DSIP-Like KND Peptide Reduces Brain Infarction in C57Bl/6 and Reduces Myocardial Infarction in SD Rats When Administered during Reperfusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Myocardial Infarction Model
2.2.1. Experimental Design-Groups and Doses
2.2.2. Procedure of Myocardial Infarction Simulation
2.2.3. Determination of the Area at Risk, the Infarction Size, the Total Area of the Left Ventricle
2.3. Cerebral Stroke Model
2.3.1. Experimental Design—Groups and Doses
2.3.2. Procedure of Acute Focal Stroke Simulation in Mice
2.3.3. Determination of the Volume of Cerebral Infarction
2.4. Preliminary Studies with the Introduction of DSIP and KND during the Occlusion Period
3. Results
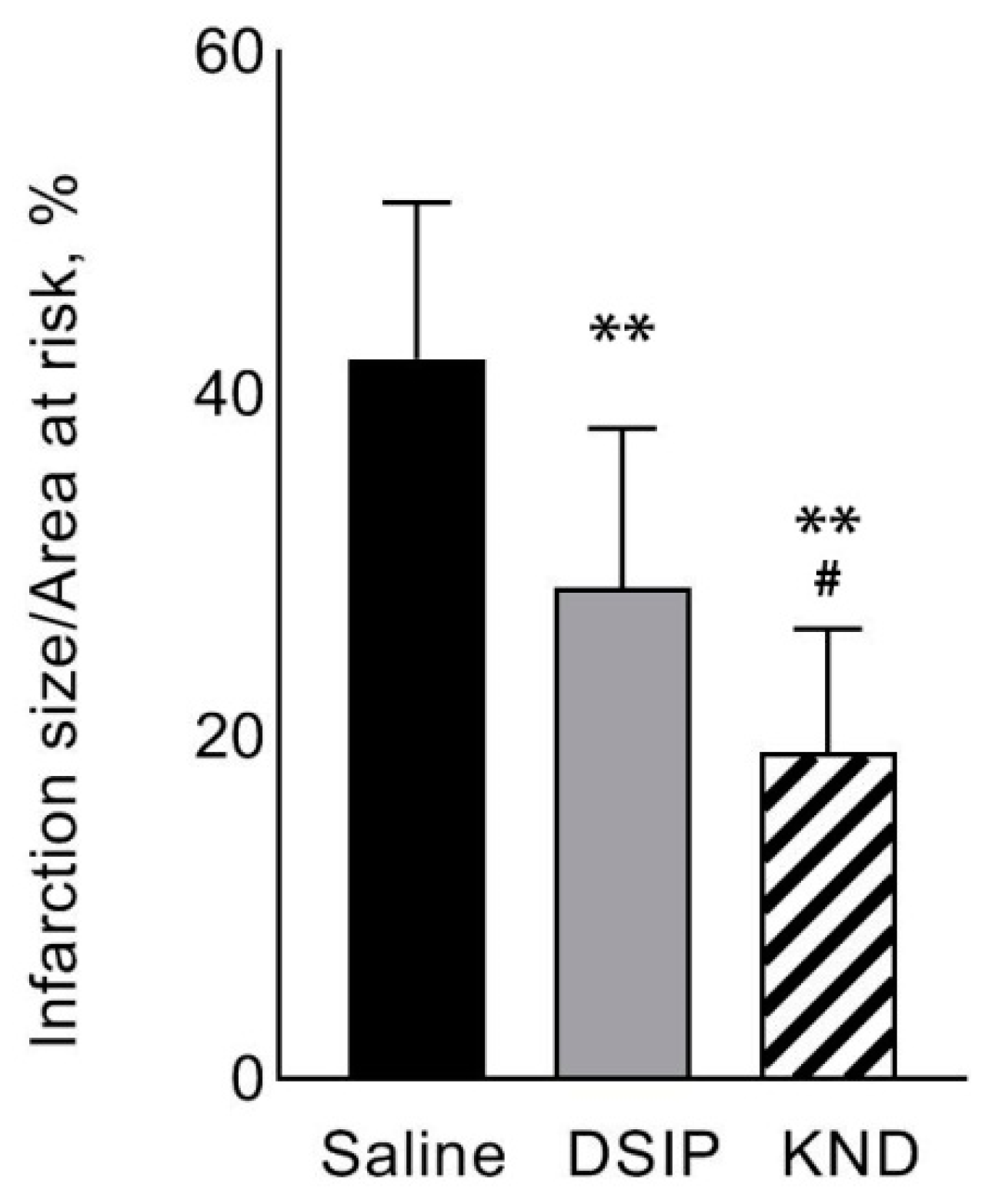
3.1. Heart Infarction
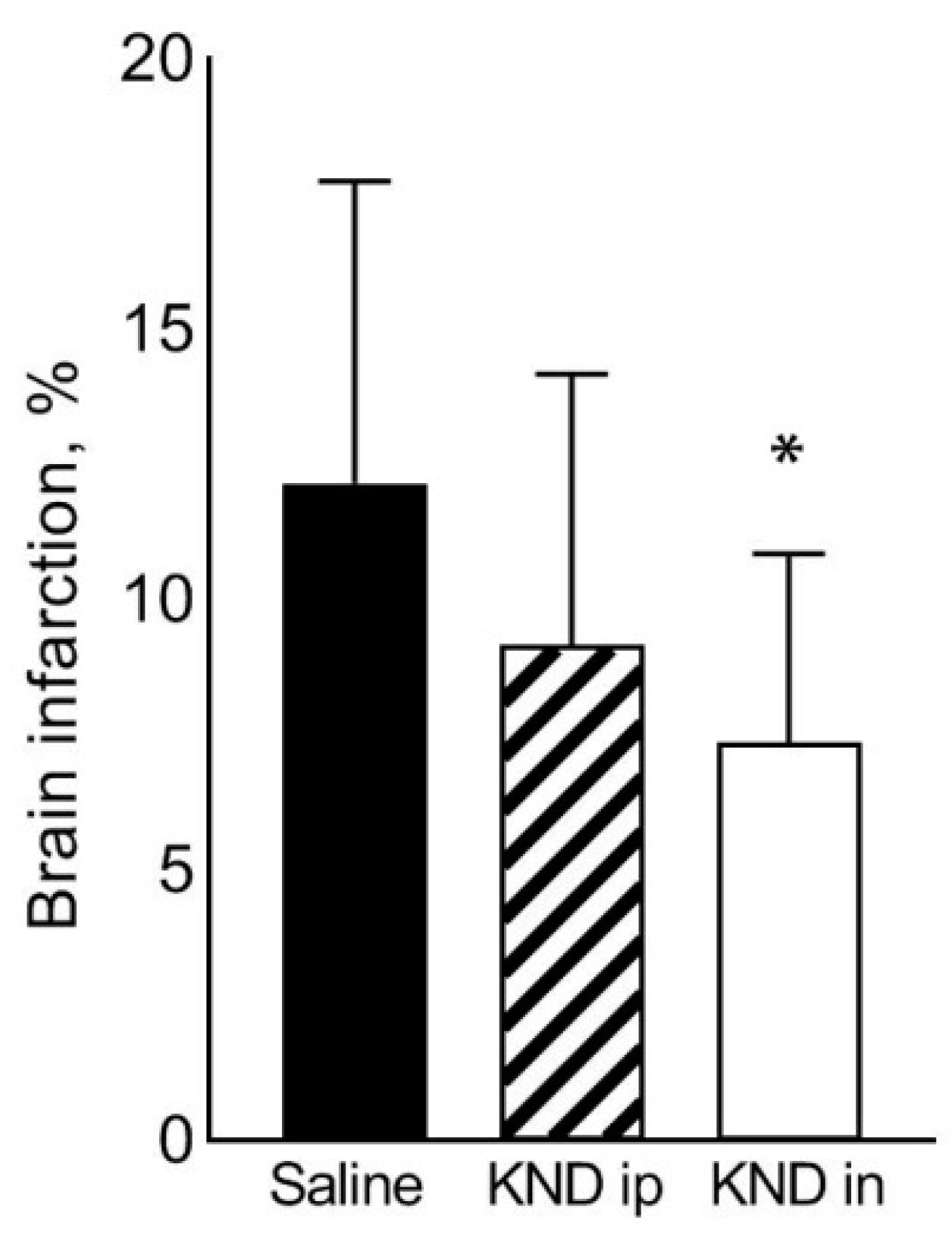
3.2. Stroke
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Raw Data on Heart Infarction
| Animal Number | Area at Risk (pxl) | Infarct Size (pxl) | IS/AAR (%) | AAR/LV (%) |
|---|---|---|---|---|
| 1 | 183,178 | 96,205 | 52.5 | 39.7 |
| 2 | 197,700 | 96,841 | 49.0 | 41.9 |
| 3 | 147,014 | 44,366 | 30.2 | 38.4 |
| 4 | 185,200 | 78,850 | 42.6 | 35.1 |
| 5 | 120,950 | 43,561 | 36.0 | 33.0 |
| Mean ± SD | 166,808 ± 31,841 | 71,965 ± 26,563 | 42.1 ±9.2 | 37.6 ± 3.6 |
| Animal Number | Area at Risk (pxl) | Infarct Size (pxl) | IS/AAR (%) | AAR/LV (%) |
|---|---|---|---|---|
| 6 | 131,333 | 29,030 | 22.1 | 38.7 |
| 7 | 141,290 | 20,690 | 14.6 | 50.7 |
| 8 | 125,025 | 54,818 | 43.8 | 16.4 |
| 9 | 157,725 | 43,169 | 27.4 | 36.19 |
| 10 | 168,022 | 60,736 | 36.1 | 42.46 |
| 11 | 179,456 | 63,629 | 35.5 | 30.84 |
| 12 | 144,298 | 33,708 | 23.4 | 32.4 |
| 13 | 190,906 | 50,938 | 26.7 | 44.6 |
| Mean ± SD | 154,757 ± 17,965 | 44,590 ± 16,871 | 28.7 ± 11.5 | 36.5 ± 17.4 |
| Animal Number | Area at Risk, pxl | Infarct Size, (pxl) | IS/AAR, % | AAR/LV, % |
|---|---|---|---|---|
| 14 | 160,999 | 45,037 | 28.0 | 37.9 |
| 15 | 145,299 | 25,736 | 17.7 | 29.0 |
| 16 | 172,489 | 40,656 | 23.6 | 39.9 |
| 17 | 219,653 | 46,644 | 21.2 | 44.5 |
| 18 | 195,348 | 51,709 | 26.5 | 43.7 |
| 19 | 142,787 | 10,276 | 7.2 | 39.9 |
| 20 | 134,033 | 22,997 | 17.2 | 30.6 |
| 21 | 128,607 | 14,278 | 11.1 | 32.2 |
| Mean ± SD | 162.402 ± 29.248 | 32.167 ± 9.892 | 19.1 ± 4.1 | 37.2 ± 6.2 |
Appendix A.2. Raw Data on Cerebral Stroke
| Animal Number | Right Hemisphere Volume (mm3) | Right Hemisphere Damage Volume (mm3) | Right Hemisphere Damage Volume (%) |
|---|---|---|---|
| 1 | 0.229 | 0.022 | 9.657 |
