Development and Carcinogenesis: Roles of GATA Factors in the Sympathoadrenal and Urogenital Systems
Abstract
1. Introduction
2. Review Design
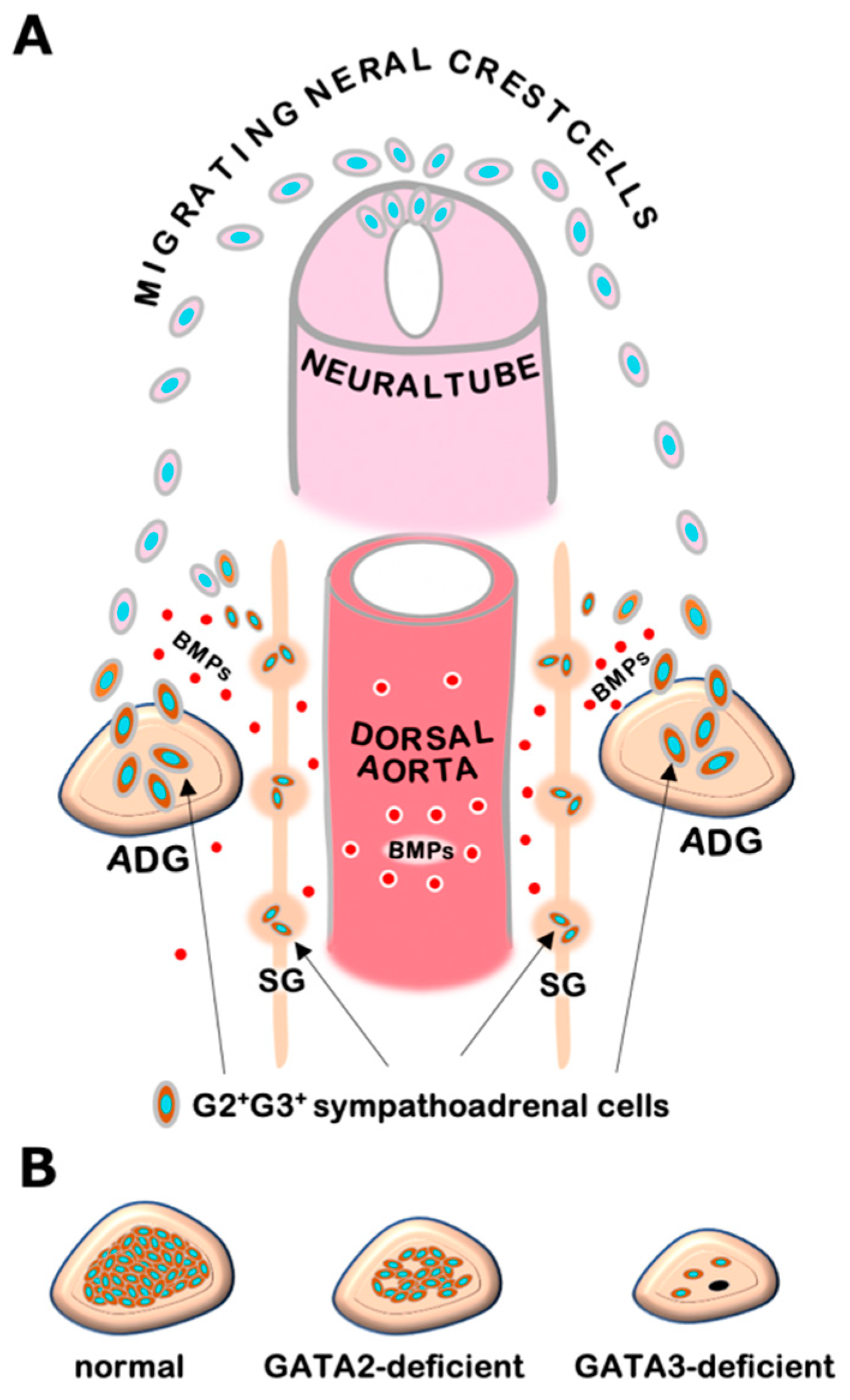
3. GATA2 and GATA3 in Sympathetic and Adrenal Chromaffin Cell Development
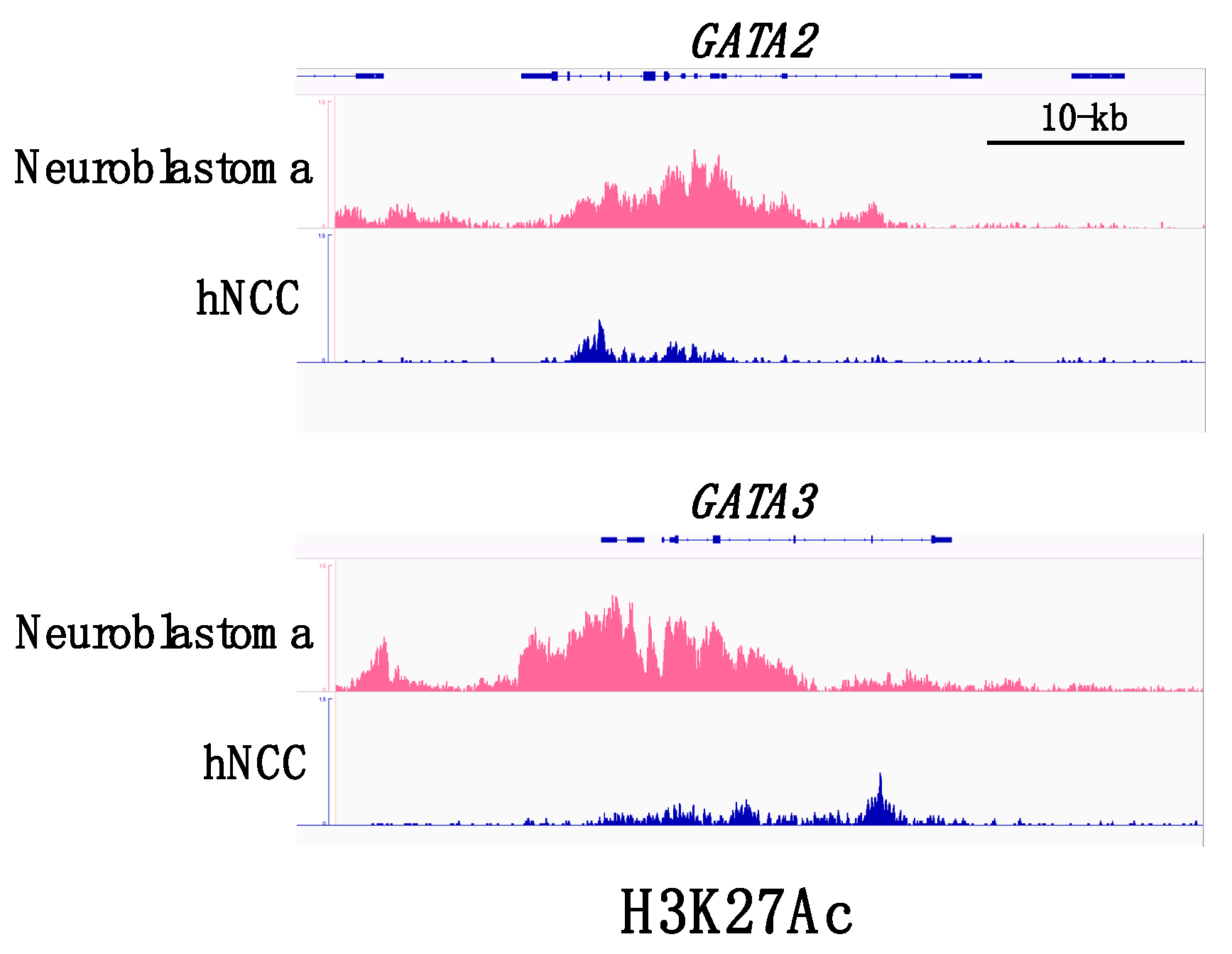
4. Neuroblastomas and GATA Factors
5. GATA3 and DNA Methylation in Neuroblastoma
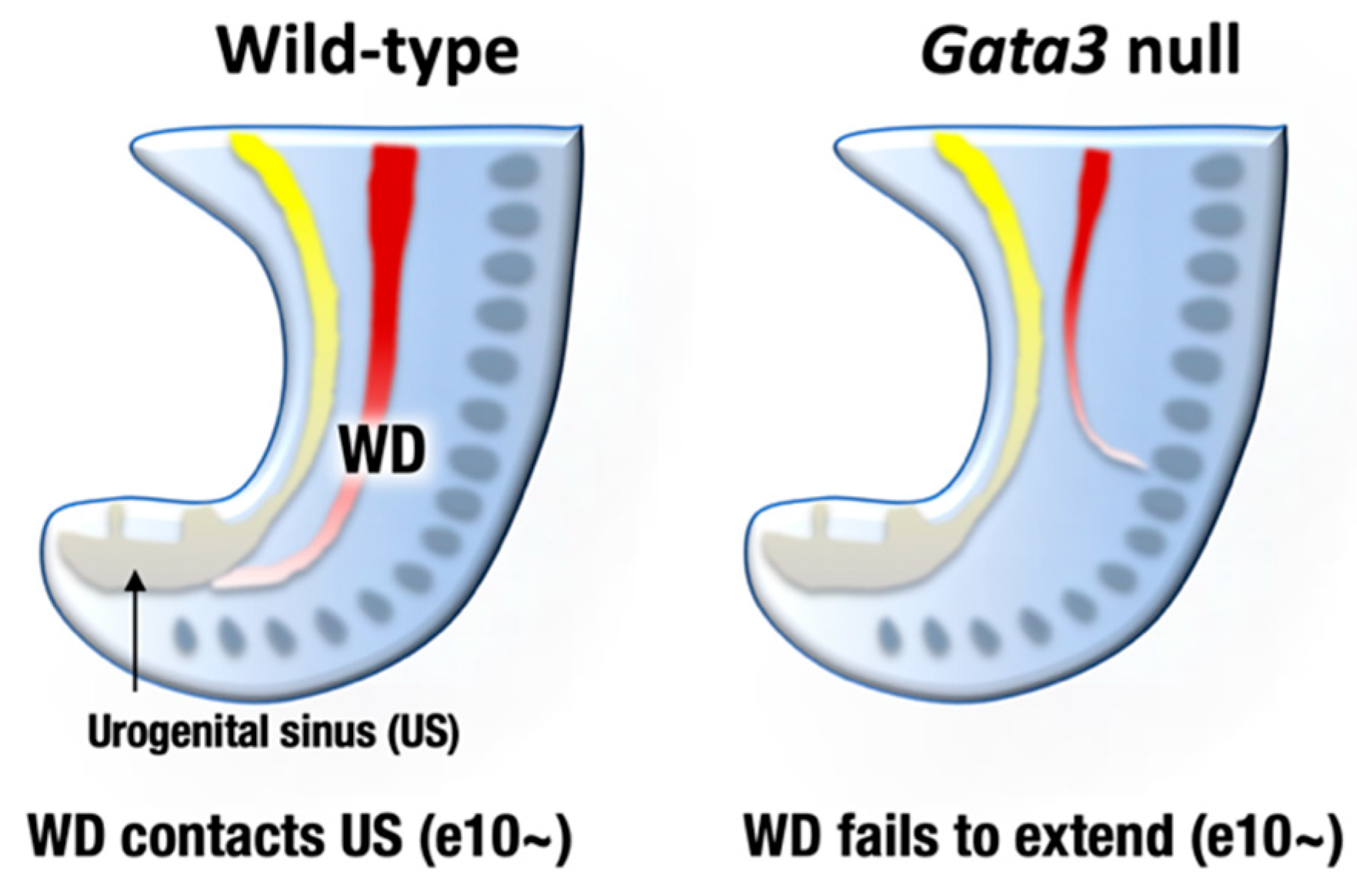
6. GATA2 and GATA3 in Urogenital Development
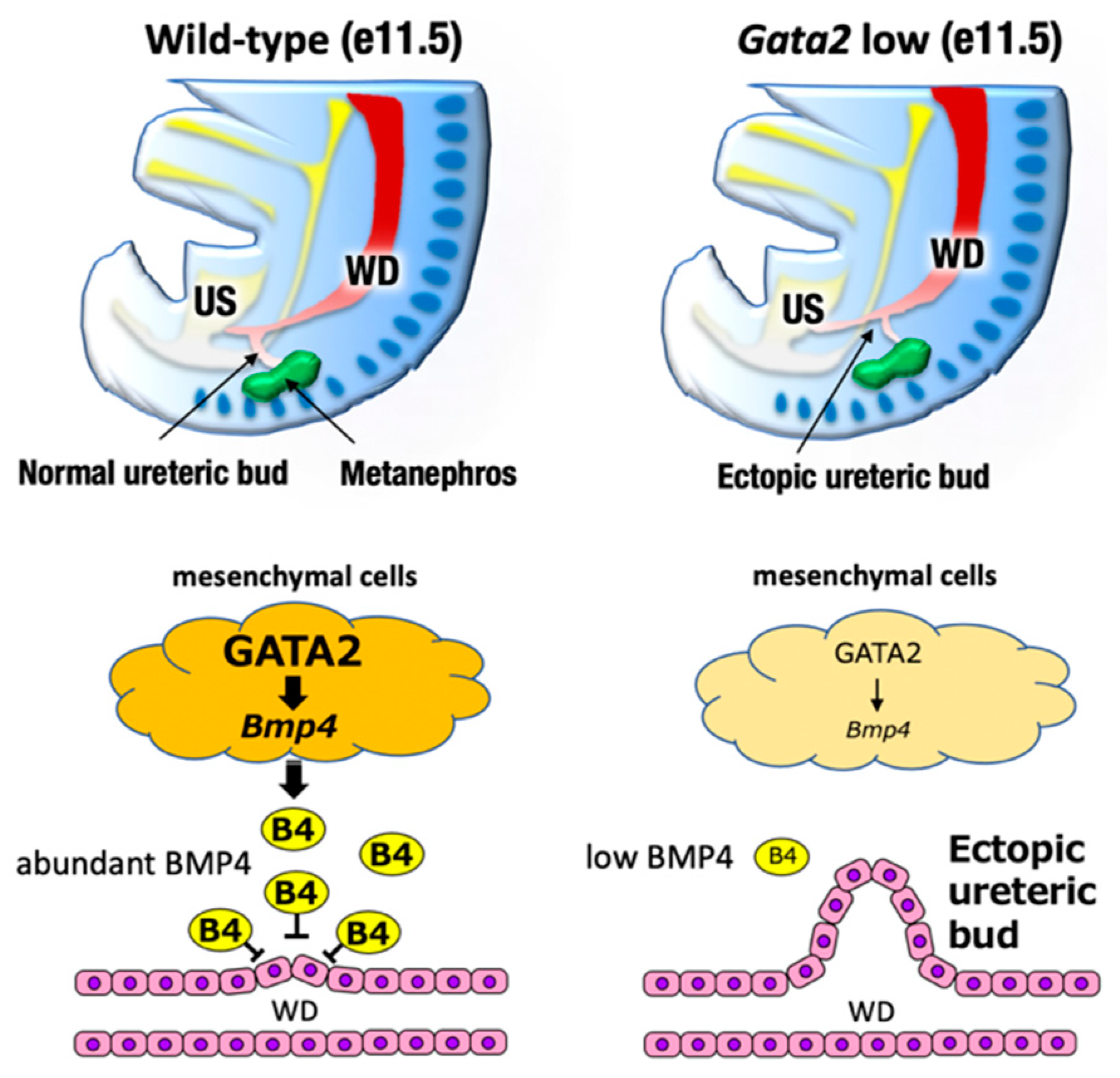
7. GATA2 and Hydronephrosis
8. GATA3 and Urogenital Cancers
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moriguchi, T.; Yamamoto, M. A regulatory network governing Gata1 and Gata2 gene transcription orchestrates erythroid lineage differentiation. Int. J. Hematol. 2014, 100, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.; Sanchez-Ferras, O.; Bouchard, M. GATA transcription factors in development and disease. Development 2018, 145, dev164384. [Google Scholar] [CrossRef]
- Lim, K.-C.; Hosoya, T.; Brandt, W.; Ku, C.-J.; Hosoya-Ohmura, S.; Camper, S.A.; Yamamoto, M.; Engel, J.D. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J. Clin. Investig. 2012, 122, 3705–3717. [Google Scholar] [CrossRef]
- Ostergaard, P.; Simpson, M.A.; Connell, F.C.; Steward, C.G.; Brice, G.; Woollard, W.J.; Dafou, D.; Kilo, T.; Smithson, S.; Lunt, P.; et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 2011, 43, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Spinner, M.A.; Sanchez, L.A.; Hsu, A.P.; Shaw, P.A.; Zerbe, C.S.; Calvo, K.R.; Arthur, D.C.; Gu, W.; Gould, C.M.; Brewer, C.C.; et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014, 123, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Kuroha, T.; Moriguchi, T.; Cummings, D.; Maillard, I.; Lim, K.C.; Engel, J.D. GATA-3 is required for early T lineage pro-genitor development. J. Exp. Med. 2009, 206, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Maillard, I.; Engel, J.D. From the cradle to the grave: Activities of GATA-3 throughout T-cell development and dif-ferentiation. Immunol. Rev. 2010, 238, 110–125. [Google Scholar] [CrossRef]
- Murga-Zamalloa, C.; Wilcox, R.A. GATA-3 in T-cell lymphoproliferative disorders. IUBMB Life 2020, 72, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-C.; Lakshmanan, G.; Crawford, S.E.; Gu, Y.; Grosveld, F.; Engel, J.D. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat. Genet. 2000, 25, 209–212. [Google Scholar] [CrossRef]
- Kouros-Mehr, H.; Slorach, E.M.; Sternlicht, M.D.; Werb, Z. GATA-3 Maintains the Differentiation of the Luminal Cell Fate in the Mammary Gland. Cell 2006, 127, 1041–1055. [Google Scholar] [CrossRef]
- Moriguchi, T.; Takako, N.; Hamada, M.; Maeda, A.; Fujioka, Y.; Kuroha, T.; Huber, R.E.; Hasegawa, S.L.; Rao, A.; Yamamoto, M.; et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 2006, 133, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Lim, K.-C.; Engel, J.D. Transcription Factor Networks SpecifySympathetic and Adrenal Chromaffin Cell Differen-tiation. Funct. Dev. Embryol. 2007, 1, 130–135. [Google Scholar]
