Preoperative Localization for Primary Hyperparathyroidism: A Clinical Review
Abstract
1. Introduction
1.1. Clinical Relevance of Primary Hyperparathyroidism
1.2. Clinically Relevant Embryology of the Parathyroid Glands
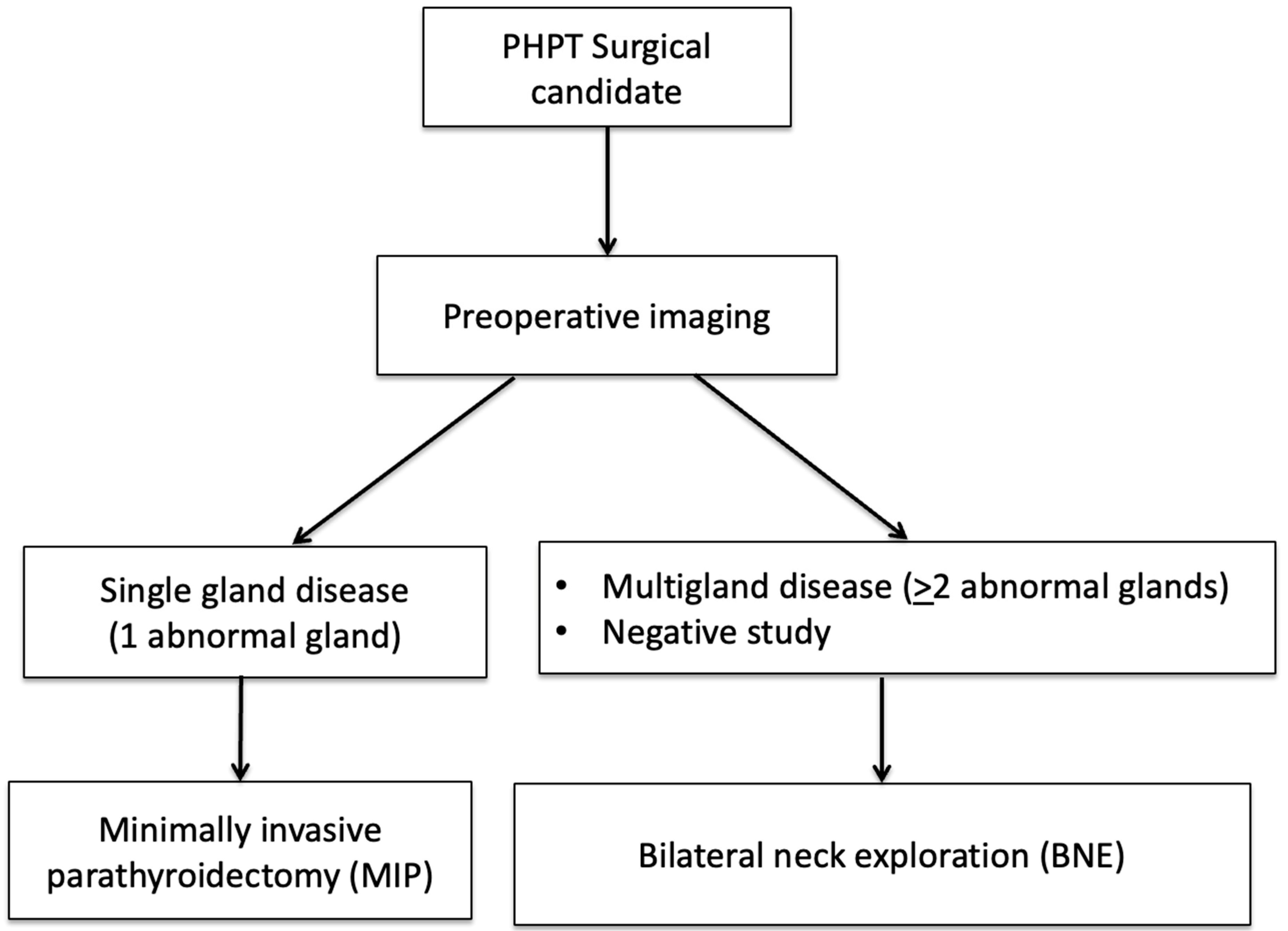
1.3. Role of Imaging in PHPT
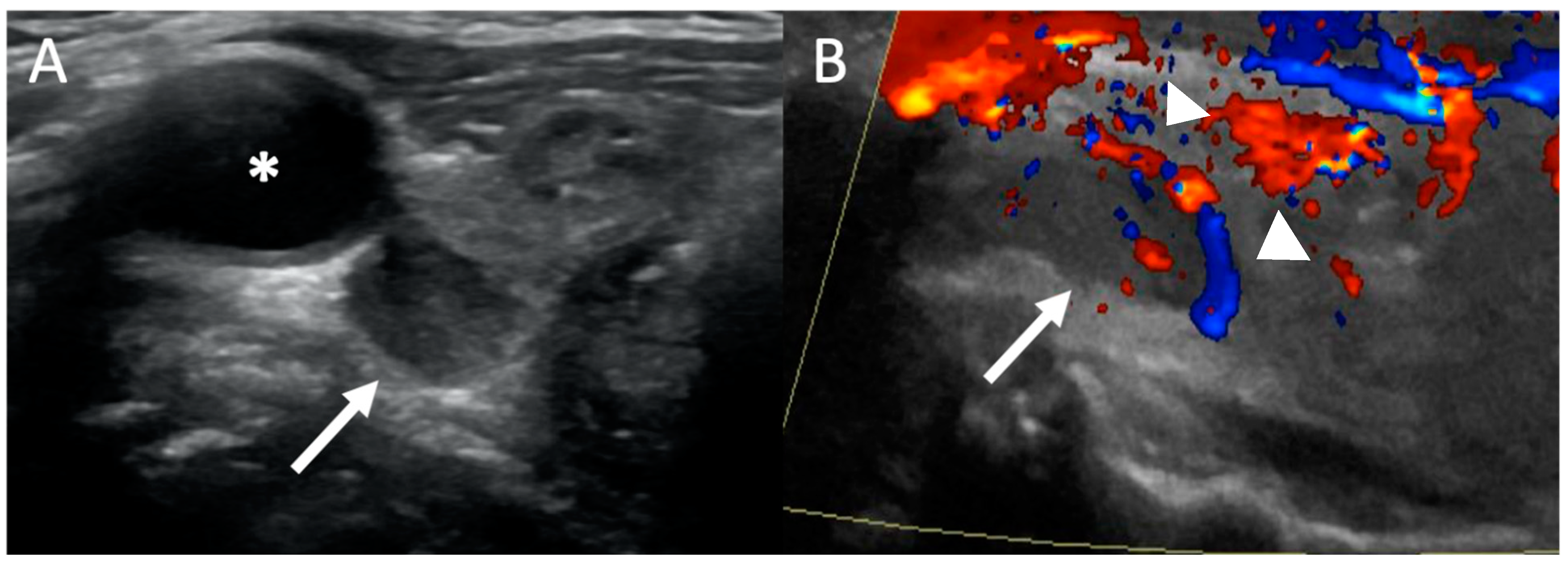
2. Ultrasound
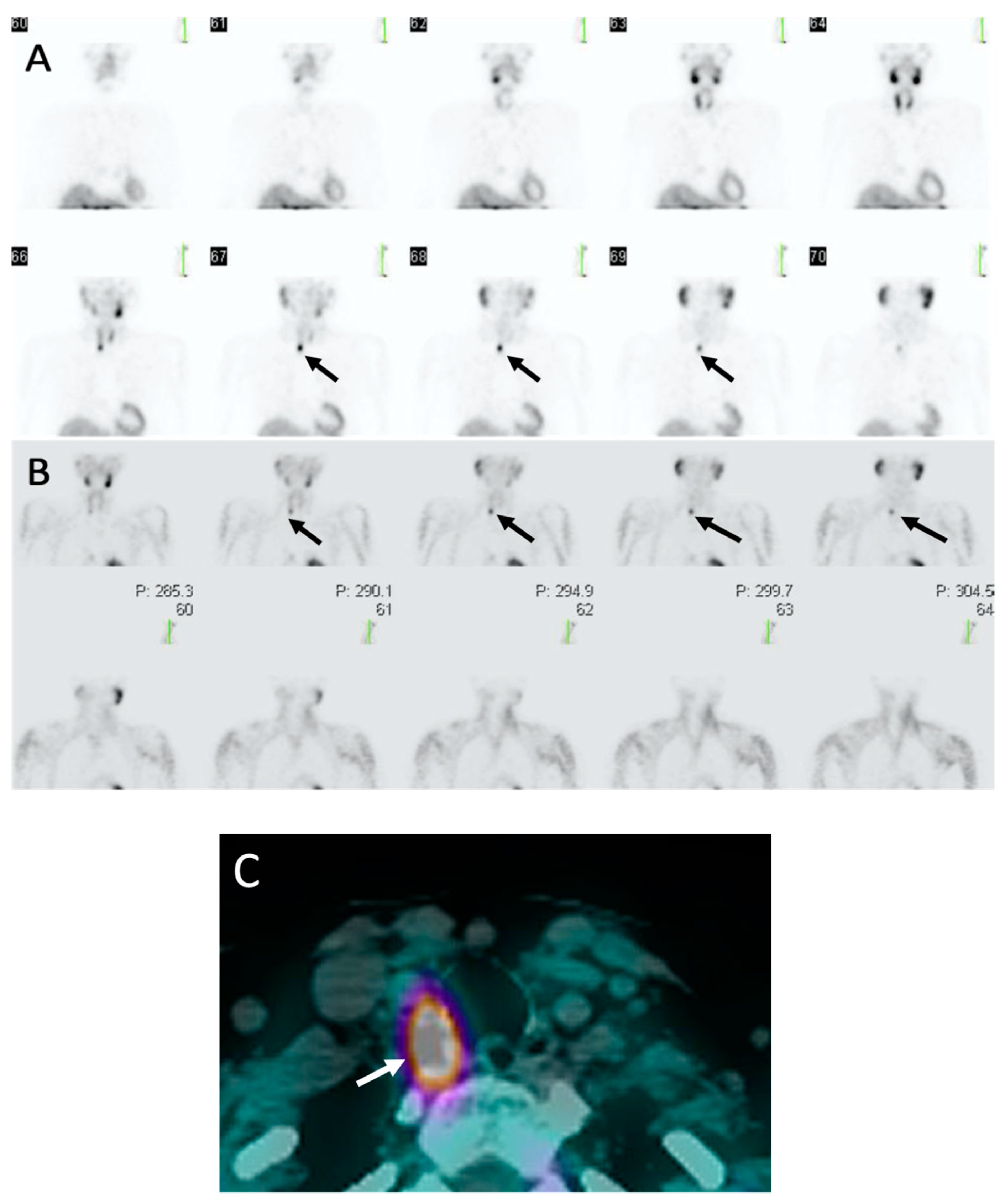
3. Sestamibi Scintigraphy
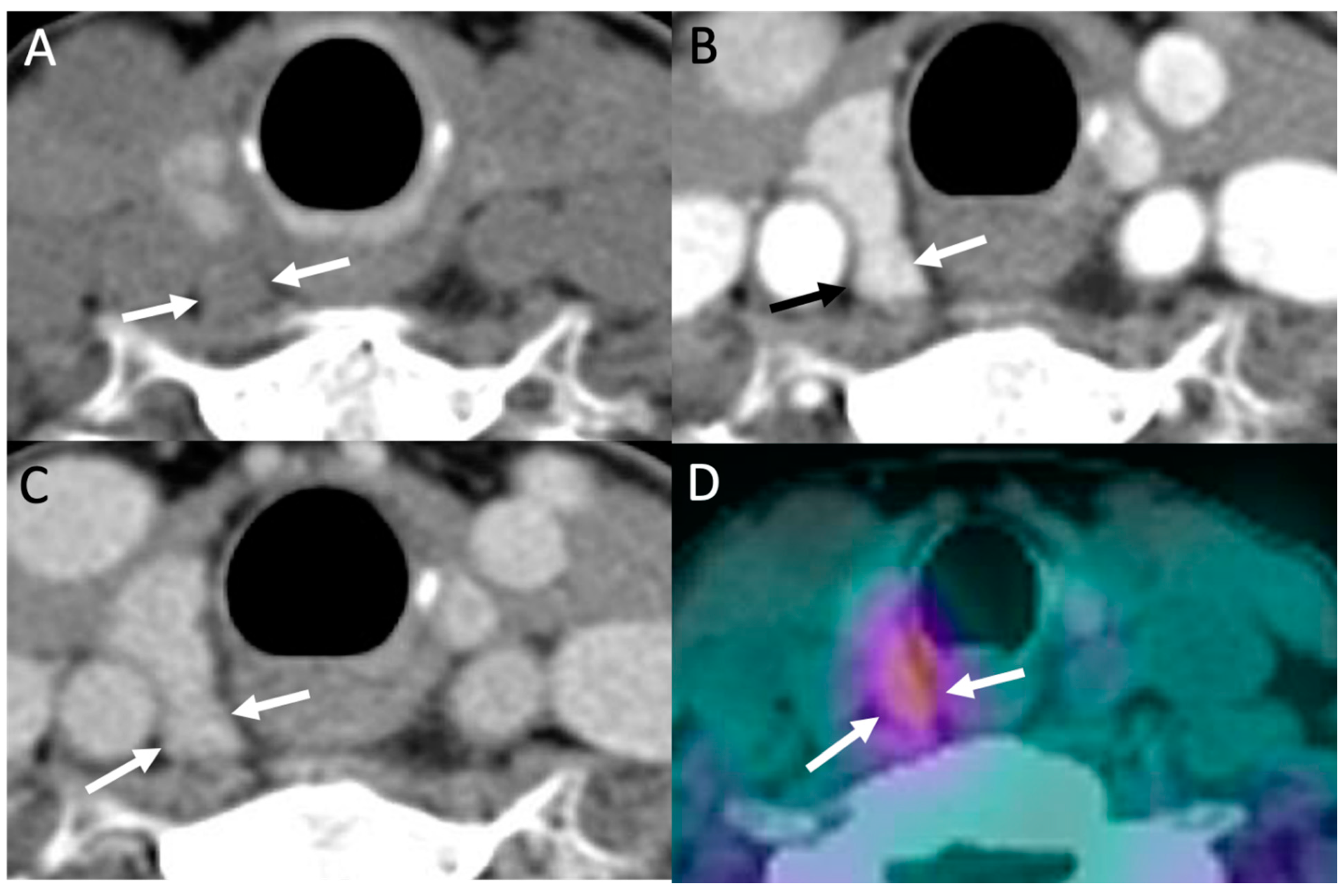
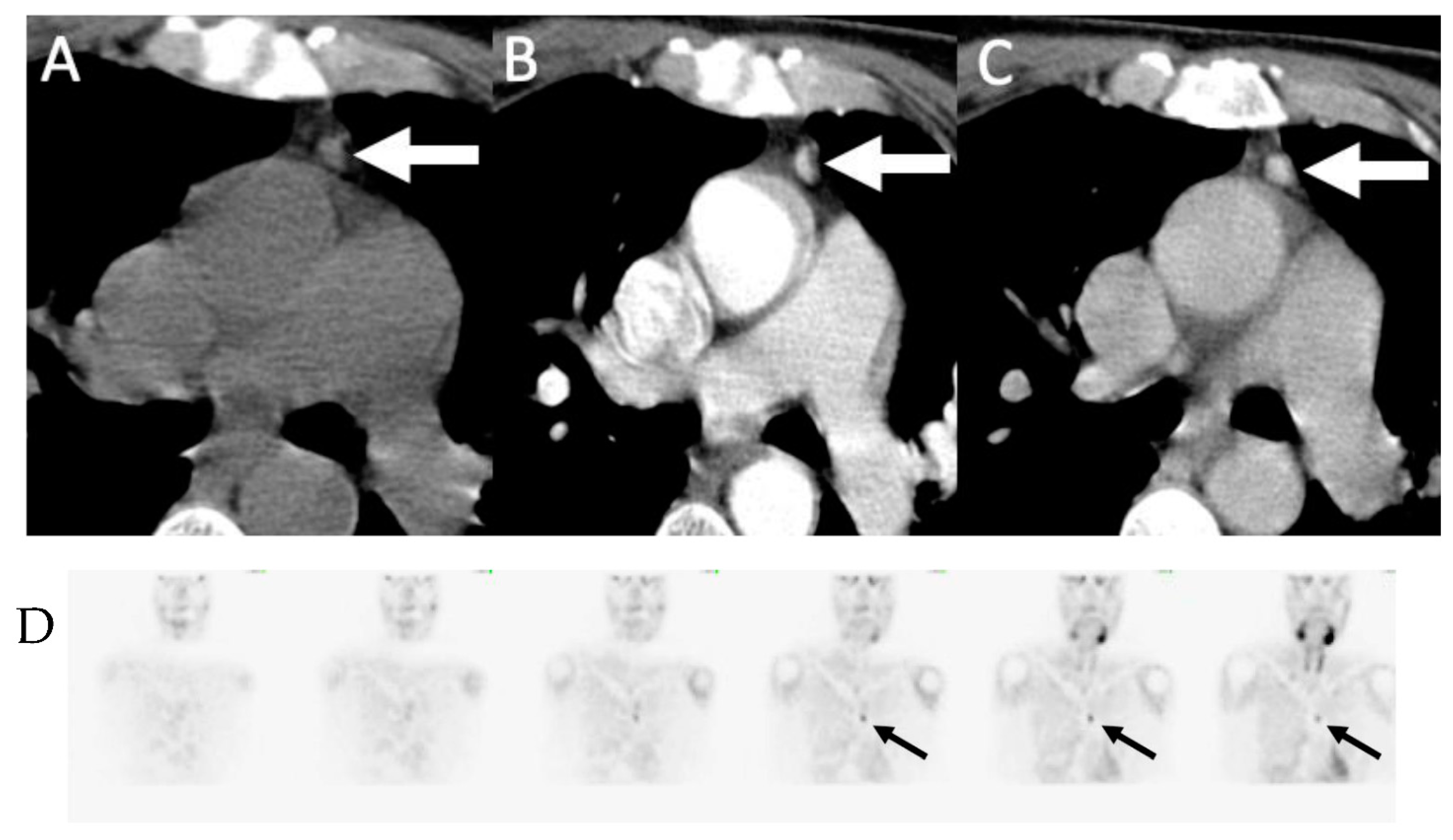
4. Parathyroid 4D-CT
5. Parathyroid Venous Sampling
6. Other Newer Modalities
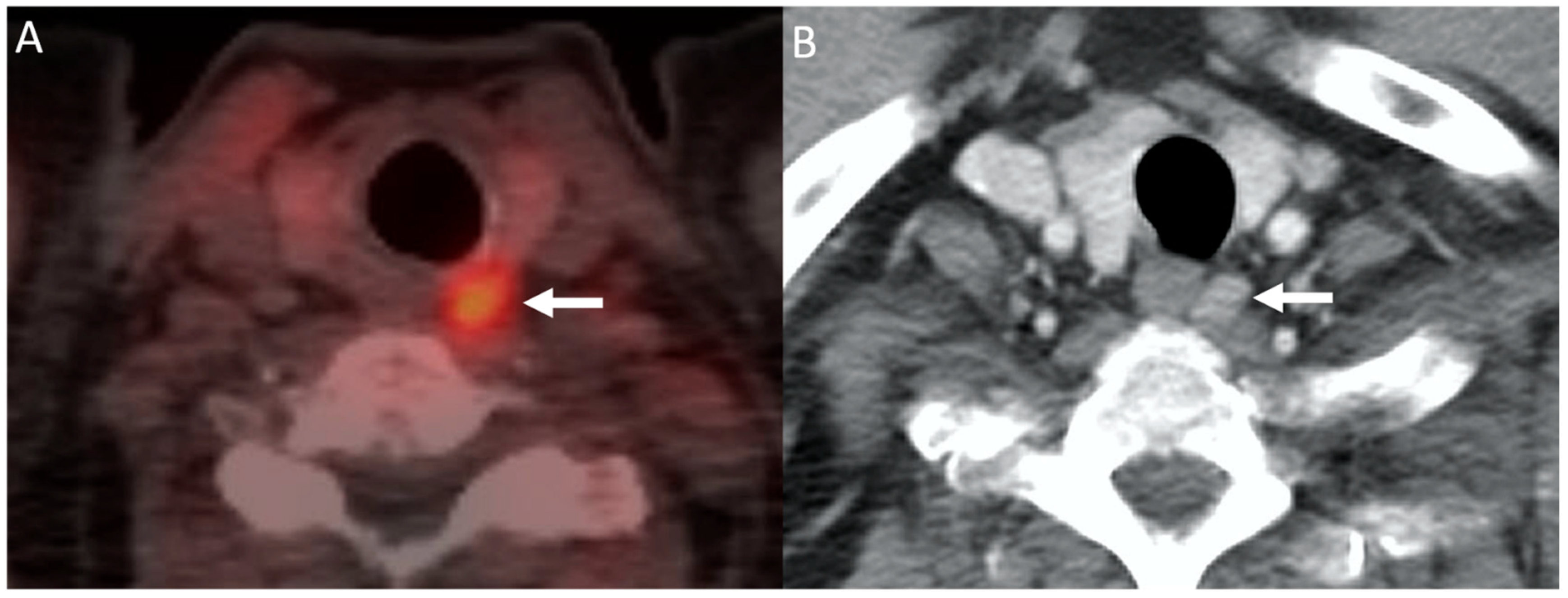
6.1. Choline PET/CT
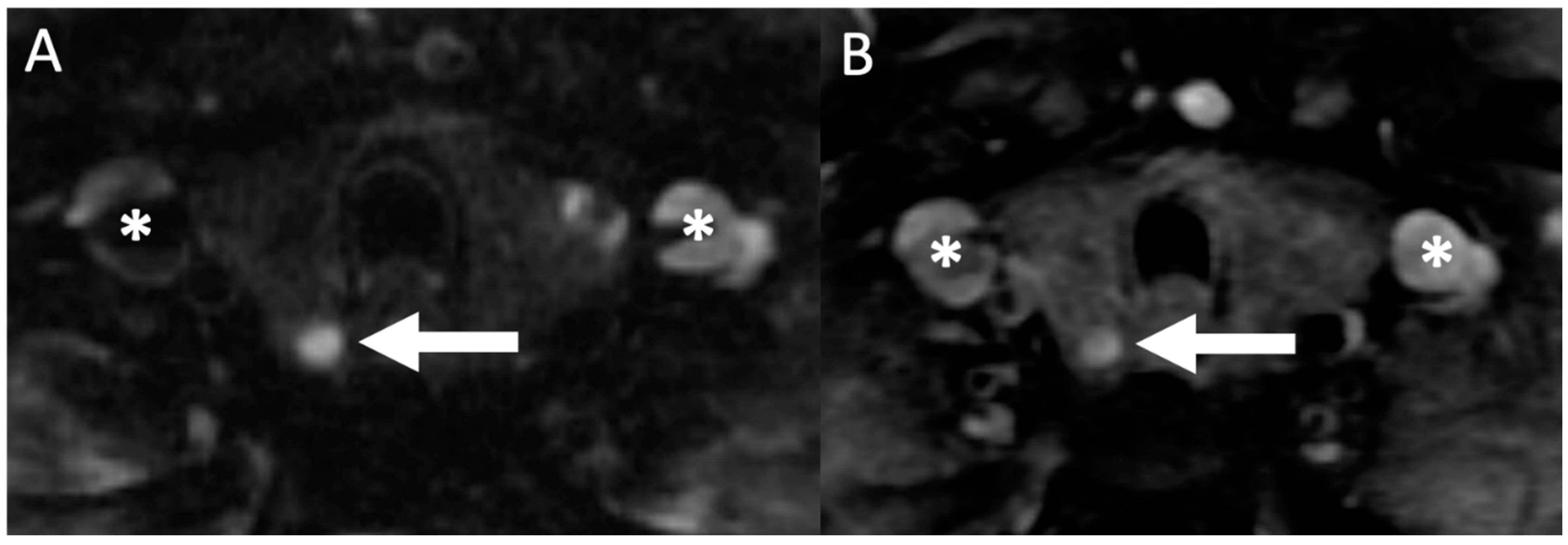
6.2. MRI
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adami, S.; Marcocci, C.; Gatti, D. Epidemiology of primary hyperparathyroidism in Europe. J. Bone Miner. Res. 2002, 17, 18. [Google Scholar]
- Khosla, S.; Melton, J. Fracture risk in primary hyperparathyroidism. J. Bone Miner. Res. 2002, 17, N103–N107. [Google Scholar] [PubMed]
- Vignali, E.; Viccica, G.; Diacinti, D.; Cetani, F.; Cianferotti, L.; Ambrogini, E.; Banti, C.; Del Fiacco, R.; Bilezikian, J.P.; Pinchera, A.; et al. Morphometric Vertebral Fractures in Postmenopausal Women with Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2009, 94, 2306–2312. [Google Scholar] [CrossRef]
- Cipriani, C.; Biamonte, F.; Costa, A.G.; Zhang, C.; Biondi, P.; Diacinti, D.; Pepe, J.; Piemonte, S.; Scillitani, A.; Minisola, S.; et al. Prevalence of Kidney Stones and Vertebral Fractures in Primary Hyperparathyroidism Using Imaging Technology. J. Clin. Endocrinol. Metab. 2015, 100, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, S.J.; Shane, E.; De La Cruz, L.; Dempster, D.W.; Feldman, F.; Seldin, D.; Jacobs, T.P.; Siris, E.S.; Cafferty, M.; Parisien, M.V.; et al. Skeletal disease in primary hyperparathyroidism. J. Bone Miner. Res. 2009, 4, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.-K.D.; Yeh, R.; Kuo, J.H.; McManus, C.; Lee, J.A.; Bilezikian, J.P. Pre-operative localization of abnormal parathyroid tissue by 99mTc-sestamibi in primary hyperparathyroidism using four-quadrant site analysis: An evaluation of the predictive value of vitamin D deficiency. Endocrine 2018, 60, 36–45. [Google Scholar] [CrossRef]
- Dropkin, B.M.; Moses, R.A.; Sharma, D.; Pais, V.M. The Natural History of Nonobstructing Asymptomatic Renal Stones Managed with Active Surveillance. J. Urol. 2015, 193, 1265–1269. [Google Scholar] [CrossRef]
- Siperstein, A.; Berber, E.; Mackey, R.; Alghoul, M.; Wagner, K.; Milas, M. Prospective evaluation of sestamibi scan, ultrasonography, and rapid PTH to predict the success of limited exploration for sporadic primary hyperparathyroidism. Surgery 2004, 136, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Hwang, J.; Reiff, E.; Duh, Q.-Y.; Clark, O.H. Predictors of Single-Gland vs Multigland Parathyroid Disease in Primary Hyperparathyroidism. Arch. Surg. 2006, 141, 777–782. [Google Scholar] [CrossRef]
- Akerström, G.; Malmaeus, J.; Bergström, R. Surgical anatomy of human parathyroid glands. Surgery 1984, 95, 14–21. [Google Scholar]
- Taterra, D.; Wong, L.M.; Vikse, J.; Sanna, B.; Pękala, P.; Walocha, J.; Cirocchi, R.; Tomaszewski, K.; Henry, B.M. The prevalence and anatomy of parathyroid glands: A meta-analysis with implications for parathyroid surgery. Langenbeck’s Arch. Surg. 2019, 404, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Phitayakorn, R.; McHenry, C.R. Incidence and location of ectopic abnormal parathyroid glands. Am. J. Surg. 2006, 191, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.K.; Sung, W.-K.; Bahl, M.; Phillips, C.D. How to Perform Parathyroid 4D CT: Tips and Traps for Technique and Interpretation. Radiology 2014, 270, 15–24. [Google Scholar] [CrossRef]