| 2 | 0.220 | 0.012 | 5.495 |
| 3 | 0.227 | 0.034 | 14.832 |
| 4 | 0.206 | 0.023 | 11.392 |
| 5 | 0.214 | 0.033 | 15.218 |
| 6 | 0.192 | 0.045 | 23.660 |
| 7 | 0.190 | 0.019 | 9.966 |
| 8 | 0.215 | 0.053 | 24.688 |
| 9 | 0.207 | 0.029 | 14.130 |
| 10 | 0.211 | 0.016 | 7.704 |
| 11 | 0.209 | 0.012 | 5.955 |
| 12 | 0.206 | 0.020 | 9.544 |
| 13 | 0.209 | 0.023 | 10.851 |
| 14 | 0.206 | 0.024 | 11.635 |
| 15 | 0.216 | 0.014 | 6.290 |
| 16 | 0.218 | 0.029 | 13.456 |
| Mean ± SD | 0.211 ± 0.011 | 0.026 ± 0.011 | 12.155 ± 5.592 |
| Animal Number | Right Hemisphere Volume, (mm3) | Right Hemisphere Damage Volume, (mm3) | Right Hemisphere Damage Volume (%) |
|---|---|---|---|
| 17 | 0.203 | 0.001 | 0.604 |
| 18 | 0.211 | 0.011 | 5.195 |
| 19 | 0.216 | 0.014 | 6.555 |
| 20 | 0.207 | 0.021 | 10.393 |
| 21 | 0.214 | 0.027 | 12.603 |
| 22 | 0.196 | 0.013 | 6.437 |
| 23 | 0.205 | 0.024 | 11.816 |
| 24 | 0.200 | 0.040 | 19.801 |
| 25 | 0.218 | 0.030 | 13.628 |
| 26 | 0.211 | 0.022 | 10.505 |
| 27 | 0.208 | 0.013 | 6.325 |
| 28 | 0.207 | 0.013 | 6.056 |
| Mean ± SD | 0.208 ± 0.007 | 0.019 ± 0.010 | 9.160 ± 5.011 |
| Animal Number | Right Hemisphere Volume (mm3) | Right Nemisphere Damage Volume (mm3) | Right Hmisphere Damage Volume (%) |
|---|---|---|---|
| 29 | 0.209 | 0.011 | 5.250 |
| 30 | 0.224 | 0.014 | 6.180 |
| 31 | 0.213 | 0.019 | 9.144 |
| 32 | 0.218 | 0.023 | 10.762 |
| 33 | 0.221 | 0.014 | 6.136 |
| 34 | 0.206 | 0.028 | 13.427 |
| 35 | 0.220 | 0.012 | 5.477 |
| 36 | 0.210 | 0.005 | 2.519 |
| Mean ± SD | 0.215 ± 0.007 | 0.016 ± 0.007 | 7.362 ± 3.503 |
References
- Mikhaleva, I.I.; Prudchenko, I.A.; Onoprienko, L.V.; Lobanov, A.V.; Rodionov, A.N.; Tukhovskaya, E.A.; Khokhlova, O.N.; Shykhutdinova, E.R.; Murashev, A.N.; Ivanov, V.T. Synthesis and Biological Properties of a Number of Analogs of DSIP and Its KND Endogenous Prototype. Russ. J. Bioorganic Chem. 2018, 44, 631–639. [Google Scholar] [CrossRef]
- Mammalian Gene Collection (MGC) Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl. Acad. Sci. USA 2002, 99, 16899–16903. [Google Scholar] [CrossRef] [PubMed]
- Mikhaleva, I.I.; Prudchenko, I.A.; Ivanov, V.T.; Voitenkov, V.B. JmjC-domain-containing histone demethylases of the JMJD1B type as putative precursors of endogenous DSIP. Peptides 2011, 32, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Konorova, I.L.; Gannushkina, I.V.; Koplik, E.V.; Antelava, A.L. Deltaran prevents an adverse effect of emotional stress on the course of cerebral ischemia in low-resistant animals. Bull. Exp. Biol. Med. 2006, 141, 564–566. [Google Scholar] [CrossRef]