- Moriguchi, T.; Yu, L.; Otsuki, A.; Ainoya, K.; Lim, K.-C.; Yamamoto, M.; Engel, J.D. Gata3Hypomorphic Mutant Mice Rescued with a Yeast Artificial Chromosome Transgene Suffer a Glomerular Mesangial Cell Defect. Mol. Cell. Biol. 2016, 36, 2272–2281. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Hoshino, T.; Rao, A.; Yu, L.; Takai, J.; Uemura, S.; Ise, K.; Nakamura, Y.; Lim, K.-C.; Shimizu, R.; et al. A Gata3 3′ Distal Otic Vesicle Enhancer Directs Inner Ear-Specific Gata3 Expression. Mol. Cell. Biol. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Grigorieva, I.V.; Mirczuk, S.; Gaynor, K.U.; Nesbit, M.A.; Grigorieva, E.F.; Wei, Q.; Ali, A.; Fairclough, R.J.; Stacey, J.M.; Stechman, M.J.; et al. Gata3-deficient mice develop parathyroid abnormalities due to dysregulation of the parathyroid-specific transcription factor Gcm2. J. Clin. Investig. 2010, 120, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Terunuma, T.; Takai, J.; Uemura, S.; Nakamura, Y.; Hamada, M.; Takahashi, S.; Yamamoto, M.; Engel, J.D.; Moriguchi, T. Spiral ganglion cell degeneration-induced deafness as a consequence of reduced GATA factor activity. Genes Cells 2019, 24, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.D.D.; Sirisena, N.D.; Kumanan, T.; Sujanitha, V.; Strelow, V.; Yamamoto, R.; Wieczorek, S.; Dissanayake, V.H.W. Hypoparathyroidism, Sensorineural deafness and renal disease (Barakat syndrome) caused by a reduced gene dosage in GATA3: A case report and review of literature. BMC Endocr. Disord. 