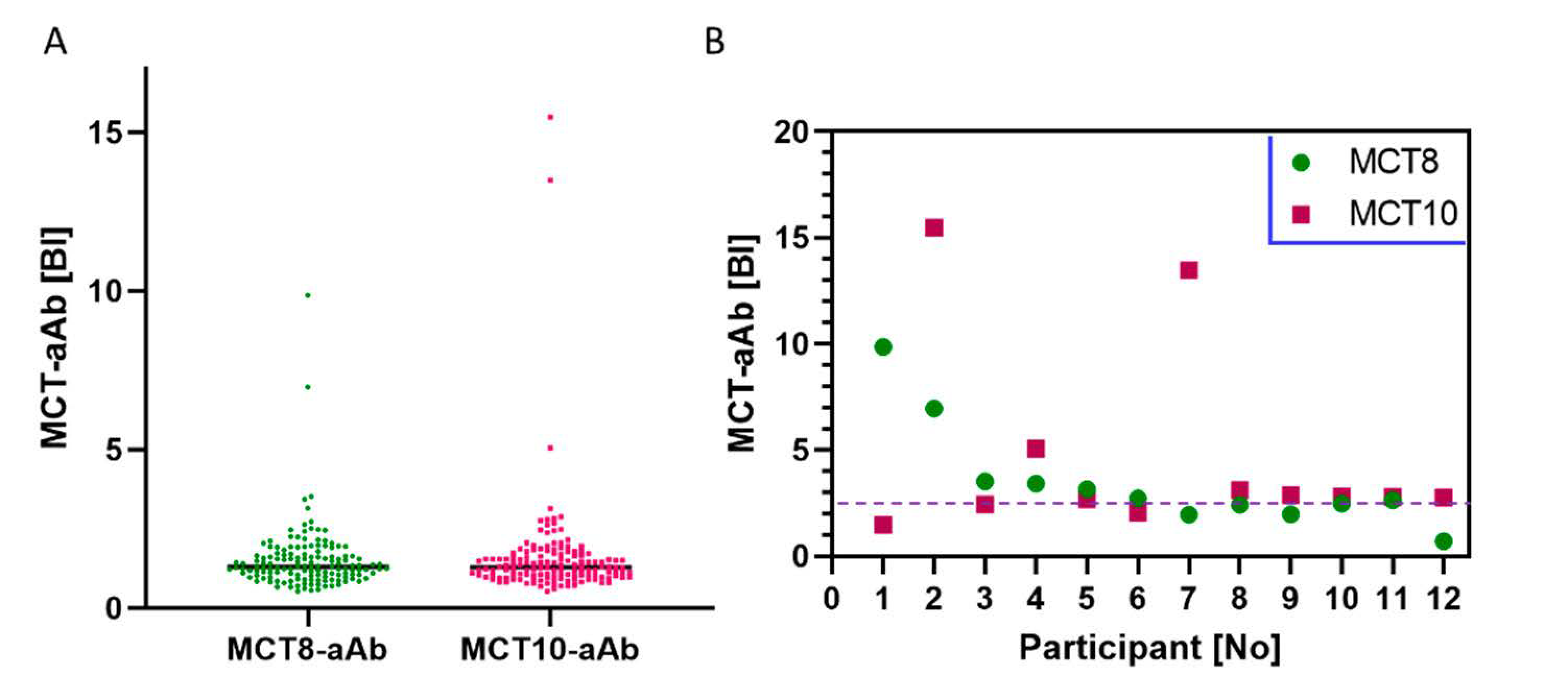
3.5. Prevalence of MCT8-aAb and MCT10-aAb in Thyroid Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernal, J.; Guadaño-Ferraz, A.; Morte, B. Erratum: Thyroid hormone transporters—Functions and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, U.; Johannes, J.; Bayer, D.; Braun, D. Structure and Function of Thyroid Hormone Plasma Membrane Transporters. Eur. Thyroid J. 2014, 3, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Friesema, E.C.; Grueters, A.; Biebermann, H.; Krude, H.; von Moers, A.; Reeser, M.; Barrett, T.G.; Mancilla, E.E.; Svensson, J.; Kester, M.H.; et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 2004, 364, 1435–1437. [Google Scholar] [CrossRef]
- Dumitrescu, A.M.; Liao, X.-H.; Best, T.B.; Brockmann, K.; Refetoff, S. A Novel Syndrome Combining Thyroid and Neurological Abnormalities Is Associated with Mutations in a Monocarboxylate Transporter Gene. Am. J. Hum. Genet. 2004, 74, 168–175. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Stevenson, R.E. The MCT8 thyroid hormone transporter and Allan–Herndon–Dudley syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 307–321. [Google Scholar] [CrossRef]
- Groeneweg, S.; Van Geest, F.S.; Abacı, A.; Alcantud, A.; Ambegaonkar, G.P.; Armour, C.M.; Bakhtiani, P.; Barca, D.; Bertini, E.S.; Van Beynum, I.M.; et al. Disease characteristics of MCT8 deficiency: An international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol. 2020, 8, 594–605. [Google Scholar] [CrossRef]
- Grijota-Martínez, C.; Bárez-López, S.; Gómez-Andrés, D.; Guadaño-Ferraz, A. MCT8 Deficiency: The Road to Therapies for a Rare Disease. Front. Neurosci. 2020, 14, 380. [Google Scholar] [CrossRef]
- Groeneweg, S.; Peeters, R.P.; Moran, C.; Stoupa, A.; Auriol, F.; Tonduti, D.; Dica, A.; Paone, L.; Rozenkova, K.; Malikova, J.; et al. Effectiveness and safety of the tri-iodothyronine analogue Triac in children and adults with MCT8 deficiency: An international, single-arm, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019, 7, 695–706. [Google Scholar] [CrossRef]
- Fu, J.; Korwutthikulrangsri, M.; Ramos-Platt, L.; Pierson, T.M.; Liao, X.-H.; Refetoff, S.; Weiss, R.E.; Dumitrescu, A.M. Sorting Variants of Unknown Significance Identified by Whole Exome Sequencing: Genetic and Laboratory Investigations of Two Novel MCT8 Variants. Thyroid 2020, 30, 463–465. [Google Scholar] [CrossRef]
- Braun, D.; Lelios, I.; Krause, G.; Schweizer, U. Histidines in Potential Substrate Recognition Sites Affect Thyroid Hormone Transport by Monocarboxylate Transporter 8 (MCT8). Endocrinology 2013, 154, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Morshed, S.A.; Davies, T.F. Graves’ Disease Mechanisms: The Role of Stimulating, Blocking, and Cleavage Region TSH Receptor Antibodies. Horm. Metab. Res. 2015, 47, 727–734. [Google Scholar] [CrossRef]
- McLachlan, S.M.; Rapoport, B. Thyrotropin-Blocking Autoantibodies and Thyroid-Stimulating Autoantibodies: Potential Mechanisms Involved in the Pendulum Swinging from Hypothyroidism to Hyperthyroidism or Vice Versa. Thyroid 2013, 23, 14–24. [Google Scholar] [CrossRef]
