Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways
Abstract
1. Introduction
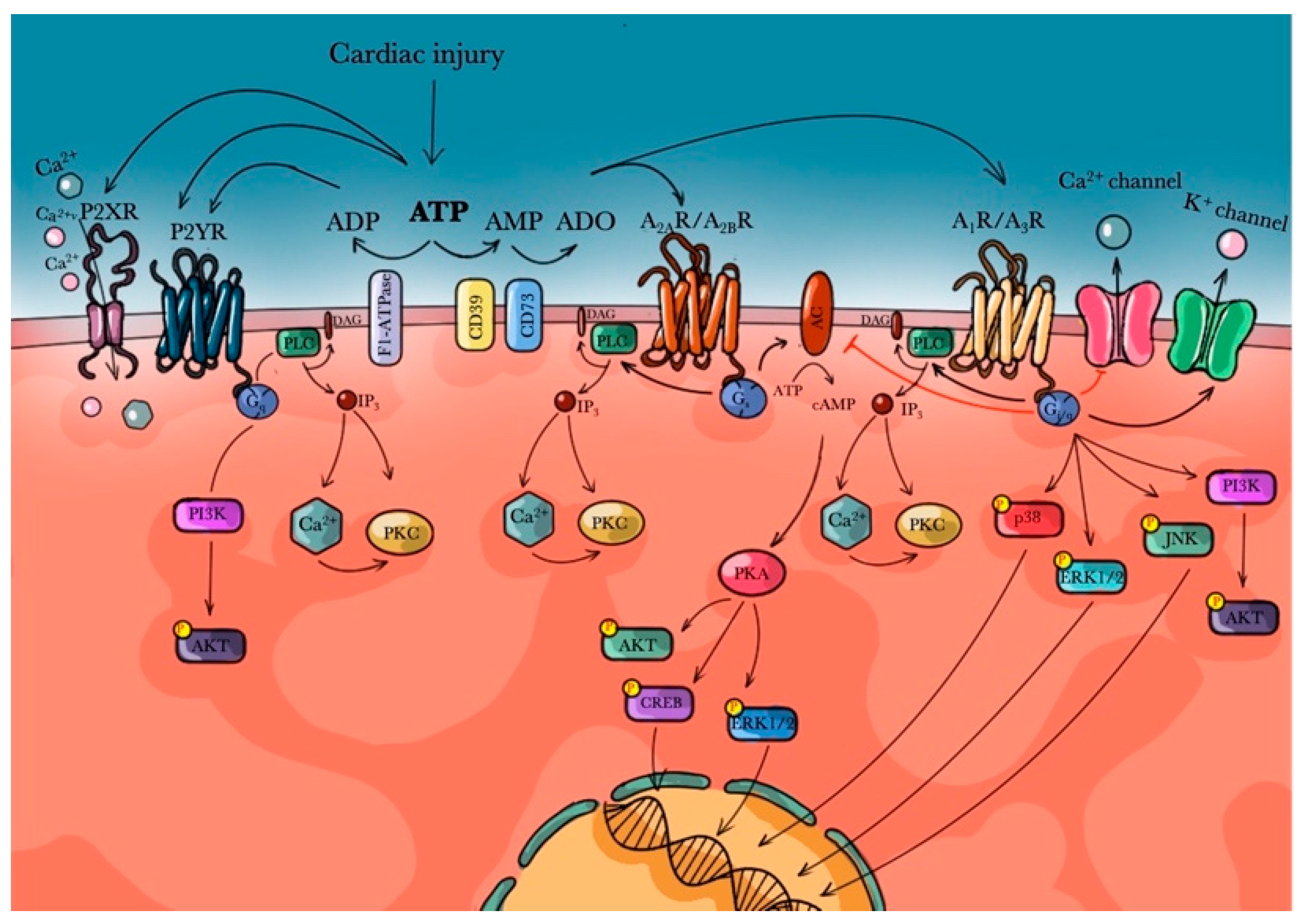
2. Purinergic Receptors
3. Adenosine Receptors
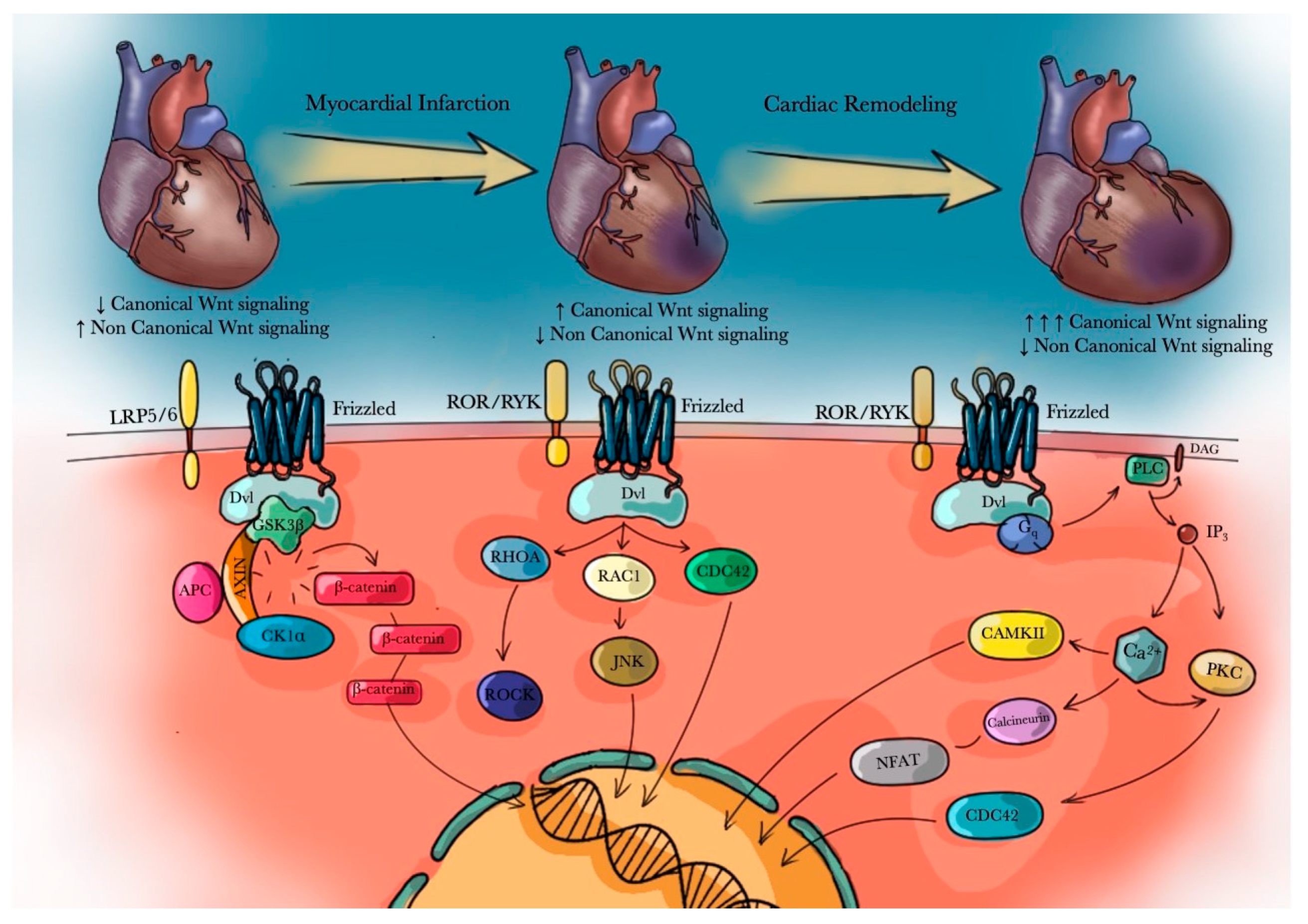
4. Wnt/β-Catenin Signaling Pathway Role in Myocardial Infarction (MI)
5. Adenosine Agonists and Antagonists
5.1. A1R
5.2. A2AR
5.3. A2BR
5.4. A3R
6. The Therapeutic Benefits of Wnt Pathway Inhibitors
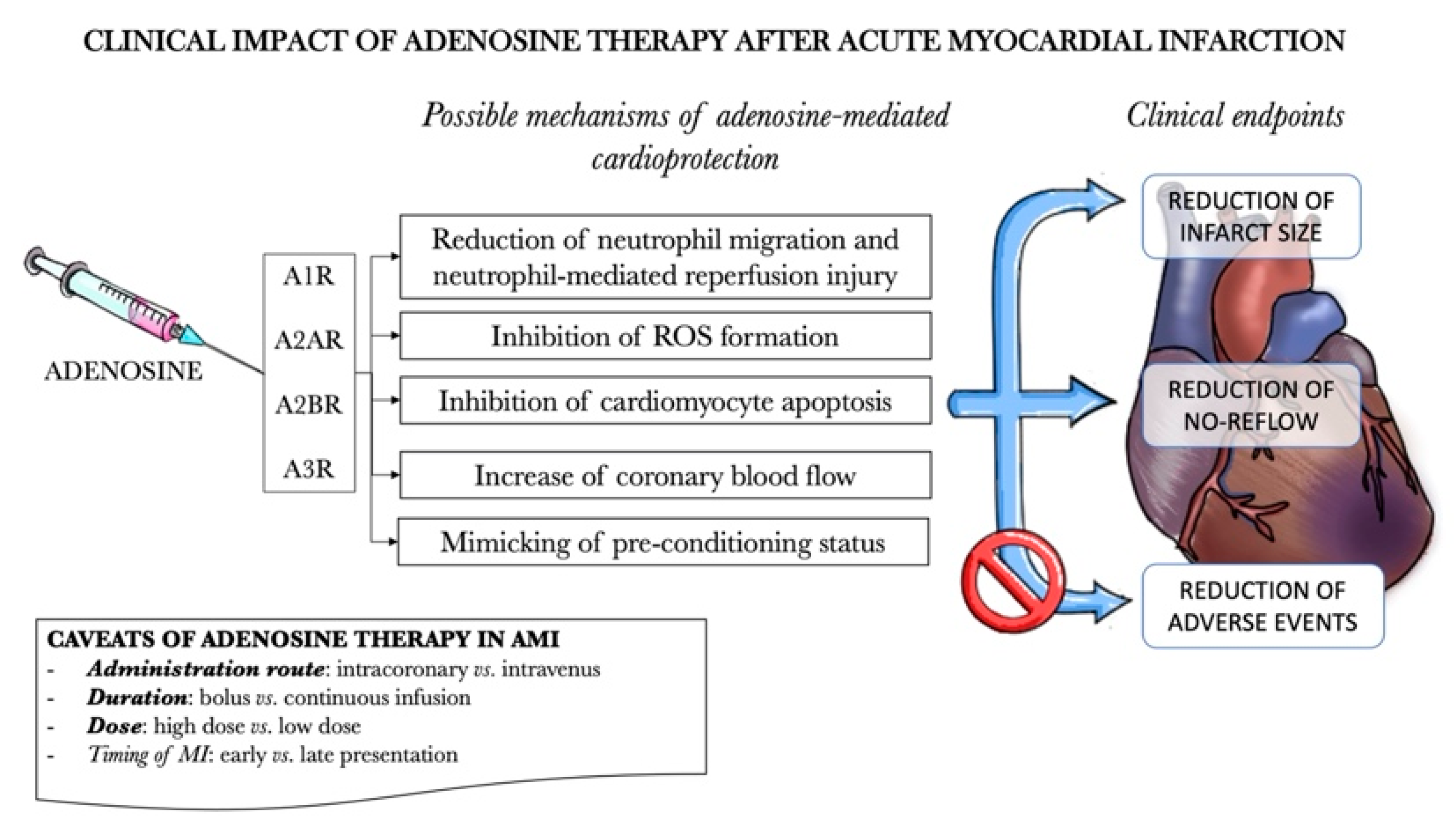
7. Clinical Outlook–Clinical Studies for Adenosine Receptor Agonists/Antagonists in Humans with Acute Myocardial Infarction
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carney, R.M.; Freedland, K.E. Depression and Coronary Heart Disease. Nat. Rev. Cardiol. 2017, 14, 145–155. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.; De Boer, R.A. From Inflammation to Fibrosis—Molecular and Cellular Mechanisms of Myocardial Tissue Remodelling and Perspectives on Differential Treatment Opportunities. Curr. Hear. Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Kurose, H.; Mangmool, S. Myofibroblasts and Inflammatory Cells as Players of Cardiac Fibrosis. Arch. Pharmacal Res. 2016, 39, 1100–1113. [Google Scholar] [CrossRef]
- Volders, P.G.; Willems, I.E.; Cleutjens, J.P.; Aren, J.-W.; Havenith, M.G.; Daemen, M.J. Interstitial Collagen is Increased in the Non-Infarcted Human Myocardium After Myocardial Infarction. J. Mol. Cell. Cardiol. 1993, 25, 1317–1323. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef]
- Fu, W.-B.; Wang, W.E.; Zeng, C.-Y. Wnt Signaling Pathways in Myocardial Infarction and the Therapeutic Effects of Wnt Pathway Inhibitors. Acta Pharmacol. Sin. 2019, 40, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Moore, X.-L.; Dart, A.M.; Wang, L.-M. Systemic Inflammatory Response Following Acute Myocardial Infarction. J. Geriatr. Cardiol. 2015, 12, 305–312. [Google Scholar] [PubMed]
- Hermans, K.C.M.; Daskalopoulos, E.P.; Blankesteijn, W.M. Interventions in Wnt Signaling as a Novel Therapeutic Approach to Improve Myocardial Infarct Healing. Fibrogenesis Tissue Repair 2012, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, M.I.F.J.; Goumans, M.-J.; Van Middelaar, B.; Clevers, H.; Doevendans, P.A.; Sluijter, J. Active Wnt Signaling in Response to Cardiac Injury. Basic Res. Cardiol. 2010, 105, 631–641. [Google Scholar] [CrossRef]
- Działo, E.; Tkacz, K.; Błyszczuk, P. Crosstalk Between TGF-β and WNT Signalling Pathways during Cardiac Fibrogenesis. Acta Biochim. Pol. 2018, 65, 341–349. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Hua, Y.; Chen, H.; Yang, H.; Zhang, T.; Huang, G.; Fan, H.; Tan, Z.; Huang, X.; et al. Aldehyde Dehydrogenase-2 Protects against Myocardial Infarction-Related Cardiac Fibrosis through Modulation of the Wnt/β-Catenin Signaling Pathway. Ther. Clin. Risk Manag. 2015, 11, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Luo, M.; Zhang, Y.; Wilkes, D.C.; Ge, G.; Grieskamp, T.; Yamada, C.; Liu, T.-C.; Huang, G.; Basson, C.T.; et al. Secreted Frizzled-Related Protein 2 Is a Procollagen C Proteinase Enhancer with a Role in Fibrosis Associated with Myocardial Infarction. Nat. Cell Biol. 2008, 11, 46–55. [Google Scholar] [CrossRef]
- Matsushima, K.; Suyama, T.; Takenaka, C.; Nishishita, N.; Ikeda, K.; Ikada, Y.; Sawa, Y.; Jakt, L.M.; Mori, H.; Kawamata, S. Secreted Frizzled Related Protein 4 Reduces Fibrosis Scar Size and Ameliorates Cardiac Function After Ischemic Injury. Tissue Eng. Part A 2010, 16, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.T.; Kohn, A.D.; De Ferrari, G.V.; Kaykas, A. WNT and β-Catenin Signalling: Diseases and Therapies. Nat. Rev. Genet. 2004, 5, 691–701. [Google Scholar] [CrossRef]
- Sklepkiewicz, P.; Shiomi, T.; Kaur, R.; Sun, J.; Kwon, S.; Mercer, B.; Bodine, P.; Schermuly, R.T.; George, I.; Schulze, P.C.; et al. Loss of Secreted Frizzled-Related Protein-1 Leads to Deterioration of Cardiac Function in Mice and Plays a Role in Human Cardiomyopathy. Circ. Hear. Fail. 2015, 8, 362–372. [Google Scholar] [CrossRef]
- Barandon, L.; Couffinhal, T.; Ezan, J.; Dufourcq, P.; Costet, P.; Alzieu, P.; Leroux, L.; Moreau, C.; Dare, D.; Duplàa, C. Reduction of Infarct Size and Prevention of Cardiac Rupture in Transgenic Mice Overexpressing FrzA. Circulation 2003, 108, 2282–2289. [Google Scholar] [CrossRef]
- Błyszczuk, P.; Müller-Edenborn, B.; Valenta, T.; Osto, E.; Stellato, M.; Behnke, S.; Glatz, K.; Basler, K.; Lüscher, T.F.; Distler, O.; et al. Transforming Growth Factor-β-Dependent Wnt Secretion Controls Myofibroblast Formation and Myocardial Fibrosis Progression in Experimental Autoimmune Myocarditis. Eur. Hear. J. 2016, 38, 1413–1425. [Google Scholar] [CrossRef]
- Olsson, R.A.; Pearson, J.D. Cardiovascular Purinoceptors. Physiol. Rev. 1990, 70, 761–845. [Google Scholar] [CrossRef]
- Shryock, J.C.; Belardinelli, L. Adenosine and Adenosine Receptors in the Cardiovascular System: Biochemistry, Physiology, and Pharmacology. Am. J. Cardiol. 1997, 79, 2–10. [Google Scholar] [CrossRef]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine Receptors and the Heart: Role in Regulation of Coronary Blood Flow and Cardiac Electrophysiology. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 161–188. [Google Scholar]