- Wang, C.-A. The Anatomic Basis of Parathyroid Surgery. Ann. Surg. 1976, 183, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.; Tay, Y.-K.; Dercle, L.; Bandeira, L.; Parekh, M.; Bilezikian, J. A Simple Formula to Estimate Parathyroid Weight on 4D-CT, Predict Pathologic Weight, and Diagnose Parathyroid Adenoma in Patients with Primary Hyperparathyroidism. Am. J. Neuroradiol. 2020. [Google Scholar] [CrossRef]
- Bilezikian, J.P. Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2018, 103, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Udelsman, R.; Åkerström, G.; Biagini, C.; Duh, Q.-Y.; Miccoli, P.; Niederle, B.; Tonelli, F. The Surgical Management of Asymptomatic Primary Hyperparathyroidism: Proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab. 2014, 99, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.-Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Zafereo, M.; Yu, J.; Angelos, P.; Brumund, K.; Chuang, H.H.; Goldenberg, D.; Lango, M.; Perrier, N.; Randolph, G.; Shindo, M.L.; et al. American Head and Neck Society Endocrine Surgery Section update on parathyroid imaging for surgical candidates with primary hyperparathyroidism. Head Neck 2019, 41, 2398–2409. [Google Scholar] [CrossRef]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in Parathyroid Imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 151–166. [Google Scholar] [CrossRef]
- Yeh, R.; Tay, Y.-K.D.; Tabacco, G.; Dercle, L.; Kuo, J.H.; Bandeira, L.; McManus, C.; Leung, D.K.; Lee, J.A.; Bilezikian, J.P. Diagnostic Performance of 4D CT and Sestamibi SPECT/CT in Localizing Parathyroid Adenomas in Primary Hyperparathyroidism. Radiology 2019, 291, 469–476. [Google Scholar] [CrossRef]
- American Institute of Ultrasound in Medicine AIUM Practice Guideline for the Performance of a Thyroid and Parathyroid Ultrasound Examination. J. Ultrasound Med. 2003, 22, 1126–1130. [CrossRef] [PubMed]
- Wolf, R.J.; Cronan, J.J.; Monchik, J.M. Color Doppler sonography: An adjunctive technique in assessment of parathyroid adenomas. J. Ultrasound Med. 1994, 13, 303–308. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moloudi, F.; Ghasemi-Rad, M. The role of colour Doppler ultrasonography in the preoperative localization of parathyroid adenomas. Endocr. J. 2012, 59, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.T.; Yip, L.; Tublin, M.E. Sonography of intrathyroid parathyroid adenomas: Are there distinctive features that allow for preoperative identification? Eur. J. Radiol. 2013, 82, e22–e27. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Wang, T.S.; Farrokhyar, F.; Roman, S.A.; Sosa, J.A. A Meta-analysis of Preoperative Localization Techniques for Patients with Primary Hyperparathyroidism. Ann. Surg. Oncol. 2011, 19, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.Q.; Holcombe, J.M.; Rackley, A.A.; Tanner, R.M.; Giles, W.H. Negative Sestamibi Scans Predict Lower Likelihood of Surgical Referral in Patients with Primary Hyperparathyroidism. Am. Surg. 2018, 84, 1264–1268. [Google Scholar] [CrossRef]
- De Feo, M.L.; Colagrande, S.; Biagini, C.; Tonarelli, A.; Bisi, G.; Vaggelli, L.; Borrelli, D.; Cicchi, P.; Tonelli, F.; Amorosi, A.; et al. Parathyroid Glands: Combination of99mTc MIBI Scintigraphy and US for Demonstration of Parathyroid Glands and Nodules. Radiology 2000, 214, 393–402. [Google Scholar] [CrossRef]
- Solorzano, C.C.; Carneiro-Pla, D.M.; Irvin, G.L. Surgeon-Performed Ultrasonography as the Initial and Only Localizing Study in Sporadic Primary Hyperparathyroidism. J. Am. Coll. Surg. 2006, 202, 18–24. [Google Scholar] [CrossRef]
- Ruda, J.M.; Hollenbeak, C.S.; Stack, B.C. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol. Neck Surg. 2005, 132, 359–372. [Google Scholar] [CrossRef]