- Goichot, B.; Leenhardt, L.; Massart, C.; Raverot, V.; Tramalloni, J.; Iraqi, H. Diagnostic procedure in suspected Graves’ disease. Ann. d’Endocrinologie 2018, 79, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Diana, T. TSH Receptor Antibody Functionality and Nomenclature. Front. Endocrinol. 2017, 8, 28. [Google Scholar] [CrossRef]
- Marmouch, H.; Jenzri, H.; Ben Abdallah, B.; Tahri, S.; Charrada, I.; Khochtali, I. Obesity in Association of Autoimmune Thyroid Diseases and Type 2 Diabetes. Metabolism 2021, 116, 38. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-H.; Wang, B.; Yao, Q.-M.; Li, Q.; Jia, X.; Zhang, J.-A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef]
- Tsigalou, C.; Vallianou, N.; Dalamaga, M. Autoantibody Production in Obesity: Is There Evidence for a Link Between Obesity and Autoimmunity? Curr. Obes. Rep. 2020, 9, 245–254. [Google Scholar] [CrossRef]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016, 20, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, M.; Kazemian, E.; Ramezani-Tehrani, F.; Tohidi, M.; Azizi, F.; Khalili, D.; Rahmati, M.; Amouzegar, A. Assessment of the simultaneous effect of hypothyroidism and thyroid autoimmunity with gestational diabetes on the incidence of type 2 diabetes. BMC Endocr. Disord. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef]
- Bau, A.-M.; Ernert, A.; Krude, H.; Wiegand, S. Hormonal regulatory mechanisms in obese children and adolescents after previous weight reduction with a lifestyle intervention: Maintain - paediatric part - a RCT from 2009-15. BMC Obes. 2016, 3, 29. [Google Scholar] [CrossRef]
- Mehl, S.; Sun, Q.; Görlich, C.L.; Hackler, J.; Kopp, J.F.; Renko, K.; Mittag, J.; Schwerdtle, T.; Schomburg, L. Cross-sectional analysis of trace element status in thyroid disease. J. Trace Elements Med. Biol. 2020, 58, 126430. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B.; Dehina, N.; Welsink, T.; Schwiebert, C.; Morgenthaler, N.G.; Köhrle, J.; Eckstein, A.; Schomburg, L. Autoantibodies to the IGF1 Receptor in Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 2013, 98, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, A.-M.; Mehl, S.; Renko, K.; Kasim, R.H.; Schaefer, J.-A.; Minich, W.B.; Schomburg, L. Re-visiting autoimmunity to sodium-iodide symporter and pendrin in thyroid disease. Eur. J. Endocrinol. 2020, 183, 571–580. [Google Scholar] [CrossRef]
- Schwiebert, C.; Kühnen, P.; Becker, N.-P.; Welsink, T.; Keller, T.; Minich, W.B.; Wiegand, S.; Schomburg, L. Antagonistic Autoantibodies to Insulin-Like Growth Factor-1 Receptor Associate with Poor Physical Strength. Int. J. Mol. Sci. 2020, 21, 463. [Google Scholar] [CrossRef]
- Johannes, J.; Jayarama-Naidu, R.; Meyer, F.; Wirth, E.K.; Schweizer, U.; Schomburg, L.; Köhrle, J.; Renko, K. Silychristin, a Flavonolignan Derived From the Milk Thistle, Is a Potent Inhibitor of the Thyroid Hormone Transporter MCT8. Endocrinology 2016, 157, 1694–1701. [Google Scholar] [CrossRef]