- Shandra, A.A.; Godlevskii, L.S.; Brusentsov, A.I.; Vast’Yanov, R.S.; Karlyuga, V.A.; Dzygal, A.F.; Nikel, B. Effects of delta-sleep-inducing peptide in cerebral ischemia in rats. Neurosci. Behav. Physiol. 1998, 28, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Warltier, D.C.; Zyvoloski, M.G.; Gross, G.J.; Hardman, H.F.; Brooks, H.L. Determination of Experimental myocardial infarct size. J. Pharmacol. Methods 1981, 6, 199–210. [Google Scholar] [CrossRef]
- Vivaldi, M.T.; Kloner, R.A.; Schoen, F.J. Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am. J. Pathol. 1985, 121, 522–530. [Google Scholar] [PubMed]
- Valtchanova-Matchouganska, A.; Ojewole, J.A.O. Mechanisms of opioid delta (delta) and kappa (kappa) receptors’ cardioprotection in ischaemic preconditioning in a rat model of myocardial infarction. Cardiovasc. J. S. Afr. Off. J. South. Afr. Card. Soc. S. Afr. Soc. Card. Pr. 2003, 14, 73–80. [Google Scholar]
- Zhang, Y.; Irwin, M.G.; Wong, T.M. Remifentanil Preconditioning Protects against Ischemic Injury in the Intact Rat Heart. J. Am. Soc. Anesthesiol. 2004, 101, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Lishmanov, I.B.; Headrick, J.P.; Pei, J.-M.; Hanus, L.; Krylatov, A.V.; Naryzhnaia, N.V. Perspective of creation of drugs on basis of opioids increasing cardiac tolerance to pathogenic impact of ischemia reperfusion. Eksp Klin Farmakol. 2012, 75, 22–28. [Google Scholar] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Dygert, L.; Toborek, M. Induction of Ischemic Stroke and Ischemia-reperfusion in Mice Using the Middle Artery Occlusion Technique and Visualization of Infarct Area. J. Vis. Exp. 2017, e54805. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, T.I.; Maiboroda, E.A.; Mikhaleva, I.I.; Prudchenko, I.A. Mechanism of geroprotective action of delta-sleep inducing peptide. Adv. Gerontol. 2011, 1, 328–339. [Google Scholar] [CrossRef]
- Kutilin, D.S.; Bondarenko, T.I.; Kornienko, I.V.; Mikhaleva, I.I. Effect of Delta Sleep-Inducing Peptide on the Expression of Antioxidant Enzyme Genes in the Brain and Blood of Rats during Physiological Aging. Bull. Exp. Biol. Med. 2014, 157, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Umriukhin, P.E. Cycloheximide prevents inhibition of expression of immediate early gene c-fos in paraventricular nuclei of rat hypothalamus produced by delta sleep-inducing peptide. Bull. Exp. Biol. Med. 2002, 134, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Grigor’Ev, V.V.; Ivanova, T.A.; Kustova, E.A.; Petrova, L.N.; Serkova, T.P.; Bachurin, S.O. Effects of delta sleep-inducing peptide on pre- and postsynaptic glutamate and postsynaptic GABA receptors in neurons of the cortex, hippocampus, and cerebellum in rats. Bull. Exp. Biol. Med. 2006, 142, 186–188. [Google Scholar] [CrossRef]
