The Real Need for Regenerative Medicine in the Future of Congenital Heart Disease Treatment
Abstract
1. Introduction
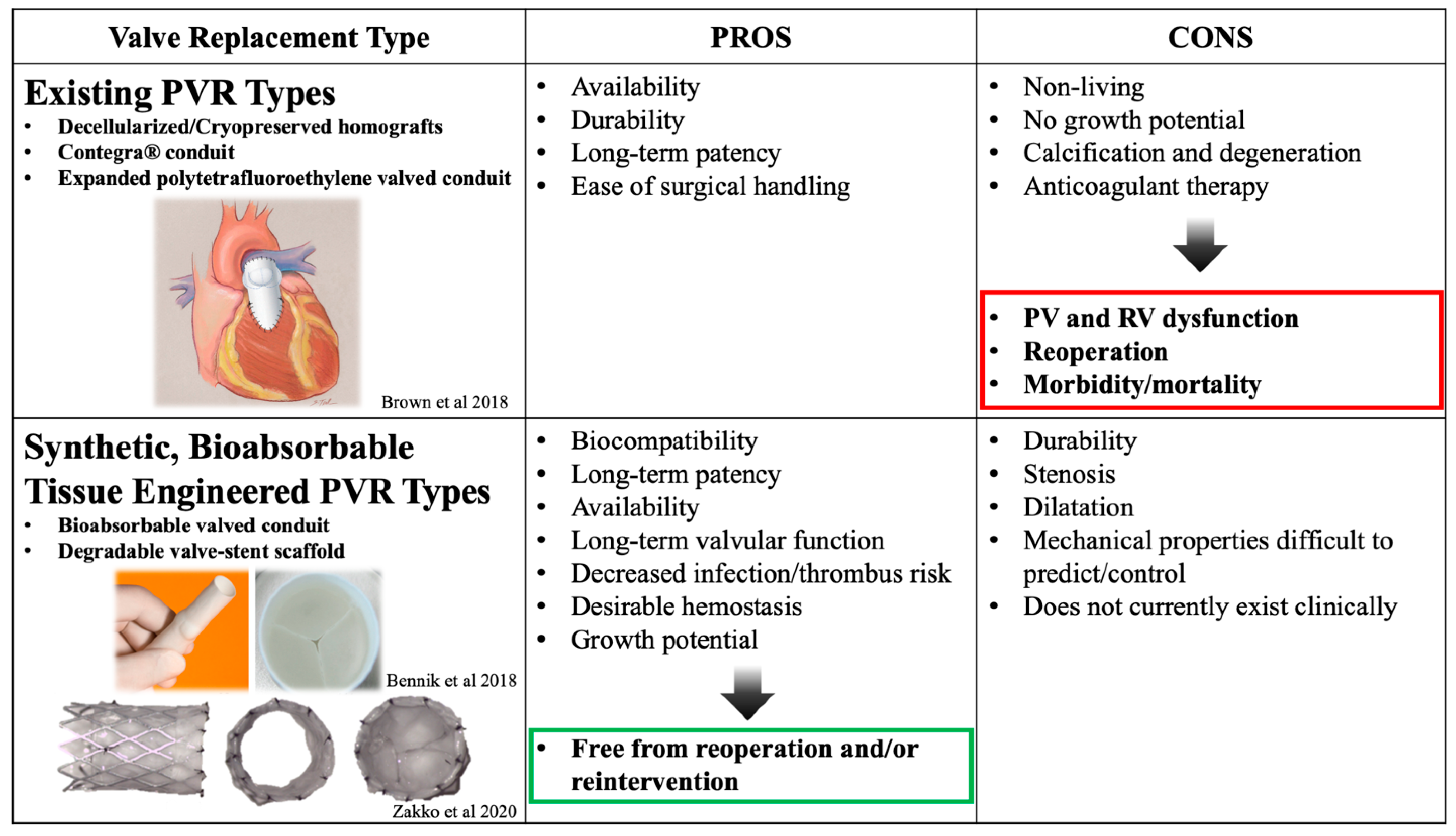
2. Tissue-Engineered Pulmonary Valve
3. Tissue-Engineered Patch
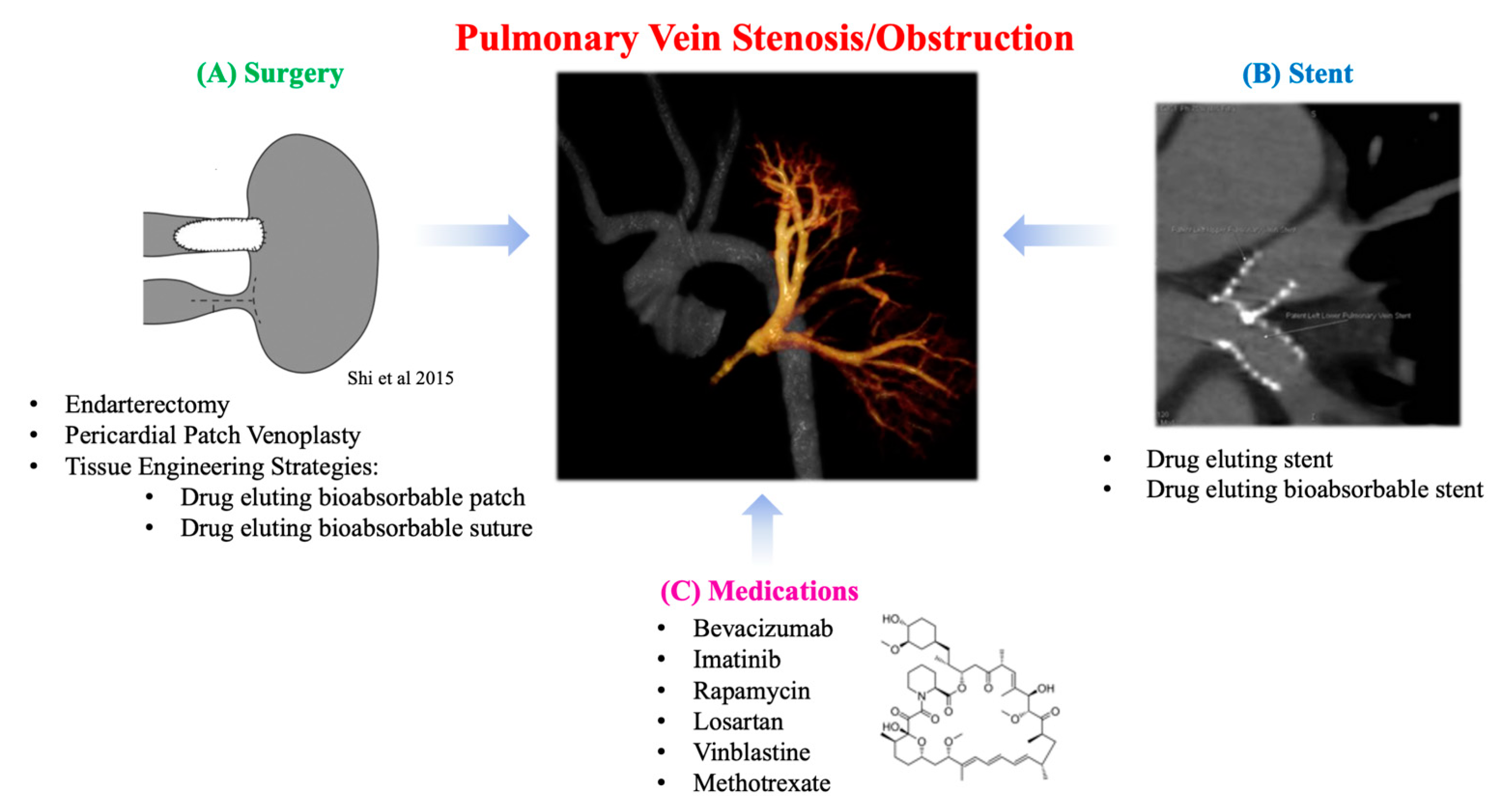
4. Regenerative Medicine Solutions for Pulmonary Vein Stenosis
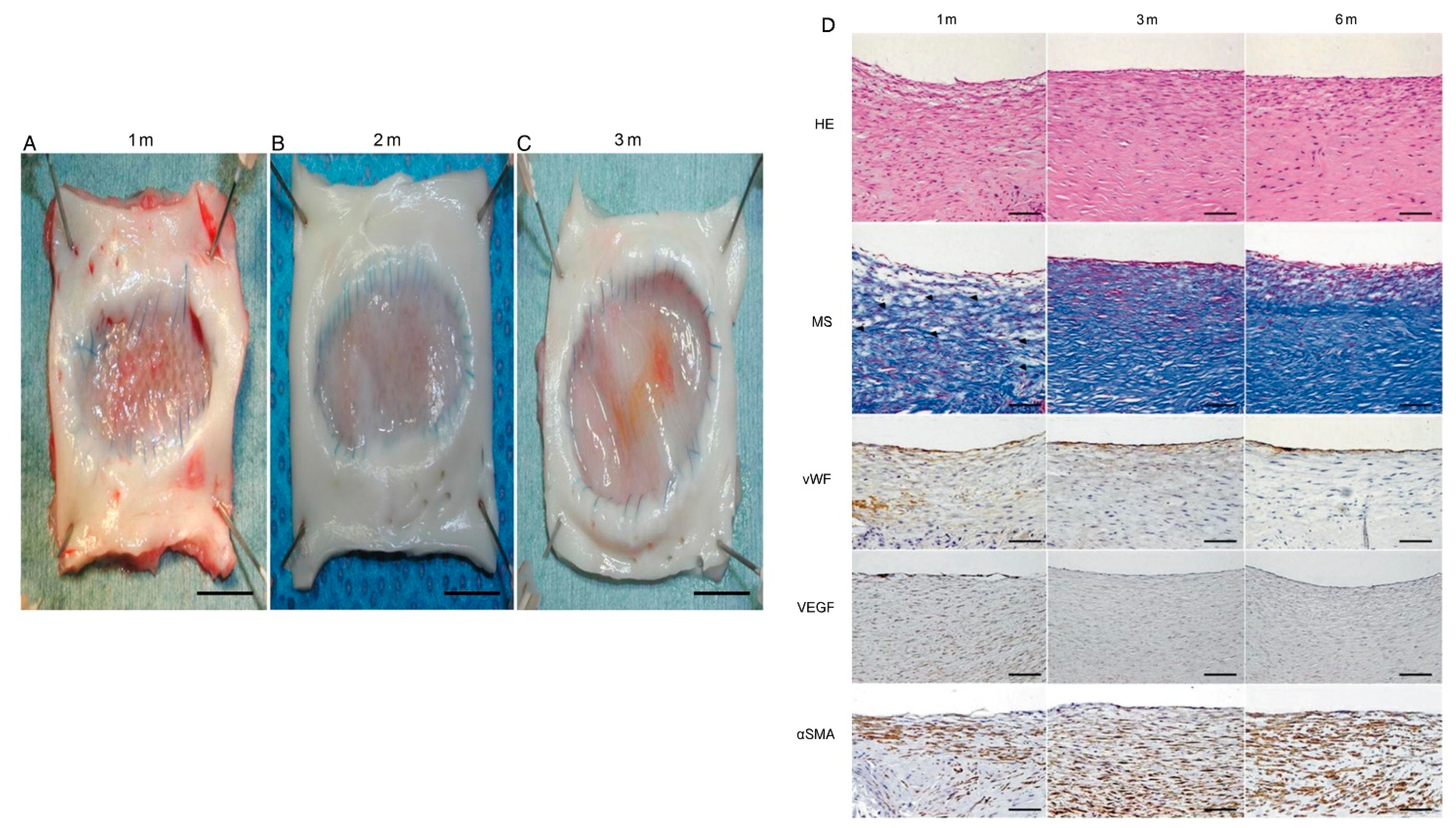
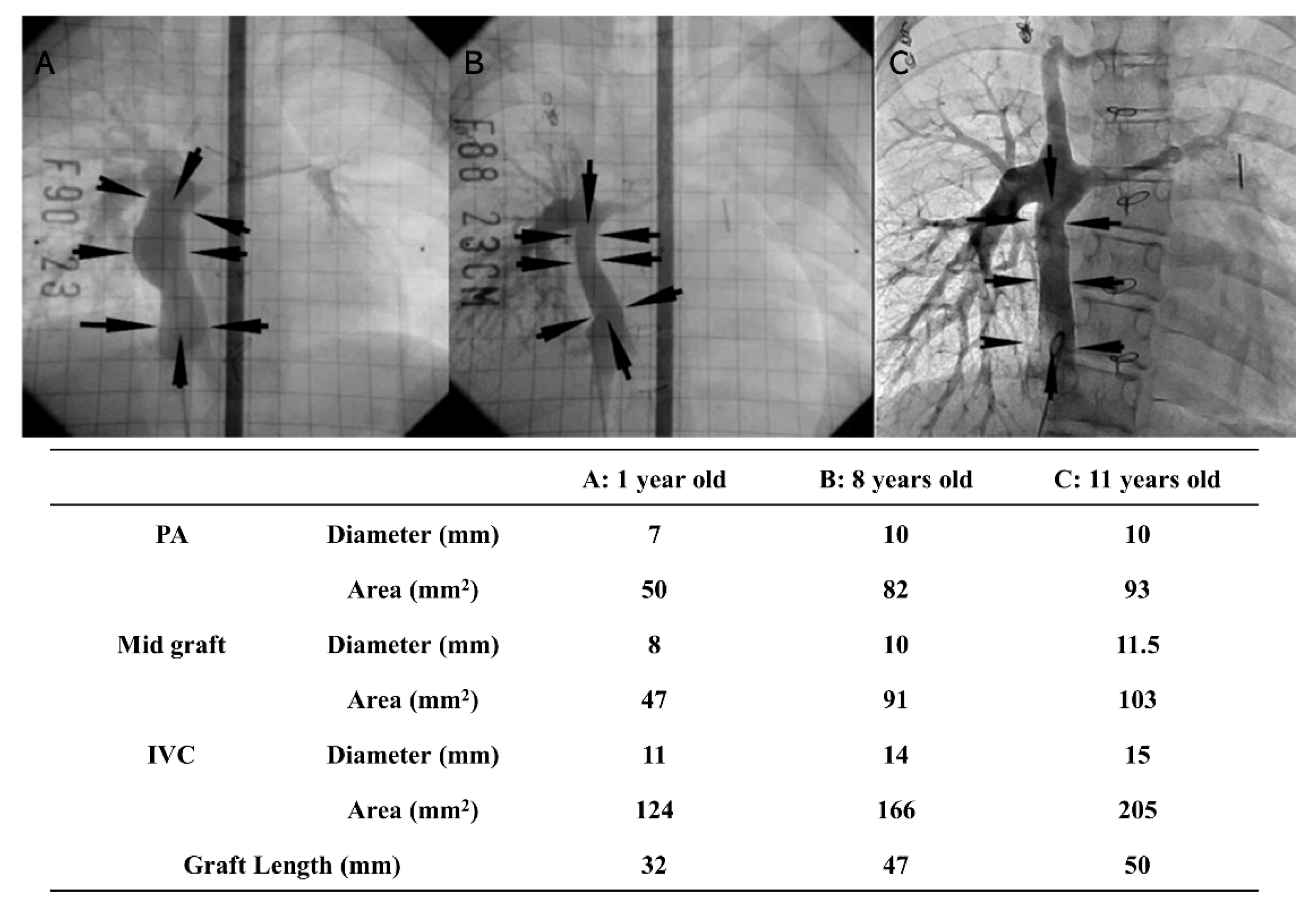
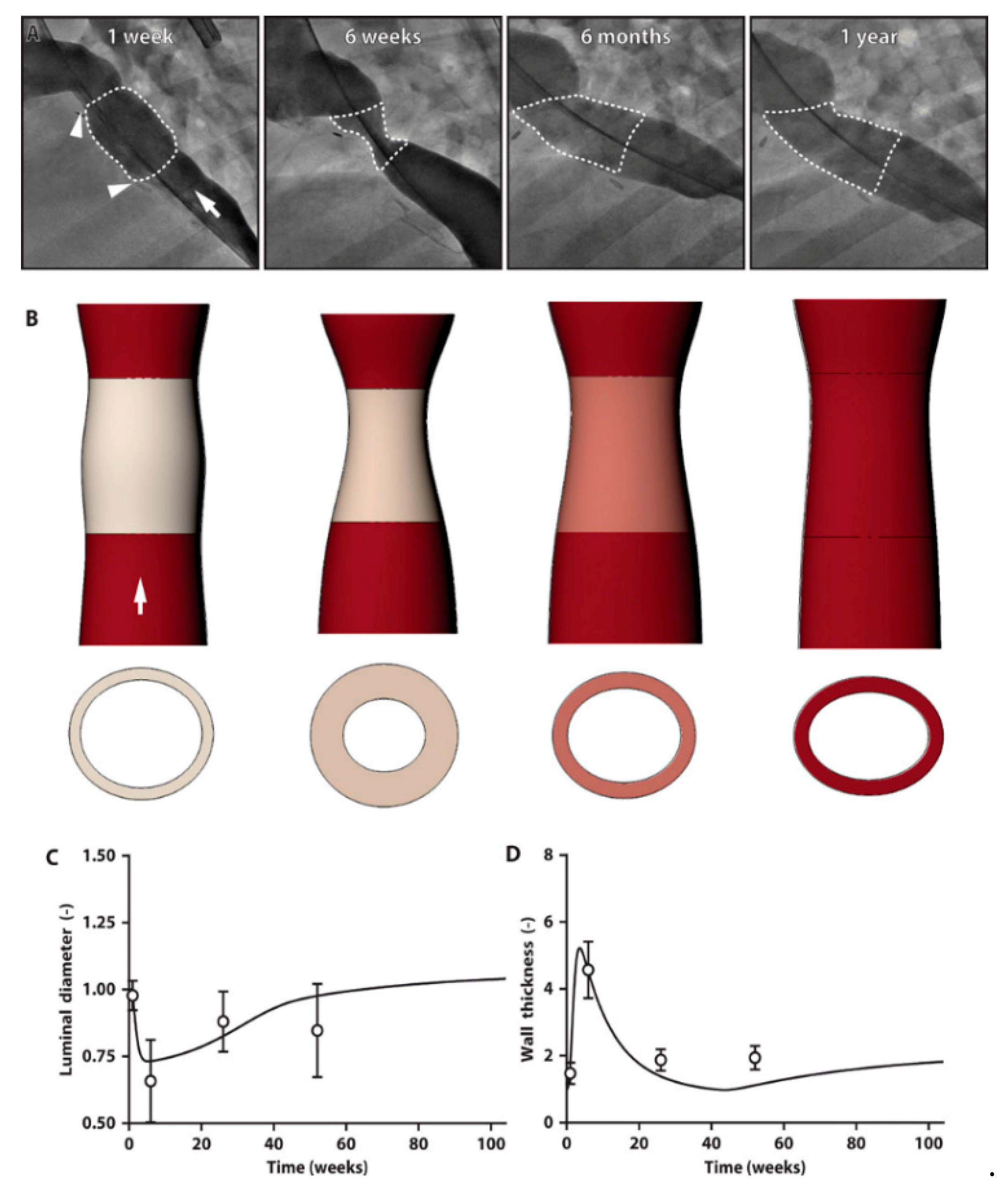
5. Tissue-Engineered Vascular Graft
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosure
Abbreviations
| CHD | Congenital Heart Disease |
| (e)PTFE | (expanded) PolyTetraFluoroEthylene |
| PV | Pulmonary Valve |
| RV | Right Ventricle |
| ECM | ExtraCellular Matrix |
| PVR | Pulmonary Valve Replacement |
| RVOT | Right Ventricular Outflow Tract |
| TEPV | Tissue-Engineered Pulmonary Valves |
| PLCL | PolyLactide-co-epsilon-CaproLactone |
| PCL | PolyCaproLactone |
| PGA | PolyGlycolic Acid |
| PLLA | Poly-L-Lactide Acid |
| PVS | Pulmonary Vein Stenosis |
| BMS | Bare Metal Stent |
| DES | Drug-Eluting Stent |
| TGF-β | Transforming Growth Factor-β |
| TAPVC | Total Anomalous Pulmonary Venous Connection |
| TEVG | Tissue-Engineered Vascular Graft |
References
- Van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Adachi, I.; Yagihara, T.; Kagisaki, K.; Hagino, I.; Ishizaka, T.; Koh, M.; Uemura, H.; Kitamura, S. Fontan operation with a viable and growing conduit using pedicled autologous pericardial roll: Serial changes in conduit geometry. J. Thorac. Cardiovasc. Surg. 2005, 130, 1517–1522.e1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.; Hwang, S.W.; Lim, H.G.; Kim, S.-J.; Lee, J.Y.; Shim, W.-S.; Kim, W.-H. Midterm follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur. J. Cardio-Thoracic Surg. 2007, 31, 1008–1012. [Google Scholar] [CrossRef]
- Monagle, P.; Cochrane, A.; McCrindle, B.; Benson, L.; Williams, W.; Andrew, M. Editorial: Thromboembolic Complications After Fontan Procedures—The Role Of Prophylactic Anticoagulation. J. Thorac. Cardiovasc. Surg. 1998, 115, 493–498. [Google Scholar] [CrossRef]
- Limongi, T.; Brigo, L.; Tirinato, L.; Pagliari, F.; Gandin, A.; Contessotto, P.; Giugni, A.; Brusatin, G. Three-dimensionally two-photon lithography realized vascular grafts. Biomed. Mater. 2021, 16, 035013. [Google Scholar] [CrossRef]
- Limongi, T.; Tirinato, L.; Pagliari, F.; Giugni, A.; Allione, M.; Perozziello, G.; Candeloro, P.; Di Fabrizio, E. Fabrication and Applications of Micro/Nanostructured Devices for Tissue Engineering. Nano-Micro Lett. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Shinoka, T.; Shum-Tim, D.; Ma, P.X.; Tanel, R.E.; Isogai, N.; Langer, R.; Vacanti, J.P.; Mayer, J.E. Creation Of Viable Pulmonary Artery Autografts Through Tissue Engineering. J. Thorac. Cardiovasc. Surg. 1998, 115, 536–546. [Google Scholar] [CrossRef]
- Drews, J.D.; Miyachi, H.; Shinoka, T. Tissue-engineered vascular grafts for congenital cardiac disease: Clinical experience and current status. Trends Cardiovasc. Med. 2017, 27, 521–531. [Google Scholar] [CrossRef]
- Tara, S.; Rocco, K.A.; Hibino, N.; Sugiura, T.; Kurobe, H.; Breuer, C.K.; Shinoka, T. Vessel Bioengineering. Circ. J. 2014, 78, 12–19. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef] [PubMed]
- Ambastha, C.; Bittle, G.J.; Morales, D.; Parchment, N.; Saha, P.; Mishra, R.; Sharma, S.; Vasilenko, A.; Gunasekaran, M.; Al-Suqi, M.T.; et al. Regenerative medicine therapy for single ventricle congenital heart disease. Transl. Pediatr. 2018, 7, 176–187. [Google Scholar] [CrossRef]