- Talukder, M.H.; Morrison, R.R.; Ledent, C.; Mustafa, S.J. Endogenous Adenosine Increases Coronary Flow by Activation of Both A2A and A2B Receptors in Mice. J. Cardiovasc. Pharmacol. 2003, 41, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.E.; Teng, B.; Morrison, R.R.; Schnermann, J.; Mustafa, S.J. Role of A1 Adenosine Receptor in the Regulation of Coronary Flow. Am. J. Physiol. Circ. Physiol. 2006, 291, H467–H472. [Google Scholar] [CrossRef]
- Fernandez, P.; Jara, C.; Aguilera, V.; Caviedes, L.; Diaz, F.; Radojkovic, C.; Veas, C.; Lamperti, L.; Escudero, C.; Aguayo, C. Adenosine A2A and A3 Receptors Are Involved in the Human Endothelial Progenitor Cells Migration. J. Cardiovasc. Pharmacol. 2012, 59, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M. P2Y12 Receptors: Structure and Function. J. Thromb. Haemost. 2015, 13, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Gerczuk, P.Z.; Kloner, R.A. An Update on Cardioprotection. J. Am. Coll. Cardiol. 2012, 59, 969–978. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Bøtker, H.E.; Condorelli, G.; Ferdinandy, P.; Garcia-Dorado, D.; Heusch, G.; Lecour, S.; Van Laake, L.W.; Madonna, R.; Ruiz-Meana, M.; et al. Translating Cardioprotection for Patient Benefit: Position Paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2013, 98, 7–27. [Google Scholar] [CrossRef] [PubMed]
- García-Dorado, D.; Otaegui, I.; Palomares, J.R.; Evangelista, A.; Pineda, V.; Salmeron, R.R.; Gimeno, F.; Aviles, F.F.; Román, A.S.; Del Blanco, B.G. Primary Results of the PROMISE Trial: Myocardial Protection with Intracoronary Adenosine Given before Reperfusion in Patients with STEMI. Eur. Hear. J. 2013, 34, 3736. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, N.-L.; Hu, Z.-Y.; Wang, F.; Chen, L.; Zhang, Y.; Chen, S.-L. Three Hours Continuous Injection of Adenosine Improved Left Ventricular Function and Infarct Size in Patients with ST-Segment Elevation Myocardial Infarction. Chin. Med. J. 2012, 125, 1713–1719. [Google Scholar]
- Kupka, D.; Sibbing, D. P2Y12 Receptor Inhibitors: An Evolution in Drug Design to Prevent Arterial Thrombosis. Expert Opin. Drug Metab. Toxicol. 2018, 14, 303–315. [Google Scholar] [CrossRef]
- Ralevic, V.; Burnstock, G. Receptors for Purines and Pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- Burnstock, G. Purine and Pyrimidine Receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Nucleotide-and Nucleoside-Converting Ectoenzymes: Important Modulators of Purinergic Signalling Cascade. Biochim. Biophys. Acta (BBA)—Bioenerg. 2008, 1783, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Arslan, G.; Halldner, L.; Kull, B.; Schulte, G.; Wasserman, W. Structure and Function of Adenosine Receptors and Their Genes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 364–374. [Google Scholar] [CrossRef]
- Williams, A.C.; Forrester, T. Possible Source of Adenosine Triphosphate Released from Rat Myocytes in Response to Hypoxia and Acidosis. Cardiovasc. Res. 1983, 17, 301–312. [Google Scholar] [CrossRef]
- De Young, M.B.; Scarpa, A. ATP Receptor-Induced ca2+ Transients in Cardiac Myocytes: Sources of Mobilized Ca2+. Am. J. Physiol. Physiol. 1989, 257, C750–C758. [Google Scholar] [CrossRef]
- Vial, C.; Owen, P.; Opie, L.; Posel, D. Significance of Release of Adenosine Triphosphate and Adenosine Induced by Hypoxia or Adrenaline in Perfused Rat Heart. J. Mol. Cell. Cardiol. 1987, 19, 187–197. [Google Scholar] [CrossRef]
- Brass, L.F.; Manning, D.R.; Cichowski, K.; Abrams, C.S. Signaling through G Proteins in Platelets: To the Integrins and Beyond. Thromb. Haemost. 1997, 78, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Sato, Y.; Uemura, A.; Narita, Y.; Tozaki-Saitoh, H.; Nakaya, M.; Ide, T.; Suzuki, K.; Inoue, K.; Nagao, T.; et al. P2Y6 Receptor-Gα12/13 Signalling in Cardiomyocytes Triggers Pressure Overload-Induced Cardiac Fibrosis. EMBO J. 2008, 27, 3104–3115. [Google Scholar] [CrossRef]
- McIntosh, V.J.; Lasley, R.D. Adenosine Receptor-Mediated Cardioprotection. J. Cardiovasc. Pharmacol. Ther. 2011, 17, 21–33. [Google Scholar] [CrossRef]
- Woodiwiss, A.J.; Honeyman, T.W.; Fenton, R.A.; Dobson, J.G. Adenosine A2a-Receptor Activation Enhances Cardiomyocyte Shortening via Ca2+-Independent and -Dependent Mechanisms. Am. J. Physiol. Content 1999, 276, H1434–H1441. [Google Scholar] [CrossRef]
- Hopkins, S.V.; Goldie, R. A Species Difference in the Uptake of Adenosine by Heart. Biochem. Pharmacol. 1971, 20, 3359–3365. [Google Scholar] [CrossRef]
- Stangl, V.; Baumann, G.; Felix, S.B. Negative Inotropic Mediators Released from the Heart after Myocardial Ischaemia–Reperfusion. Cardiovasc. Res. 2002, 53, 12–30. [Google Scholar] [CrossRef]
- James, T.N. The Chronotropic Action of ATP and Related Compounds Studied by Direct Perfusion of the Sinus Node. J. Pharmacol. Exp. Ther. 1965, 149, 233–247. [Google Scholar]
- Zheng, J.S.; Boluyt, M.O.; O’Neill, L.; Crow, M.T.; Lakatta, E.G. Extracellular ATP Induces Immediate-Early Gene Expression but Not Cellular Hypertrophy in Neonatal Cardiac Myocytes. Circ. Res. 1994, 74, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Shiode, N.; Teragawa, H.; Hirao, H.; Yamada, T.; Yamagata, T.; Matsuura, H.; Kajiyama, G. Adenosine 5’-Triphosphate Induced Dilation of Human Coronary Microvessels In Vivo. Intern. Med. 1999, 38, 324–329. [Google Scholar] [CrossRef]
- Hansmann, G.; Ihling, C.; Pieske, B.; Bültmann, R. Nucleotide-Evoked Relaxation of Human Coronary Artery. Eur. J. Pharmacol. 1998, 359, 59–67. [Google Scholar] [CrossRef]