- Khvatova, E.M.; Samartzev, V.N.; Zagoskin, P.P.; Prudchenko, I.A.; Mikhaleva, I.I. Delta sleep inducing peptide (DSIP): Effect on respiration activity in rat brain mitochondria and stress protective potency under experimental hypoxia. Peptides 2003, 24, 307–311. [Google Scholar] [CrossRef]
- Koplik, E.V.; Umryukhin, P.E.; Konorova, I.L.; Terekhina, O.L.; Mikhaleva, I.I.; Gannushkina, I.V.; Sudakov, K.V. Delta sleep-inducing peptide and Deltaran: Potential approaches to antistress protection. Neurosci. Behav. Physiol. 2008, 38, 953–957. [Google Scholar] [CrossRef]
- Dovedova, E.L.; Khrustalev, D.A.; Khudoerkov, R.M. Effect of Delta-Sleep-Inducing Peptide on Activity of Enzymes of Biogenic Amine Metabolism in the Brain of Wistar and August Rats. Bull. Exp. Biol. Med. 2005, 140, 514–516. [Google Scholar] [CrossRef]
- Allen, K.L.; Almeida, A.; Bates, T.E.; Clark, J.B. Changes of Respiratory Chain Activity in Mitochondrial and Synaptosomal Fractions Isolated from the Gerbil Brain after Graded Ischaemia. J. Neurochem. 2002, 64, 2222–2229. [Google Scholar] [CrossRef]
- Borutaite, V.; Mildaziene, V.; Brown, G.C.; Brand, M.D. Control and kinetic analysis of ischemia-damaged heart mitochondria: Which parts of the oxidative phosphorylation system are affected by ischemia? Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 1995, 1272, 154–158. [Google Scholar] [CrossRef]
- McCully, J.D.; Wakiyama, H.; Cowan, U.B.; Federman, M.; Parker, R.A.; Levitsky, S. Diazoxide amelioration of myocardial injury and mitochondrial damage during cardiac surgery. Ann. Thorac. Surg. 2002, 74, 2138–2146. [Google Scholar] [CrossRef][Green Version]
- Levitsky, S.; Laurikka, J.; Stewart, R.D.; Campos, C.T.; Lahey, S.J.; McCully, J.D. Mitochondrial DNA deletions in coronary artery bypass grafting patients. Eur. J. Cardio-Thoracic Surg. 2003, 24, 777–784. [Google Scholar] [CrossRef]
- Hsieh, Y.-J.; Wakiyama, H.; Levitsky, S.; McCully, J.D. Cardioplegia and Diazoxide Modulate STAT3 Activation and DNA Binding. Ann. Thorac. Surg. 2007, 84, 1272–1278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCully, J.D.; Levitsky, S.; Del Nido, P.J.; Cowan, D.B. Mitochondrial transplantation for therapeutic use. Clin. Transl. Med. 2016, 5, 16. [Google Scholar] [CrossRef]