- Dzilic, E.; Doppler, S.; Lange, R.; Krane, M. Regenerative Medicine for the Treatment of Congenital Heart Disease. Cardiovasc. Regen. Med. 2019, 207–221. [Google Scholar] [CrossRef]
- Schoen, F.J. Evolving Concepts of Cardiac Valve Dynamics. Circulation 2008, 118, 1864–1880. [Google Scholar] [CrossRef]
- Feltes, T.F.; Bacha, E.; Beekman, R.H.; Cheatham, J.P.; Feinstein, J.A.; Gomes, A.S.; Hijazi, Z.M.; Ing, F.F.; De Moor, M.; Morrow, W.R.; et al. Indications for Cardiac Catheterization and Intervention in Pediatric Cardiac Disease. Circulation 2011, 123, 2607–2652. [Google Scholar] [CrossRef]
- Drossner, D.M.; Mahle, W.T. A Management Strategy for Mild Valvar Pulmonary Stenosis. Pediatr. Cardiol. 2008, 29, 649–652. [Google Scholar] [CrossRef]
- Cavalcanti, P.E.F.; Sá, M.P.B.O.; Santos, C.A.; Esmeraldo, I.M.; de Escobar, R.R.; de Menezes, A.M.; de Azevedo, O.M.; Silva, F.P.D.V.; Lins, R.F.D.A.; Lima, R.D.C. Pulmonary Valve Replacement After Operative Repair of Tetralogy of Fallot. J. Am. Coll. Cardiol. 2013, 62, 2227–2243. [Google Scholar] [CrossRef]
- Geva, T.; Gauvreau, K.; Powell, A.J.; Cecchin, F.; Rhodes, J.; Geva, J.; del Nido, P. Randomized Trial of Pulmonary Valve Replacement With and Without Right Ventricular Remodeling Surgery. Circulation 2010, 122, S201–S208. [Google Scholar] [CrossRef]
- Fathallah, M.; Krasuski, R.A. Pulmonic Valve Disease: Review of Pathology and Current Treatment Options. Curr. Cardiol. Rep. 2017, 19, 108. [Google Scholar] [CrossRef]
- Geva, T. Indications and Timing of Pulmonary Valve Replacement After Tetralogy of Fallot Repair. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2006, 9, 11–22. [Google Scholar] [CrossRef]
- Oechslin, E.N.; Harrison, D.A.; Harris, L.; Downar, E.; Webb, G.D.; Siu, S.S.; Williams, W.G. Reoperation in adults with repair of tetralogy of fallot: Indications and outcomes. J. Thorac. Cardiovasc. Surg. 1999, 118, 245–251. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamagishi, M.; Miyazaki, T. Current status of right ventricular outflow tract reconstruction: Complete translation of a review article originally published in Kyobu Geka 2014; 67: 65–77. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Caldarone, C.A.; McCrindle, B.W.; Van Arsdell, G.S.; Coles, J.G.; Webb, G.; Freedom, R.M.; Williams, W.G. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J. Thorac. Cardiovasc. Surg. 2000, 120, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Butany, J.; Feng, T.; Luk, A.; Law, K.; Suri, R.; Nair, V. Modes of Failure in Explanted Mitroflow Pericardial Valves. Ann. Thorac. Surg. 2011, 92, 1621–1627. [Google Scholar] [CrossRef]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Acar, P.; Le Bidois, J.; Sidi, D.; Kachaner, J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000, 356, 1403–1405. [Google Scholar] [CrossRef]
- Cheatham, S.L.; Holzer, R.J.; Chisolm, J.L.; Cheatham, J.P. The medtronic melody® transcatheter pulmonary valve implanted at 24-mm diameter-it works. Catheter. Cardiovasc. Interv. 2013, 82, 816–823. [Google Scholar] [CrossRef]
- Zahn, E.M.; Hellenbrand, W.E.; Lock, J.E.; McElhinney, D.B. Implantation of the Melody Transcatheter Pulmonary Valve in Patients With a Dysfunctional Right Ventricular Outflow Tract Conduit. J. Am. Coll. Cardiol. 2009, 54, 1722–1729. [Google Scholar] [CrossRef]
- Ewert, P.; Horlick, E.; Berger, F. First implantation of the CE-marked transcatheter Sapien pulmonic valve in Europe. Clin. Res. Cardiol. 2011, 100, 85–87. [Google Scholar] [CrossRef][Green Version]
