Noncoding RNAs Associated with Therapeutic Resistance in Pancreatic Cancer
Abstract
1. Introduction
1.1. Noncoding RNAs
1.2. Mechanisms of Therapeutic Resistance
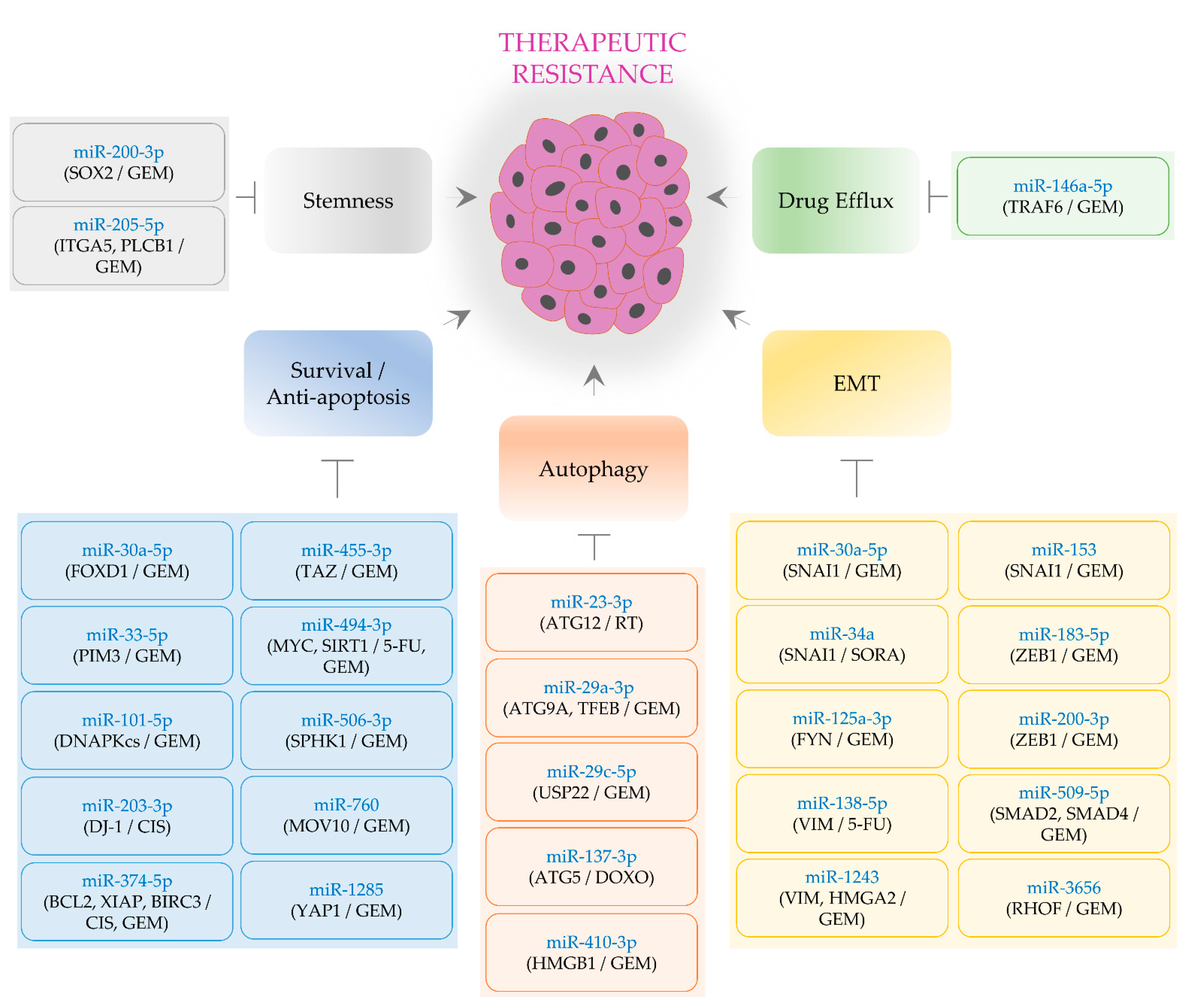
2. Oncogenic miRNAs Conferring Therapeutic Resistance
2.1. EMT-Regulating MiRNAs
2.1.1. MiR-10a-5p
2.1.2. MiR-125a-5p
2.1.3. MiR-221-3p
2.1.4. MiR-223-3p
2.1.5. MiR-301-3p
2.2. Stemness-Regulating MiRNAs
2.2.1. MiR-21-5p and MiR-221-3p
2.2.2. MiR-1246
2.3. Cell Survival- and Apoptosis-Regulating MiRNAs
2.3.1. MiR-17-5p, MiR-21-5p, MiR-301-3p, and MiR-320a
2.3.2. MiR-29-3p
2.3.3. MiR-135-5p
2.3.4. MiR-181c-5p
2.3.5. MiR-223-3p
2.3.6. MiR-296-5p
2.3.7. MiR-342-3p
2.3.8. MiR-1266-5p
2.4. An MiRNA Associated with Drug Efflux
MiR-331-3p
3. Tumor-Suppressive MiRNAs Alleviating Therapeutic Resistance
3.1. EMT-Regulating MiRNAs
3.1.1. MiR-30a-5p
3.1.2. MiR-34a
3.1.3. MiR-125a-3p
3.1.4. MiR-138-5p and MiR-153
3.1.5. MiR-183-5p and MiR-200-3p
3.1.6. MiR-509-5p and MiR-1243
3.1.7. MiR-3656
3.2. Stemness-Regulating MiRNAs
3.2.1. MiR-200-3p
3.2.2. MiR-205-5p
3.3. Cell Survival- and Apoptosis-Regulating MiRNAs
3.3.1. MiR-30a-5p
3.3.2. MiR-33-5p, MiR-101-5p, MiR-203-3p, and MiR-506-3p
3.3.3. MiR-374-5p
3.3.4. MiR-455-3p and MiR-1285
3.3.5. MiR-494-3p
3.3.6. MiR-760
3.4. Autophagy-Inhibiting MiRNAs
3.4.1. MiR-23-3p and MiR-137-3p
3.4.2. MiR-29a-3p
3.4.3. MiR-29c-5p
3.4.4. MiR-410-3p
3.5. MiRNAs Regulating Drug Efflux
MiR-146a-5p
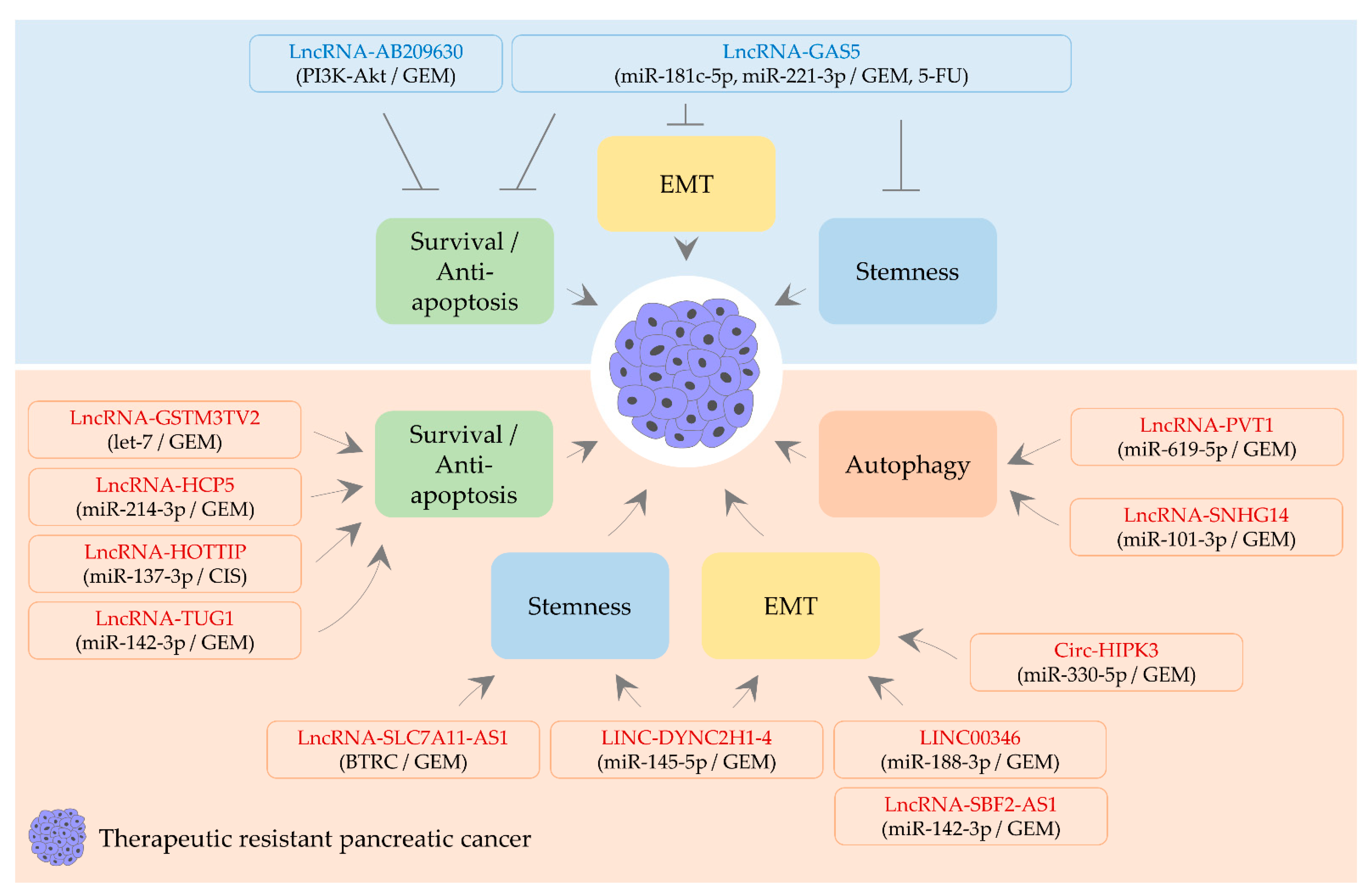
4. CircRNA, lncRNA, and Therapeutic Resistance
4.1. LncRNAs Alleviating Therapeutic Resistance
4.1.1. LncRNA-AB209630
4.1.2. LncRNA-GAS5
4.2. A circRNA and LncRNAs Aggravating Therapeutic Resistance
4.2.1. Circ-HIPK3
4.2.2. LINC00346
4.2.3. LINC-DYNC2H1-4
4.2.4. LncRNA-GSTM3TV2
4.2.5. LncRNA-HCP5 and lncRNA-HOTTIP
4.2.6. LncRNA-PVT1
4.2.7. LncRNA-SBF2-AS1
4.2.8. LncRNA-SLC7A11-AS1
4.2.9. LncRNA-SNHG14
4.2.10. LncRNA-TUG1
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunner, M.; Wu, Z.; Krautz, C.; Pilarsky, C.; Grutzmann, R.; Weber, G.F. Current clinical strategies of pancreatic cancer treatment and open molecular questions. Int. J. Mol. Sci. 2019, 20, 4543. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Mejia, I.; Bodapati, S.; Chen, K.T.; Diaz, B. Pancreatic adenocarcinoma invasiveness and the tumor microenvironment: From biology to clinical trials. Biomedicines 2020, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Pottler, M.; Lan, B.; Grutzmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in pancreatic cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Son, S.W.; Moeng, S.; Choi, S.Y.; Park, J.K. The role of noncoding rnas in the regulation of anoikis and anchorage-independent growth in cancer. Int. J. Mol. Sci. 2021, 22, 627. [Google Scholar] [CrossRef]
- Taniue, K.; Akimitsu, N. The functions and unique features of lncrnas in cancer development and tumorigenesis. Int. J. Mol. Sci. 2021, 22, 632. [Google Scholar] [CrossRef]
- Rawat, M.; Kadian, K.; Gupta, Y.; Kumar, A.; Chain, P.S.G.; Kovbasnjuk, O.; Kumar, S.; Parasher, G. Microrna in pancreatic cancer: From biology to therapeutic potential. Genes 2019, 10, 752. [Google Scholar] [CrossRef]
- Limb, C.; Liu, D.S.K.; Veno, M.T.; Rees, E.; Krell, J.; Bagwan, I.N.; Giovannetti, E.; Pandha, H.; Strobel, O.; Rockall, T.A.; et al. The role of circular rnas in pancreatic ductal adenocarcinoma and biliary-tract cancers. Cancers 2020, 12, 3250. [Google Scholar] [CrossRef]
- Schuster, S.L.; Hsieh, A.C. The untranslated regions of mrnas in cancer. Trends Cancer 2019, 5, 245–262. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and functions of long non-coding rnas at multiple regulatory levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding rnas and its biological functions. Nat. Rev. Mol. Cell Biol. 2020, 22, 96–118. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Chen, Q.; Ge, W.; Meng, L.; Huang, X.; Shen, P.; Yuan, H.; Shi, G.; Miao, Y.; et al. Long noncoding rna sox2ot promotes the proliferation of pancreatic cancer by binding to fus. Int. J. Cancer 2020, 147, 175–188. [Google Scholar] [CrossRef]
- Song, S.; Yu, W.; Lin, S.; Zhang, M.; Wang, T.; Guo, S.; Wang, H. Lncrna adpgk-as1 promotes pancreatic cancer progression through activating zeb1-mediated epithelial-mesenchymal transition. Cancer Biol. Ther. 2018, 19, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding rna anril modulates ribosomal rna maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular rnas are a large class of animal rnas with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009, 69, 5820–5828. [Google Scholar] [CrossRef] [PubMed]
- Gaianigo, N.; Melisi, D.; Carbone, C. Emt and treatment resistance in pancreatic cancer. Cancers 2017, 9, 122. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Lin, Y.; Wang, X.; Cui, X.; Zhang, Z.; Xian, G.; Qin, C. Novel crosstalk between klf4 and zeb1 regulates gemcitabine resistance in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2017, 51, 1239–1248. [Google Scholar] [CrossRef]
- Nguyen, A.M.; Zhou, J.; Sicairos, B.; Sonney, S.; Du, Y. Upregulation of cd73 confers acquired radioresistance and is required for maintaining irradiation-selected pancreatic cancer cells in a mesenchymal state. Mol. Cell Proteom. 2020, 19, 375–389. [Google Scholar] [CrossRef]
- Di Carlo, C.; Brandi, J.; Cecconi, D. Pancreatic cancer stem cells: Perspectives on potential therapeutic approaches of pancreatic ductal adenocarcinoma. World J. Stem Cells 2018, 10, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, H.; Rong, Y.; Zhu, K.; Zhu, Z.; Tang, Z.; Xiong, C.; Tao, J. Hypoxia potentiates gemcitabine-induced stemness in pancreatic cancer cells through akt/notch1 signaling. J. Exp. Clin. Cancer Res. 2018, 37, 291. [Google Scholar] [CrossRef]
- Rodriguez-Aznar, E.; Wiesmuller, L.; Sainz, B., Jr.; Hermann, P.C. Emt and stemness-key players in pancreatic cancer stem cells. Cancers 2019, 11, 1136. [Google Scholar] [CrossRef]
- Kaushik, G.; Seshacharyulu, P.; Rauth, S.; Nallasamy, P.; Rachagani, S.; Nimmakayala, R.K.; Vengoji, R.; Mallya, K.; Chirravuri-Venkata, R.; Singh, A.B.; et al. Selective inhibition of stemness through egfr/foxa2/sox9 axis reduces pancreatic cancer metastasis. Oncogene 2020, 40, 848–862. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Zhang, K.; Yu, X.; Lin, H.; Wu, W.; Peng, Y.; Sun, J. Prom2 promotes gemcitabine chemoresistance via activating the akt signaling pathway in pancreatic cancer. Exp. Mol. Med. 2020, 52, 409–422. [Google Scholar] [CrossRef]
- Boucher, M.J.; Morisset, J.; Vachon, P.H.; Reed, J.C.; Laine, J.; Rivard, N. Mek/erk signaling pathway regulates the expression of bcl-2, bcl-x(l), and mcl-1 and promotes survival of human pancreatic cancer cells. J. Cell Biochem. 2000, 79, 355–369. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, S.; Guo, J.; Chen, H.; Greenblatt, D.Y.; Kleeff, J.; Liao, Q.; Chen, G.; Friess, H.; Leung, P.S. Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. J. Surg. Res. 2006, 136, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, F.; Zheng, C.; Sun, S.; Jiang, Y. Knockdown of clusterin sensitizes pancreatic cancer cells to gemcitabine chemotherapy by erk1/2 inactivation. J. Exp. Clin. Cancer Res. 2012, 31, 73. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, X.; Jiang, L.; Zhang, L.; Xiang, M.; Ren, H. Foxm1 induced paclitaxel resistance via activation of the foxm1/phb1/raf-mek-erk pathway and enhancement of the abca2 transporter. Mol. Ther. Oncolytics 2019, 14, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.J.; Gorski, S.M. Molecular mechanisms underlying autophagy-mediated treatment resistance in cancer. Cancers 2019, 11, 1775. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Zhao, Y.; Zhang, M.; He, R.Z.; Shi, X.H.; Guo, X.J.; Shi, C.J.; Peng, F.; Wang, M.; Shen, M.; et al. Inhibition of autophagy by deguelin sensitizes pancreatic cancer cells to doxorubicin. Int. J. Mol. Sci. 2017, 18, 370. [Google Scholar] [CrossRef]
- Ropolo, A.; Catrinacio, C.; Renna, F.J.; Boggio, V.; Orquera, T.; Gonzalez, C.D.; Vaccaro, M.I. A novel e2f1-ep300-vmp1 pathway mediates gemcitabine-induced autophagy in pancreatic cancer cells carrying oncogenic kras. Front. Endocrinol. 2020, 11, 411. [Google Scholar] [CrossRef]
- Hashimoto, D.; Blauer, M.; Hirota, M.; Ikonen, N.H.; Sand, J.; Laukkarinen, J. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. Eur. J. Cancer 2014, 50, 1382–1390. [Google Scholar] [CrossRef]
- Endo, S.; Nakata, K.; Sagara, A.; Koikawa, K.; Ando, Y.; Kibe, S.; Takesue, S.; Nakayama, H.; Abe, T.; Okumura, T.; et al. Autophagy inhibition enhances antiproliferative effect of salinomycin in pancreatic cancer cells. Pancreatology 2017, 17, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Li, Y.; Lan, X.; Ai, L. Microrna-10a-5p suppresses cancer proliferation and division in human cervical cancer by targeting bdnf. Exp. Ther. Med. 2017, 14, 6147–6151. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wu, H.; Li, Y.; Zhang, Y.; Liu, M.; Li, X.; Tang, H. Mir-10a suppresses colorectal cancer metastasis by modulating the epithelial-to-mesenchymal transition and anoikis. Cell Death Dis. 2017, 8, e2739. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Huang, H.; Feng, M.; Yang, G.; Zheng, S.; You, L.; Zheng, L.; Hu, Y.; Zhang, T.; Zhao, Y. Mir-10a-5p targets tfap2c to promote gemcitabine resistance in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 76. [Google Scholar] [CrossRef]