- Rousou, A.J.; Ericsson, M.; Federman, M.; Levitsky, S.; McCully, J.D. Opening of mitochondrial KATP channels enhances cardioprotection through the modulation of mitochondrial matrix volume, calcium accumulation, and respiration. Am. J. Physiol. Circ. Physiol. 2004, 287, H1967–H1976. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Karmazyn, M. Relationship between oxidative phosphorylation and adenine nucleotide translocase activity of two populations of cardiac mitochondria and mechanical recovery of ischemic hearts following reperfusion. Can. J. Physiol. Pharmacol. 1989, 67, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.D.; Balaban, R.S. Mitochondrial F1-ATPase activity of canine myocardium: Effects of hypoxia and stimulation. Am. J. Physiol. Circ. Physiol. 1994, 266, H2396–H2403. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, G.; Zweier, J.; Duilio, C.; Kuppusamy, P.; Santoro, G.; Elia, P.; Tritto, I.; Cirillo, P.; Condorelli, M.; Chiariello, M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 1993, 268, 18532–18541. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Slabe, T.J.; Stoll, M.S.K.; Minkler, P.E.; Hoppel, C.L. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am. J. Physiol. Circ. Physiol. 2001, 280, H2770–H2778. [Google Scholar] [CrossRef] [PubMed]
- Lesnefsky, E.J.; Tandler, B.; Ye, J.; Slabe, T.J.; Turkaly, J.; Hoppel, C.L. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am. J. Physiol. Content 1997, 273, H1544–H1554. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.C.; Strey, F.; Talbert, L. Investigation of the essential boundary layer phospholipids of cytochrome c oxidase using Triton X-100 delipidation. Biochemistry 1980, 19, 3656–3661. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V.; Jekabsone, A.; Morkuniene, R.; Brown, G.C. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J. Mol. Cell. Cardiol. 2003, 35, 357–366. [Google Scholar] [CrossRef]
- Reed, D.R.G.A.J.C. Mitochondria and Apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Moghaddas, S.; Tandler, B.; Kerner, J.; Hoppel, C.L. Mitochondrial Dysfunction in Cardiac Disease: Ischemia–Reperfusion, Aging, and Heart Failure. J. Mol. Cell. Cardiol. 2001, 33, 1065–1089. [Google Scholar] [CrossRef]
- Weiss, J.N.; Korge, P.; Honda, H.M.; Ping, P. Role of the Mitochondrial Permeability Transition in Myocardial Disease. Circ. Res. 2003, 93, 292–301. [Google Scholar] [CrossRef]