- Berber, E.; Parikh, R.T.; Ballem, N.; Garner, C.N.; Milas, M.; Siperstein, A.E. Factors Contributing to Negative Parathyroid Localization: An Analysis of 1000 patients. Surgery 2008, 144, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Mihai, R.; Simon, D.; Hellman, P. Imaging for primary hyperparathyroidism—an evidence-based analysis. Langenbecks Arch. Chir. 2009, 394, 765–784. [Google Scholar] [CrossRef] [PubMed]
- Eslamy, H.K.; Ziessman, H.A. Parathyroid Scintigraphy in Patients with Primary Hyperparathyroidism:99mTc Sestamibi SPECT and SPECT/CT. Radiographics 2008, 28, 1461–1476. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R.; Schalin-Jäntti, C.; Caldarella, C.; Ceriani, L.; Giovanella, L.; Eisele, D.W. Detection rate of 99m Tc-MIBI single photon emission computed tomography (SPECT)/CT in preoperative planning for patients with primary hyperparathyroidism: A meta-analysis. Head Neck 2016, 38, E2159–E2172. [Google Scholar] [CrossRef]
- Krakauer, M.; Wieslander, B.; Myschetzky, P.S.; Lundstrøm, A.; Bacher, T.; Sørensen, C.H.; Trolle, W.; Nygaard, B.; Bennedbæk, F.N. A Prospective Comparative Study of Parathyroid Dual-Phase Scintigraphy, Dual-Isotope Subtraction Scintigraphy, 4D-CT, and Ultrasonography in Primary Hyperparathyroidism. Clin. Nucl. Med. 2016, 41, 93–100. [Google Scholar] [CrossRef]
- Lavely, W.C.; Goetze, S.; Friedman, K.P.; Leal, J.P.; Zhang, Z.; Garret-Mayer, E.; Dackiw, A.P.; Tufano, R.P.; Zeiger, M.A.; Ziessman, H.A. Comparison of SPECT/CT, SPECT, and Planar Imaging with Single- and Dual-Phase 99mTc-Sestamibi Parathyroid Scintigraphy. J. Nucl. Med. 2007, 48, 1084–1089. [Google Scholar] [CrossRef]
- Sinha, P.; Oates, M.E. Parathyroid Scintigraphy: How Does It Fit In? Radiology 2019, 291, 477–478. [Google Scholar] [CrossRef]
- Hoang, J.K.; Reiman, R.E.; Nguyen, G.B.; Januzis, N.; Chin, B.B.; Lowry, C.; Yoshizumi, T.T. Lifetime Attributable Risk of Cancer From Radiation Exposure During Parathyroid Imaging: Comparison of 4D CT and Parathyroid Scintigraphy. Am. J. Roentgenol. 2015, 204, W579–W585. [Google Scholar] [CrossRef]
- Lubitz, C.C.; Stephen, A.E.; Hodin, R.A.; Pandharipande, P.V. Preoperative Localization Strategies for Primary Hyperparathyroidism: An Economic Analysis. Ann. Surg. Oncol. 2012, 19, 4202–4209. [Google Scholar] [CrossRef]
- Rodgers, S.E.; Hunter, G.J.; Hamberg, L.M.; Schellingerhout, D.; Doherty, D.B.; Ayers, G.D.; Shapiro, S.E.; Edeiken, B.S.; Truong, M.T.; Evans, D.B.; et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 2006, 140, 932–941. [Google Scholar] [CrossRef]
- Kelly, H.; Hamberg, L.; Hunter, G. 4D-CT for Preoperative Localization of Abnormal Parathyroid Glands in Patients with Hyperparathyroidism: Accuracy and Ability to Stratify Patients by Unilateral versus Bilateral Disease in Surgery-Naïve and Re-Exploration Patients. Am. J. Neuroradiol. 2014, 35, 176–181. [Google Scholar] [CrossRef]
- Starker, L.F.; Mahajan, A.; Björklund, P.; Sze, G.; Udelsman, R.; Carling, T. 4D Parathyroid CT as the Initial Localization Study for Patients with De Novo Primary Hyperparathyroidism. Ann. Surg. Oncol. 2010, 18, 1723–1728. [Google Scholar] [CrossRef]
- Day, K.M.; Elsayed, M.; Beland, M.D.; Monchik, J.M. The utility of 4-dimensional computed tomography for preoperative localization of primary hyperparathyroidism in patients not localized by sestamibi or ultrasonography. Surgery 2015, 157, 534–539. [Google Scholar] [CrossRef]
- Hamidi, M.; Sullivan, M.; Hunter, G.; Hamberg, L.; Cho, N.L.; Gawande, A.A.; Doherty, G.M.; Moore, F.D.; Nehs, M.A. 4D-CT is Superior to Ultrasound and Sestamibi for Localizing Recurrent Parathyroid Disease. Ann. Surg. Oncol. 2018, 25, 1403–1409. [Google Scholar] [CrossRef]
- Eichhorn-Wharry, L.I.; Carlin, A.M.; Talpos, G.B. Mild hypercalcemia: An indication to select 4-dimensional computed tomography scan for preoperative localization of parathyroid adenomas. Am. J. Surg. 2011, 201, 334–338. [Google Scholar] [CrossRef]