- Kim, W.; Kim, E.; Lee, S.; Kim, D.; Chun, J.; Park, K.H.; Youn, H.; Youn, B. Tfap2c-mediated upregulation of tgfbr1 promotes lung tumorigenesis and epithelial-mesenchymal transition. Exp. Mol. Med. 2016, 48, e273. [Google Scholar] [CrossRef]
- Gu, J.; Wang, D.; Zhang, J.; Zhu, Y.; Li, Y.; Chen, H.; Shi, M.; Wang, X.; Shen, B.; Deng, X.; et al. Gfralpha2 prompts cell growth and chemoresistance through down-regulating tumor suppressor gene pten via mir-17-5p in pancreatic cancer. Cancer Lett. 2016, 380, 434–441. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Ischenko, I.; Bao, Q.; Schwarz, B.; Niess, H.; Wang, Y.; Renner, A.; Mysliwietz, J.; Jauch, K.W.; et al. Antisense inhibition of microrna-21 and microrna-221 in tumor-initiating stem-like cells modulates tumorigenesis, metastasis, and chemotherapy resistance in pancreatic cancer. Target. Oncol. 2015, 10, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, W.; Wang, L.; Zhang, Y.; Zhang, X.; Chen, M.; Wang, F.; Yu, J.; Ma, Y.; Sun, G. Microrna-21 induces 5-fluorouracil resistance in human pancreatic cancer cells by regulating pten and pdcd4. Cancer Med. 2016, 5, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Tomimaru, Y.; Eguchi, H.; Hama, N.; Wada, H.; Kawamoto, K.; Kobayashi, S.; Mori, M.; Doki, Y. Microrna-29a induces resistance to gemcitabine through the wnt/beta-catenin signaling pathway in pancreatic cancer cells. Int. J. Oncol. 2013, 43, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, Z.; Wang, X.; Xu, P.; Zhao, L.; Qian, J. Mir-125a regulates chemo-sensitivity to gemcitabine in human pancreatic cancer cells through targeting a20. Acta Biochim. Biophys. Sin. 2016, 48, 202–208. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, S.; Shen, J.; Guo, L.; Sun, Y.; Zhu, Y.; Ma, Z.; Zhang, X.; Hu, Y.; Xiao, W.; et al. The mir-135b-bmal1-yy1 loop disturbs pancreatic clockwork to promote tumourigenesis and chemoresistance. Cell Death Dis. 2018, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, M.; Xu, S.; Guo, X.; Jiang, J. Upregulation of mir-181c contributes to chemoresistance in pancreatic cancer by inactivating the hippo signaling pathway. Oncotarget 2015, 6, 44466–44479. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Q.; Wang, J.F.; Chen, D.H.; Ma, X.S.; Yang, W.; Zhe, T.; Dang, X.W. Long non-coding rna gas5 antagonizes the chemoresistance of pancreatic cancer cells through down-regulation of mir-181c-5p. Biomed. Pharmacother. 2018, 97, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zou, D.; Wei, X.; Wang, L.; Zhang, Y.; Liu, S.; Si, Y.; Zhao, H.; Wang, F.; Yu, J.; et al. Mirna-221-3p desensitizes pancreatic cancer cells to 5-fluorouracil by targeting rb1. Tumor Biol. 2016, 37, 16053–16063. [Google Scholar] [CrossRef]
- Liu, B.; Wu, S.; Ma, J.; Yan, S.; Xiao, Z.; Wan, L.; Zhang, F.; Shang, M.; Mao, A. Lncrna gas5 reverses emt and tumor stem cell-mediated gemcitabine resistance and metastasis by targeting mir-221/socs3 in pancreatic cancer. Mol. Ther. Nucleic Acids 2018, 13, 472–482. [Google Scholar] [CrossRef]
- Ma, J.; Fang, B.; Zeng, F.; Ma, C.; Pang, H.; Cheng, L.; Shi, Y.; Wang, H.; Yin, B.; Xia, J.; et al. Down-regulation of mir-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget 2015, 6, 1740–1749. [Google Scholar] [CrossRef]
- Ma, J.; Zeng, F.; Ma, C.; Pang, H.; Fang, B.; Lian, C.; Yin, B.; Zhang, X.; Wang, Z.; Xia, J. Synergistic reversal effect of epithelial-to-mesenchymal transition by mir-223 inhibitor and genistein in gemcitabine-resistant pancreatic cancer cells. Am. J. Cancer Res. 2016, 6, 1384–1395. [Google Scholar]
- Huang, R.; Song, X.; Wang, C.M. Mir-223 regulates cddp resistance in pancreatic cancer via targeting foxo3a. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7892–7898. [Google Scholar] [PubMed]
- Okazaki, J.; Tanahashi, T.; Sato, Y.; Miyoshi, J.; Nakagawa, T.; Kimura, T.; Miyamoto, H.; Fujino, Y.; Nakamura, F.; Takehara, M.; et al. Microrna-296-5p promotes cell invasion and drug resistance by targeting bcl2-related ovarian killer, leading to a poor prognosis in pancreatic cancer. Digestion 2020, 101, 794–806. [Google Scholar] [CrossRef]
- Funamizu, N.; Lacy, C.R.; Parpart, S.T.; Takai, A.; Hiyoshi, Y.; Yanaga, K. Microrna-301b promotes cell invasiveness through targeting tp63 in pancreatic carcinoma cells. Int. J. Oncol. 2014, 44, 725–734. [Google Scholar] [CrossRef]
- Zhang, K.D.; Hu, B.; Cen, G.; Yang, Y.H.; Chen, W.W.; Guo, Z.Y.; Wang, X.F.; Zhao, Q.; Qiu, Z.J. Mir-301a transcriptionally activated by hif-2alpha promotes hypoxia-induced epithelial-mesenchymal transition by targeting tp63 in pancreatic cancer. World J. Gastroenterol. 2020, 26, 2349–2373. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, K.; Luo, G.; Cen, G.; Cao, J.; Huang, K.; Qiu, Z. Downregulation of mir-301a-3p sensitizes pancreatic cancer cells to gemcitabine treatment via pten. Am. J. Transl. Res. 2017, 9, 1886–1895. [Google Scholar]
- Wang, W.; Zhao, L.; Wei, X.; Wang, L.; Liu, S.; Yang, Y.; Wang, F.; Sun, G.; Zhang, J.; Ma, Y.; et al. Microrna-320a promotes 5-fu resistance in human pancreatic cancer cells. Sci. Rep. 2016, 6, 27641. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Chen, X.; Tian, X.; Han, Z.; Liu, M.; Zou, Y.; Huang, S.; Chen, A.; Cheng, X.; Deng, J.; et al. Mir-331-3p links to drug resistance of pancreatic cancer cells by activating wnt/beta-catenin signal via st7l. Technol. Cancer Res. Treat. 2020, 19, 1533033820945801. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Fan, Z.; Du, G.; Wang, H. Leptin-elicited mirna-342-3p potentiates gemcitabine resistance in pancreatic ductal adenocarcinoma. Biochem. Biophys. Res. Commun. 2019, 509, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Eguchi, H.; Nagano, H.; Konno, M.; Tomimaru, Y.; Wada, H.; Hama, N.; Kawamoto, K.; Kobayashi, S.; Nishida, N.; et al. Microrna-1246 expression associated with ccng2-mediated chemoresistance and stemness in pancreatic cancer. Br. J. Cancer 2014, 111, 1572–1580. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, D.; Wu, X.; Lin, X.; Ye, L.; Lin, C.; Wu, S.; Zhu, J.; Peng, X.; Song, L. Mir-1266 contributes to pancreatic cancer progression and chemoresistance by the stat3 and nf-kappab signaling pathways. Mol. Ther. Nucleic Acids 2018, 11, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yin, L.; Wu, J.; Gu, J.J.; Ding, K.; Zhang, N.; Du, M.Y.; Qian, L.X.; Lu, Z.W.; He, X. Tnfaip3 inhibits migration and invasion in nasopharyngeal carcinoma by suppressing epithelial mesenchymal transition. Neoplasma 2017, 64, 389–394. [Google Scholar] [CrossRef]
- Wang, X.; Ma, C.; Zong, Z.; Xiao, Y.; Li, N.; Guo, C.; Zhang, L.; Shi, Y. A20 inhibits the motility of hcc cells induced by tnf-alpha. Oncotarget 2016, 7, 14742–14754. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Lu, Z.; Zhang, H.; Zhuang, N.; Wang, B.; Song, Z.; Chen, G.; Huang, C.; Xu, D.; et al. Mir-127 promotes emt and stem-like traits in lung cancer through a feed-forward regulatory loop. Oncogene 2017, 36, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.F.; Zhou, C.F.; Wu, X.G.; He, L.N.; Wu, L.F.; Chen, X.J.; Yan, R.M.; Zhong, M.; Yu, Y.H.; Liang, L.; et al. Microrna-221-3p, a twist2 target, promotes cervical cancer metastasis by directly targeting thbs2. Cell Death Dis. 2017, 8, 3220. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hong, X.; Lai, J.; Cheng, L.; Cheng, Y.; Yao, M.; Wang, R.; Hu, N. Exosomal microrna-221-3p confers adriamycin resistance in breast cancer cells by targeting pik3r1. Front. Oncol. 2020, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Xu, X.; Song, Q.; Xu, Y.; Tai, Y.; Goodman, S.B.; Bi, W.; Xu, M.; Jiao, S.; Maloney, W.J.; et al. Mir-223-3p inhibits human osteosarcoma metastasis and progression by directly targeting cdh6. Mol. Ther. 2018, 26, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, W.; Zhang, C.; Wen, S.; Huangfu, H.; Kang, J.; Wang, B. Hsa-mir-301a-3p acts as an oncogene in laryngeal squamous cell carcinoma via target regulation of smad4. J. Cancer 2015, 6, 1260–1275. [Google Scholar] [CrossRef] [PubMed]
- Nam, R.K.; Benatar, T.; Wallis, C.J.; Amemiya, Y.; Yang, W.; Garbens, A.; Naeim, M.; Sherman, C.; Sugar, L.; Seth, A. Mir-301a regulates e-cadherin expression and is predictive of prostate cancer recurrence. Prostate 2016, 76, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, T.; Jin, R.; Zhao, H.; Hu, J.; Feng, B.; Zang, L.; Zheng, M.; Wang, M. Microrna-301a promotes migration and invasion by targeting tgfbr2 in human colorectal cancer. J. Exp. Clin. Cancer Res. 2014, 33, 113. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kanwar, S.S.; Patel, B.B.; Oh, P.S.; Nautiyal, J.; Sarkar, F.H.; Majumdar, A.P. Microrna-21 induces stemness by downregulating transforming growth factor beta receptor 2 (tgfbetar2) in colon cancer cells. Carcinogenesis 2012, 33, 68–76. [Google Scholar] [CrossRef]