- Andersen, S.; Bruun, N.H.; Pedersen, K.M.; Laurberg, P. Biologic Variation is Important for Interpretation of Thyroid Function Tests. Thyroid 2003, 13, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, R.; Midgley, J.E.M.; Larisch, R.; Dietrich, J.W. Functional and Symptomatic Individuality in the Response to Levothyroxine Treatment. Front. Endocrinol. 2019, 10, 664. [Google Scholar] [CrossRef]
- Panicker, V. Genetics of Thyroid Function and Disease. Clin. Biochem. Rev. 2011, 32, 165–175. [Google Scholar]
- Medici, M.; Visser, W.E.; Visser, T.J.; Peeters, R.P. Genetic Determination of the Hypothalamic-Pituitary-Thyroid Axis: Where Do We Stand? Endocr. Rev. 2015, 36, 214–244. [Google Scholar] [CrossRef]
- Brigante, G.; Spaggiari, G.; Santi, D.; Cioni, K.; Gnarini, V.; Diazzi, C.; Pignatti, E.; Casarini, L.; Marino, M.; Tuttelmann, F.; et al. The TRHR Gene Is Associated with Hypothalamo-Pituitary Sensitivity to Levothyroxine. Eur. Thyroid J. 2014, 3, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kazukauskiene, N.; Skiriute, D.; Gustiene, O.; Burkauskas, J.; Zaliunaite, V.; Mickuviene, N.; Brozaitiene, J. Importance of Thyroid Hormone level and Genetic Variations in Deiodinases for Patients after Acute Myocardial Infarction: A Longitudinal Observational Study. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Hoftijzer, H.C.; Heemstra, K.A.; Visser, T.J.; Le Cessie, S.; Peeters, R.P.; Corssmit, E.P.M.; Smit, J.W.A. The Type 2 Deiodinase ORFa-Gly3Asp Polymorphism (rs12885300) Influences the Set Point of the Hypothalamus-Pituitary-Thyroid Axis in Patients Treated for Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Van Der Deure, W.M.; Peeters, R.P.; Visser, T.J. Molecular aspects of thyroid hormone transporters, including MCT8, MCT10, and OATPs, and the effects of genetic variation in these transporters. J. Mol. Endocrinol. 2009, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Teumer, A.; Chaker, L.; Groeneweg, S.; Li, Y.; Di Munno, C.; Barbieri, C.; Schultheiss, U.T.; Traglia, M.; Ahluwalia, T.S.; Akiyama, M.; et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Van Gucht, A.L.; Moran, C.; Meima, M.E.; Visser, W.E.; Chatterjee, K.; Visser, T.J.; Peeters, R.P. Resistance to Thyroid Hormone due to Heterozygous Mutations in Thyroid Hormone Receptor Alpha. Curr. Top. Dev. Biol. 2017, 125, 337–355. [Google Scholar] [CrossRef]
- Fujisawa, H.; Gagné, J.; Dumitrescu, A.M.; Refetoff, S. Very Severe Resistance to Thyroid Hormone beta in One of Three Affected Members of a Family with a Novel Mutation in the THRB Gene. Thyroid 2019, 29, 1518–1520. [Google Scholar] [CrossRef]