2019, 19, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.C.; Thakker, R.V. Hypoparathyroidism, deafness, and renal dysplasia syndrome: 20 Years after the identification of the first GATA3 mutations. Hum. Mutat. 2020, 41, 1341–1350. [Google Scholar] [CrossRef]
- Rohrer, H. Transcriptional control of differentiation and neurogenesis in autonomic ganglia. Eur. J. Neurosci. 2011, 34, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Tsarovina, K.; Pattyn, A.; Stubbusch, J.; Müller, F.; van der Wees, J.; Schneider, C.; Brunet, J.F.; Rohrer, H. Essential role of Gata tran-scription factors in sympathetic neuron development. Development 2004, 131, 4775–4786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Y.; Quaife, C.J.; Palmiter, R.D. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are re-quired for mouse fetal development. Nature 1995, 374, 640–643. [Google Scholar] [CrossRef]
- Thomas, S.A.; Matsumoto, A.M.; Palmiter, R.D. Noradrenaline is essential for mouse fetal development. Nat. Cell Biol. 1995, 374, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Morita, S.; Sawada, H.; Mizuguchi, T.; Yamada, K.; Nagatsu, I.; Hata, T.; Watanabe, Y.; Fujita, K.; Nagatsu, T. Targeted Disruption of the Tyrosine Hydroxylase Locus Results in Severe Catecholamine Depletion and Perinatal Lethality in Mice. J. Biol. Chem. 1995, 270, 27235–27243. [Google Scholar] [CrossRef]