- Chatterjee, A.; Bajaj, N.S.; McMahon, W.S.; Cribbs, M.G.; White, J.S.; Mukherjee, A.; Law, M.A. Transcatheter Pulmonary Valve Implantation: A Comprehensive Systematic Review and Meta-Analyses of Observational Studies. J. Am. Hear. Assoc. 2017, 6, e006432. [Google Scholar] [CrossRef]
- Suradi, H.S.; Hijazi, Z.M. Percutaneous pulmonary valve implantation. Glob. Cardiol. Sci. Pract. 2015, 2015, 23. [Google Scholar] [CrossRef]
- Stulak, J.M.; Mora, B.N.; Said, S.M.; Schaff, H.V.; Dearani, J.A. Mechanical Pulmonary Valve Replacement. Semin. Thorac. Cardiovasc. Surgery: Pediatr. Card. Surg. Annu. 2016, 19, 82–89. [Google Scholar] [CrossRef]
- Sacks, M.S.; Schoen, F.J.; Mayer, J.E. Bioengineering Challenges for Heart Valve Tissue Engineering. Annu. Rev. Biomed. Eng. 2009, 11, 289–313. [Google Scholar] [CrossRef]
- Yacoub, M.H.; Takkenberg, J.J.M. Will heart valve tissue engineering change the world? Nat. Clin. Pract. Neurol. 2005, 2, 60–61. [Google Scholar] [CrossRef]
- Shinoka, T.; Breuer, C.K.; Tanel, R.E.; Zund, G.; Miura, T.; Ma, P.X.; Langer, R.; Vacanti, J.P.; Mayer, J.E. Tissue engineering heart valves: Valve leaflet replacement study in a lamb model. Ann. Thorac. Surg. 1995, 60, S513–S516. [Google Scholar] [CrossRef]
- Gottlieb, D.; Kunal, T.; Emani, S.; Aikawa, E.; Brown, D.W.; Powell, A.J.; Nedder, A.; Engelmayr, G.C.; Melero-Martin, J.M.; Sacks, M.S.; et al. In vivo monitoring of function of autologous engineered pulmonary valve. J. Thorac. Cardiovasc. Surg. 2010, 139, 723–731. [Google Scholar] [CrossRef]
- Theodoridis, K.; Tudorache, I.; Calistru, A.; Cebotari, S.; Meyer, T.; Sarikouch, S.; Bara, C.; Brehm, R.; Haverich, A.; Hilfiker, A. Successful matrix guided tissue regeneration of decellularized pulmonary heart valve allografts in elderly sheep. Biomaterials 2015, 52, 221–228. [Google Scholar] [CrossRef]
- Iop, L.; Bonetti, A.; Naso, F.; Rizzo, S.; Cagnin, S.; Bianco, R.; Lin, C.D.; Martini, P.; Poser, H.; Franci, P.; et al. Decellularized Allogeneic Heart Valves Demonstrate Self-Regeneration Potential after a Long-Term Preclinical Evaluation. PLoS ONE 2014, 9, e99593. [Google Scholar] [CrossRef]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2021, 18, 92–116. [Google Scholar] [CrossRef]
- Jana, S.; Tefft, B.; Spoon, D.; Simari, R. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 2014, 10, 2877–2893. [Google Scholar] [CrossRef]
- Brown, J.W. Polytetrafluoroethylene valved conduits for right ventricle–pulmonary artery reconstruction: Do they outperform xenografts and allografts? J. Thorac. Cardiovasc. Surg. 2018, 155, 2577–2578. [Google Scholar] [CrossRef]
- Bennink, G.; Torii, S.; Brugmans, M.; Cox, M.; Svanidze, O.; Ladich, E.; Carrel, T.; Virmani, R. A novel restorative pulmonary valved conduit in a chronic sheep model: Mid-term hemodynamic function and histologic assessment. J. Thorac. Cardiovasc. Surg. 2018, 155, 2591–2601.e3. [Google Scholar] [CrossRef] [PubMed]
- Zakko, J.; Blum, K.M.; Drews, J.D.; Wu, Y.-L.; Hatoum, H.; Russell, M.; Gooden, S.; Heitkemper, M.; Conroy, O.; Kelly, J.; et al. Development of Tissue Engineered Heart Valves for Percutaneous Transcatheter Delivery in a Fetal Ovine Model. JACC Basic Transl. Sci. 2020, 5, 815–828. [Google Scholar] [CrossRef]
- Weber, B.; Dijkman, P.E.; Scherman, J.; Sanders, B.; Emmert, M.Y.; Grünenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Franz, T.; et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 2013, 34, 7269–7280. [Google Scholar] [CrossRef]