- Shin, B.; Saeed, M.Y.; Esch, J.J.; Guariento, A.; Blitzer, D.; Moskowitzova, K.; Ramirez-Barbieri, G.; Orfany, A.; Thedsanamoorthy, J.K.; Cowan, D.B.; et al. A Novel Biological Strategy for Myocardial Protection by Intracoronary Delivery of Mitochondria: Safety and Efficacy. JACC Basic Transl. Sci. 2019, 4, 871–888. [Google Scholar] [CrossRef]
- McCully, J.D.; Cowan, D.B.; Pacak, C.A.; Toumpoulis, I.K.; Dayalan, H.; Levitsky, S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Circ. Physiol. 2009, 296, H94–H105. [Google Scholar] [CrossRef]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef]
- Kaza, A.K.; Wamala, I.; Friehs, I.; Kuebler, J.D.; Rathod, R.H.; Berra, I.; Ericsson, M.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg. 2017, 153, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.B.; Yao, R.; Akurathi, V.; Snay, E.R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Ericsson, M.; Friehs, I.; Wu, Y.; Levitsky, S.; et al. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS ONE 2016, 11, e0160889. [Google Scholar] [CrossRef]
- Pacak, C.A.; Preble, J.M.; Kondo, H.; Seibel, P.; Levitsky, S.; Del Nido, P.J.; Cowan, D.B.; McCully, J.D. Actin-dependent mitochondrial internalization in cardiomyocytes: Evidence for rescue of mitochondrial function. Biol. Open 2015, 4, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.B.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D. Transit and integration of extracellular mitochondria in human heart cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McCully, J.D.; Cowan, D.B.; Emani, S.M.; del Nido, P.J. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion 2017, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; del Nido, P.J.; McCully, J.D. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Pomfrett, C.J.D.; Dolling, S.; Anders, N.R.K.; Glover, D.G.; Bryan, A.; Pollard, B.J. Delta sleep-inducing peptide alters bispectral index, the electroencephalogram and heart rate variability when used as an adjunct to isoflurane anaesthesia. Eur. J. Anaesthesiol. 2009, 26, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.L.; Masson, N.; Dunne, K.; Flashman, E.; Kawamura, A. The Activity of JmjC Histone Lysine Demethylase KDM4A is Highly Sensitive to Oxygen Concentrations. ACS Chem. Biol. 2017, 12, 1011–1019. [Google Scholar] [CrossRef]
- Batie, M.; Rocha, S. JmjC histone demethylases act as chromatin oxygen sensors. Mol. Cell. Oncol. 2019, 6, 1608501. [Google Scholar] [CrossRef]