- Watanabe-Asaka, T.; Hayashi, M.; Engel, J.D.; Kawai, Y.; Moriguchi, T. GATA2 functions in adrenal chromaffin cells. Genes Cells 2020. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis Primers. 2016, 2, 16078. [Google Scholar] [CrossRef] [PubMed]
- Hoene, V.; Fischer, M.; Ivanova, A.; Wallach, T.; Berthold, F.; Dame, C. GATA factors in human neuroblastoma: Distinctive expression patterns in clinical subtypes. Br. J. Cancer 2009, 101, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chng, W.-J.; Zhou, J. Super-enhancers: Critical roles and therapeutic targets in hematologic malignancies. J. Hematol. Oncol. 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Behera, G.; Chhabra, G.; Mishra, P.; Sable, M. Pediatric neuroblastic tumors: A critical evaluation of cytomorphological features for risk stratification on aspiration cytology. Diagn. Cytopathol. 2020, 48, 464–474. [Google Scholar] [CrossRef]
- Lakshmanan, G.; Lieuw, K.H.; Lim, K.-C.; Gu, Y.; Grosveld, F.; Engel, J.D.; Karis, A. Localization of Distant Urogenital System-, Central Nervous System-, and Endocardium-Specific Transcriptional Regulatory Elements in the GATA-3 Locus. Mol. Cell. Biol. 1999, 19, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Charlet, J.; Tomari, A.; Dallosso, A.R.; Szemes, M.; Kaselova, M.; Curry, T.J.; Almutairi, B.; Etchevers, H.C.; McConville, C.; Malik, K.T.A.; et al. Genome-wide DNA methylation analysis identifiesMEGF10as a novel epigenetically repressed candidate tumor suppressor gene in neuroblastoma. Mol. Carcinog. 2017, 56, 1290–1301. [Google Scholar] [CrossRef]
