The ElonginB/C-Cullin5-SOCS-Box-Complex Is a Potential Biomarker for Growth Hormone Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Sample Preparation
2.3. Assays
2.4. Statistics
3. Results
3.1. Patient Characteristics
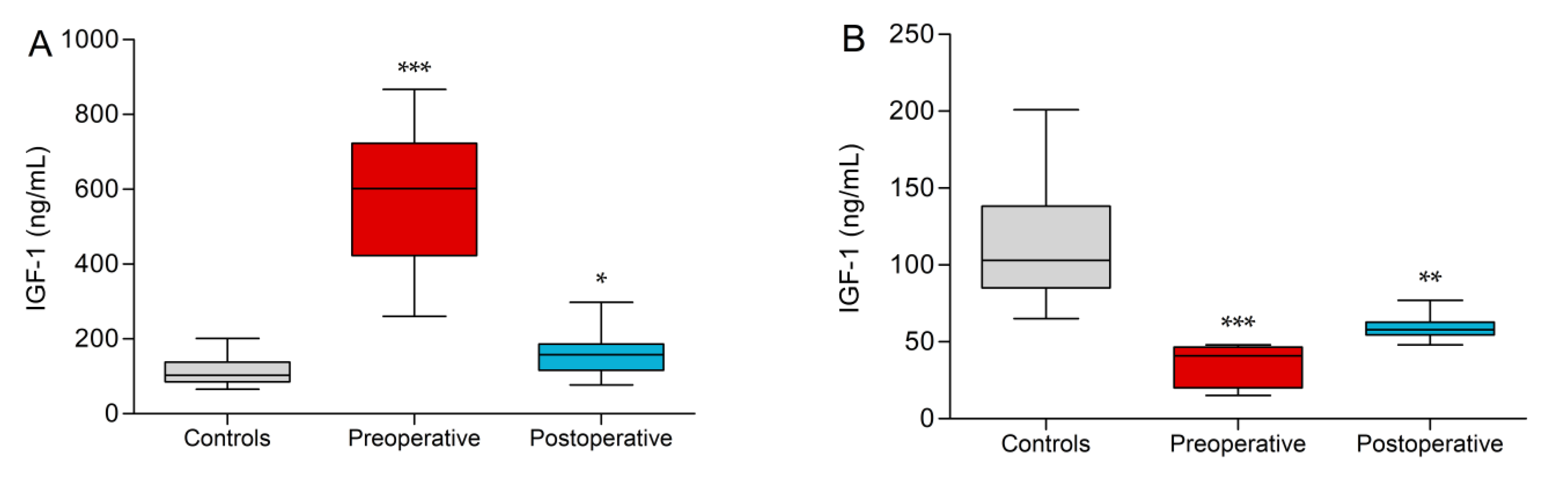
3.2. IGF-1
3.3. ECS-Complex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezzat, S.; Asa, S.L.; Couldwell, W.T.; Barr, C.E.; Dodge, W.E.; Vance, M.L.; McCutcheon, I.E. The prevalence of pituitary adenomas. Cancer 2004, 101, 613–619. [Google Scholar] [CrossRef]
- Day, P.F.; Loto, M.G.; Glerean, M.; Picasso, M.F.R.; Lovazzano, S.; Giunta, D.H. Incidence and prevalence of clinically relevant pituitary adenomas: Retrospective cohort study in a Health Management Organization in Buenos Aires, Argentina. Arch. Endocrinol. Metab. 2016, 60, 554–561. [Google Scholar] [CrossRef]
- Sesmilo, G. Epidemiología de la acromegalia en España. [Epidemiology of acromegaly in Spain]. Endocrinol. Nutr. 2013, 60, 470–474. [Google Scholar] [CrossRef]
- Burton, T.; Le Nestour, E.; Neary, M.; Ludlam, W.H. Incidence and prevalence of acromegaly in a large US health plan database. Pituitary 2016, 19, 262–267. [Google Scholar] [CrossRef]
- Fernandez, A.; Karavitaki, N.; Wass, J.A.H. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.; Ferraù, F.; Ragonese, M.; Curtò, L.; Torre, M.L.; Magistri, M.; Marchese, A.; Alibrandi, A.; Trimarchi, F. Increased prevalence of acromegaly in a highly polluted area. Eur. J. Endocrinol. 2010, 163, 509–513. [Google Scholar] [CrossRef]
- Dal, J.; Feldt-Rasmussen, U.; Andersen, M.; Kristensen, L.Ø.; Laurberg, P.; Pedersen, L.; Dekkers, O.M.; Sørensen, H.T.; Jorgensen, J.O.L. Acromegaly incidence, prevalence, complications and long-term prognosis: A nationwide cohort study. Eur. J. Endocrinol. 2016, 175, 181–190. [Google Scholar] [CrossRef]
- Regal, M.; Páramo, C.; Sierra, J.M.; García-Mayor, R.V. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin. Endocrinol. 2001, 55, 735–740. [Google Scholar] [CrossRef]
- Parent, A.D.; Bebin, J.; Smith, R.R. Incidental pituitary adenomas. J. Neurosurg. 1981, 54, 228–231. [Google Scholar] [CrossRef]
- Mestron, A.; Webb, S.M.; Astorga, R.; Benito, P.; Catala, M.; Gaztambide, S.; Gomez, J.-M.; Halperin, I.; Lucas-Morante, T.; Moreno, B.; et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur. J. Endocrinol. 2004, 151, 439–446. [Google Scholar] [CrossRef]
- Swearingen, B.; Barker, F.G.; Katznelson, L.; Biller, B.M.K.; Grinspoon, S.; Klibanski, A.; Moayeri, N.; Black, P.M.; Zervas, N.T. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J. Clin. Endocrinol. Metab. 1998, 83, 3419–3426. [Google Scholar] [CrossRef]