- Motta, S.E.; Lintas, V.; Fioretta, E.S.; Dijkman, P.E.; Putti, M.; Caliskan, E.; Biefer, H.R.C.; Lipiski, M.; Sauer, M.; Cesarovic, N.; et al. Human cell-derived tissue-engineered heart valve with integrated Valsalva sinuses: Towards native-like transcatheter pulmonary valve replacements. NPJ Regen. Med. 2019, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, P.E.; Driessen-Mol, A.; Frese, L.; Hoerstrup, S.P.; Baaijens, F.P. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 2012, 33, 4545–4554. [Google Scholar] [CrossRef]
- Kluin, J.; Talacua, H.; Smits, A.I.; Emmert, M.Y.; Brugmans, M.C.; Fioretta, E.S.; Dijkman, P.E.; Söntjens, S.H.; Duijvelshoff, R.; Dekker, S.; et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant—From material design to 12 months follow-up in sheep. Biomaterials 2017, 125, 101–117. [Google Scholar] [CrossRef]
- Fioretta, E.S.; Lintas, V.; Mallone, A.; Motta, S.E.; von Boehmer, L.; Dijkman, P.E.; Cesarovic, N.; Caliskan, E.; Biefer, H.R.C.; Lipiski, M.; et al. Differential Leaflet Remodeling of Bone Marrow Cell Pre-Seeded Versus Nonseeded Bioresorbable Transcatheter Pulmonary Valve Replacements. JACC Basic Transl. Sci. 2020, 5, 15–31. [Google Scholar] [CrossRef]
- Lee, Y.-U.; Yi, T.; James, I.; Tara, S.; Stuber, A.J.; Shah, K.V.; Lee, A.Y.; Sugiura, T.; Hibino, N.; Shinoka, T.; et al. Transplantation of Pulmonary Valve Using a Mouse Model of Heterotopic Heart Transplantation. J. Vis. Exp. 2014, e51695. [Google Scholar] [CrossRef]
- Ing, F.F.; Khan, A.; Kobayashi, D.; Hagler, D.J.; Forbes, T.J. Pulmonary artery stents in the recent era. Catheter. Cardiovasc. Interv. 2014, 84, 1123–1130. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Ii, R.J.G.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Health Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef]
- Soliman, O.I.; Miyazaki, Y.; AbdelGhani, M.; Brugmans, M.; Witsenburg, M.; Onuma, Y.; Cox, M.; Serruys, P.W. Midterm performance of a novel restorative pulmonary valved conduit: Preclinical results. EuroIntervention 2017, 13, e1418–e1427. [Google Scholar] [CrossRef]
- Dohmen, P.M. Clinical results of implanted tissue engineered heart valves. HSR Prc. Intensive Care Cardiovasc. Anesth 2012, 4, 225–231. [Google Scholar]
- Emmert, M.Y.; Schmitt, B.A.; Loerakker, S.; Sanders, B.; Spriestersbach, H.; Fioretta, E.S.; Bruder, L.; Brakmann, K.; Motta, S.E.; Lintas, V.; et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci. Transl. Med. 2018, 10, eaan4587. [Google Scholar] [CrossRef]
- Holubec, T.; Caliskan, E.; Sündermann, S.H.; Starck, C.T.; Plass, A.; Bettex, D.; Falk, V.; Maisano, F. The Use of Extracellular Matrix Patches in Cardiac Surgery. J. Card. Surg. 2014, 30, 145–148. [Google Scholar] [CrossRef]
- Vitanova, K.; Cleuziou, J.; Hörer, J.; Von Ohain, J.P.; Vogt, M.; Schreiber, C.; Lange, R. Which type of conduit to choose for right ventricular outflow tract reconstruction in patients below 1 year of age? Eur. J. Cardio-Thorac. Surg. 2014, 46, 961–966. [Google Scholar] [CrossRef]
- Pok, S.; Jacot, J.G. Biomaterials Advances in Patches for Congenital Heart Defect Repair. J. Cardiovasc. Transl. Res. 2011, 4, 646–654. [Google Scholar] [CrossRef]
- Iwai, S.; Sawa, Y.; Ichikawa, H.; Taketani, S.; Uchimura, E.; Chen, G.; Hara, M.; Miyake, J.; Matsuda, H. Biodegradable polymer with collagen microsponge serves as a new bioengineered cardiovascular prosthesis. J. Thorac. Cardiovasc. Surg. 2004, 128, 472–479. [Google Scholar] [CrossRef]