- Bahl, M.; Sepahdari, A.R.; Sosa, J.A.; Hoang, J.K. Parathyroid Adenomas and Hyperplasia on Four-dimensional CT Scans: Three Patterns of Enhancement Relative to the Thyroid Gland Justify a Three-Phase Protocol. Radiology 2015, 277, 454–462. [Google Scholar] [CrossRef]
- Beland, M.D.; Mayo-Smith, W.W.; Grand, D.J.; Machan, J.T.; Monchik, J.M. Dynamic MDCT for Localization of Occult Parathyroid Adenomas in 26 Patients with Primary Hyperparathyroidism. Am. J. Roentgenol. 2011, 196, 61–65. [Google Scholar] [CrossRef]
- Lubitz, C.C.; Hunter, G.J.; Hamberg, L.M.; Parangi, S.; Ruan, D.; Gawande, A.; Gaz, R.D.; Randolph, G.W.; Moore, F.D.; Hodin, R.A.; et al. Accuracy of 4-dimensional computed tomography in poorly localized patients with primary hyperparathyroidism. Surgery 2010, 148, 1129–1138. [Google Scholar] [CrossRef]
- Kutler, D.I.; Moquete, R.; Kazam, E.; Kuhel, W.I. Parathyroid localization with modified 4D-computed tomography and ultrasonography for patients with primary hyperparathyroidism. Laryngoscope 2011, 121, 1219–1224. [Google Scholar] [CrossRef]
- Bahl, M.; Muzaffar, M.; Vij, G.; Sosa, J.; Choudhury, K.R.; Hoang, J. Prevalence of the Polar Vessel Sign in Parathyroid Adenomas on the Arterial Phase of 4D CT. Am. J. Neuroradiol. 2013, 35, 578–581. [Google Scholar] [CrossRef]
- Wan, Q.-C.; Li, J.-F.; Tang, L.-L.; Lv, J.; Xie, L.-J.; Li, J.-P.; Qin, L.-P.; Cheng, M.-H. Comparing the diagnostic accuracy of 4D CT and 99mTc-MIBI SPECT/CT for localizing hyperfunctioning parathyroid glands. Nucl. Med. Commun. 2020. [Google Scholar] [CrossRef]
- Hoang, J.K.; Williams, K.; Gaillard, F.; Dixon, A.; Sosa, J.A. Parathyroid 4D-CT. Otolaryngol. Neck Surg. 2016, 155, 956–960. [Google Scholar] [CrossRef]
- Mahajan, A.; Starker, L.F.; Ghita, M.; Udelsman, R.; Brink, J.A.; Carling, T. Parathyroid Four-Dimensional Computed Tomography: Evaluation of Radiation Dose Exposure during Preoperative Localization of Parathyroid Tumors in Primary Hyperparathyroidism. World J. Surg. 2012, 36, 1335–1339. [Google Scholar] [CrossRef]
- Lebastchi, A.H.; Aruny, J.E.; Donovan, P.I.; Quinn, C.E.; Callender, G.G.; Carling, T.; Udelsman, R. Real-Time Super Selective Venous Sampling in Remedial Parathyroid Surgery. J. Am. Coll. Surg. 2015, 220, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Taslakian, B.; Trerotola, S.O.; Sacks, B.; Oklu, R.; Deipolyi, A. The Essentials of Parathyroid Hormone Venous Sampling. Cardiovasc. Interv. Radiol. 2017, 40, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, K.; Toraih, E.A.; Haddad, A.B.; Farag, M.; Randolph, G.W.; Kandil, E. Selective parathyroid venous sampling in primary hyperparathyroidism: A systematic review and meta-analysis. Laryngoscope 2018, 128, 2662–2667. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, M.; Yamada, T.; Shinjo, Y.; Morimoto, T.; Kumano, R.; Yagihashi, K.; Katabami, T.; Nakajima, Y. Selective venous sampling supports localization of adenoma in primary hyperparathyroidism. Acta Radiol. Open 2018, 7. [Google Scholar] [CrossRef]
- Sun, P.Y.; Thompson, S.M.; Andrews, J.C.; Wermers, R.A.; McKenzie, T.J.; Richards, M.L.; Farley, D.R.; Thompson, G.B. Selective Parathyroid Hormone Venous Sampling in Patients with Persistent or Recurrent Primary Hyperparathyroidism and Negative, Equivocal or Discordant Noninvasive Imaging. World J. Surg. 2016, 40, 2956–2963. [Google Scholar] [CrossRef]
- Cherry, S.R. The 2006 Henry N. Wagner Lecture: Of mice and men (and positrons)—Advances in PET imaging technology. J. Nucl. Med. 2006, 47, 1735–1745. [Google Scholar]
- Messa, C.; Bettinardi, V.; Picchio, M.; Pelosi, E.; Landoni, C.; Gianolli, L.; Gilardi, M.C.; Fazio, F. PET/CT in diagnostic oncology. Q. J. Nucl. Med. Mol. Imaging 2004, 48, 66–75. [Google Scholar]
- Visioni, A.; Kim, J. Positron Emission Tomography for Benign and Malignant Disease. Surg. Clin. North Am. 2011, 91, 249–266. [Google Scholar] [CrossRef]