- Almutairi, B.; Charlet, J.; Dallosso, A.R.; Szemes, M.; Etchevers, H.C.; Malik, K.T.A.; Brown, K.W. Epigenetic deregulation of GATA3 in neuroblastoma is associated with increased GATA3 protein expression and with poor outcomes. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Krug, N.; Hohlfeld, J.M.; Kirsten, A.-M.; Kornmann, O.; Beeh, K.M.; Kappeler, D.; Korn, S.; Ignatenko, S.; Timmer, W.; Rogon, C.; et al. Allergen-Induced Asthmatic Responses Modified by a GATA3-Specific DNAzyme. N. Engl. J. Med. 2015, 372, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, J.J.; Ebus, M.E.; Koster, J.; Santo, E.; Geerts, D.; Versteeg, R.; Caron, H.N. Cyclin D1 is a direct transcriptional target of GATA3 in neuroblastoma tumor cells. Oncogene 2010, 29, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Dressler, G.R. Tubulogenesis in the developing mammalian kidney. Trends Cell Biol. 2002, 12, 390–395. [Google Scholar] [CrossRef]
- Habiba, M.; Heyn, R.; Bianchi, P.; Brosens, I.; Benagiano, G. The development of the human uterus: Morphogenesis to menarche. Hum. Reprod. Updat. 2021, 27, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.; Suzuki, N.; Lewton, J.; Yamamoto, M.; Engel, J.D. Multiple, distant Gata2 enhancers specify temporally and tis-sue-specific patterning in the developing urogenital system. Mol. Cell Biol. 2004, 24, 10263–10276. [Google Scholar] [CrossRef]