- Burnstock, G. Hypoxia, Endothelium, and Purines. Drug Dev. Res. 1993, 28, 301–305. [Google Scholar] [CrossRef]
- Malmsjö, M.; Bergdahl, A.; Möller, S.; Zhao, X.-H.; Sun, X.-Y.; Hedner, T.; Edvinsson, L.; Erlinge, D. Congestive Heart Failure Induces Downregulation of P2X1-Receptors in Resistance Arteries. Cardiovasc. Res. 1999, 43, 219–227. [Google Scholar] [CrossRef][Green Version]
- Malmsjö, M.; Hou, M.; Harden, T.K.; Pendergast, W.; Pantev, E.; Edvinsson, L.; Erlinge, D. Characterization of Contractile P2 Receptors in Human Coronary Arteries by Use of the Stable Pyrimidines Uridine 5’-O-Thiodiphosphate and Uridine 5’-O-3-Thiotriphosphate. J. Pharmacol. Exp. Ther. 2000, 293, 755–760. [Google Scholar] [PubMed]
- Burnstock, G. Short-and Long-Term (Trophic) Purinergic Signalling. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150422. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling and Vascular Cell Proliferation and Death. Arter. Thromb. Vasc. Biol. 2002, 22, 364–373. [Google Scholar] [CrossRef]
- Burnstock, G.; Verkhratsky, A. Long-Term (Trophic) Purinergic Signalling: Purinoceptors Control Cell Proliferation, Differentiation and Death. Cell Death Dis. 2010, 1, e9. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.T.; Abbracchio, M.P. Trophic Roles of Purines and Pyrimidines. In Purinergic and Pyrimidinergic Signalling I; Springer Nature: Berlin, Germany, 2001; pp. 305–338. [Google Scholar]
- Jagroop, I.A.; Burnstock, G.; Mikhailidis, D.P. Both the ADP Receptors P2Y1 and P2Y12, Play a Role in Controlling Shape Change in Human Platelets. Platelets 2003, 14, 15–20. [Google Scholar] [CrossRef]
- Farndale, R.W.; Siljander, P.R.-M.; Onley, D.J.; Sundaresan, P.; Knight, C.G.; Barnes, M.J. Collagen-Platelet Interactions: Recognition and Signalling. Biochem. Soc. Symp. 2003, 70, 81–94. [Google Scholar] [CrossRef]
- Rozalski, M.; Nocun, M.; Watala, C. Adenosine Diphosphate Receptors on Blood Platelets: Potential New Targets for Antiplatelet Therapy. Acta Biochim. Pol. 2005, 52, 411–415. [Google Scholar] [PubMed]
- Guieu, R.; Deharo, J.-C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020, 9, 1366. [Google Scholar] [CrossRef]
- Shaikh, G.; Cronstein, B.N. Signaling Pathways Involving Adenosine A2a and A2b Receptors in Wound Healing and Fibrosis. Purinergic Signal. 2016, 12, 191–197. [Google Scholar] [CrossRef]
- Braun, O.Ö.; Lu, D.; Aroonsakool, N.; Insel, P.A. Uridine Triphosphate (UTP) Induces Profibrotic Responses in Cardiac Fibroblasts by Activation of P2Y2 Receptors. J. Mol. Cell. Cardiol. 2010, 49, 362–369. [Google Scholar] [CrossRef]
- Feng, J.; Armillei, M.K.; Yu, A.S.; Liang, B.T.; Runnels, L.W.; Yue, L. Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases. J. Cardiovasc. Dev. Dis. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Gillespie, D.G.; Jackson, E.K. Adenosine Inhibits Collagen and Protein Synthesis in Cardiac Fibroblasts. Hypertension 1998, 31, 943–948. [Google Scholar] [CrossRef]
- Headrick, J.P.; Ashton, K.J.; Rose’Meyer, R.B.; Peart, J.N. Cardiovascular Adenosine Receptors: Expression, Actions and Interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Moser, G.H.; Schrader, J.; Deussen, A. Turnover of Adenosine in Plasma of Human and Dog Blood. Am. J. Physiol. Physiol. 1989, 256, C799–C806. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.; Gaudry, M.; Ruf, J.; Guieu, R. Recent Advances in the Role of the Adenosinergic System in Coronary Artery Disease. Cardiovasc. Res. 2020, 275. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, P.G.W.; Wohlhueter, R.M.; Kraupp, M. Adenosine Uptake, Transport, and Metabolism in Human Erythrocytes. J. Cell. Physiol. 1985, 125, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.D.; Mihaylova-Todorova, S.; Westfall, T.D.; Sneddon, P.; Kennedy, C.; Bjur, R.A.; Westfall, D.P. Neuronal Release of Soluble Nucleotidases and Their Role in Neurotransmitter Inactivation. Nat. Cell Biol. 1997, 387, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.; Ramkumar, V. Adenosine Receptors: Expression, Function and Regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Keely, S.L. Activation of cAMP-Dependent Protein Kinase without a Corresponding Increase in Phosphorylase Activity. Res. Commun. Chem. Pathol. Pharmacol. 1977, 18, 283–290. [Google Scholar] [PubMed]
- Varani, K.; Laghi-Pasini, F.; Camurri, A.; Capecchi, P.L.; Maccherini, M.; Diciolla, F.; Ceccatelli, L.; Lazzerini, P.E.; Ulouglu, C.; Cattabeni, F.; et al. Changes of Peripheral A 2A Adenosine Receptors in Chronic Heart Failure and Cardiac Transplantation. FASEB J. 2002, 17, 280–282. [Google Scholar] [CrossRef]
- Gariboldi, V.; Vairo, D.; Guieu, R.; Marlinge, M.; Ravis, E.; Lagier, D.; Mari, A.; Thery, E.; Collart, F.; Gaudry, M.; et al. Expressions of Adenosine A2a Receptors in Coronary Arteries and Peripheral Blood Mononuclear Cells Are Correlated in Coronary Artery Disease Patients. Int. J. Cardiol. 2017, 230, 427–431. [Google Scholar] [CrossRef]
- Hussain, T.; Mustafa, S.J. Regulation of Adenosine Receptor System in Coronary Artery: Functional Studies and cAMP. Am. J. Physiol. Circ. Physiol. 1993, 264, H441–H447. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, W.-J.; Linnemann, J.R.; Brouwer, P.M.; Bos, J.L.; Rehmann, H. Rap1 and Rap2 Antagonistically Control Endothelial Barrier Resistance. PLoS ONE 2013, 8, e57903. [Google Scholar] [CrossRef]