- Kluijfhout, W.P.; Pasternak, J.D.; Drake, F.T.; Beninato, T.; Gosnell, J.E.; Shen, W.T.; Duh, Q.-Y.; Allen, I.E.; Vriens, M.R.; De Keizer, B.; et al. Use of PET tracers for parathyroid localization: A systematic review and meta-analysis. Langenbeck’s Arch. Surg. 2016, 401, 925–935. [Google Scholar] [CrossRef]
- Evangelista, L.; Sorgato, N.; Torresan, F.; Boschin, I.M.; Pennelli, G.; Saladini, G.; Piotto, A.; Rubello, M.; Pelizzo, M.R. FDG-PET/CT and parathyroid carcinoma: Review of literature and illustrative case series. World J. Clin. Oncol. 2011, 2, 348–354. [Google Scholar] [CrossRef]
- Vallabhajosula, S. 18F-Labeled Positron Emission Tomographic Radiopharmaceuticals in Oncology: An Overview of Radiochemistry and Mechanisms of Tumor Localization. Semin. Nucl. Med. 2007, 37, 400–419. [Google Scholar] [CrossRef]
- Ishizuka, T.; Kajita, K.; Kamikubo, K.; Komaki, T.; Miura, K.; Nagao, S.; Nozawa, Y. Phospholipid/Ca2+-dependent Protein Kinase Activity in Human Parathyroid Adenoma. Endocrinol. Jpn. 1987, 34, 965–968. [Google Scholar] [CrossRef]
- Hara, T.; Kosaka, N.; Kishi, H. PET imaging of prostate cancer using carbon-11-choline. J. Nucl. Med. 1998, 39, 990–995. [Google Scholar]
- Quak, E.; Lheureux, S.; Reznik, Y.; Bardet, S.; Aide, N. F18-Choline, a Novel PET Tracer for Parathyroid Adenoma? J. Clin. Endocrinol. Metab. 2013, 98, 3111–3112. [Google Scholar] [CrossRef] [PubMed]
- Welle, C.L.; Cullen, E.L.; Peller, P.J.; Lowe, V.J.; Murphy, R.C.; Johnson, G.B.; Binkovitz, L.A. 11C-Choline PET/CT in Recurrent Prostate Cancer and Nonprostatic Neoplastic Processes. Radiographics 2016, 36, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Elaraj, D. Current Status and Treatment of Primary Hyperparathyroidism. Perm. J. 2008, 12, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Parvinian, A.; Martin-Macintosh, E.L.; Goenka, A.H.; Durski, J.M.; Mullan, B.P.; Kemp, B.J.; Johnson, G.B. 11C-Choline PET/CT for Detection and Localization of Parathyroid Adenomas. Am. J. Roentgenol. 2018, 210, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Burgess, A.; Huchet, V.; Lefèvre, M.; Tassart, M.; Ohnona, J.; Kerrou, K.; Balogova, S.; Talbot, J.-N.; Périé, S. Is 18F-Fluorocholine-Positron Emission Tomography/Computerized Tomography a New Imaging Tool for Detecting Hyperfunctioning Parathyroid Glands in Primary or Secondary Hyperparathyroidism? J. Clin. Endocrinol. Metab. 2014, 99, 4531–4536. [Google Scholar] [CrossRef] [PubMed]
- Amadou, C.; Bera, G.; Ezziane, M.; Chami, L.; Delbot, T.; Rouxel, A.; Leban, M.; Herve, G.; Menegaux, F.; Leenhardt, L.; et al. 18F-Fluorocholine PET/CT and Parathyroid 4D Computed Tomography for Primary Hyperparathyroidism: The Challenge of Reoperative Patients. World J. Surg. 2019, 43, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Piccardo, A.; Imperiale, A.; Strobel, K.; Kaufmann, P.A.; Prior, J.O.; Giovanella, L. Diagnostic performance of choline PET for detection of hyperfunctioning parathyroid glands in hyperparathyroidism: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 751–765. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Y.; Huo, L.; Hu, Y.; Wang, O.; Liu, H.; Chang, X.; Liu, Y.; Xing, X.; Li, F.; et al. Preoperative Localization of Adenomas in Primary Hyperparathyroidism: The Value of 11C-Choline PET/CT in Patients with Negative or Discordant Findings on Ultrasonography and 99mTc-Sestamibi SPECT/CT. J. Nucl. Med. 2019, 61, 584–589. [Google Scholar] [CrossRef]
- DeGrado, T.R.; E Reiman, R.; Price, D.T.; Wang, S.; Coleman, R.E. Pharmacokinetics and radiation dosimetry of 18F-fluorocholine. J. Nucl. Med. 2002, 43, 92–96. [Google Scholar]
- Kluijfhout, W.P.; Vorselaars, W.M.; Vriens, M.R.; Rinkes, I.H.B.; Valk, G.D.; De Keizer, B. Enabling minimal invasive parathyroidectomy for patients with primary hyperparathyroidism using Tc-99m-sestamibi SPECT–CT, ultrasound and first results of 18F-fluorocholine PET–CT. Eur. J. Radiol. 2015, 84, 1745–1751. [Google Scholar] [CrossRef]