- Roscigno, G.; Quintavalle, C.; Donnarumma, E.; Puoti, I.; Diaz-Lagares, A.; Iaboni, M.; Fiore, D.; Russo, V.; Todaro, M.; Romano, G.; et al. Mir-221 promotes stemness of breast cancer cells by targeting dnmt3b. Oncotarget 2016, 7, 580–592. [Google Scholar] [CrossRef]
- Lin, S.S.; Peng, C.Y.; Liao, Y.W.; Chou, M.Y.; Hsieh, P.L.; Yu, C.C. Mir-1246 targets ccng2 to enhance cancer stemness and chemoresistance in oral carcinomas. Cancers 2018, 10, 272. [Google Scholar] [CrossRef]
- Pasca di Magliano, M.; Biankin, A.V.; Heiser, P.W.; Cano, D.A.; Gutierrez, P.J.; Deramaudt, T.; Segara, D.; Dawson, A.C.; Kench, J.G.; Henshall, S.M.; et al. Common activation of canonical wnt signaling in pancreatic adenocarcinoma. PLoS ONE 2007, 2, e1155. [Google Scholar] [CrossRef]
- Ram Makena, M.; Gatla, H.; Verlekar, D.; Sukhavasi, S.; KPandey, M.; CPramanik, K. Wnt/beta-catenin signaling: The culprit in pancreatic carcinogenesis and therapeutic resistance. Int. J. Mol. Sci. 2019, 20, 4242. [Google Scholar] [CrossRef] [PubMed]
- Gurney, A.; Axelrod, F.; Bond, C.J.; Cain, J.; Chartier, C.; Donigan, L.; Fischer, M.; Chaudhari, A.; Ji, M.; Kapoun, A.M.; et al. Wnt pathway inhibition via the targeting of frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 11717–11722. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Cancilla, B.; Yeung, V.P.; Cattaruzza, F.; Chartier, C.; Murriel, C.L.; Cain, J.; Tam, R.; Cheng, C.Y.; Evans, J.W.; et al. Wnt antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci. Adv. 2017, 3, e1700090. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Jiu, M.; You, Y.; Feng, Y.; Xin, R.; Liu, X.; Mo, L.; Nie, Y. Mir29a3p represses proliferation and metastasis of gastric cancer cells via attenuating has3 levels. Mol. Med. Rep. 2018, 17, 8145–8152. [Google Scholar]
- Jiang, W.; Zhao, S.; Jiang, X.; Zhang, E.; Hu, G.; Hu, B.; Zheng, P.; Xiao, J.; Lu, Z.; Lu, Y.; et al. The circadian clock gene bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016, 371, 314–325. [Google Scholar] [CrossRef]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Tsumura, H. Microrna-181c prevents apoptosis by targeting of fas receptor in ewing’s sarcoma cells. Cancer Cell Int. 2018, 18, 37. [Google Scholar] [CrossRef]
- Chen, X.; Gu, W.; Wang, Q.; Fu, X.; Wang, Y.; Xu, X.; Wen, Y. C-myc and bcl-2 mediate yap-regulated tumorigenesis in oscc. Oncotarget 2018, 9, 668–679. [Google Scholar] [CrossRef]
- LeBlanc, L.; Lee, B.K.; Yu, A.C.; Kim, M.; Kambhampati, A.V.; Dupont, S.M.; Seruggia, D.; Ryu, B.U.; Orkin, S.H.; Kim, J. Yap1 safeguards mouse embryonic stem cells from excessive apoptosis during differentiation. Elife 2018, 7, e40167. [Google Scholar] [CrossRef]
- Li, N.; Cheng, C.; Wang, T. Mir-181c-5p mitigates tumorigenesis in cervical squamous cell carcinoma via targeting glycogen synthase kinase 3beta interaction protein (gskip). Onco Targets Ther. 2020, 13, 4495–4505. [Google Scholar] [CrossRef]
- Ruan, J.; Lou, S.; Dai, Q.; Mao, D.; Ji, J.; Sun, X. Tumor suppressor mir-181c attenuates proliferation, invasion, and self-renewal abilities in glioblastoma. Neuroreport 2015, 26, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Llambi, F.; Wang, Y.M.; Victor, B.; Yang, M.; Schneider, D.M.; Gingras, S.; Parsons, M.J.; Zheng, J.H.; Brown, S.A.; Pelletier, S.; et al. Bok is a non-canonical bcl-2 family effector of apoptosis regulated by er-associated degradation. Cell 2016, 165, 421–433. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Glozak, M.A.; Ma, Y.; Cress, W.D. Bok, bcl-2-related ovarian killer, is cell cycle-regulated and sensitizes to stress-induced apoptosis. J. Biol. Chem. 2006, 281, 22729–22735. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, A.M.; Chalfant, M.C.; Gorden, L.D.; VanSaun, M.N. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS ONE 2015, 10, e0126686. [Google Scholar] [CrossRef]
- Chen, C.; Chang, Y.C.; Liu, C.L.; Liu, T.P.; Chang, K.J.; Guo, I.C. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin d1 and down-regulating bax via a janus kinase 2-linked pathway. Endocr. Relat. Cancer 2007, 14, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, P.V.; Rampoldi, A.; Harbuzariu, A.; Gonzalez-Perez, R.R. Leptin signaling and cancer chemoresistance: Perspectives. World J. Clin. Oncol. 2017, 8, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, X.; Guo, B. Klf6 induces apoptosis in prostate cancer cells through up-regulation of atf3. J. Biol. Chem. 2008, 283, 29795–29801. [Google Scholar] [CrossRef]
- Ito, G.; Uchiyama, M.; Kondo, M.; Mori, S.; Usami, N.; Maeda, O.; Kawabe, T.; Hasegawa, Y.; Shimokata, K.; Sekido, Y. Kruppel-like factor 6 is frequently down-regulated and induces apoptosis in non-small cell lung cancer cells. Cancer Res. 2004, 64, 3838–3843. [Google Scholar] [CrossRef]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: Nf-kappab turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Al Zaid Siddiquee, K.; Turkson, J. Stat3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008, 18, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Weber, C.K.; Greten, T.F.; Schneider, G.; Wagner, M.; Adler, G.; Schmid, R.M. Stat3 and nf-kappab activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology 2002, 123, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Munoz, A.R.; Pingali, S.; Payton-Stewart, F.; Chan, D.E.; Freeman, J.W.; Ghosh, R.; Kumar, A.P. Downregulation of stat3/nf-kappab potentiates gemcitabine activity in pancreatic cancer cells. Mol. Carcinog. 2017, 56, 402–411. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.C.; Wang, C.; Liu, X.; Hu, F.C.; Xie, N.; Lu, L.; Chen, X.; Huang, H.Z. Overexpression of beta-catenin induces cisplatin resistance in oral squamous cell carcinoma. Biomed. Res. Int. 2016, 2016, 5378567. [Google Scholar]
- Chen, Z.; Huang, C.; Ma, T.; Jiang, L.; Tang, L.; Shi, T.; Zhang, S.; Zhang, L.; Zhu, P.; Li, J.; et al. Reversal effect of quercetin on multidrug resistance via fzd7/beta-catenin pathway in hepatocellular carcinoma cells. Phytomedicine 2018, 43, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.Y.; Zhang, W.; Zeng, X.; Liu, C.Q. Inhibition of wnt/beta-catenin signaling downregulates p-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013, 104, 1303–1308. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, H.; Liao, Y.; Wu, W.; Liu, L.; Liu, L.; Wu, Y.; Sun, F.; Lin, H.W. Inhibition of wnt/beta-catenin pathway reverses multi-drug resistance and emt in oct4(+)/nanog(+) nsclc cells. Biomed. Pharmacother. 2020, 127, 110225. [Google Scholar] [CrossRef]
- Vesel, M.; Rapp, J.; Feller, D.; Kiss, E.; Jaromi, L.; Meggyes, M.; Miskei, G.; Duga, B.; Smuk, G.; Laszlo, T.; et al. Abcb1 and abcg2 drug transporters are differentially expressed in non-small cell lung cancers (nsclc) and expression is modified by cisplatin treatment via altered wnt signaling. Respir. Res. 2017, 18, 52. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Yu, M. Mechanism of low expression of mir-30a-5p on epithelial-mesenchymal transition and metastasis in ovarian cancer. DNA Cell Biol. 2019, 38, 341–351. [Google Scholar] [CrossRef]
- Chung, Y.H.; Li, S.C.; Kao, Y.H.; Luo, H.L.; Cheng, Y.T.; Lin, P.R.; Tai, M.H.; Chiang, P.H. Mir-30a-5p inhibits epithelial-to-mesenchymal transition and upregulates expression of tight junction protein claudin-5 in human upper tract urothelial carcinoma cells. Int. J. Mol. Sci. 2017, 18, 1826. [Google Scholar] [CrossRef]
- Park, Y.R.; Kim, S.L.; Lee, M.R.; Seo, S.Y.; Lee, J.H.; Kim, S.H.; Kim, I.H.; Lee, S.O.; Lee, S.T.; Kim, S.W. Microrna-30a-5p (mir-30a) regulates cell motility and emt by directly targeting oncogenic tm4sf1 in colorectal cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1915–1927. [Google Scholar] [CrossRef]
- Wang, T.; Chen, G.; Ma, X.; Yang, Y.; Chen, Y.; Peng, Y.; Bai, Z.; Zhang, Z.; Pei, H.; Guo, W. Mir-30a regulates cancer cell response to chemotherapy through snai1/irs1/akt pathway. Cell Death Dis. 2019, 10, 153. [Google Scholar] [CrossRef]
- Xiong, G.; Liu, C.; Yang, G.; Feng, M.; Xu, J.; Zhao, F.; You, L.; Zhou, L.; Zheng, L.; Hu, Y.; et al. Long noncoding rna gstm3tv2 upregulates lat2 and olr1 by competitively sponging let-7 to promote gemcitabine resistance in pancreatic cancer. J. Hematol. Oncol. 2019, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, J.; Zhang, L.; Zhu, Z.; Fan, J.; Chen, L.; Zhuang, L.; Luo, J.; Chen, H.; Liu, L.; et al. Microrna 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology 2013, 145, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.J.; Willy, J.A.; Quirin, K.A.; Wek, R.C.; Korc, M.; Yin, X.M.; Kota, J. Novel role of mir-29a in pancreatic cancer autophagy and its therapeutic potential. Oncotarget 2016, 7, 71635–71650. [Google Scholar] [CrossRef]
- Huang, L.; Hu, C.; Cao, H.; Wu, X.; Wang, R.; Lu, H.; Li, H.; Chen, H. Microrna-29c increases the chemosensitivity of pancreatic cancer cells by inhibiting usp22 mediated autophagy. Cell Physiol. Biochem. 2018, 47, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jia, S.; Ding, G.; Zhang, M.; Yu, W.; Wu, Z.; Cao, L. Down-regulation of mir-30a-5p is associated with poor prognosis and promotes chemoresistance of gemcitabine in pancreatic ductal adenocarcinoma. J. Cancer 2019, 10, 5031–5040. [Google Scholar] [CrossRef]