- Boularan, C.; Gales, C. Cardiac cAMP: Production, Hydrolysis, Modulation and Detection. Front. Pharmacol. 2015, 6, 203. [Google Scholar] [CrossRef]
- Rekik, M.; Mustafa, J.S. Modulation of A2A Adenosine Receptors and Associated Gαs Proteins by ZM 241385 Treatment of Porcine Coronary Artery. J. Cardiovasc. Pharmacol. 2003, 42, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Shubeita, H.E.; Martinson, E.A.; Van Bilsen, M.; Chien, K.R.; Brown, J.H. Transcriptional Activation of the Cardiac Myosin Light Chain 2 and Atrial Natriuretic Factor Genes by Protein Kinase C in Neonatal Rat Ventricular Myocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1305–1309. [Google Scholar] [CrossRef]
- Shen, J.; Halenda, S.P.; Sturek, M.; Wilden, P.A. Cell-Signaling Evidence for Adenosine Stimulation of Coronary Smooth Muscle Proliferation via the A 1 Adenosine Receptor. Circ. Res. 2005, 97, 574–582. [Google Scholar] [CrossRef]
- Li, J.M.; Fenton, R.A.; Cutler, B.S.; Dobson, J.G. Adenosine Enhances Nitric Oxide Production by Vascular Endothelial Cells. Am. J. Physiol. Physiol. 1995, 269, C519–C523. [Google Scholar] [CrossRef]
- Yoshioka, K.; Saitoh, O.; Nakata, H. Heteromeric Association Creates a P2Y-Like Adenosine Receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7617–7622. [Google Scholar] [CrossRef] [PubMed]
- Ginés, S.; Hillion, J.; Torvinen, M.; Le Crom, S.; Casadó, V.; Canela, E.I.; Rondin, S.; Lew, J.Y.; Watson, S.; Zoli, M.; et al. Dopamine D1 and Adenosine A1 Receptors form Functionally Interacting Heteromeric Complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 8606–8611. [Google Scholar]
- Arellano, R.O.; Garay, E.; Vázquez-Cuevas, F. Functional Interaction between Native G protein-Coupled Purinergic Receptors in Xenopus follicles. Proc. Natl. Acad. Sci. USA 2009, 106, 16680–16685. [Google Scholar] [CrossRef]
- Canals, M.; Marcellino, D.; Fanelli, F.; Ciruela, F.; De Benedetti, P.; Goldberg, S.R.; Neve, K.; Fuxe, K.; Agnati, L.F.; Woods, A.S.; et al. Adenosine A2A-Dopamine D2 Receptor-Receptor Heteromerization. J. Biol. Chem. 2003, 278, 46741–46749. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Karcz-Kubicha, M.; Hope, B.T.; Popoli, P.; Burgueño, J.; Gutiérrez, M.A.; Casadó, V.; Fuxe, K.; Goldberg, S.R.; Lluis, C.; et al. Synergistic Interaction between Adenosine A2A and Glutamate mGlu5 Receptors: Implications for Striatal Neuronal Function. Proc. Natl. Acad. Sci. USA 2002, 99, 11940–11945. [Google Scholar] [CrossRef] [PubMed]
- Headrick, J.P.; Peart, J.N.; Reichelt, M.E.; Haseler, L.J. Adenosine and Its Receptors in the Heart: Regulation, Retaliation and Adaptation. Biochim. et Biophys. Acta (BBA)—Biomembr. 2011, 1808, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt Signaling Pathway in Development and Disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Duchartre, Y.; Kim, Y.; Kahn, M. The Wnt Signaling Pathway in Cancer. Crit. Rev. Oncol. 2016, 99, 141–149. [Google Scholar] [CrossRef]
- Nakamura, T.; Hamada, F.; Ishidate, T.; Anai, K.-I.; Kawahara, K.; Toyoshima, K.; Akiyama, T. Axin, an Inhibitor of the Wnt Signalling Pathway, Interacts with β-Catenin, GSK-3β and APC and Reduces the β-Catenin Level. Genes Cells 1998, 3, 395–403. [Google Scholar] [CrossRef]
- Schulte, G.; Bryja, V. The Frizzled Family of Unconventional G-Protein-Coupled Receptors. Trends Pharmacol. Sci. 2007, 28, 518–525. [Google Scholar] [CrossRef]
- Kimelman, D.; Xu, W. β-Catenin Destruction Complex: Insights and Questions from a Structural Perspective. Oncogene 2006, 25, 7482–7491. [Google Scholar] [CrossRef]
- Veeman, M.T.; Axelrod, J.D.; Moon, R.T. A Second Canon. Dev. Cell 2003, 5, 367–377. [Google Scholar] [CrossRef]
- Teo, J.-L.; Kahn, M. The Wnt Signaling Pathway in Cellular Proliferation and Differentiation: A Tale of Two Coactivators. Adv. Drug Deliv. Rev. 2010, 62, 1149–1155. [Google Scholar] [CrossRef]
- Pahnke, A.; Conant, G.; Huyer, L.D.; Radisic, M.; Feric, N.; Radisic, M. The Role of Wnt Regulation in Heart Development, Cardiac Repair and Disease: A Tissue Engineering Perspective. Biochem. Biophys. Res. Commun. 2016, 473, 698–703. [Google Scholar] [CrossRef]
- LeCarpentier, Y.; Schussler, O.; Hébert, J.-L.; Vallée, A. Multiple Targets of the Canonical WNT/β-Catenin Signaling in Cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen Synthase Kinase-3 (GSK3): Regulation, Actions, and Diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Z.; Miao, J.; Du, S.; Ai, S.; Xu, E.; Feng, M.; Song, J.; Guan, W. Adenosine Interaction with Adenosine Receptor A2a Promotes Gastric Cancer Metastasis by Enhancing PI3K–AKT–mTOR Signaling. Mol. Biol. Cell 2019, 30, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Campos-Contreras, A.D.R.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Purinergic Signaling in the Hallmarks of Cancer. Cells 2020, 9, 1612. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef]
- Gordon, M.D.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed]
- Malbon, C.C.; Kühl, M.; Sheldahl, L.C.; Moon, R.T. Ca2+/Calmodulin-dependent Protein Kinase II Is Stimulated by Wnt and Frizzled Homologs and Promotes Ventral Cell Fates in Xenopus. J. Biol. Chem. 2000, 275, 12701–12711. [Google Scholar] [CrossRef]