- Fischli, S.; Suter-Widmer, I.; Nguyen, B.T.; Muller, W.; Metzger, J.; Strobel, K.; Grünig, H.; Henzen, C. The Significance of 18F-Fluorocholine-PET/CT as Localizing Imaging Technique in Patients with Primary Hyperparathyroidism and Negative Conventional Imaging. Front. Endocrinol. 2018, 8, 380. [Google Scholar] [CrossRef]
- Erbil, Y.; Barbaros, U.; Yanik, B.T.; Salmaslioğlu, A.; Tunaci, M.; Adalet, I.; Bozbora, A.; Özarmağan, S. Impact of Gland Morphology and Concomitant Thyroid Nodules on Preoperative Localization of Parathyroid Adenomas. Laryngoscope 2006, 116, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Barbaros, U.; Erbil, Y.; Salmashoğlu, A.; Issever, H.; Aral, F.; Tunacı, M.; Özarmağan, S. The characteristics of concomitant thyroid nodules cause false-positive ultrasonography results in primary hyperparathyroidism. Am. J. Otolaryngol. 2009, 30, 239–243. [Google Scholar] [CrossRef]
- Thanseer, N.; Bhadada, S.K.; Sood, A.; Mittal, B.R.; Behera, A.; Gorla, A.K.R.; Kalathoorakathu, R.R.; Singh, P.; Dahiya, D.; Saikia, U.N.; et al. Comparative Effectiveness of Ultrasonography, 99mTc-Sestamibi, and 18F-Fluorocholine PET/CT in Detecting Parathyroid Adenomas in Patients With Primary Hyperparathyroidism. Clin. Nucl. Med. 2017, 42, e491–e497. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Balogova, S.; Burgess, A.; Ohnona, J.; Huchet, V.; Kerrou, K.; Lefèvre, M.; Tassart, M.; Montravers, F.; Périé, S.; et al. A Pilot Comparison of 18F-fluorocholine PET/CT, Ultrasonography and 123I/99mTc-sestaMIBI Dual-Phase Dual-Isotope Scintigraphy in the Preoperative Localization of Hyperfunctioning Parathyroid Glands in Primary or Secondary Hyperparathyroidism. Medicine 2015, 94, e1701. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.; Ghosh, S.; Nowell, S.; Clark, D. Increased Mortality in Patients with Primary Hyperparathyroidism: Does Surgery Make A Difference? Endocr. Pr. 2019, 25, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Ravelli, I.; Magnani, F.; Iacobone, M.; Giraudo, C.; Camozzi, V.; Spimpolo, A.; Cecchin, D. 18F-choline PET/CT and PET/MRI in primary and recurrent hyperparathyroidism: A systematic review of the literature. Ann. Nucl. Med. 2020, 34, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Noltes, M.E.; Kruijff, S.; Noordzij, W.; Telenga, E.D.; García, D.V.; Trofimiuk-Müldner, M.; Opalińska, M.; Hubalewska-Dydejczyk, A.; Luurtsema, G.; Dierckx, R.A.J.O.; et al. Optimization of parathyroid 11C-choline PET protocol for localization of parathyroid adenomas in patients with primary hyperparathyroidism. EJNMMI Res. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Broos, W.A.; van der Zant, F.M.; Knol, R.J.; Wondergem, M. Choline PET/CT in parathyroid imaging. Nucl. Med. Commun. 2019, 40, 96–105. [Google Scholar] [CrossRef]
- Frank, E.; Bs, W.W.; Fujimoto, S.; Filho, P.D.A.; Inman, J.; Simental, A. Surgery versus Imaging in Non-Localizing Primary Hyperparathyroidism: A Cost-Effectiveness Model. Laryngoscope 2020, 130, 963. [Google Scholar] [CrossRef]
- Huber, G.F.; Hüllner, M.; Schmid, C.; Brunner, A.; Sah, B.; Vetter, D.; Kaufmann, P.A.; Von Schulthess, G.K. Benefit of 18F-fluorocholine PET imaging in parathyroid surgery. Eur. Radiol. 2018, 28, 2700–2707. [Google Scholar] [CrossRef]
- Piccardo, A.; Trimboli, P.; Rutigliani, M.; Puntoni, M.; Foppiani, L.; Bacigalupo, L.; Crescenzi, A.; Bottoni, G.; Treglia, G.; Paparo, F.; et al. Additional value of integrated 18F-choline PET/4D contrast-enhanced CT in the localization of hyperfunctioning parathyroid glands and correlation with molecular profile. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 766–775. [Google Scholar] [CrossRef]