- Liang, C.; Yu, X.J.; Guo, X.Z.; Sun, M.H.; Wang, Z.; Song, Y.; Ni, Q.X.; Li, H.Y.; Mukaida, N.; Li, Y.Y. Microrna-33a-mediated downregulation of pim-3 kinase expression renders human pancreatic cancer cells sensitivity to gemcitabine. Oncotarget 2015, 6, 14440–14455. [Google Scholar] [CrossRef]
- Ma, Y.; Chai, N.; Jiang, Q.; Chang, Z.; Chai, Y.; Li, X.; Sun, H.; Hou, J.; Linghu, E. DNA methyltransferase mediates the hypermethylation of the microrna 34a promoter and enhances the resistance of patient-derived pancreatic cancer cells to molecular targeting agents. Pharmacol. Res. 2020, 160, 105071. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, P.; Wang, C.; Xin, B. Snhg14 enhances gemcitabine resistance by sponging mir-101 to stimulate cell autophagy in pancreatic cancer. Biochem. Biophys. Res. Commun. 2019, 510, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; He, Y.; Wang, Y.; Chen, W.; Hu, B.; Gu, Y. Micorrna-101 silences DNA-pkcs and sensitizes pancreatic cancer cells to gemcitabine. Biochem. Biophys. Res. Commun. 2017, 483, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ji, L.; Ke, M.; Ou, Z.; Tang, N.; Li, Y. Mir-125a-3p is responsible for chemosensitivity in pdac by inhibiting epithelial-mesenchymal transition via fyn. Biomed. Pharmacother. 2018, 106, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Huang, F.Z.; Xu, H.B.; Sun, J.C.; Wang, C.F. Microrna-137 inhibits autophagy and chemosensitizes pancreatic cancer cells by targeting atg5. Int. J. Biochem. Cell Biol. 2019, 111, 63–71. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, Q.; Dong, Z.; Hu, J.; Ma, Z. Lncrna hottip participates in cisplatin resistance of tumor cells by regulating mir-137 expression in pancreatic cancer. Onco Targets Ther. 2020, 13, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, M.; Chen, M.; Huang, Y.; Jiang, J. Upregulation of microrna1385p inhibits pancreatic cancer cell migration and increases chemotherapy sensitivity. Mol. Med. Rep. 2015, 12, 5135–5140. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Zhu, Y.D.; Xie, G.Q.; Zhang, K.; Sheng, J.; Zhu, Z.F.; Ning, Z.Y.; Chen, H.; Chen, Z.; Meng, Z.Q.; et al. Long non-coding sbf2-as1 acting as a competing endogenous rna to sponge microrna-142-3p to participate in gemcitabine resistance in pancreatic cancer via upregulating twf1. Aging 2019, 11, 8860–8878. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Li, K.; Gong, L.; Yang, Q.; Huang, X.; Hong, C.; Ding, M.; Yang, H. Linc-dync2h1-4 promotes emt and csc phenotypes by acting as a sponge of mir-145 in pancreatic cancer cells. Cell Death Dis. 2017, 8, e2924. [Google Scholar] [CrossRef]
- Meng, Q.; Liang, C.; Hua, J.; Zhang, B.; Liu, J.; Zhang, Y.; Wei, M.; Yu, X.; Xu, J.; Shi, S. A mir-146a-5p/traf6/nf-kb p65 axis regulates pancreatic cancer chemoresistance: Functional validation and clinical significance. Theranostics 2020, 10, 3967–3979. [Google Scholar] [CrossRef]
- Liu, F.; Liu, B.; Qian, J.; Wu, G.; Li, J.; Ma, Z. Mir-153 enhances the therapeutic effect of gemcitabine by targeting snail in pancreatic cancer. Acta Biochim. Biophys. Sin. 2017, 49, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, C.; Ning, Z.; Hua, Y.; Li, Y.; Chen, L.; Liu, L.; Chen, Z.; Meng, Z. Long non-coding rna linc00346 promotes pancreatic cancer growth and gemcitabine resistance by sponging mir-188-3p to derepress brd4 expression. J. Exp. Clin. Cancer Res. 2019, 38, 60. [Google Scholar] [CrossRef]
- Ma, C.; Huang, T.; Ding, Y.C.; Yu, W.; Wang, Q.; Meng, B.; Luo, S.X. Microrna-200c overexpression inhibits chemoresistance, invasion and colony formation of human pancreatic cancer stem cells. Int. J. Clin. Exp. Pathol. 2015, 8, 6533–6539. [Google Scholar]
- Du, S.L.; Xu, L.Y.; Gao, P.; Liu, Q.S.; Lu, F.F.; Mo, Z.H.; Fan, Z.Z.; Cheng, X.L.; Dong, Z.H. Mir-203 regulates dj-1 expression and affects proliferation, apoptosis and ddp resistance of pancreatic cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8833–8840. [Google Scholar]
- Chaudhary, A.K.; Mondal, G.; Kumar, V.; Kattel, K.; Mahato, R.I. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microrna-205. Cancer Lett. 2017, 402, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Dong, L.; Xia, L.; Zhu, H.; Li, Z.; Yu, X. Long noncoding rna hcp5 regulates pancreatic cancer gemcitabine (gem) resistance by sponging hsa-mir-214-3p to target hdgf. Onco Targets Ther. 2019, 12, 8207–8216. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, L.; Dong, L.; Wang, J.; Xiao, Q.; Yu, X.; Zhu, H. Circhipk3 promotes gemcitabine (gem) resistance in pancreatic cancer cells by sponging mir-330-5p and targets rassf1. Cancer Manag. Res. 2020, 12, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, X.; Sui, G.; Chen, S.; Yu, M.; Zhang, P. Downregulation of mir-374b-5p promotes chemotherapeutic resistance in pancreatic cancer by upregulating multiple anti-apoptotic proteins. Int. J. Oncol. 2018, 52, 1491–1503. [Google Scholar] [CrossRef]
- Schreiber, R.; Mezencev, R.; Matyunina, L.V.; McDonald, J.F. Evidence for the role of microrna 374b in acquired cisplatin resistance in pancreatic cancer cells. Cancer Gene Ther. 2016, 23, 241–245. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, D.; Wei, A.; Ke, N.; Wang, Y.; Tang, J.; He, S.; Hu, W.; Liu, X. Microrna-410-3p attenuates gemcitabine resistance in pancreatic ductal adenocarcinoma by inhibiting hmgb1-mediated autophagy. Oncotarget 2017, 8, 107500–107512. [Google Scholar] [CrossRef]
- Zhan, T.; Huang, X.; Tian, X.; Chen, X.; Ding, Y.; Luo, H.; Zhang, Y. Downregulation of microrna-455-3p links to proliferation and drug resistance of pancreatic cancer cells via targeting taz. Mol. Ther. Nucleic Acids 2018, 10, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Zhu, S.; Zhang, J.G.; Yang, M.; Qin, Q.; Deng, S.C.; Wang, B.; Tian, K.; Liu, L.; et al. Ectopic expression of mir-494 inhibited the proliferation, invasion and chemoresistance of pancreatic cancer by regulating sirt1 and c-myc. Gene Ther. 2015, 22, 729–738. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Li, W.; Yin, L.; Guo, S.; Xu, X.; Ouyang, Y.; Zhao, Z.; Liu, S.; Tian, Y.; et al. Downregulated mir-506 expression facilitates pancreatic cancer progression and chemoresistance via sphk1/akt/nf-kappab signaling. Oncogene 2016, 35, 5501–5514. [Google Scholar] [CrossRef]
- Hiramoto, H.; Muramatsu, T.; Ichikawa, D.; Tanimoto, K.; Yasukawa, S.; Otsuji, E.; Inazawa, J. Mir-509-5p and mir-1243 increase the sensitivity to gemcitabine by inhibiting epithelial-mesenchymal transition in pancreatic cancer. Sci. Rep. 2017, 7, 4002. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Wan, L.; Chen, R.; Chen, F. Mir-509-5p inhibits cellular proliferation and migration via targeting mdm2 in pancreatic cancer cells. Onco Targets Ther. 2017, 10, 4455–4464. [Google Scholar] [CrossRef]
- Zhou, C.; Yi, C.; Yi, Y.; Qin, W.; Yan, Y.; Dong, X.; Zhang, X.; Huang, Y.; Zhang, R.; Wei, J.; et al. Lncrna pvt1 promotes gemcitabine resistance of pancreatic cancer via activating wnt/beta-catenin and autophagy pathway through modulating the mir-619-5p/pygo2 and mir-619-5p/atg14 axes. Mol. Cancer 2020, 19, 118. [Google Scholar] [CrossRef]
- Yang, D.; Hu, Z.; Xu, J.; Tang, Y.; Wang, Y.; Cai, Q.; Zhu, Z. Mir-760 enhances sensitivity of pancreatic cancer cells to gemcitabine through modulating integrin beta1. Biosci. Rep. 2019, 39, BSR20192358. [Google Scholar] [CrossRef]
- Huang, H.; Xiong, G.; Shen, P.; Cao, Z.; Zheng, L.; Zhang, T.; Zhao, Y. Microrna-1285 inhibits malignant biological behaviors of human pancreatic cancer cells by negative regulation of yap1. Neoplasma 2017, 64, 358–366. [Google Scholar] [CrossRef]
- Yang, R.M.; Zhan, M.; Xu, S.W.; Long, M.M.; Yang, L.H.; Chen, W.; Huang, S.; Liu, Q.; Zhou, J.; Zhu, J.; et al. Mir-3656 expression enhances the chemosensitivity of pancreatic cancer to gemcitabine through modulation of the rhof/emt axis. Cell Death Dis. 2017, 8, e3129. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, Y.; Cheng, Y.S. Mir-34a inhibits pancreatic cancer progression through snail1-mediated epithelial-mesenchymal transition and the notch signaling pathway. Sci. Rep. 2017, 7, 38232. [Google Scholar] [CrossRef]
- Jia, C.W.; Sun, Y.; Zhang, T.T.; Lu, Z.H.; Chen, J. Effects of mir-125a-5p on cell proliferation, apoptosis and cell cycle of pancreatic cancer cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016, 38, 415–421. [Google Scholar]
- Yan, Q.; Hu, D.; Li, M.; Chen, Y.; Wu, X.; Ye, Q.; Wang, Z.; He, L.; Zhu, J. The serum microrna signatures for pancreatic cancer detection and operability evaluation. Front. Bioeng. Biotechnol. 2020, 8, 379. [Google Scholar] [CrossRef]
- Zhao, C.; Ling, X.; Li, X.; Hou, X.; Zhao, D. Microrna-138-5p inhibits cell migration, invasion and emt in breast cancer by directly targeting rhbdd1. Breast Cancer 2019, 26, 817–825. [Google Scholar] [CrossRef]