- Iwai, S.; Sawa, Y.; Taketani, S.; Torikai, K.; Hirakawa, K.; Matsuda, H. Novel Tissue-Engineered Biodegradable Material for Reconstruction of Vascular Wall. Ann. Thorac. Surg. 2005, 80, 1821–1827. [Google Scholar] [CrossRef]
- Bernabei, M.; Margaryan, R.; Arcieri, L.; Bianchi, G.; Pak, V.; Murzi, B. Aortic arch reconstruction in newborns with an autologous pericardial patch: Contemporary results. Interact. Cardiovasc. Thorac. Surg. 2012, 16, 282–285. [Google Scholar] [CrossRef]
- Morell, V.O.; Wearden, P.A. Experience with Bovine Pericardium for the Reconstruction of the Aortic Arch in Patients Undergoing a Norwood Procedure. Ann. Thorac. Surg. 2007, 84, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, H.M.; Ashburn, D.A.; Konstantinov, I.E.; De Oliviera, N.C.; Benson, L.; Williams, W.G.; Van Arsdell, G.S. Interdigitating arch reconstruction eliminates recurrent coarctation after the Norwood procedure. J. Thorac. Cardiovasc. Surg. 2005, 130, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Knyshov, G.V.; Sitar, L.L.; Glagola, M.D.; Atamanyuk, M.Y. Aortic aneurysms at the site of the repair of coarctation of the aorta: A review of 48 patients. Ann. Thorac. Surg. 1996, 61, 935–939. [Google Scholar] [CrossRef]
- Liu, J.; Itatani, K.; Shiurba, R.; Miyakoshi, T.; Qian, Y.; Murakami, A.; Miyaji, K.; Umezu, M. Image-based computational hemodynamics of distal aortic arch recoarctation following the Norwood procedure. In Proceedings of the 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI), Shanghai, China, 15–17 October 2011; Volume 1, pp. 318–323. [Google Scholar]
- Cohen, M.; Fuster, V.; Steele, P.M.; Driscoll, D.; McGoon, D.C. Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction. Circulation 1989, 80, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Backer, C.L.; Mavroudis, C.; Zias, E.A.; Amin, Z.; Weigel, T.J. Repair of coarctation with resection and extended end-to-end anastomosis. Ann. Thorac. Surg. 1998, 66, 1365–1370. [Google Scholar] [CrossRef]
- Daniels, S.R. Repair of coarctation of the aorta and hypertension: Does age matter. Lancet 2001, 358, 89. [Google Scholar] [CrossRef]
- Toro-Salazar, O.H.; Steinberger, J.; Thomas, W.; Rocchini, A.P.; Carpenter, B.; Moller, J.H. Long-term follow-up of patients after coarctation of the aorta repair. Am. J. Cardiol. 2002, 89, 541–547. [Google Scholar] [CrossRef]
- Coady, M.A.; Rizzo, J.A.; Goldstein, L.J.; Elefteriades, J.A. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol. Clin. 1999, 17, 615–635. [Google Scholar] [CrossRef]
- Wainwright, J.M.; Hashizume, R.; Fujimoto, K.L.; Remlinger, N.T.; Pesyna, C.; Wagner, W.R.; Tobita, K.; Gilbert, T.W.; Badylak, S.F. Right Ventricular Outflow Tract Repair with a Cardiac Biologic Scaffold. Cells Tissues Organs 2012, 195, 159–170. [Google Scholar] [CrossRef]
- Mewhort, H.E.; Turnbull, J.D.; Meijndert, H.C.; Ngu, J.M.; Fedak, P.W. Epicardial infarct repair with basic fibroblast growth factor–enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J. Thorac. Cardiovasc. Surg. 2014, 147, 1650–1659. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.H.; Nathan, M.; Emani, S.; Baird, C.; Del Nido, P.J.; Gauvreau, K.; Harris, M.; Sanders, S.P.; Padera, R.F. Preliminary experience with porcine intestinal submucosa (CorMatrix) for valve reconstruction in congenital heart disease: Histologic evaluation of explanted valves. J. Thorac. Cardiovasc. Surg. 2014, 148, 2216–2225.e1. [Google Scholar] [CrossRef]