- Pretet, V.; Rotania, M.; Helali, M.; Ignat, M.; Vix, M.; Imperiale, A. 18F-Fluorocholine PET and Multiphase CT Integrated in Dual Modality PET/4D-CT for Preoperative Evaluation of Primary Hyperparathyroidism. J. Clin. Med. 2020, 9, 2005. [Google Scholar] [CrossRef]
- Berger, A. How does it work?: Magnetic resonance imaging. BMJ 2002, 324, 35. [Google Scholar] [CrossRef]
- Bley, T.A.; Wieben, O.; François, C.J.; Brittain, J.H.; Reeder, S.B. Fat and water magnetic resonance imaging. J. Magn. Reson. Imaging 2009, 31, 4–18. [Google Scholar] [CrossRef]
- Currie, S.; Hoggard, N.; Craven, I.J.; Hadjivassiliou, M.; Wilkinson, I.D. Understanding MRI: Basic MR physics for physicians. Postgrad. Med J. 2012, 89, 209–223. [Google Scholar] [CrossRef]
- Jacobs, M.A.; Ibrahim, T.S.; Ouwerkerk, R. MR Imaging: Brief Overview and Emerging Applications. Radiographics 2007, 27, 1213–1229. [Google Scholar] [CrossRef]
- Grayev, A.M.; Gentry, L.R.; Hartman, M.J.; Chen, H.; Perlman, S.B.; Reeder, S.B. Presurgical Localization of Parathyroid Adenomas with Magnetic Resonance Imaging at 3.0 T: An Adjunct Method to Supplement Traditional Imaging. Ann. Surg. Oncol. 2011, 19, 981–989. [Google Scholar] [CrossRef]
- Kluijfhout, W.P.; Venkatesh, S.; Beninato, T.; Vriens, M.R.; Duh, Q.-Y.; Wilson, D.M.; Hope, T.A.; Suh, I. Performance of magnetic resonance imaging in the evaluation of first-time and reoperative primary hyperparathyroidism. Surgery 2016, 160, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, R.; Tuda, S.; Lamster, E.; Meyer, A.; Roediger, H.; Stier, A.; Conrad, E.; Basche, S.; Klisch, J.; Vogl, T. Dynamic magnetic resonance angiography for localization of hyperfunctioning parathyroid glands in the reoperative neck. Eur. J. Radiol. 2012, 81, 3371–3377. [Google Scholar] [CrossRef]
- Stanisz, G.J.; Odrobina, E.E.; Pun, J.; Escaravage, M.; Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reson. Med. 2005, 54, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.M.; Smith, M.P.; Pedrosa, I.; Lenkinski, R.E.; Rofsky, N.M. Body MR Imaging at 3.0 T: Understanding the Opportunities and Challenges. Radiographics 2007, 27, 1445–1462. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, B.; Argirò, R.; Diacinti, D.; Iannarelli, A.; Bezzi, M.; Cipriani, C.; Pisani, D.; Cipolla, V.; De Felice, C.; Minisola, S.; et al. MR appearance of parathyroid adenomas at 3 T in patients with primary hyperparathyroidism: What radiologists need to know for pre-operative localization. Eur. Radiol. 2015, 26, 664–673. [Google Scholar] [CrossRef]
- Merchavy, S.; Luckman, J.; Guindy, M.; Segev, Y.; Khafif, A. 4D MRI for the Localization of Parathyroid Adenoma. Otolaryngol. Neck Surg. 2015, 154, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Nael, K.; Hur, J.; Bauer, A.; Khan, R.; Sepahdari, A.; Inampudi, R.; Guerrero, M. Dynamic 4D MRI for Characterization of Parathyroid Adenomas: Multiparametric Analysis. Am. J. Neuroradiol. 2015, 36, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Argirò, R.; Diacinti, D.; Sacconi, B.; Iannarelli, A.; Diacinti, D.; Cipriani, C.; Pisani, D.; Romagnoli, E.; Biffoni, M.; Di Gioia, C.; et al. Diagnostic accuracy of 3T magnetic resonance imaging in the preoperative localisation of parathyroid adenomas: Comparison with ultrasound and 99mTc-sestamibi scans. Eur. Radiol. 2018, 28, 4900–4908. [Google Scholar] [CrossRef]
- Yildiz, S.; Aralasmak, A.; Yetis, H.; Kilicarslan, R.; Sharifov, R.; Alkan, A.; Toprak, H. MRI findings and utility of DWI in the evaluation of solid parathyroid lesions. La Radiol. Med. 2019, 124, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Gotway, M.B.; Higgins, C.B. MR IMAGING OF THE THYROID AND PARATHYROID GLANDS. Magn. Reson. Imaging Clin. North Am. 2000, 8, 163–182. [Google Scholar] [CrossRef]