- Xu, W.; Chen, B.; Ke, D.; Chen, X. Microrna-138-5p targets the nfib-snail1 axis to inhibit colorectal cancer cell migration and chemoresistance. Cancer Cell Int. 2020, 20, 475. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, Q.; Zhang, J.; Yu, J.; Chen, W.; Zhang, Z. Downregulation of mir-153 contributes to epithelial-mesenchymal transition and tumor metastasis in human epithelial cancer. Carcinogenesis 2013, 34, 539–549. [Google Scholar] [CrossRef]
- Xia, W.; Ma, X.; Li, X.; Dong, H.; Yi, J.; Zeng, W.; Yang, Z. Mir-153 inhibits epithelial-to-mesenchymal transition in hepatocellular carcinoma by targeting snail. Oncol. Rep. 2015, 34, 655–662. [Google Scholar] [CrossRef]
- Yu, C.; Wang, M.; Li, Z.; Xiao, J.; Peng, F.; Guo, X.; Deng, Y.; Jiang, J.; Sun, C. Microrna-138-5p regulates pancreatic cancer cell growth through targeting foxc1. Cell Oncol. 2015, 38, 173–181. [Google Scholar] [CrossRef]
- Bai, Z.; Sun, J.; Wang, X.; Wang, H.; Pei, H.; Zhang, Z. Microrna-153 is a prognostic marker and inhibits cell migration and invasion by targeting snai1 in human pancreatic ductal adenocarcinoma. Oncol. Rep. 2015, 34, 595–602. [Google Scholar] [CrossRef]
- Zammarchi, F.; Morelli, M.; Menicagli, M.; Di Cristofano, C.; Zavaglia, K.; Paolucci, A.; Campani, D.; Aretini, P.; Boggi, U.; Mosca, F.; et al. Klf4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am. J. Pathol. 2011, 178, 361–372. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Z.; Wang, J.; Zhou, L.; Zhang, J.; Yao, B.; Dou, J.; Qiu, Z.; Huang, C. Kruppel-like factor 4 inhibits pancreatic cancer epithelial-to-mesenchymal transition and metastasis by down-regulating caveolin-1 expression. Cell Physiol. Biochem. 2018, 46, 238–252. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Bai, Y.; Chen, M.; Cheng, D.; Wu, M.; Xia, J. Rhof promotes hepatocellular carcinoma metastasis by altering the metabolic status of cancer cells via rab3d. Hepatology 2020. [Google Scholar] [CrossRef]
- Feng, Z.M.; Qiu, J.; Chen, X.W.; Liao, R.X.; Liao, X.Y.; Zhang, L.P.; Chen, X.; Li, Y.; Chen, Z.T.; Sun, J.G. Essential role of mir-200c in regulating self-renewal of breast cancer stem cells and their counterparts of mammary epithelium. BMC Cancer 2015, 15, 645. [Google Scholar] [CrossRef]
- Karimi Dermani, F.; Amini, R.; Saidijam, M.; Najafi, R. Mir-200c, a tumor suppressor that modulate the expression of cancer stem cells markers and epithelial-mesenchymal transition in colorectal cancer. J. Cell Biochem. 2018, 119, 6288–6295. [Google Scholar] [CrossRef]
- Rahimi, M.; Sharifi-Zarchi, A.; Zarghami, N.; Geranpayeh, L.; Ebrahimi, M.; Alizadeh, E. Down-regulation of mir-200c and up-regulation of mir-30c target both stemness and metastasis genes in breast cancer. Cell J. 2020, 21, 467–478. [Google Scholar]
- Xu, R.; Zhu, X.; Chen, F.; Huang, C.; Ai, K.; Wu, H.; Zhang, L.; Zhao, X. Lncrna xist/mir-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018, 18, 41. [Google Scholar] [CrossRef]
- Lu, Y.X.; Yuan, L.; Xue, X.L.; Zhou, M.; Liu, Y.; Zhang, C.; Li, J.P.; Zheng, L.; Hong, M.; Li, X.N. Regulation of colorectal carcinoma stemness, growth, and metastasis by an mir-200c-sox2-negative feedback loop mechanism. Clin. Cancer Res. 2014, 20, 2631–2642. [Google Scholar] [CrossRef]
- Fu, H.; Gu, Y.H.; Yang, Y.N.; Liao, S.; Wang, G.H. Mir-200b/c family inhibits renal fibrosis through modulating epithelial-to-mesenchymal transition via targeting fascin-1/cd44 axis. Life Sci. 2020, 252, 117589. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, G.; Qian, Y.W.; Li, Y.W. Down-regulation of mir-205 promotes stemness of hepatocellular carcinoma cells by targeting plcbeta1 and increasing cd24 expression. Neoplasma 2015, 62, 567–573. [Google Scholar] [CrossRef][Green Version]
- Xiao, Y.; Li, Y.; Tao, H.; Humphries, B.; Li, A.; Jiang, Y.; Yang, C.; Luo, R.; Wang, Z. Integrin alpha5 down-regulation by mir-205 suppresses triple negative breast cancer stemness and metastasis by inhibiting the src/vav2/rac1 pathway. Cancer Lett. 2018, 433, 199–209. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Xu, X.; He, X.; Wang, G.; Fan, C.; Zheng, Q.; Li, F. Mir-205/runx2 axis negatively regulates cd44(+)/cd24(-) breast cancer stem cell activity. Am. J. Cancer Res. 2020, 10, 1871–1887. [Google Scholar]
- Massihnia, D.; Avan, A.; Funel, N.; Maftouh, M.; van Krieken, A.; Granchi, C.; Raktoe, R.; Boggi, U.; Aicher, B.; Minutolo, F.; et al. Phospho-akt overexpression is prognostic and can be used to tailor the synergistic interaction of akt inhibitors with gemcitabine in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 9. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.M.; Meinkoth, J.; Pittman, R.N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 2000, 151, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Kodama, R.; Moritomo, S.; Hoshino, T.; Mizushima, T. Zidovudine, an anti-viral drug, resensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine by inhibition of the akt-gsk3beta-snail pathway. Cell Death Dis. 2015, 6, e1795. [Google Scholar] [CrossRef]
- Chen, D.; Niu, M.; Jiao, X.; Zhang, K.; Liang, J.; Zhang, D. Inhibition of akt2 enhances sensitivity to gemcitabine via regulating puma and nf-kappab signaling pathway in human pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 2012, 13, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Kim, Y.; Lim, S.; Kang, H.G.; Kim, D.H.; Park, J.W.; Cheong, W.; Kong, H.K.; Han, W.; Park, W.Y.; et al. Mir-374a-5p promotes tumor progression by targeting arrb1 in triple negative breast cancer. Cancer Lett. 2019, 454, 224–233. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Tang, J.; Gong, C. Microrna-374b-5p functions as a tumor suppressor in non-small cell lung cancer by targeting foxp1 and predicts prognosis of cancer patients. Onco Targets Ther. 2020, 13, 4229–4237. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, G.; Zheng, W.; Xue, Q.; Wei, D.; Zheng, Y.; Yuan, J. Mir-454-3p and mir-374b-5p suppress migration and invasion of bladder cancer cells through targetting zeb2. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Kim, M.K.; Jang, J.W.; Bae, S.C. DNA binding partners of yap/taz. BMB Rep. 2018, 51, 126–133. [Google Scholar] [CrossRef]
- Nguyen, C.D.K.; Yi, C. Yap/taz signaling and resistance to cancer therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar] [CrossRef]
- Kitagawa, K.; Moriya, K.; Kaji, K.; Saikawa, S.; Sato, S.; Nishimura, N.; Namisaki, T.; Akahane, T.; Mitoro, A.; Yoshiji, H. Atorvastatin augments gemcitabine-mediated anti-cancer effects by inhibiting yes-associated protein in human cholangiocarcinoma cells. Int. J. Mol. Sci. 2020, 21, 7588. [Google Scholar] [CrossRef]
- Buchholz, M.; Schatz, A.; Wagner, M.; Michl, P.; Linhart, T.; Adler, G.; Gress, T.M.; Ellenrieder, V. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of nfatc1 and the ca2+/calcineurin signaling pathway. EMBO J. 2006, 25, 3714–3724. [Google Scholar] [CrossRef]
- Jin, J.; Chu, Z.; Ma, P.; Meng, Y.; Yang, Y. Sirt1 promotes the proliferation and metastasis of human pancreatic cancer cells. Tumor Biol. 2017, 39, 1010428317691180. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Peng, J.; Xie, B.; Shou, Q.; Wang, J. Silencing c-myc enhances the antitumor activity of bufalin by suppressing the hif-1alpha/sdf-1/cxcr4 pathway in pancreatic cancer cells. Front. Pharmacol. 2020, 11, 495. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, J.; Zhang, J.G.; Qin, Q.; Chen, Q.; Yin, T.; Deng, S.C.; Liu, Y.; Liu, L.; Wang, B.; et al. Sirt1 rnai knockdown induces apoptosis and senescence, inhibits invasion and enhances chemosensitivity in pancreatic cancer cells. Gene Ther. 2011, 18, 920–928. [Google Scholar] [CrossRef]
- Lin, H.; Huang, Z.P.; Liu, J.; Qiu, Y.; Tao, Y.P.; Wang, M.C.; Yao, H.; Hou, K.Z.; Gu, F.M.; Xu, X.F. Mir-494-3p promotes pi3k/akt pathway hyperactivation and human hepatocellular carcinoma progression by targeting pten. Sci. Rep. 2018, 8, 10461. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.P.; Metcalfe, A.D.; Romer, L.H.; Streuli, C.H. Integrin-mediated survival signals regulate the apoptotic function of bax through its conformation and subcellular localization. J. Cell Biol. 2000, 149, 431–446. [Google Scholar] [CrossRef]
- Brannon, A., III; Drouillard, D.; Steele, N.; Schoettle, S.; Abel, E.V.; Crawford, H.C.; Pasca di Magliano, M. Beta 1 integrin signaling mediates pancreatic ductal adenocarcinoma resistance to mek inhibition. Sci. Rep. 2020, 10, 11133. [Google Scholar] [CrossRef]
- Grzesiak, J.J.; Tran Cao, H.S.; Burton, D.W.; Kaushal, S.; Vargas, F.; Clopton, P.; Snyder, C.S.; Deftos, L.J.; Hoffman, R.M.; Bouvet, M. Knockdown of the beta(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. Int. J. Cancer 2011, 129, 2905–2915. [Google Scholar] [CrossRef]
- He, Q.; Zhao, L.; Liu, X.; Zheng, J.; Liu, Y.; Liu, L.; Ma, J.; Cai, H.; Li, Z.; Xue, Y. Mov10 binding circ-dicer1 regulates the angiogenesis of glioma via mir-103a-3p/mir-382-5p mediated zic4 expression change. J. Exp. Clin. Cancer Res. 2019, 38, 9. [Google Scholar] [CrossRef]
- Walczak, M.; Martens, S. Dissecting the role of the atg12-atg5-atg16 complex during autophagosome formation. Autophagy 2013, 9, 424–425. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Zhou, H.; Wang, W.; Luo, Y.; Yang, H.; Yi, H. Inhibition of autophagy promotes cisplatin-induced apoptotic cell death through atg5 and beclin 1 in a549 human lung cancer cells. Mol. Med. Rep. 2018, 17, 6859–6865. [Google Scholar] [CrossRef]