- Gao, L.; Sun, H. A novel human-derived tissue-engineered patch for vascular reconstruction. J. Mol. Cell. Cardiol. 2020, 140, 53. [Google Scholar] [CrossRef]
- Gao, L.-P.; Du, M.-J.; Lv, J.-J.; Schmull, S.; Huang, R.-T.; Li, J. Use of human aortic extracellular matrix as a scaffold for construction of a patient-specific tissue engineered vascular patch. Biomed. Mater. 2017, 12, 065006. [Google Scholar] [CrossRef]
- Cho, S.-W.; Jeon, O.; Lim, J.E.; Gwak, S.-J.; Kim, S.-S.; Choi, C.Y.; Kim, D.-I.; Kim, B.-S. Preliminary experience with tissue engineering of a venous vascular patch by using bone marrow–derived cells and a hybrid biodegradable polymer scaffold. J. Vasc. Surg. 2006, 44, 1329–1340. [Google Scholar] [CrossRef]
- Cho, S.-W.; Park, H.J.; Ryu, J.H.; Kim, S.H.; Kim, Y.H.; Choi, C.Y.; Lee, M.-J.; Kim, J.-S.; Jang, I.-S.; Kim, D.-I.; et al. Vascular patches tissue-engineered with autologous bone marrow-derived cells and decellularized tissue matrices. Biomaterials 2005, 26, 1915–1924. [Google Scholar] [CrossRef]
- Ichihara, Y.; Shinoka, T.; Matsumura, G.; Ikada, Y.; Yamazaki, K. A new tissue-engineered biodegradable surgical patch for high-pressure systems. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 768–776. [Google Scholar] [CrossRef][Green Version]
- Caldarone, C.A.; Najm, H.K.; Kadletz, M.; Smallhorn, J.F.; Freedom, R.M.; Williams, W.G.; Coles, J.G. Relentless pulmonary vein stenosis after repair of total anomalous pulmonary venous drainage. Ann. Thorac. Surg. 1998, 66, 1514–1519. [Google Scholar] [CrossRef]
- Friesen, C.L.H.; Zurakowski, D.; Thiagarajan, R.R.; Forbess, J.M.; del Nido, P.J.; Mayer, J.E.; Jonas, R.A. Total Anomalous Pulmonary Venous Connection: An Analysis of Current Management Strategies in a Single Institution. Ann. Thorac. Surg. 2005, 79, 596–606. [Google Scholar] [CrossRef]
- Porres, D.V.; Morenza, Ó.P.; Pallisa, E.; Roque, A.; Andreu, J.; Martínez, M. Learning from the Pulmonary Veins. Radiographics 2013, 33, 999–1022. [Google Scholar] [CrossRef]
- Pazos-López, P.; García-Rodríguez, C.; Guitián-González, A.; Paredes-Galán, E.; Álvarez-Moure, M.Á.D.L.G.; Rodríguez-Álvarez, M.; Baz-Alonso, J.A.; Teijeira-Fernández, E.; Calvo-Iglesias, F.E.; Íñiguez-Romo, A. Pulmonary vein stenosis: Etiology, diagnosis and management. World J. Cardiol. 2016, 8, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhu, Z.; Chen, H.; Zhang, H.; Zheng, J.; Liu, J. Surgical repair for primary pulmonary vein stenosis: Single-institution, midterm follow-up. J. Thorac. Cardiovasc. Surg. 2015, 150, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Devaney, E.J.; Chang, A.C.; Ohye, R.G.; Bove, E.L. Management of Congenital and Acquired Pulmonary Vein Stenosis. Ann. Thorac. Surg. 2006, 81, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Mendeloff, E.N.; Spray, T.L.; Huddleston, C.B.; Bridges, N.D.; Canter, C.B.; Mallory, G.B.; Malloryjr, G. Lung transplantation for congenital pulmonary vein stenosis. Ann. Thorac. Surg. 1995, 60, 903–907. [Google Scholar] [CrossRef]
- Tomita, H.; Watanabe, K.; Yazaki, S.; Kimura, K.; Ono, Y.; Yagihara, T.; Echigo, S. Stent Implantation and Subsequent Dilatation for Pulmonary Vein Stenosis in Pediatric Patients: Maximizing Effectiveness. Circ. J. 2003, 67, 187–190. [Google Scholar] [CrossRef]
- Khan, A.; Qureshi, A.M.; Justino, H. Comparison of drug eluting versus bare metal stents for pulmonary vein stenosis in childhood. Catheter. Cardiovasc. Interv. 2019, 94, 233–242. [Google Scholar] [CrossRef]