- Ntali, G.; Karavitaki, N. Recent advances in the management of acromegaly. F1000Research 2015, 4, 1426. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Biermasz, N.R.; Pereira, A.M.; Romijn, J.A.; Vandenbroucke, J.P. Mortality in acromegaly: A metaanalysis. J. Clin. Endocrinol. Metab. 2008, 93, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Hill, D.M.; Lowy, C.; Fraser, T.R. Mortality in acromegaly. Q. J. Med. 1970, 39, 1–16. [Google Scholar]
- Cook, D.M.; Yuen, K.C.; Biller, B.M.; Kemp, S.F.; Vance, M.L. American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients—2009 update. Endocr. Pract. 2009, 15, 1–29. [Google Scholar] [CrossRef]
- Badaru, A.; Wilson, D.M. Alternatives to growth hormone stimulation testing in children. Trends Endocrinol. Metab. 2004, 15, 252–258. [Google Scholar] [CrossRef]
- Zadik, Z.; Chalew, S.A.; Gilula, Z.; Kowarski, A.A. Reproducibility of growth hormone testing procedures: A comparison between 24-hour integrated concentration and pharmacological stimulation. J. Clin. Endocrinol. Metab. 1990, 71, 1127–1130. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Zhao, Y.; Yan, Y.; Liu, Y.; Cai, J. Diagnostic value of serum IGF-1 and IGFBP-3 in growth hormone deficiency: A systematic review with meta-analysis. Eur. J. Pediatr. 2014, 174, 419–427. [Google Scholar] [CrossRef]
- Höybye, C.; Wahlström, E.; Tollet-Egnell, P.; Norstedt, G. Metabolomics: A tool for the diagnosis of GH deficiency and for monitoring GH replacement? Endocr. Connect. 2014, 3, 200–206. [Google Scholar] [CrossRef]
- Svensson, J.; Johannsson, G.; Bengtsson, B.-A. Insulin-like growth factor-I in growth hormone-deficient adults: Relationship to population-based normal values, body composition and insulin tolerance test. Clin. Endocrinol. 1997, 46, 579–586. [Google Scholar] [CrossRef]
- Hilding, A.; Hall, K.; Wivall-Helleryd, I.-L.; Sääf, M.; Melin, A.-L.; Thorén, M. Serum levels of insulin-like growth factor I in 152 patients with growth hormone deficiency, aged 19–82 years, in relation to those in healthy subjects. J. Clin. Endocrinol. Metab. 1999, 84, 2013–2019. [Google Scholar] [CrossRef]
- Pokrajac, A.; Wark, G.; Ellis, A.R.; Wear, J.; Wieringa, G.E.; Trainer, P.J. Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin. Endocrinol. 2007, 67, 65–70. [Google Scholar] [CrossRef]
- Chanson, P.; Arnoux, A.; Mavromati, M.; Brailly-Tabard, S.; Massart, C.; Young, J.; Piketty, M.-L.; Souberbielle, J.-C.; for the VARIETE Investigators. Reference values for IGF-I serum concentrations: Comparison of six immunoassays. J. Clin. Endocrinol. Metab. 2016, 101, 3450–3458. [Google Scholar] [CrossRef]
- Murray, P.G.; Dattani, M.T.; Clayton, P.E. Controversies in the diagnosis and management of growth hormone deficiency in childhood and adolescence. Arch. Dis. Child. 2015, 101, 96–100. [Google Scholar] [CrossRef]