- Ma, J.; Weng, L.; Jia, Y.; Liu, B.; Wu, S.; Xue, L.; Yin, X.; Mao, A.; Wang, Z.; Shang, M. Ptbp3 promotes malignancy and hypoxia-induced chemoresistance in pancreatic cancer cells by atg12 up-regulation. J. Cell Mol. Med. 2020, 24, 2917–2930. [Google Scholar] [CrossRef]
- Judith, D.; Jefferies, H.B.J.; Boeing, S.; Frith, D.; Snijders, A.P.; Tooze, S.A. Atg9a shapes the forming autophagosome through arfaptin 2 and phosphatidylinositol 4-kinase iiibeta. J. Cell Biol. 2019, 218, 1634–1652. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. Tfeb links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Wang, A.; Liang, J.; Xie, Y.; Liu, J.; Yan, Q.; Wang, Z. Usp22 promotes epithelial-mesenchymal transition via the fak pathway in pancreatic cancer cells. Oncol. Rep. 2014, 32, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, S.; Norgard, R.J.; Yan, F.; Yamazoe, T.; Blanco, A.; Stanger, B.Z. Tumor cell-intrinsic usp22 suppresses antitumor immunity in pancreatic cancer. Cancer Immunol. Res. 2020, 8, 282–291. [Google Scholar] [CrossRef]
- Liang, J.X.; Ning, Z.; Gao, W.; Ling, J.; Wang, A.M.; Luo, H.F.; Liang, Y.; Yan, Q.; Wang, Z.Y. Ubiquitinspecific protease 22induced autophagy is correlated with poor prognosis of pancreatic cancer. Oncol. Rep. 2014, 32, 2726–2734. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J., III; et al. Endogenous hmgb1 regulates autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef]
- Kuramitsu, Y.; Wang, Y.; Kitagawa, T.; Tokuda, K.; Akada, J.; Tokunaga, M.; Nakamura, K. High-mobility group box 1 and mitogen-activated protein kinase activated protein kinase-2 are up-regulated in gemcitabine-resistant pancreatic cancer cells. Anticancer Res. 2015, 35, 3861–3865. [Google Scholar]
- Chen, X.; Zhang, L.; Jiang, Y.; Song, L.; Liu, Y.; Cheng, F.; Fan, X.; Cao, X.; Gong, A.; Wang, D.; et al. Radiotherapy-induced cell death activates paracrine hmgb1-tlr2 signaling and accelerates pancreatic carcinoma metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 77. [Google Scholar] [CrossRef]
- Chen, Q.; Bian, Y.; Zeng, S. Involvement of ap-1 and nf-kappab in the up-regulation of p-gp in vinblastine resistant caco-2 cells. Drug Metab. Pharmacokinet. 2014, 29, 223–226. [Google Scholar] [CrossRef]
- Bentires-Alj, M.; Barbu, V.; Fillet, M.; Chariot, A.; Relic, B.; Jacobs, N.; Gielen, J.; Merville, M.P.; Bours, V. Nf-kappab transcription factor induces drug resistance through mdr1 expression in cancer cells. Oncogene 2003, 22, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Sun, Y. Long non-coding rna ab209630 suppresses cell proliferation and metastasis in human hepatocellular carcinoma. Exp. Ther. Med. 2017, 14, 3419–3424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, J.; Li, M.; Yu, W.; Li, W.; Wang, J.; Xiang, X.; Li, G.; Pan, X.; Lei, D. Ab209630, a long non-coding rna decreased expression in hypopharyngeal squamous cell carcinoma, influences proliferation, invasion, metastasis, and survival. Oncotarget 2016, 7, 14628–14638. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Na, L.; Yu, J.; Huang, L.; Meng, Z.Q.; Chen, Z.; Chen, H.; Ming, L.L.; Hua, Y.Q. Lncrna ab209630 inhibits gemcitabine resistance cell proliferation by regulating pi3k/akt signaling in pancreatic ductal adenocarcinoma. Cancer Biomark. 2018, 22, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, C.; Sun, Z. Long non-coding rna linc00346, linc00578, linc00673, linc00671, linc00261, and snhg9 are novel prognostic markers for pancreatic cancer. Am. J. Transl. Res. 2018, 10, 2648–2658. [Google Scholar]
- Zhao, L.; Kong, H.; Sun, H.; Chen, Z.; Chen, B.; Zhou, M. Lncrna-pvt1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate mir-448. J. Cell Physiol. 2018, 233, 4044–4055. [Google Scholar] [CrossRef]
- Yang, Q.; Li, K.; Huang, X.; Zhao, C.; Mei, Y.; Li, X.; Jiao, L.; Yang, H. Lncrna slc7a11-as1 promotes chemoresistance by blocking scf(beta-trcp)-mediated degradation of nrf2 in pancreatic cancer. Mol. Ther. Nucleic Acids 2020, 19, 974–985. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Zhang, L.; Cheng, L.; Li, X. Lncrna tug1 promoted viability and associated with gemcitabine resistant in pancreatic ductal adenocarcinoma. J. Pharmacol. Sci. 2018, 137, 116–121. [Google Scholar] [CrossRef]
- Yu, Y.; Hann, S.S. Novel tumor suppressor lncrna growth arrest-specific 5 (gas5) in human cancer. Onco Targets Ther. 2019, 12, 8421–8436. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Q.; Wang, J.F.; Chen, D.H.; Ma, X.S.; Wu, Y.; Tang, Z.; Dang, X.W. Long non-coding rna gas5 suppresses pancreatic cancer metastasis through modulating mir-32-5p/pten axis. Cell Biosci. 2017, 7, 66. [Google Scholar] [CrossRef]
- Jin, W. Role of jak/stat3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef]
- Zeng, K.; Chen, X.; Xu, M.; Liu, X.; Hu, X.; Xu, T.; Sun, H.; Pan, Y.; He, B.; Wang, S. Circhipk3 promotes colorectal cancer growth and metastasis by sponging mir-7. Cell Death Dis. 2018, 9, 417. [Google Scholar] [CrossRef]
- Lai, J.; Xin, J.; Fu, C.; Zhang, W. Circhipk3 promotes proliferation and metastasis and inhibits apoptosis of renal cancer cells by inhibiting mir-485-3p. Cancer Cell Int. 2020, 20, 248. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, F.; Xiao, X.; Xie, F.; Tao, D.; Huang, C.; Liu, D.; Wang, M.; Wang, L.; Zeng, F.; et al. Circhipk3 sponges mir-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017, 18, 1646–1659. [Google Scholar] [CrossRef]
- Yang, C.; Zheng, J.; Liu, X.; Xue, Y.; He, Q.; Dong, Y.; Wang, D.; Li, Z.; Liu, L.; Ma, J.; et al. Role of ankhd1/linc00346/znf655 feedback loop in regulating the glioma angiogenesis via staufen1-mediated mrna decay. Mol. Ther. Nucleic Acids 2020, 20, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, B.; Zhang, L.; Cui, M.; Sun, B. Silencing of long noncoding rna linc00346 inhibits the tumorigenesis of colorectal cancer through targeting microrna-148b. Onco Targets Ther. 2020, 13, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Pu, T.; Zhang, Y.; Wang, J.; Zhao, Y. Long non-coding rna linc00346 contributes to cisplatin resistance in nasopharyngeal carcinoma by repressing mir-342-5p. Open Biol. 2020, 10, 190286. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, Y.; Sun, H.; Zhang, Y.; Yang, J.; Brasier, A.R. Brd4 mediates nf-kappab-dependent epithelial-mesenchymal transition and pulmonary fibrosis via transcriptional elongation. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L1183–L1201. [Google Scholar] [CrossRef]
- Mei, L.L.; Wang, W.J.; Qiu, Y.T.; Xie, X.F.; Bai, J.; Shi, Z.Z. Mir-145-5p suppresses tumor cell migration, invasion and epithelial to mesenchymal transition by regulating the sp1/nf-kappab signaling pathway in esophageal squamous cell carcinoma. Int. J. Mol. Sci. 2017, 18, 1833. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Zhu, Y.; Li, Y.; Zhang, Y.; Wang, Y.; Li, X.; Xie, X.; Wang, J.; Huang, M.; et al. Circptn sponges mir-145-5p/mir-330-5p to promote proliferation and stemness in glioma. J. Exp. Clin. Cancer Res. 2019, 38, 398. [Google Scholar] [CrossRef]
- Feng, M.; Xiong, G.; Cao, Z.; Yang, G.; Zheng, S.; Qiu, J.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. Lat2 regulates glutamine-dependent mtor activation to promote glycolysis and chemoresistance in pancreatic cancer. J. Exp. Clin. Cancer Res. 2018, 37, 274. [Google Scholar] [CrossRef] [PubMed]
- Khaidakov, M.; Mitra, S.; Kang, B.Y.; Wang, X.; Kadlubar, S.; Novelli, G.; Raj, V.; Winters, M.; Carter, W.C.; Mehta, J.L. Oxidized ldl receptor 1 (olr1) as a possible link between obesity, dyslipidemia and cancer. PLoS ONE 2011, 6, e20277. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Zeng, W.; Wan, C.; Duan, S.; Jiang, S. Microrna-137 promotes apoptosis in ovarian cancer cells via the regulation of xiap. Br. J. Cancer 2017, 116, 66–76. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, W.; Zhang, J.; Ge, L.; Zhang, Y.; Jiang, X.; Peng, W.; Wang, D.; Gong, A.; Xu, M. Long noncoding rna pvt1 promotes epithelialmesenchymal transition via the tgfbeta/smad pathway in pancreatic cancer cells. Oncol. Rep. 2018, 40, 1093–1102. [Google Scholar]
- Bockhorn, J.; Dalton, R.; Nwachukwu, C.; Huang, S.; Prat, A.; Yee, K.; Chang, Y.F.; Huo, D.; Wen, Y.; Swanson, K.E.; et al. Microrna-30c inhibits human breast tumour chemotherapy resistance by regulating twf1 and il-11. Nat. Commun. 2013, 4, 1393. [Google Scholar] [CrossRef]
- Li, Y.; He, Q.; Wen, X.; Hong, X.; Yang, X.; Tang, X.; Zhang, P.; Lei, Y.; Sun, Y.; Zhang, J.; et al. Ezh2-dnmt1-mediated epigenetic silencing of mir-142-3p promotes metastasis through targeting zeb2 in nasopharyngeal carcinoma. Cell Death Differ. 2019, 26, 1089–1106. [Google Scholar] [CrossRef]