- Cory, M.J.; Ooi, Y.K.; Kelleman, M.S.; Vincent, R.N.; Kim, D.W.; Petit, C.J. Reintervention Is Associated With Improved Survival in Pediatric Patients With Pulmonary Vein Stenosis. JACC: Cardiovasc. Interv. 2017, 10, 1788–1798. [Google Scholar] [CrossRef]
- Masaki, N.; Adachi, O.; Katahira, S.; Saiki, Y.; Horii, A.; Kawamoto, S.; Saiki, Y. Progression of vascular remodeling in pulmonary vein obstruction. J. Thorac. Cardiovasc. Surg. 2020, 160, 777–790.e5. [Google Scholar] [CrossRef]
- Kato, H.; Fu, Y.Y.; Zhu, J.; Wang, L.; Aafaqi, S.; Rahkonen, O.; Slorach, C.; Traister, A.; Leung, C.H.; Chiasson, D.; et al. Pulmonary vein stenosis and the pathophysiology of “upstream” pulmonary veins. J. Thorac. Cardiovasc. Surg. 2014, 148, 245–253. [Google Scholar] [CrossRef]
- Zhu, J.; Ide, H.; Fu, Y.Y.; Teichert, A.-M.; Kato, H.; Weisel, R.D.; Maynes, J.T.; Coles, J.G.; Caldarone, C.A. Losartan ameliorates “upstream” pulmonary vein vasculopathy in a piglet model of pulmonary vein stenosis. J. Thorac. Cardiovasc. Surg. 2014, 148, 2550–2558. [Google Scholar] [CrossRef]
- Rehman, M.; Jenkins, K.J.; Juraszek, A.L.; Connor, J.A.; Gauvreau, K.; Muneeb, M.; Sena, L.M.; Colan, S.D.; Saia, T.; Kieran, M.W. A Prospective Phase II Trial of Vinblastine and Methotrexate in Multivessel Intraluminal Pulmonary Vein Stenosis in Infants and Children. Congenit. Hear. Dis. 2011, 6, 608–623. [Google Scholar] [CrossRef]
- Quinonez, L.G.; Gauvreau, K.; Borisuk, M.; Ireland, C.; Marshall, A.M.; Mayer, J.E.; Jenkins, K.J.; Fynn-Thompson, F.E.; Baird, C.W. Outcomes of surgery for young children with multivessel pulmonary vein stenosis. J. Thorac. Cardiovasc. Surg. 2015, 150, 911–917. [Google Scholar] [CrossRef]
- Vanderlaan, R.D.; Rome, J.; Hirsch, R.; Ivy, D.; Caldarone, C.A. Pulmonary vein stenosis: Treatment and challenges. J. Thorac. Cardiovasc. Surg. 2020. [Google Scholar] [CrossRef]
- Callahan, R.; Kieran, M.W.; Baird, C.W.; Colan, S.D.; Gauvreau, K.; Ireland, C.M.; Marshall, A.C.; Sena, L.M.; Vargas, S.O.; Jenkins, K.J. Adjunct Targeted Biologic Inhibition Agents to Treat Aggressive Multivessel Intraluminal Pediatric Pulmonary Vein Stenosis. J. Pediatr. 2018, 198, 29–35.e5. [Google Scholar] [CrossRef]
- Zilberman, M.; Eberhart, R.C. DRUG-ELUTING BIORESORBABLE STENTS FOR VARIOUS APPLICATIONS. Annu. Rev. Biomed. Eng. 2006, 8, 153–180. [Google Scholar] [CrossRef]
- Padmakumar, S.; Joseph, J.; Neppalli, M.H.; Mathew, S.E.; Nair, S.V.; Shankarappa, S.A.; Menon, D. Electrospun Polymeric Core–sheath Yarns as Drug Eluting Surgical Sutures. ACS Appl. Mater. Interfaces 2016, 8, 6925–6934. [Google Scholar] [CrossRef]
- Fontan, F.; Kirklin, J.W.; Fernandez, G.; Costa, F.; Naftel, D.C.; Tritto, F.; Blackstone, E.H. Outcome after a “perfect” Fontan operation. Circulation 1990, 81, 1520–1536. [Google Scholar] [CrossRef]
- Giannico, S.; Hammad, F.; Amodeo, A.; Michielon, G.; Drago, F.; Turchetta, A.; Di Donato, R.; Sanders, S.P. Clinical Outcome of 193 Extracardiac Fontan Patients. J. Am. Coll. Cardiol. 2006, 47, 2065–2073. [Google Scholar] [CrossRef]
- Van Son, J.A.; Reddy, V.; Hanley, F.L. Extracardiac modification of the Fontan operation without use of prosthetic material. J. Thorac. Cardiovasc. Surg. 1995, 110, 1766–1768. [Google Scholar] [CrossRef][Green Version]
- Khairy, P.; Poirier, N. Is the Extracardiac Conduit the Preferred Fontan Approach for Patients With Univentricular Hearts? Circulation 2012, 126, 2516–2525. [Google Scholar] [CrossRef]