- Fu, J.; Dumitrescu, A.M. Inherited defects in thyroid hormone cell-membrane transport and metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.M.; Liao, X.-H.; Abdullah, M.S.Y.; Lado-Abeal, J.; Majed, F.A.; Moeller, L.C.; Boran, G.; Schomburg, L.; Weiss, R.E.; Refetoff, S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat. Genet. 2005, 37, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, R.; Yamada, M.; Horiguchi, K.; Ishii, S.; Hashimoto, K.; Okada, S.; Satoh, T.; Mori, M. Aberrant Histone Modifications at the Thyrotropin-Releasing Hormone Gene in Resistance to Thyroid Hormone: Analysis of F455S Mutant Thyroid Hormone Receptor. Endocrinology 2009, 150, 3425–3432. [Google Scholar] [CrossRef]
- Khan, M.S.; Pandith, A.A.; Masoodi, S.R.; Wani, K.A.; Hussain, M.U.; Mudassar, S. Epigenetic silencing of TSHR gene in thyroid cancer patients in relation to their BRAF V600E mutation status. Endocrine 2014, 47, 449–455. [Google Scholar] [CrossRef]
- Ling, Y.; Shi, X.; Wang, Y.; Ling, X.; Li, Q. Down-regulation of thyroid hormone receptor beta1 gene expression in gastric cancer involves promoter methylation. Biochem. Biophys. Res. Commun. 2014, 444, 147–152. [Google Scholar] [CrossRef]
- Hernandez, A.; Stohn, J.P. The Type 3 Deiodinase: Epigenetic Control of Brain Thyroid Hormone Action and Neurological Function. Int. J. Mol. Sci. 2018, 19, 1804. [Google Scholar] [CrossRef]
- Brix, T.H.; Hegedüs, L.; Weetman, A.P.; Kemp, H.E. Pendrin and NIS antibodies are absent in healthy individuals and are rare in autoimmune thyroid disease: Evidence from a Danish twin study. Clin. Endocrinol. 2014, 81, 440–444. [Google Scholar] [CrossRef]
- Andersen, S.; Pedersen, K.M.; Bruun, N.H.; Laurberg, P. Narrow Individual Variations in Serum T4and T3in Normal Subjects: A Clue to the Understanding of Subclinical Thyroid Disease. J. Clin. Endocrinol. Metab. 2002, 87, 1068–1072. [Google Scholar] [CrossRef]
- Sørgjerd, E.P.; Skorpen, F.; Kvaløy, K.; Midthjell, K.; Grill, V. Prevalence of ZnT8 antibody in relation to phenotype andSLC30A8polymorphism in adult autoimmune diabetes. Results from the HUNT study, Norway. Autoimmunity 2012, 46, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, P.; Lampasona, V.; Landherr, U.; Koczwara, K.; Krause, S.; Grallert, H.; Winkler, C.; Pflüger, M.; Illig, T.; Bonifacio, E.; et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009, 52, 1881–1888. [Google Scholar] [CrossRef]
- Kawasaki, E.; Nakamura, K.; Kuriya, G.; Satoh, T.; Kuwahara, H.; Kobayashi, M.; Abiru, N.; Yamasaki, H.; Eguchi, K. Autoantibodies to Insulin, Insulinoma-Associated Antigen-2, and Zinc Transporter 8 Improve the Prediction of Early Insulin Requirement in Adult-Onset Autoimmune Diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 707–713. [Google Scholar] [CrossRef]
- Burke, G.W., 3rd; Vendrame, F.; Virdi, S.K.; Ciancio, G.; Chen, L.; Ruiz, P.; Messinger, S.; Reijonen, H.K.; Pugliese, A. Lessons From Pancreas Transplantation in Type 1 Diabetes: Recurrence of Islet Autoimmunity. Curr. Diabetes Rep. 2015, 15, 121. [Google Scholar] [CrossRef]