- Yuen, K.C.; Tritos, N.A.; Samson, S.L.; Hoffman, A.R.; Katznelson, L. American Association of Clinical Endocrinologists and American College of Endocrinology Disease state clinical review: Update on growth hormone stimulation testing and proposed revised cut-point for the glucagon stimulation test in the diagnosis of adult growth hormone deficiency. Endocr. Pract. 2016, 22, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Lima, T.H.; Vasques, G.A.; Nakaguma, M.; Brito, L.P.; Mendonça, B.B.; Arnhold, I.J.; Jorge, A.A.L. A Bayesian approach to diagnose growth hormone deficiency in children: Insulin-like growth factor type 1 is valuable for screening and IGF-binding protein type 3 for confirmation. Horm. Res. Paediatr. 2020, 93, 197–205. [Google Scholar] [CrossRef]
- Fernandez-Perez, L.; Novoa, J.; Ståhlberg, N.; Santana-Farré, R.; Boronat, M.; Marrero, D.; Henriquez-Hernandez, L.A.; Norstedt, G.; Flores-Morales, A. The effect of in vivo growth hormone treatment on blood gene expression in adults with growth hormone deficiency reveals potential biomarkers to monitor growth hormone therapy. Clin. Endocrinol. 2009, 72, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Setser, J.; Zhao, H.; Stannard, B.; Haluzik, M.; Glatt, V.; Bouxsein, M.L.; Kopchick, J.J.; Leroith, D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1–deficient mice. J. Clin. Investig. 2004, 113, 96–105. [Google Scholar] [CrossRef]
- Ertl, D.-A.; Chen, J.; Gleiss, A.; Janu, D.; Sagmeister, S.; Raimann, A.; Riedl, S.; Haeusler, G. Diagnostic value of serum acid-labile subunit alone and in combination with IGF-I and IGFBP-3 in the diagnosis of growth hormone deficiency. Horm. Res. Paediatr. 2020, 93, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Neidert, M.C.; Sze, L.; Zwimpfer, C.; Sarnthein, J.; Seifert, B.; Frei, K.; Leske, H.; Rushing, E.J.; Schmid, C.; Bernays, R.-L. Soluble α-klotho: A novel serum biomarker for the activity of GH-producing pituitary adenomas. Eur. J. Endocrinol. 2013, 168, 575–583. [Google Scholar] [CrossRef]
- Bahl, N.; Stone, G.; McLean, M.; Ho, K.K.Y.; Birzniece, V. Decorin, a growth hormone-regulated protein in humans. Eur. J. Endocrinol. 2018, 178, 145–152. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Jorgensen, J.O.L.; Christensen, B.; Sackmann-Sala, L.; Krusenstjerna-Hafstrøm, T.; Jara, A.; Okada, S.; Kopchick, J.J. Identification of new biomarkers of low-dose GH replacement therapy in GH-deficient patients. J. Clin. Endocrinol. Metab. 2011, 96, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, S.; Keay, N.; Ehrnborg, C.; Cittadini, A.; Rosen, T.; Dall, R.; Boroujerdi, M.A.; Bassett, E.E.; Healy, M.L.; Pentecost, C.; et al. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: A double blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2000, 85, 1505–1512. [Google Scholar] [CrossRef]