- Yang, J.; Harris, A.L.; Davidoff, A.M. Hypoxia and Hormone-Mediated Pathways Converge at the Histone Demethylase KDM4B in Cancer. Int. J. Mol. Sci. 2018, 19, 240. [Google Scholar] [CrossRef]
- Healy, S.; Khan, P.; Davie, J.R. Immediate early response genes and cell transformation. Pharmacol. Ther. 2013, 137, 64–77. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, J.S.; Céspedes, I.C.; Abrão, R.O.; Dos Santos, T.B.; Diniz, L.; De Britto, L.R.G.; Bratfisch, R.C.S.; Ortolani, D.; Melo-Thomas, L.; Da Silva, R.C.B.; et al. Chronic unpredictable mild stress alters an anxiety-related defensive response, Fos immunoreactivity and hippocampal adult neurogenesis. Behav. Brain Res. 2013, 250, 81–90. [Google Scholar] [CrossRef]
- Imbe, H.; Kimura, A.; Donishi, T.; Kaneoke, Y. Repeated forced swim stress enhances CFA-evoked thermal hyperalgesia and affects the expressions of pCREB and c-Fos in the insular cortex. Neurosci. 2014, 259, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ohmomo, H.; Shutoh, F.; Nogami, H.; Hisano, S. Presentation of noise during acute restraint stress attenuates expression of immediate early genes and arginine vasopressin in the hypothalamic paraventricular nucleus but not corticosterone secretion in rats. Neurosci. Res. 2015, 96, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.J. Measurement of Immediate-Early Gene Activation-c-fosand Beyond. J. Neuroendocr. 2008, 20, 665–672. [Google Scholar] [CrossRef]
- Sudakov, K.V.; Umryukhin, P.E.; Koplik, E.V.; Anokhin, K.V. Expression of the c-fos gene during emotional stress in rats: The clocking effect of delta sleep-inducing peptide. Neurosci. Behav. Physiol. 2001, 31, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Umriukhin, P.; Koplik, E.; Sudakov, K. Dizocilpine and cycloheximide prevent inhibition of c-Fos gene expression by delta sleep-inducing peptide in the paraventricular nucleus of the hypothalamus in rats with different resistance to emotional stress. Neurosci. Lett. 2012, 506, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Aĭvazian, L.M.; Zakharian, G.V.; Melkonian, M.M. Shift in the content of immune cytokines in heart of mice under acoustic stress conditions and delta-sleep inducing peptide application. Georgian Med. News 2008, 158, 45–48. [Google Scholar]
- Voĭtenkov, V.B.; Popovich, I.G.; Arutiunian, A.V.; I Oparina, T.; Prokopenko, V.M. Effect of delta-sleep inducing peptide on free-radical processes in the brain and liver of mice during various light regimens. Adv. Gerontol. Uspekhi Gerontol. 2008, 21, 53–55. [Google Scholar]