- Xu, T.; He, B.S.; Pan, B.; Pan, Y.Q.; Sun, H.L.; Liu, X.X.; Xu, X.N.; Chen, X.X.; Zeng, K.X.; Xu, M.; et al. Mir-142-3p functions as a tumor suppressor by targeting rac1/pak1 pathway in breast cancer. J. Cell Physiol. 2020, 235, 4928–4940. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Angelova, P.R.; Holmstrom, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ros production by mitochondria and nadph oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. Scf/{beta}-trcp promotes glycogen synthase kinase 3-dependent degradation of the nrf2 transcription factor in a keap1-independent manner. Mol. Cell Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef]
- Deng, W.; Vanderbilt, D.B.; Lin, C.C.; Martin, K.H.; Brundage, K.M.; Ruppert, J.M. Sox9 inhibits beta-trcp-mediated protein degradation to promote nuclear gli1 expression and cancer stem cell properties. J. Cell Sci. 2015, 128, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhou, H.; Yao, W.; Meng, L.; Lang, B. Lncrna tug1 promotes cisplatin resistance by regulating ccnd2 via epigenetically silencing mir-194-5p in bladder cancer. Mol. Ther. Nucleic Acids 2019, 16, 257–271. [Google Scholar] [CrossRef]
- MacKenzie, T.N.; Mujumdar, N.; Banerjee, S.; Sangwan, V.; Sarver, A.; Vickers, S.; Subramanian, S.; Saluja, A.K. Triptolide induces the expression of mir-142-3p: A negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol. Cancer Ther. 2013, 12, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, A.V.; Blake, D.R.; Gilbert, T.S.K.; Ng, S.; Hostetter, G.; Azam, S.H.; Ozkan-Dagliyan, I.; Gautam, P.; Bryant, K.L.; Pearce, K.H.; et al. Kras suppression-induced degradation of myc is antagonized by a mek5-erk5 compensatory mechanism. Cancer Cell 2018, 34, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.L.; Stalnecker, C.A.; Zeitouni, D.; Klomp, J.E.; Peng, S.; Tikunov, A.P.; Gunda, V.; Pierobon, M.; Waters, A.M.; George, S.D.; et al. Combination of erk and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 2019, 25, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hsia, T.C.; Yeh, M.H.; Chen, T.W.; Chen, Y.J.; Chen, J.T.; Wei, Y.L.; Tu, C.Y.; Huang, W.C. Mek inhibitors induce akt activation and drug resistance by suppressing negative feedback erk-mediated her2 phosphorylation at thr701. Mol. Oncol. 2017, 11, 1273–1287. [Google Scholar] [CrossRef]
- Hayes, T.K.; Neel, N.F.; Hu, C.; Gautam, P.; Chenard, M.; Long, B.; Aziz, M.; Kassner, M.; Bryant, K.L.; Pierobon, M.; et al. Long-term erk inhibition in kras-mutant pancreatic cancer is associated with myc degradation and senescence-like growth suppression. Cancer Cell 2016, 29, 75–89. [Google Scholar] [CrossRef]
- Moeng, S.; Son, S.W.; Lee, J.S.; Lee, H.Y.; Kim, T.H.; Choi, S.Y.; Kuh, H.J.; Park, J.K. Extracellular vesicles (evs) and pancreatic cancer: From the role of evs to the interference with ev-mediated reciprocal communication. Biomedicines 2020, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Son, S.W.; Lee, H.Y.; Moeng, S.; Kuh, H.J.; Choi, S.Y.; Park, J.K. Participation of micrornas in the treatment of cancer with phytochemicals. Molecules 2020, 25, 4701. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.A.; Moeng, S.; Sim, S.; Kuh, H.J.; Choi, S.Y.; Park, J.K. Microrna-based combinatorial cancer therapy: Effects of micrornas on the efficacy of anti-cancer therapies. Cells 2019, 9, 29. [Google Scholar] [CrossRef]
- Baldassari, F.; Zerbinati, C.; Galasso, M.; Corra, F.; Minotti, L.; Agnoletto, C.; Previati, M.; Croce, C.M.; Volinia, S. Screen for microrna and drug interactions in breast cancer cell lines points to mir-126 as a modulator of cdk4/6 and pik3ca inhibitors. Front. Genet. 2018, 9, 174. [Google Scholar] [CrossRef]
- Wang, Q.; Teng, Y.; Wang, R.; Deng, D.; You, Y.; Peng, Y.; Shao, N.; Zhi, F. The long non-coding rna snhg14 inhibits cell proliferation and invasion and promotes apoptosis by sponging mir-92a-3p in glioma. Oncotarget 2018, 9, 12112–12124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Q.; Ao, J.; Li, H.; Li, M. Role of mir-92a-3p/pten axis in regulation of pancreatic cancer cell proliferation and metastasis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 280–289. [Google Scholar]
- Han, C.; Wang, S.; Wang, H.; Zhang, J. Exosomal circ-hipk3 facilitates tumor progression and temozolomide resistance by regulating mir-421/zic5 axis in glioma. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef]
- Wang, M.; Hu, H.; Wang, Y.; Huang, Q.; Huang, R.; Chen, Y.; Ma, T.; Qiao, T.; Zhang, Q.; Wu, H.; et al. Long non-coding rna tug1 mediates 5-fluorouracil resistance by acting as a cerna of mir-197-3p in colorectal cancer. J. Cancer 2019, 10, 4603–4613. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, S.; Zhou, Y.; Liu, C.; Hu, X.; Shao, C. Microrna 421 suppresses dpc4/smad4 in pancreatic cancer. Biochem. Biophys. Res. Commun. 2011, 406, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Satoh, K.; Miura, S.; Hirota, M.; Kanno, A.; Masamune, A.; Kikuta, K.; Kume, K.; Unno, J.; Egawa, S.; et al. Mir-197 induces epithelial-mesenchymal transition in pancreatic cancer cells by targeting p120 catenin. J. Cell Physiol. 2013, 228, 1255–1263. [Google Scholar] [CrossRef] [PubMed]

| miRNA | Expression | In Vivo Experiment and/or Clinical Relevance | Ref. |
|---|---|---|---|
| miR-10a-5p | Increased in gemcitabine-resistant AsPC-1 cells. Upregulated in cancer tissues compared to matched adjacent tissues | Subcutaneous injections of AsPC-1 cells transduced with miR-10a-5p lentiviral plasmids. A positive correlation with unfavorable prognosis of patients | [37] |
| miR-17-5p | Augmented in MIAPaCa-2 cells overexpressing GFRA2. Escalated in cancer tissues | Positively correlated with poor survival | [39] |
| miR-21-5p | Upregulated in stem-like cells isolated from gemcitabine-resistant L3.6pl cells (GR-L3.6pl) | Orthotopic injections of stem-like cells from GR-L3.6pl following miR-21-5p knockdown | [40] |
| Upregulated in 5-FU-resistant PATU8988 cells | - | [41] | |
| miR-29-3p | Highly expressed in MIAPaCa-2, PSN-1, and PANC-1 cells compared to BxPC-3 cells | - | [42] |
| miR-125a-5p | Upregulated in cancer tissues from chemo-resistant patients | Inversely correlated with the level of a target gene (TNFAIP3) | [43] |
| miR-135-5p | High expression in cancer tissues compared to normal controls | Subcutaneous injections of miR-135-5p-overexpressing MIAPaCa-2 cells or miR-135-5p knockdown PANC-1 cells followed by gemcitabine treatment. Short overall survival of patients with high miR-135-5p levels | [44] |
| miR-181c-5p | High expression in cancer tissues compared to normal controls. Upregulated in gemcitabine-resistant SW1990 cells and 5-FU-resistant PATU8988 cells | Poor overall survival of patients with strong miR-181c-5p expression | [45,46] |
| miR-221-3p | Upregulated in 5-FU-resistant PATU8988 cells | Negative correlation with the overall survival of patients | [47] |
| Upregulated in stem-like cells isolated from gemcitabine-resistant L3.6pl cells (GR-L3.6pl) | Orthotopic injections of stem-like cells from GR-L3.6pl following miR-221-3p knockdown | [40] | |
| Upregulated in cancer tissues compared to normal controls | - | [48] | |
| miR-223-3p | Highly abundant in gemcitabine-resistant AsPC-1 and PANC-1 cells | - | [49] |
| Downregulated by genistein in gemcitabine-resistant AsPC-1 and BxPC-3 cells | Subcutaneous injections of gemcitabine-resistant BxPC-3 cells + intratumor injection of miR-223-3p inhibitors or genistein (15 mg/kg, oral administration) | [50] | |
| Upregulated in cisplatin-resistant BxPC-3 cells | - | [51] | |
| miR-296-5p | High expression in MIAPaCa-2, PK-8, and PK-45H cells | Negative correlation with the overall survival of patients | [52] |
| miR-301-3p | Upregulated in CFPAC-1 and BxPC-3 cells under hypoxia | Aggressive cancer behaviors and poor overall survival in patients with elevated miR-301-3p expression | [53,54] |
| Heightened in gemcitabine-resistant SW1990 and PANC-1 cells (GR-PANC-1) | Intraperitoneal injections of miR-301-3p inhibitors and gemcitabine (20 mg/kg) into mice bearing GR-PANC-1 xenografts | [55] | |
| miR-320a | Upregulated in 5-FU-resistant PATU8988 cells | - | [56] |
| miR-331-3p | Upregulated in gemcitabine-resistant PANC-1 and MIAPaCa-2 cells. Increased in plasma from patients receiving chemotherapy | - | [57] |
| miR-342-3p | Highly expressed in gemcitabine-resistant cancer tissues from patients | Intraperitoneal injections of gemcitabine (12.5 mg/kg) into orthotopic xenograft mouse models established using miR-342-3p-overexpressing MIAPaCa-2 cells | [58] |
| miR-1246 | Highly abundant in gemcitabine-resistant PANC-1 cells | Negatively correlated with the overall survival of patients | [59] |
| miR-1266-5p | Upregulated in cancer tissues compared to normal controls | Tail vein injections of miR-1266-5p inhibitors into mice bearing AsPC-1 xenograft + intraperitoneal injection of gemcitabine (50 mg/kg). Positively correlated with unfavorable prognosis of patients | [60] |
| miRNA | Expression | In Vivo Experiment and/or Clinical Relevance | Ref. |
|---|---|---|---|
| let-7 | Negatively regulated by lncRNA-GSTM3TV2 | - | [104] |
| miR-23-3p | Reduced in radioresistant PANC-1 and BxPC-3 cells | Radiotherapy (10-Gy) following the establishment of xenograft mouse models using miR-23-3p-overexpressing cells | [105] |