- Bezuska, L.; Lebetkevicius, V.; Sudikiene, R.; Liekiene, D.; Tarutis, V. 30-year experience of Fontan surgery: Single-centre’s data. J. Cardiothorac. Surg. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shin’Oka, T.; Matsumura, G.; Hibino, N.; Naito, Y.; Watanabe, M.; Konuma, T.; Sakamoto, T.; Nagatsu, M.; Kurosawa, H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 2005, 129, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Bockeria, L.A.; Svanidze, O.; Kim, A.; Shatalov, K.; Makarenko, V.; Cox, M.; Carrel, T. Total cavopulmonary connection with a new bioabsorbable vascular graft: First clinical experience. J. Thorac. Cardiovasc. Surg. 2017, 153, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436.e2. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Matsumura, G.; Miyamoto, S.; Miyachi, H.; Breuer, C.K.; Shinoka, T. Tissue-engineered Vascular Grafts in Children With Congenital Heart Disease: Intermediate Term Follow-up. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Szafron, J.M.; Khosravi, R.; Reinhardt, J.; Best, C.A.; Bersi, M.R.; Yi, T.; Breuer, C.K.; Humphrey, J.D. Immuno-driven and Mechano-mediated Neotissue Formation in Tissue Engineered Vascular Grafts. Ann. Biomed. Eng. 2018, 46, 1938–1950. [Google Scholar] [CrossRef]
- Drews, J.D.; Pepper, V.K.; Best, C.A.; Szafron, J.M.; Cheatham, J.P.; Yates, A.R.; Hor, K.N.; Zbinden, J.C.; Chang, Y.-C.; Mirhaidari, G.J.M.; et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci. Transl. Med. 2020, 12, eaax6919. [Google Scholar] [CrossRef]
- Shi, X.; He, L.; Zhang, S.-M.; Luo, J. Human iPS Cell-derived Tissue Engineered Vascular Graft: Recent Advances and Future Directions. Stem Cell Rev. Rep. 2020, 1–16. [Google Scholar] [CrossRef]
- Ferrantini, C.; Pioner, J.M.; Martella, D.; Coppini, R.; Piroddi, N.; Paoli, P.; Calamai, M.; Pavone, F.S.; Wiersma, D.S.; Tesi, C.; et al. Development of Light-Responsive Liquid Crystalline Elastomers to Assist Cardiac Contraction. Circ. Res. 2019, 124, e44–e54. [Google Scholar] [CrossRef]
| Classification of Diseases | Examples of Diagnoses | |
|---|---|---|
| RVOT Congenital Defect | Stenosis | Tetralogy of Fallot +/− absent pulmonary valve syndrome |
| Isolated pulmonary stenosis | ||
| Atresia | Pulmonary atresia +/− VSD | |
| Truncus Arteriosus | ||
| RVOT Iatrogenic Defect | Rastelli Procedure | DORV + VSD + sub-pulmonary stenosis |
| TGA or Corrected TGA + sub-pulmonary stenosis | ||
| Ross Procedure | Aortic Stenosis (congenital or acquired) | |
| Aortic Regurgitation (congenital or acquired) | ||
| Secondary Pulmonary Regurgitation | PR after repair of Tetralogy of Fallot | |
| PR after pulmonary valvuloplasty | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, Y.; Wiet, M.G.; Boe, B.A.; Shinoka, T. The Real Need for Regenerative Medicine in the Future of Congenital Heart Disease Treatment. Biomedicines 2021, 9, 478. https://doi.org/10.3390/biomedicines9050478
Matsuzaki Y, Wiet MG, Boe BA, Shinoka T. The Real Need for Regenerative Medicine in the Future of Congenital Heart Disease Treatment. Biomedicines. 2021; 9(5):478. https://doi.org/10.3390/biomedicines9050478
Chicago/Turabian StyleMatsuzaki, Yuichi, Matthew G. Wiet, Brian A. Boe, and Toshiharu Shinoka. 2021. "The Real Need for Regenerative Medicine in the Future of Congenital Heart Disease Treatment" Biomedicines 9, no. 5: 478. https://doi.org/10.3390/biomedicines9050478
APA StyleMatsuzaki, Y., Wiet, M. G., Boe, B. A., & Shinoka, T. (2021). The Real Need for Regenerative Medicine in the Future of Congenital Heart Disease Treatment. Biomedicines, 9(5), 478. https://doi.org/10.3390/biomedicines9050478