- Belykh, A.E.; Bobyntsev, I.I. Delta Sleep-Inducing Peptide: Several Biological Effects and Mechanisms of Their Development. Kursk Sci. Pract. Bull. Man His Health 2016, 1, 79–90. [Google Scholar]




| Group | Drug | Dose/Injection Volume | Route of Administration | Number of Animals |
|---|---|---|---|---|
| 1 | Vehicle (saline) | 5 mL/kg | intraperitoneally | 5 |
| 2 | KND | 150 μg/kg/5 mL | intraperitoneally | 8 |
| 3 | DSIP | 150 μg/kg/5 mL | intraperitoneally | 8 |
| Group | Drug | Dose/Injection Volume | Route of Administration | Number of Animals |
|---|---|---|---|---|
| 1 | Vehicle (saline) | 5 mL/kg | intraperitoneally | 8 |
| 5 μL/mouse | intranasally | 8 | ||
| 2 | KND | 300 μg/kg/5 mL | intraperitoneally | 12 |
| 3 | DS KND IP | 300 μg/kg in 5 μL/mouse | intranasally | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukhovskaya, E.A.; Shaykhutdinova, E.R.; Ismailova, A.M.; Slashcheva, G.A.; Prudchenko, I.A.; Mikhaleva, I.I.; Khokhlova, O.N.; Murashev, A.N.; Ivanov, V.T. DSIP-Like KND Peptide Reduces Brain Infarction in C57Bl/6 and Reduces Myocardial Infarction in SD Rats When Administered during Reperfusion. Biomedicines 2021, 9, 407. https://doi.org/10.3390/biomedicines9040407
Tukhovskaya EA, Shaykhutdinova ER, Ismailova AM, Slashcheva GA, Prudchenko IA, Mikhaleva II, Khokhlova ON, Murashev AN, Ivanov VT. DSIP-Like KND Peptide Reduces Brain Infarction in C57Bl/6 and Reduces Myocardial Infarction in SD Rats When Administered during Reperfusion. Biomedicines. 2021; 9(4):407. https://doi.org/10.3390/biomedicines9040407
Chicago/Turabian StyleTukhovskaya, Elena A., Elvira R. Shaykhutdinova, Alina M. Ismailova, Gulsara A. Slashcheva, Igor A. Prudchenko, Inessa I. Mikhaleva, Oksana N. Khokhlova, Arkady N. Murashev, and Vadim T. Ivanov. 2021. "DSIP-Like KND Peptide Reduces Brain Infarction in C57Bl/6 and Reduces Myocardial Infarction in SD Rats When Administered during Reperfusion" Biomedicines 9, no. 4: 407. https://doi.org/10.3390/biomedicines9040407
APA StyleTukhovskaya, E. A., Shaykhutdinova, E. R., Ismailova, A. M., Slashcheva, G. A., Prudchenko, I. A., Mikhaleva, I. I., Khokhlova, O. N., Murashev, A. N., & Ivanov, V. T. (2021). DSIP-Like KND Peptide Reduces Brain Infarction in C57Bl/6 and Reduces Myocardial Infarction in SD Rats When Administered during Reperfusion. Biomedicines, 9(4), 407. https://doi.org/10.3390/biomedicines9040407