- Neggers, S.J.C.M.M.; Biermasz, N.R.; Lely, A.J. What is active acromegaly and which parameters do we have? Clin. Endocrinol. 2012, 76, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Schilbach, K.; Olsson, D.S.; Boguszewski, M.C.; Bidlingmaier, M.; Johannsson, G.; Jørgensen, J.-O.L. Biomarkers of GH action in children and adults. Growth Horm. IGF Res. 2018, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morales, A.; Greenhalgh, C.J.; Norstedt, G.; Rico-Bautista, E. Negative regulation of growth hormone receptor signaling. Mol. Endocrinol. 2006, 20, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, C.J.; Rico-Bautista, E.; Lorentzon, M.; Thaus, A.L.; Morgan, P.O.; Willson, T.A.; Zervoudakis, P.; Metcalf, D.; Street, I.; Nicola, N.A.; et al. SOCS2 negatively regulates growth hormone action in vitro and in vivo. J. Clin. Investig. 2005, 115, 397–406. [Google Scholar] [CrossRef]
- Uyttendaele, I.; Lemmens, I.; Verhee, A.; De Smet, A.-S.; Vandekerckhove, J.; Lavens, D.; Peelman, F.; Tavernier, J. Mammalian protein-protein interaction trap (MAPPIT) analysis of STAT5, CIS, and SOCS2 interactions with the growth hormone receptor. Mol. Endocrinol. 2007, 21, 2821–2831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vesterlund, M.; Zadjali, F.; Persson, T.; Nielsen, M.L.; Kessler, B.M.; Norstedt, G.; Flores-Morales, A. The SOCS2 ubiquitin ligase complex regulates growth hormone receptor levels. PLoS ONE 2011, 6, e25358. [Google Scholar] [CrossRef]
- Tollet-Egnell, P.; Flores-Morales, A.; Stavréus-Evers, A.; Sahlin, L.; Norstedt, G. Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology 1999, 140, 3693–3704. [Google Scholar] [CrossRef]
- Hochberg, I.; Tran, Q.T.; Barkan, A.L.; Saltiel, A.R.; Chandler, W.F.; Bridges, D. Gene expression signature in adipose tissue of acromegaly patients. PLoS ONE 2015, 10, e0129359. [Google Scholar] [CrossRef]
- Kamura, T.; Maenaka, K.; Kotoshiba, S.; Matsumoto, M.; Kohda, D.; Conaway, R.C.; Conaway, J.W.; Nakayama, K.I. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004, 18, 3055–3065. [Google Scholar] [CrossRef]
- Deshaies, R.J.; Joazeiro, C.A. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009, 78, 399–434. [Google Scholar] [CrossRef]
- Badolato, R.; Bond, H.M.; Venuta, S.; Tenore, A.; Valerio, G.; Petrella, A.; Morrone, G.; Waters, M.J. Differential expression of surface membrane growth hormone receptor on human peripheral blood lymphocytes detected by dual fluorochrome flow cytometry. J. Clin. Endocrinol. Metab. 1994, 79, 984–990. [Google Scholar] [CrossRef]
- Gossing, W.; Radke, L.; Frohme, M.; Biering, H. ECS-Komplex—Ein neuer Biomarker bei Wachstumshormon-störungen? Wiss. Beiträge 2016, 20, 23–29. [Google Scholar] [CrossRef]
- Prentice, R.L. A generalization of the probit and logit methods for dose response curves. Biometrics 1976, 32, 761–768. [Google Scholar] [CrossRef]
- Rahman Farazi, M.M.; Rahmatullah Imon, A.H.M. Detection of outliers in gene expression data using expressed robust T test. Malays. J. Math. Sci. 2016, 10, 117–135. Available online: https://einspem.upm.edu.my/journal/fullpaper/vol10no2may/1.%20imon.pdf (accessed on 11 February 2021).