- Ooka, S.; Matsui, T.; Nishioka, K.; Kato, T. Autoantibodies to low-density-lipoprotein-receptor-related protein 2 (LRP2) in systemic autoimmune diseases. Arthritis Res. 2003, 5, R174–R180. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Saravia, S.G.M.; Haberland, A.; Bartel, S.; Araujo, R.; Valda, G.; Duchen, D.; Ramirez, I.D.; Borges, A.C.; Schimke, I. Distinct Patterns of Autoantibodies Against G-Protein–Coupled Receptors in Chagas’ Cardiomyopathy and Megacolon. Their potential impact for early risk assessment in asymptomatic Chagas’ patients. J. Am. Coll. Cardiol. 2010, 55, 463–468. [Google Scholar] [CrossRef]
- Boege, F.; Westenfeld, R.; Jahns, R. beta1AAb Determined by Peptide ELISA: A Signal in the Noise? J. Am. Coll. Cardiol. 2017, 70, 807–808. [Google Scholar] [CrossRef]
- Wallukat, G.; Wenzel, K.; Schimke, I. Analytics of Functional Autoantibodies in Patients with Chagas Disease. Methods Mol. Biol. 2019, 1955, 247–261. [Google Scholar] [CrossRef]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.-D.; Fluge, Ø.; et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef]
- Hara, H.; Hayashi, K.; Ohta, K.; Itoh, N.; Nishitani, H.; Ohta, M. Detection and characterization of blocking-type an-ti-acetylcholine receptor antibodies in sera from patients with myasthenia gravis. Clin. Chem. 1993, 39, 2053–2057. [Google Scholar] [CrossRef]
- Roper, J.; Fleming, M.E.; Long, B.; Koyfman, A. Myasthenia Gravis and Crisis: Evaluation and Management in the Emergency Department. J. Emerg. Med. 2017, 53, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Ciafaloni, E. Myasthenia Gravis and Congenital Myasthenic Syndromes. Contin. Lifelong Learn. Neurol. 2019, 25, 1767–1784. [Google Scholar] [CrossRef] [PubMed]
- Lupsa, B.C.; Chong, A.Y.; Cochran, E.K.; Soos, M.A.; Semple, R.K.; Gorden, P. Autoimmune Forms of Hypoglycemia. Medicine 2009, 88, 141–153. [Google Scholar] [CrossRef]
- Censi, S.; Mian, C.; Betterle, C. Insulin autoimmune syndrome: From diagnosis to clinical management. Ann. Transl. Med. 2018, 6, 335. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porst, T.; Johannes, J.; Gluschke, H.; Köhler, R.; Mehl, S.; Kühnen, P.; Renko, K.; Minich, W.B.; Wiegand, S.; Schomburg, L. Natural Autoimmunity to the Thyroid Hormone Monocarboxylate Transporters MCT8 and MCT10. Biomedicines 2021, 9, 496. https://doi.org/10.3390/biomedicines9050496
Porst T, Johannes J, Gluschke H, Köhler R, Mehl S, Kühnen P, Renko K, Minich WB, Wiegand S, Schomburg L. Natural Autoimmunity to the Thyroid Hormone Monocarboxylate Transporters MCT8 and MCT10. Biomedicines. 2021; 9(5):496. https://doi.org/10.3390/biomedicines9050496
Chicago/Turabian StylePorst, Theresa, Jörg Johannes, Hans Gluschke, Richard Köhler, Sebastian Mehl, Peter Kühnen, Kostja Renko, Waldemar B. Minich, Susanna Wiegand, and Lutz Schomburg. 2021. "Natural Autoimmunity to the Thyroid Hormone Monocarboxylate Transporters MCT8 and MCT10" Biomedicines 9, no. 5: 496. https://doi.org/10.3390/biomedicines9050496
APA StylePorst, T., Johannes, J., Gluschke, H., Köhler, R., Mehl, S., Kühnen, P., Renko, K., Minich, W. B., Wiegand, S., & Schomburg, L. (2021). Natural Autoimmunity to the Thyroid Hormone Monocarboxylate Transporters MCT8 and MCT10. Biomedicines, 9(5), 496. https://doi.org/10.3390/biomedicines9050496