- Gotway, M.B.; Reddy, G.P.; Webb, W.R.; Morita, E.T.; Clark, O.H.; Higgins, C.B. Comparison between MR Imaging and99mTc MIBI Scintigraphy in the Evaluation of Recurrent or Persistent Hyperparathyroidism. Radiology 2001, 218, 783–790. [Google Scholar] [CrossRef]
- Hänninen, E.L.; Vogl, T.J.; Steinmüller, T.; Ricke, J.; Neuhaus, P.; Felix, R. Preoperative Contrast-Enhanced MRI of the Parathyroid Glands in Hyperparathyroidism. Investig. Radiol. 2000, 35, 426–430. [Google Scholar] [CrossRef]
- Kabala, J. Computed tomography and magnetic resonance imaging in diseases of the thyroid and parathyroid. Eur. J. Radiol. 2008, 66, 480–492. [Google Scholar] [CrossRef]
- Ozturk, M.; Polat, A.V.; Celenk, C.; Elmali, M.; Kir, S.; Polat, C. The diagnostic value of 4D MRI at 3T for the localization of parathyroid adenomas. Eur. J. Radiol. 2019, 112, 207–213. [Google Scholar] [CrossRef]
- Memeh, K.O.; Palacios, J.E.; Khan, R.; Guerrero, M.A. Pre-Operative Localization of Parathyroid Adenoma: Performance of 4D Mri Parathyroid Protocol. Endocr. Pr. 2019, 25, 361–365. [Google Scholar] [CrossRef]
- Lou, I.; Balentine, C.; Clarkson, S.; Schneider, D.F.; Sippel, R.S.; Chen, H. How long should we follow patients after apparently curative parathyroidectomy? Surgery 2017, 161, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Madorin, C.A.; Owen, R.; Coakley, B.; Lowe, H.; Nam, K.-H.; Weber, K.; Kushnir, L.; Rios, J.; Genden, E.; Pawha, P.S.; et al. Comparison of Radiation Exposure and Cost Between Dynamic Computed Tomography and Sestamibi Scintigraphy for Preoperative Localization of Parathyroid Lesions. JAMA Surg. 2013, 148, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.; Lubin, J.H.; Shore, R.E.; Mabuchi, K.; Modan, B.; Pottern, L.M.; Schneider, A.B.; Tucker, M.A.; Boice, J.D. Thyroid Cancer after Exposure to External Radiation: A Pooled Analysis of Seven Studies. Radiat. Res. 1995, 141, 259–277. [Google Scholar] [CrossRef]
- Becker, J.; Patel, V.; Johnson, K.; Guerrero, M.; Klein, R.; Ranvier, G.; Owen, R.; Pawha, P.; Nael, K. 4D–Dynamic Contrast-Enhanced MRI for Preoperative Localization in Patients with Primary Hyperparathyroidism. Am. J. Neuroradiol. 2020, 41, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Kroeker, R.; Kipfer, H.D.; Lin, C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J. Magn. Reson. Imaging 2012, 36, 483–491. [Google Scholar] [CrossRef]
- Breuer, F.A.; Blaimer, M.; Heidemann, R.M.; Mueller, M.F.; Griswold, M.A.; Jakob, P.M. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn. Reson. Med. 2005, 53, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, S.; Marsden, P.K. PET-MRI: A review of challenges and solutions in the development of integrated multimodality imaging. Phys. Med. Biol. 2015, 60, R115–R154. [Google Scholar] [CrossRef]
- Kluijfhout, W.P.; Pasternak, J.D.; Gosnell, J.E.; Shen, W.T.; Duh, Q.-Y.; Vriens, M.R.; De Keizer, B.; Hope, T.A.; Glastonbury, C.M.; Pampaloni, M.H.; et al. 18F Fluorocholine PET/MR Imaging in Patients with Primary Hyperparathyroidism and Inconclusive Conventional Imaging: A Prospective Pilot Study. Radiology 2017, 284, 460–467. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, D.; Das, J.P.; Yeh, R. Preoperative Localization for Primary Hyperparathyroidism: A Clinical Review. Biomedicines 2021, 9, 390. https://doi.org/10.3390/biomedicines9040390
Tay D, Das JP, Yeh R. Preoperative Localization for Primary Hyperparathyroidism: A Clinical Review. Biomedicines. 2021; 9(4):390. https://doi.org/10.3390/biomedicines9040390
Chicago/Turabian StyleTay, Donovan, Jeeban P. Das, and Randy Yeh. 2021. "Preoperative Localization for Primary Hyperparathyroidism: A Clinical Review" Biomedicines 9, no. 4: 390. https://doi.org/10.3390/biomedicines9040390
APA StyleTay, D., Das, J. P., & Yeh, R. (2021). Preoperative Localization for Primary Hyperparathyroidism: A Clinical Review. Biomedicines, 9(4), 390. https://doi.org/10.3390/biomedicines9040390