- Ishitani, T.; Kishida, S.; Hyodo-Miura, J.; Ueno, N.; Yasuda, J.; Waterman, M.; Shibuya, H.; Moon, R.T.; Ninomiya-Tsuji, J.; Matsumoto, K. The TAK1-NLK Mitogen-Activated Protein Kinase Cascade Functions in the Wnt-5a/Ca2+ Pathway to Antagonize Wnt/β-Catenin Signaling. Mol. Cell. Biol. 2003, 23, 131–139. [Google Scholar] [CrossRef] [PubMed]
- De, A. Wnt/Ca2+ Signaling Pathway: A Brief Overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef]
- Gessert, S.; Kuehl, M. The Multiple Phases and Faces of Wnt Signaling During Cardiac Differentiation and Development. Circ. Res. 2010, 107, 186–199. [Google Scholar] [CrossRef]
- Goliasch, G.; Wiesbauer, F.; Kastl, S.; Katsaros, K.M.; Blessberger, H.; Maurer, G.; Schillinger, M.; Huber, K.; Wojta, J.; Speidl, W.S. Premature Myocardial Infarction Is Associated with Low Serum Levels of Wnt-1. Atherosclerosis 2012, 222, 251–256. [Google Scholar] [CrossRef]
- Cleutjens, J.P.; Blankesteijn, W.; Daemen, M.J.; Smits, J.F. The Infarcted Myocardium Simply Dead Tissue, or a Lively Target for Therapeutic Interventions. Cardiovasc. Res. 1999, 44, 232–241. [Google Scholar] [CrossRef]
- Borne, S.W.M.V.D.; Diez, J.; Blankesteijn, W.M.; Verjans, J.; Hofstra, L.; Narula, J. Myocardial Remodeling after Infarction: The Role of Myofibroblasts. Nat. Rev. Cardiol. 2009, 7, 30–37. [Google Scholar] [CrossRef]
- Daskalopoulos, E.P.; Janssen, B.J.; Blankesteijn, W.M. Myofibroblasts in the Infarct Area: Concepts and Challenges. Microsc. Microanal. 2012, 18, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; Lopez, E.F.; Lindsey, M.L. Macrophage Roles Following Myocardial Infarction. Int. J. Cardiol. 2008, 130, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Malsin, E.S.; Kim, S.; Lam, A.P.; Gottardi, C.J. Macrophages as a Source and Recipient of Wnt Signals. Front. Immunol. 2019, 10, 1813. [Google Scholar] [CrossRef]
- Aisagbonhi, O.; Rai, M.; Ryzhov, S.; Atria, N.; Feoktistov, I.; Hatzopoulos, A.K. Experimental Myocardial Infarction Triggers Canonical Wnt Signaling and Endothelial-to-Mesenchymal Transition. Dis. Model. Mech. 2011, 4, 469–483. [Google Scholar] [CrossRef]
- Pereira, C.; Schaer, D.J.; Bachli, E.B.; Kurrer, M.O.; Schoedon, G. Wnt5A/CaMKII Signaling Contributes to the Inflammatory Response of Macrophages and Is a Target for the Antiinflammatory Action of Activated Protein C and Interleukin-10. Arter. Thromb. Vasc. Biol. 2008, 28, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Headrick, J.P.; Lasley, R.D. Adenosine Receptors and Reperfusion Injury of the Heart. In Botulinum Toxin Therapy; Springer Nature: Berlin, Germany, 2009; pp. 189–214. [Google Scholar]
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z.-G. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Lasley, R.D. Adenosine Receptor-Mediated Cardioprotection—Current Limitations and Future Directions. Front. Pharmacol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.-G.; Tchilibon, S.; Duong, H.T.; Joshi, B.V.; Sonin, D.; Liang, B.T. Semirational Design of (North)-Methanocarba Nucleosides as Dual Acting A1and A3Adenosine Receptor Agonists: Novel Prototypes for Cardioprotection. J. Med. Chem. 2005, 48, 8103–8107. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Zhong, H.; Mezzaroma, E.; Van Tassell, B.W.; Kannan, H.; Zeng, D.; Belardinelli, L.; Voelkel, N.F.; Abbate, A. GS-6201, a Selective Blocker of the A2BAdenosine Receptor, Attenuates Cardiac Remodeling after Acute Myocardial Infarction in the Mouse. J. Pharmacol. Exp. Ther. 2012, 343, 587–595. [Google Scholar] [CrossRef]
- Stone, T.W.; Ceruti, S.; Abbracchio, M.P. Adenosine Receptors and Neurological Disease: Neuroprotection and Neurodegeneration. In Botulinum Toxin Therapy; Springer Nature: Berlin, Germany, 2009; Volume 193, pp. 535–587. [Google Scholar]
- Román, V.; Keijser, J.N.; Luiten, P.G.; Meerlo, P. Repetitive Stimulation of Adenosine A1 Receptors in Vivo: Changes in Receptor Numbers, G-Proteins and A1 Receptor Agonist-Induced Hypothermia. Brain Res. 2008, 1191, 69–74. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Civan, M.M. Ocular Purine Receptors as Drug Targets in the Eye. J. Ocul. Pharmacol. Ther. 2016, 32, 534–547. [Google Scholar] [CrossRef]
- El Zein, E.; Zablocki, J. A1 Adenosine Receptor Agonists and Their Potential Therapeutic Applications. Expert Opin. Investig. Drugs 2008, 17, 1901–1910. [Google Scholar] [CrossRef]
- Corino, V.D.A.; Sandberg, F.; Mainardi, L.T.; Platonov, P.G.; Sörnmo, L. Noninvasive Characterization of Atrioventricular Conduction in Patients with Atrial Fibrillation. J. Electrocardiol. 2015, 48, 938–942. [Google Scholar] [CrossRef]
- Mason, P.K.; DiMarco, J.P. New Pharmacological Agents for Arrhythmias. Circ. Arrhythmia Electrophysiol. 2009, 2, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Albrecht-Küpper, B.E.; Leineweber, K.; Nell, P.G. Partial Adenosine A1 Receptor Agonists for Cardiovascular Therapies. Purinergic Signal. 2011, 8, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kiesman, W.F.; ElZein, E.; Zablocki, J. A1 Adenosine Receptor Antagonists, Agonists, and Allosteric Enhancers. In Handbook of Experimental Pharmacology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 25–58. [Google Scholar]