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Kuncheva, L. Combining Pattern Classifiers: Methods and Algorithms, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004. [Google Scholar]
- Bonapart, I.E.; Van Domburg, R.; ten Have, S.M.T.H.; De Herder, W.W.; Erdman, R.A.M.; Janssen, J.A.M.J.L.; Van Der Lely, A.J. The ‘bio-assay’ quality of life might be a better marker of disease activity in acromegalic patients than serum total IGF-I concentrations. Eur. J. Endocrinol. 2005, 152, 217–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leung, K.-C.; Johannsson, G.; Leong, G.M.; Ho, K.K.Y. Estrogen regulation of growth hormone action. Endocr. Rev. 2004, 25, 693–721. [Google Scholar] [CrossRef] [PubMed]
- Leong, G.M.; Moverare, S.; Brce, J.; Doyle, N.; Sjögren, K.; Dahlman-Wright, K.; Gustafsson, J.-A.; Ho, K.K.Y.; Ohlsson, C.; Leung, K.-C. Estrogen up-regulates hepatic expression of suppressors of cytokine signaling-2 and -3 in vivo and in vitro. Endocrinology 2004, 145, 5525–5531. [Google Scholar] [CrossRef] [PubMed]
- Favre, H.; Benhamou, A.; Finidori, J.; Kelly, P.A.; Edery, M. Dual effects of suppressor of cytokine signaling (SOCS-2) on growth hormone signal transduction. FEBS Lett. 1999, 453, 63–66. [Google Scholar] [CrossRef]
- Greenhalgh, C.J.; Metcalf, D.; Thaus, A.L.; Corbin, J.E.; Uren, R.T.; Morgan, P.O.; Fabri, L.J.; Zhang, J.-G.; Martin, H.M.; Willson, T.A.; et al. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J. Biol. Chem. 2002, 277, 40181–40184. [Google Scholar] [CrossRef] [PubMed]
- Dobie, R.; Macrae, V.E.; Huesa, C.; van’t Hof, R.; Ahmed, S.F.; Farquharson, C. Direct stimulation of bone mass by increased GH signalling in the osteoblasts of Socs2−/− mice. J. Endocrinol. 2014, 223, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Neggers, S.J.C.M.M.; van Aken, M.O.; De Herder, W.W.; Feelders, R.A.; Janssen, J.A.M.J.L.; Badia, X.; Webb, S.M.; van der Lely, A.J. Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomant. J. Clin. Endocrinol. Metab. 2008, 93, 3853–3859. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gossing, W.; Radke, L.; Biering, H.; Diederich, S.; Mai, K.; Frohme, M. The ElonginB/C-Cullin5-SOCS-Box-Complex Is a Potential Biomarker for Growth Hormone Disorders. Biomedicines 2021, 9, 201. https://doi.org/10.3390/biomedicines9020201
Gossing W, Radke L, Biering H, Diederich S, Mai K, Frohme M. The ElonginB/C-Cullin5-SOCS-Box-Complex Is a Potential Biomarker for Growth Hormone Disorders. Biomedicines. 2021; 9(2):201. https://doi.org/10.3390/biomedicines9020201
Chicago/Turabian StyleGossing, Wilhelm, Lars Radke, Henrik Biering, Sven Diederich, Knut Mai, and Marcus Frohme. 2021. "The ElonginB/C-Cullin5-SOCS-Box-Complex Is a Potential Biomarker for Growth Hormone Disorders" Biomedicines 9, no. 2: 201. https://doi.org/10.3390/biomedicines9020201
APA StyleGossing, W., Radke, L., Biering, H., Diederich, S., Mai, K., & Frohme, M. (2021). The ElonginB/C-Cullin5-SOCS-Box-Complex Is a Potential Biomarker for Growth Hormone Disorders. Biomedicines, 9(2), 201. https://doi.org/10.3390/biomedicines9020201