| miR-29a-3p | Low expression in PANC-1, BxPC-3, MIAPaCa-2, and COLO357 cells compared to normal pancreatic ductal epithelial cells | - | [106] |
| miR-29c-5p | - | Intraperitoneal injections of gemcitabine (50 mg/kg) into mice bearing miR-29c-5p-overexpressing PANC-1 cells | [107] |
| miR-30a-5p | Downregulated in gemcitabine-resistant SW1990 cells | Subcutaneous injections of miR-30a-5p-overexpressing SW1990 cells followed by gemcitabine treatment (50 mg/kg) | [103] |
| Low expression in cancer cell lines (PANC-1, MIAPaCa-2, and BxPC-3) and cancer tissues | Injections of miR-30a-5p into BxPC-3 xenografts followed by gemcitabine administration (100 mg/kg). Poor overall survival of patients with low miR-30a-5p expression | [108] | |
| miR-33-5p | Lowered in plasma and cancer tissues from patients | Poor overall survival of patients with low miR-33-5p expression | [109] |
| miR-34a | Promoter is highly methylated in cancer tissues compared to paired normal tissues | Oral administration of sorafenib (1.0 mg/kg) in mice bearing xenografts of miR-34a-overexpressing PANC-1 cells | [110] |
| miR-101-3p | Downregulated in cancer tissues | - | [111] |
| miR-101-5p | Lowered in gemcitabine-resistant cancer tissues | - | [112] |
| miR-125a-3p | Reduced in gemcitabine-treated PATU8988 and PANC-1 cells | - | [113] |
| miR-137-3p | Decreased by doxorubicin treatments in PANC-1 cells. Low expression in cancer cell lines (PANC-1, HS766T, AsPC-1) | Intravenous injections of doxorubicin (5 mg/kg) in mice bearing xenografts of miR-137-overexpressing PANC-1 cells | [114,115] |
| miR-138-5p | Downregulated in primary cancer tissues compared to normal controls | - | [116] |
| miR-142-3p | Lowered in gemcitabine-resistant PANC-1 and AsPC-1 cells | - | [117] |
| miR-145-5p | Downregulated in gemcitabine-resistant BxPC-3 cells | - | [118] |
| miR-146a-5p | Decreased in cancer tissues compared to adjacent normal tissues | Intra-tumoral injections of miR-146a-5p + intraperitoneal injection of gemcitabine (20 mg/kg). Short overall survival of patients with low miR-146a-5p expression | [119] |
| miR-153 | Downregulated in cancer tissues compared to normal tissues. Low expression in gemcitabine-resistant PANC-1, Capan-2, and AsPC-1 cells | Intraperitoneal injections of gemcitabine (50 mg/kg) in mice bearing xenografts of miR-153-overexpressing AsPC-1 cells. Unfavorable overall survival of patients with low miR-153 expression | [120] |
| miR-183-5p | Reduced in PANC-1 and BxPC-3 cells following gemcitabine exposure | Intraperitoneal injections of gemcitabine (80 mg/kg) in mice bearing xenografts of KLF4-overexpressing PANC-1 cells | [18] |
| miR-188-3p | - | Poor overall survival of patients with low miR-188-3p expression | [121] |
| miR-200-3p | Reduced in PANC-1 and BxPC-3 cells following gemcitabine exposure | Intraperitoneal injections of gemcitabine (80 mg/kg) in mice bearing xenografts of KLF4-overexpressing PANC-1 cells | [18] |
| Low expression in CD24+/CD44+/epithelial-specific antigen (ESA)+ CSCs | - | [122] | |
| miR-203-3p | Downregulated in cisplatin-resistant SW1990 cells | - | [123] |
| miR-205-5p | Decreased in primary cancer lesions | Intravenous injections of gemcitabine-conjugated micelles into mice bearing xenografts of miR-205-5p-overexpressing MIAPaCa-2 cells | [124] |
| miR-214-3p | Downregulated in gemcitabine-resistant cancer tissues | - | [125] |
| miR-330-5p | Reduced in cancer tissues compared to tissues of normal pancreas | - | [126] |
| miR-374-5p | Repressed in cancer tissues compared to adjacent normal tissues | Intraperitoneal injections of gemcitabine (50 mg/kg) into xenograft mouse models established using miR-374-5p-overexpressing AsPC-1 cells | [127] |
| Downregulated in cisplatin-resistant BxPC-3 cells | - | [128] | |
| miR-410-3p | Downregulated in human cancer xenografts from gemcitabine-treated mice | Low miR-410-3p expression is correlated with short overall survival of patients | [129] |
| miR-455-3p | Decreased in cell lines (PANC-1 and MIAPaCa-2 cells) and cancer tissues | - | [130] |
| miR-494-3p | Downregulated in cancer tissues compared to tissues of normal pancreas | Low miR-494-3p expression is correlated with distant metastasis and poor overall survival of patients | [131] |
| miR-506-3p | Low expression in cancer tissues compared to normal controls | Short overall survival of patients with low miR-506-3p expression | [132] |
| miR-509-5p | Downregulated in cancer tissues compared to noncancerous adjacent tissues | Worse overall survival of patients with low miR-509-5p levels | [133,134] |
| miR-619-5p | Reduced in gemcitabine-treated PANC-1 cells | - | [135] |
| miR-760 | Low expression in SW1990, AsPC-1, PANC-1, and BxPC-3 cells compared to normal pancreatic ductal epithelial cells | - | [136] |
| miR-1243 | - | Venous invasion, a clinicopathological characteristic, is associated with the expression of miR-1243 | [133] |
| miR-1285 | Dropped in gemcitabine-resistant AsPC-1 and MIAPaCa-2 cells | - | [137] |
| miR-3656 | Reduced in gemcitabine-resistant PANC-1 cells. Downregulated in cancer tissues compared to noncancerous tissues | Subcutaneous injections of PANC-1 cells overexpressing miR-3656 + intraperitoneal injections of gemcitabine (15 mg/kg). Poor patient prognosis is correlated with low miR-3656 levels | [138] |
| LncRNA | Expression | In Vivo Experiment and/or Clinical Relevance | Ref. |
|---|---|---|---|
| Circ-HIPK3 | Abundant in gemcitabine-resistant cancer tissues | Poor overall survival of patients with high circ-HIPK3 expression | [126] |
| LINC00346 | Highly expressed in cancer tissues as well as serum from patients | Intraperitoneal injections of gemcitabine (100 mg/kg) in mice bearing xenografts of LINC00346-depleted PANC-1 cells | [121,195] |
| LINC-DYNC2H1-4 | Upregulated in gemcitabine-resistant BxPC-3 cells. Increased in cancer tissues compared to adjacent normal tissues | - | [118] |
| LncRNA-AB209630 | Reduced in cancer tissues compared to adjacent normal controls | Poor patient prognosis is associated with low lncRNA-AB209630 levels | [194] |
| LncRNA-GAS5 | Downregulated in gemcitabine-resistant SW1990 cells and 5-FU-resistnat PATU8988 cells | - | [46] |
| Downregulated in cancer tissues compared to normal tissues | Intraperitoneal injections of gemcitabine (125 mg/kg) in mice bearing xenografts of lncRNA-GAS5-overexpressing PANC-1 cells. Intravenous injections of lncRNA-GAS5-overexpressing cells for metastasis analysis | [48] | |
| LncRNA-GSTM3TV2 | Upregulated in gemcitabine-resistant AsPC-1 and MIAPaCa-2 cells | Intraperitoneal injections of gemcitabine (25 mg/kg) in mice bearing xenografts of lncRNA-GSTM3TV2-overexpressing AsPC-1 cells. Poor survival rate of patients is associated with high expression of lncRNA-GSTM3TV2 | [104] |
| LncRNA-HCP5 | High expression is detected in gemcitabine-resistant SW1990 and PANC-1 cells. Upregulated in cancer tissues compared to normal tissues | Poor survival rate of patients is associated with high expression of lncRNA-HCP5 | [125] |
| LncRNA-HOTTIP | Increased in cisplatin-resistant PANC-1, HS766T, and AsPC-1 cells | - | [115] |
| LncRNA-PVT1 | Overexpressed in cancer tissues compared to adjacent pancreatic tissues | Intraperitoneal injections of gemcitabine (50 mg/kg) in mice bearing xenografts of PANC-1 cells stably expressing lncRNA- PVT1. Correlated with vascular infiltration and distant metastasis. Poor overall survival of patients with high lncRNA-PVT1 expression | [135,196] |
| LncRNA-SBF2-AS1 | Abundantly expressed in gemcitabine-resistant AsPC-1 and PANC-1 cells. High expression is detected in cancer tissues compared to adjacent normal tissues | High expression is correlated with lymph node metastasis and poor overall survival of patients | [117] |
| LncRNA-SLC7A11-AS1 | Highly expressed in gemcitabine-resistant BxPC-3 cells. Upregulated in cancer tissues compared to adjacent normal tissues | Intraperitoneal injections of gemcitabine (50 mg/kg) in mice bearing xenografts of lncRNA-SLC7A11-AS1-depleted PANC-1 cells. Negatively correlated with overall survival of patients | [197] |
| LncRNA-SNHG14 | Higher in cancer tissues than normal tissues | - | [111] |
| LncRNA-TUG1 | Overexpressed in several cell lines (PANC-1, PANC-28, BxPC-3, and SW1990) and cancer tissues | - | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, S.W.; Song, M.G.; Yun, B.D.; Park, J.K. Noncoding RNAs Associated with Therapeutic Resistance in Pancreatic Cancer. Biomedicines 2021, 9, 263. https://doi.org/10.3390/biomedicines9030263
Son SW, Song MG, Yun BD, Park JK. Noncoding RNAs Associated with Therapeutic Resistance in Pancreatic Cancer. Biomedicines. 2021; 9(3):263. https://doi.org/10.3390/biomedicines9030263
Chicago/Turabian StyleSon, Seung Wan, Mun Gyu Song, Ba Da Yun, and Jong Kook Park. 2021. "Noncoding RNAs Associated with Therapeutic Resistance in Pancreatic Cancer" Biomedicines 9, no. 3: 263. https://doi.org/10.3390/biomedicines9030263
APA StyleSon, S. W., Song, M. G., Yun, B. D., & Park, J. K. (2021). Noncoding RNAs Associated with Therapeutic Resistance in Pancreatic Cancer. Biomedicines, 9(3), 263. https://doi.org/10.3390/biomedicines9030263