- Voors, A.A.; Bax, J.J.; Hernandez, A.F.; Wirtz, A.B.; Pap, A.F.; Ferreira, A.C.; Senni, M.; Van Der Laan, M.; Butler, J. For the Pantheon Investigators Safety and Efficacy of the Partial Adenosine A1 Receptor Agonist Neladenoson Bialanate in Patients with Chronic Heart Failure with Reduced Ejection Fraction: A Phase IIb, Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Hear. Fail. 2019, 21, 1426–1433. [Google Scholar] [CrossRef]
- Greene, S.J.; Sabbah, H.N.; Butler, J.; Voors, A.A.; Albrecht-Kuepper, B.E.; Duengen, H.-D.; Dinh, W.; Gheorghiade, M. Partial Adenosine A1 Receptor Agonism: A Potential New Therapeutic Strategy for Heart Failure. Hear. Fail. Rev. 2016, 21, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Meibom, D.; Albrecht-Küpper, B.; Diedrichs, N.; Hübsch, W.; Kast, R.; Krämer, T.; Krenz, U.; Lerchen, H.-G.; Mittendorf, J.; Nell, P.G.; et al. Neladenoson Bialanate Hydrochloride: A Prodrug of a Partial Adenosine A1Receptor Agonist for the Chronic Treatment of Heart Diseases. ChemMedChem 2017, 12, 728–737. [Google Scholar] [CrossRef]
- Voors, A.A.; Duengen, H.-D.; Senni, M.; Nodari, S.; Agostoni, P.; Ponikowski, P.; Bax, J.J.; Butler, J.; Kim, R.J.; Dorhout, B.; et al. Safety and Tolerability of Neladenoson Bialanate, a Novel Oral Partial Adenosine A1 Receptor Agonist, in Patients with Chronic Heart Failure. J. Clin. Pharmacol. 2017, 57, 440–451. [Google Scholar] [CrossRef]
- Chuo, C.H.; Devine, S.M.; Scammells, P.J.; Krum, H.; Christopoulos, A.; May, L.T.; White, P.J.; Wang, B.H. VCP746, a Novel A 1 Adenosine Receptor Biased Agonist, Reduces Hypertrophy in a Rat Neonatal Cardiac Myocyte Model. Clin. Exp. Pharmacol. Physiol. 2016, 43, 976–982. [Google Scholar] [CrossRef]
- Vecchio, E.A.; Chuo, C.H.; Baltos, J.; Ford, L.; Scammells, P.J.; Wang, B.H.; Christopoulos, A.; White, P.J.; May, L.T. The Hybrid Molecule, VCP746, Is a Potent Adenosine A2b Receptor Agonist That Stimulates Anti-fibrotic Signalling. Biochem. Pharmacol. 2016, 117, 46–56. [Google Scholar] [CrossRef]
- Urmaliya, V.B.; Pouton, C.W.; Devine, S.M.; Haynes, J.M.; Warfe, L.; Scammells, P.J.; White, P.J. A Novel Highly Selective Adenosine A1 Receptor Agonist VCP28 Reduces Ischemia Injury in a Cardiac Cell Line and Ischemia–Reperfusion Injury in Isolated Rat Hearts at Concentrations That Do Not Affect Heart Rate. J. Cardiovasc. Pharmacol. 2010, 56, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Givertz, M.M.; Massie, B.M.; Fields, T.K.; Pearson, L.L.; Dittrich, H.C. The Effects of KW-3902, an Adenosine A1-Receptor Antagonist,on Diuresis and Renal Function in Patients With Acute Decompensated Heart Failure and Renal Impairment or Diuretic Resistance. J. Am. Coll. Cardiol. 2007, 50, 1551–1560. [Google Scholar] [CrossRef]
- Nagashima, K.; Kusaka, H.; Karasawa, A. Protective Effects of KW-3902, an Adenosine A1-Receptor Antagonist, against Cisplatin-Induced Acute Renal Failure in Rats. Jpn. J. Pharmacol. 1995, 67, 349–358. [Google Scholar] [CrossRef][Green Version]
- Slawsky, M.T.; Givertz, M.M. Rolofylline: A Selective Adenosine 1 Receptor Antagonist for the Treatment of Heart Failure. Expert Opin. Pharmacother. 2009, 10, 311–322. [Google Scholar] [CrossRef]
- Greenberg, B.; Thomas, I.; Banish, D.; Goldman, S.; Havranek, E.; Massie, B.M.; Zhu, Y.; Ticho, B.; Abraham, W.T. Effects of Multiple Oral Doses of an A1 Adenosine Antagonist, BG9928, in Patients with Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 600–606. [Google Scholar] [CrossRef]
- Lasley, R.D.; Kristo, G.; Keith, B.J.; Mentzer, R.M. The A2a/A2b Receptor Antagonist ZM-241385 Blocks the Cardioprotective Effect of Adenosine Agonist Pretreatment in In Vivo Rat Myocardium. Am. J. Physiol. Circ. Physiol. 2007, 292, H426–H431. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.S.; Gabriel-Costa, D.; Sudo, R.T.; Wang, H.; Groban, L.; Ferraz, E.B.; Nascimento, J.H.M.; Fraga, C.A.M.; Barreiro, E.J.; Zapata-Sudo, G. Adenosine A2a Receptor Agonist Prevents Cardiac Remodeling and Dysfunction in Spontaneously Hypertensive Male Rats after Myocardial Infarction. Drug Des. Dev. Ther. 2017, 11, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, E.A.; White, P.J.; May, L.T. Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front. Pharmacol. 2017, 8, 243. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Everett, T.H.; Wilson, E.; Chang, R.; Zeng, D.; Belardinelli, L.; Olgin, J.E. Blockade of A2B Adenosine Receptor Reduces Left Ventricular Dysfunction and Ventricular Arrhythmias 1 Week after Myocardial Infarction in the Rat Model. Hear. Rhythm. 2014, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Phosri, S.; Arieyawong, A.; Bunrukchai, K.; Parichatikanond, W.; Nishimura, A.; Nishida, M.; Mangmool, S. Stimulation of Adenosine A2B Receptor Inhibits Endothelin-1-Induced Cardiac Fibroblast Proliferation and α-Smooth Muscle Actin Synthesis Through the cAMP/Epac/PI3K/Akt-Signaling Pathway. Front. Pharmacol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-G.; Balasubramanian, R.; Kiselev, E.; Wei, Q.; Jacobson, K.A. Probing Biased/Partial Agonism at the G Protein-Coupled A2b Adenosine Receptor. Biochem. Pharmacol. 2014, 90, 297–306. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Inoue, A.; Jacobson, K.A. On the G Protein-Coupling Selectivity of the Native A2b Adenosine Receptor. Biochem. Pharmacol. 2018, 151, 201–213. [Google Scholar] [CrossRef]
- Tian, Y.; Piras, B.A.; Kron, I.L.; French, B.A.; Yang, Z. Adenosine 2B Receptor Activation Reduces Myocardial Reperfusion Injury by Promoting Anti-Inflammatory Macrophages Differentiation via PI3K/Akt Pathway. Oxidative Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Fishman, P.; Bar-Yehuda, S.; Liang, B.T.; Jacobson, K.A. Pharmacological and Therapeutic Effects of A3 Adenosine Receptor Agonists. Drug Discov. Today 2012, 17, 359–366. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with Ischemia: A Delay of Lethal Cell Injury in Ischemic Myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef]
- Safran, N.; Shneyvays, V.; Balas, N.; Jacobson, K.A.; Nawrath, H.; Shainberg, A. Cardioprotective Effects of Adenosine A1 and A3 Receptor Activation during Hypoxia in Isolated Rat Cardiac Myocytes. Mol. Cell. Biochem. 2001, 217, 143–152. [Google Scholar] [CrossRef]
- Shneyvays, V.; Nawrath, H.; Jacobson, K.; Shainberg, A. Induction of Apoptosis in Cardiac Myocytes by an A3Adenosine Receptor Agonist. Exp. Cell Res. 1998, 243, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Shneyvays, V.; Jacobson, K.; Li, A.-H.; Nawrath, H.; Zinman, T.; Isaac, A.; Shainberg, A. Induction of Apoptosis in Rat Cardiocytes by A3 Adenosine Receptor Activation and Its Suppression by Isoproterenol. Exp. Cell Res. 2000, 257, 111–126. [Google Scholar] [CrossRef] [PubMed]
- DeNinno, M.P.; Masamune, H.; Chenard, L.K.; Dirico, K.J.; Eller, C.; Etienne, J.B.; Tickner, J.E.; Kennedy, S.P.; Knight, D.R.; Kong, J.; et al. 3‘-Aminoadenosine-5‘-Uronamides: Discovery of the First Highly Selective Agonist at the Human Adenosine A3Receptor. J. Med. Chem. 2003, 46, 353–355. [Google Scholar] [CrossRef]
- Wan, T.C.; Tampo, A.; Kwok, W.-M.; Auchampach, J.A. Ability of CP-532,903 to Protect Mouse Hearts from Ischemia/Reperfusion Injury Is Dependent on Expression of A3 Adenosine Receptors in Cardiomyoyctes. Biochem. Pharmacol. 2019, 163, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.A. Wnt Antagonism Initiates Cardiogenesis in Xenopus Laevis. Genes Dev. 2001, 15, 304–315. [Google Scholar] [CrossRef]
- Zhu, W.; Shiojima, I.; Ito, Y.; Li, Z.; Ikeda, H.; Yoshida, M.; Naito, A.T.; Nishi, J.-I.; Ueno, H.; Umezawa, A.; et al. IGFBP-4 is an Inhibitor of Canonical Wnt Signalling Required for Cardiogenesis. Nat. Cell Biol. 2008, 454, 345–349. [Google Scholar] [CrossRef]
- Wo, D.; Peng, J.; Ren, D.-N.; Qiu, L.; Chen, J.; Zhu, Y.; Yan, Y.; Yan, H.; Wu, J.; Ma, E.; et al. Opposing Roles of Wnt Inhibitors IGFBP-4 and Dkk1 in Cardiac Ischemia by Differential Targeting of LRP5/6 and β-catenin. Circulation 2016, 134, 1991–2007. [Google Scholar] [CrossRef]
- Yang, D.; Fu, W.; Li, L.; Xia, X.; Liao, Q.; Yue, R.; Chen, H.; Chen, X.; An, S.; Zeng, C.; et al. Therapeutic Effect of a Novel Wnt Pathway Inhibitor on Cardiac Regeneration after Myocardial Infarction. Clin. Sci. 2017, 131, 2919–2932. [Google Scholar] [CrossRef]
- Leach, J.P.; Heallen, T.; Zhang, M.; Rahmani, M.; Morikawa, Y.; Hill, M.C.; Segura, A.; Willerson, J.T.; Martin, J.F. Hippo Pathway Deficiency Reverses Systolic Heart Failure after Infarction. Nat. Cell Biol. 2017, 550, 260–264. [Google Scholar] [CrossRef]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, S.A.A.; Santos, C.S.X.D.C.D.; Shah, C.X.S.A.; Zhang, H.; Faber, H.Z.J.E.; Kinter, M.T.; et al. Hypoxia Induces Heart Regeneration in Adult Mice. Nat. Cell Biol. 2017, 541, 222–227. [Google Scholar] [CrossRef]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian Heart Renewal by Pre-existing Cardiomyocytes. Nat. Cell Biol. 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.E.; Li, L.; Xia, X.; Fu, W.; Liao, Q.; Lan, C.; Yang, D.; Chen, H.; Yue, R.; Zeng, C.S.; et al. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation 2017, 136, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Zhou, H.; Zhang, L.-S.; Tan, W.; Liu, Y.; Zhang, S.; Morlock, L.K.; Bao, X.; Palecek, S.P.; Feng, J.Q.; et al. Blockade to Pathological Remodeling of Infarcted Heart Tissue Using a Porcupine Antagonist. Proc. Natl. Acad. Sci. USA 2017, 114, 1649–1654. [Google Scholar] [CrossRef]
- Bastakoty, D. Temporary, Systemic Inhibition of the WNT/β-Catenin Pathway promotes Regenerative Cardiac Repair following Myocardial Infarct. Cell, Stem Cells Regen. Med. 2016, 2. [Google Scholar] [CrossRef]
- Rodriguez, J.W.; Rüegg, C. The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication. Int. J. Mol. Sci. 2020, 21, 9107. [Google Scholar] [CrossRef]
- Jimeno, A.R.; Gordon, M.; Chugh, R.; Messersmith, W.A.; Mendelson, D.; Dupont, J.; Stagg, R.; Kapoun, A.M.; Xu, L.; Uttamsingh, S.; et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 7490–7497. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.C.; Osborne, C.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. PHase Ib Clinical Trial of the Anti-frizzled Antibody Vantictumab (OMP-18r5) Plus Paclitaxel in Patients with Locally Advanced or Metastatic HER2-Negative Breast Cancer. Breast Cancer Res. Treat. 2020, 184, 53–62. [Google Scholar] [CrossRef]
- Saraswati, S.; Alfaro, M.P.; Thorne, C.A.; Atkinson, J.; Lee, E.; Young, P.P. Pyrvinium, a Potent Small Molecule Wnt Inhibitor, Promotes Wound Repair and Post-MI Cardiac Remodeling. PLoS ONE 2010, 5, e15521. [Google Scholar] [CrossRef] [PubMed]
- Laeremans, H.; Hackeng, T.M.; Van Zandvoort, M.A.M.J.; Thijssen, V.L.J.L.; Janssen, B.J.A.; Ottenheijm, H.C.J.; Smits, J.F.M.; Blankesteijn, W.M. Blocking of Frizzled Signaling with a Homologous Peptide Fragment of Wnt3a/Wnt5a Reduces Infarct Expansion and Prevents the Development of Heart Failure After Myocardial Infarction. Circulation 2011, 124, 1626–1635. [Google Scholar] [CrossRef]
- Babbitt, D.G.; Virmani, R.; Forman, M.B. Intracoronary Adenosine Administered after Reperfusion Limits Vascular Injury after Prolonged Ischemia in the Canine Model. Circulation 1989, 80, 1388–1399. [Google Scholar] [CrossRef]
- Thornton, J.D.; Liu, G.S.; Olsson, R.A.; Downey, J.M. Intravenous Pretreatment with A1-Selective Adenosine Analogues Protects the Heart against Infarction. Circulation 1992, 85, 659–665. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Puma, J.A.; Barbagelata, N.A.; DiCarli, M.F.; Leesar, M.A.; Browne, K.F.; Eisenberg, P.R.; Bolli, R.; Casas, A.C.; Molina-Viamonte, V.; et al. Adenosine as an Adjunct to Thrombolytic Therapy for Acute Myocardial Infarction. J. Am. Coll. Cardiol. 1999, 34, 1711–1720. [Google Scholar] [CrossRef]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, W.R. A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial of Adenosine as an Adjunct to Reperfusion in the Treatment of Acute Myocardial Infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Desmet, W.; Bogaert, J.; Dubois, C.; Sinnaeve, P.R.; Adriaenssens, T.; Pappas, C.; Ganame, J.; Dymarkowski, S.; Janssens, S.; Belmans, A.; et al. High-Dose Intracoronary Adenosine for Myocardial Salvage in Patients with Acute ST-Segment Elevation Myocardial Infarction. Eur. Hear. J. 2010, 32, 867–877. [Google Scholar] [CrossRef]
- Micari, A.; Belcik, T.A.; Balcells, E.A.; Powers, E.; Wei, K.; Kaul, S.; Lindner, J.R. Improvement in Microvascular Reflow and Reduction of Infarct Size with Adenosine in Patients Undergoing Primary Coronary Stenting. Am. J. Cardiol. 2005, 96, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, M.L.; Vlaar, P.J.; Vogelzang, M.; Gu, Y.L.; Kampinga, M.A.; De Smet, B.J.; Jessurun, G.A.; Anthonio, R.L.; Heuvel, A.F.V.D.; Tan, E.-S.; et al. Effect of High-Dose Intracoronary Adenosine Administration During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2009, 2, 323–329. [Google Scholar] [CrossRef]
- Nazir, S.A.; McCann, G.P.; Greenwood, J.P.; Kunadian, V.; Khan, J.N.; Mahmoud, I.Z.; Blackman, D.J.; Been, M.; Abrams, K.R.; Shipley, L.; et al. Strategies to Attenuate Micro-Vascular Obstruction during P-PCI: The Randomized Reperfusion Facilitated by Local Adjunctive Therapy in ST-Elevation Myocardial Infarction Trial. Eur. Hear. J. 2016, 37, 1910–1919. [Google Scholar] [CrossRef]
- Bulluck, H.; Sirker, A.; Loke, Y.K.; Garcia-Dorado, D.; Hausenloy, D.J. Clinical Benefit of Adenosine as an Adjunct to Reperfusion in ST-Elevation Myocardial Infarction Patients: An Updated Meta-Analysis of Randomized Controlled Trials. Int. J. Cardiol. 2016, 202, 228–237. [Google Scholar] [CrossRef]
- Yetgin, T.; Uitterdijk, A.; Hekkert, M.T.L.; Merkus, D.; Krabbendam-Peters, I.; Van Beusekom, H.M.; Falotico, R.; Serruys, P.W.; Belardi, J.A.; Van Geuns, R.-J.M.; et al. Limitation of Infarct Size and No-Reflow by Intracoronary Adenosine Depends Critically on Dose and Duration. JACC Cardiovasc. Interv. 2015, 8, 1990–1999. [Google Scholar] [CrossRef]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of Neutrophil Function by Adenosine. Arter. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of Coffee Drinking with Total and Cause-Specific Mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Maclure, M.; Muller, J.E.; Sherwood, J.B.; Mittleman, M.A. Tea Consumption and Mortality after Acute Myocardial Infarction. Circulation 2002, 105, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pathological Overproduction: The Bad Side of Adenosine. Br. J. Pharmacol. 2017, 174, 1945–1960. [Google Scholar] [CrossRef]
- Conlay, L.A.; Conant, J.A.; Debros, F.; Wurtman, R. Caffeine Alters Plasma Adenosine Levels. Nat. Cell Biol. 1997, 389, 136. [Google Scholar] [CrossRef]
- Varani, K.; Portaluppi, F.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Caffeine Alters A2a Adenosine Receptors and their Function in Human Platelets. Circulation 1999, 99, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procopio, M.C.; Lauro, R.; Nasso, C.; Carerj, S.; Squadrito, F.; Bitto, A.; Di Bella, G.; Micari, A.; Irrera, N.; Costa, F. Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways. Biomedicines 2021, 9, 204. https://doi.org/10.3390/biomedicines9020204
Procopio MC, Lauro R, Nasso C, Carerj S, Squadrito F, Bitto A, Di Bella G, Micari A, Irrera N, Costa F. Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways. Biomedicines. 2021; 9(2):204. https://doi.org/10.3390/biomedicines9020204
Chicago/Turabian StyleProcopio, Maria Cristina, Rita Lauro, Chiara Nasso, Scipione Carerj, Francesco Squadrito, Alessandra Bitto, Gianluca Di Bella, Antonio Micari, Natasha Irrera, and Francesco Costa. 2021. "Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways" Biomedicines 9, no. 2: 204. https://doi.org/10.3390/biomedicines9020204
APA StyleProcopio, M. C., Lauro, R., Nasso, C., Carerj, S., Squadrito, F., Bitto, A., Di Bella, G., Micari, A., Irrera, N., & Costa, F. (2021). Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways. Biomedicines, 9(2), 204. https://doi.org/10.3390/biomedicines9020204