- Hasegawa, S.L.; Moriguchi, T.; Rao, A.; Kuroha, T.; Engel, J.D.; Lim, K.-C. Dosage-dependent rescue of definitive nephrogenesis by a distant Gata3 enhancer. Dev. Biol. 2007, 301, 568–577. [Google Scholar] [CrossRef]
- Ainoya, K.; Moriguchi, T.; Ohmori, S.; Souma, T.; Takai, J.; Morita, M.; Chandler, K.J.; Mortlock, U.P.; Shimizu, R.; Engel, J.D.; et al. UG4 Enhancer-Driven GATA-2 and Bone Morphogenetic Protein 4 Complementation Remedies the CAKUT Phenotype in Gata2 Hypomorphic Mutant Mice. Mol. Cell. Biol. 2012, 32, 2312–2322. [Google Scholar] [CrossRef]
- Grote, D.; Boualia, S.K.; Souabni, A.; Merkel, C.; Chi, X.; Costantini, F.; Carroll, T.; Bouchard, M. Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet. 2008, 4, e1000316. [Google Scholar] [CrossRef]
- Grote, D.; Souabni, A.; Busslinger, M.; Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 2006, 133, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chia, I.; Grote, D.; Marcotte, M.; Batourina, E.; Mendelsohn, C.; Bouchard, M. Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development 2011, 138, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lim, K.; Onodera, K.; Takahashi, S.; Ohta, J.; Minegishi, N.; Tsai, F.; Orkin, S.H.; Yamamoto, M.; Engel, J.D. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J. 1998, 17, 6689–6700. [Google Scholar] [CrossRef]
- Hoshino, T.; Shimizu, R.; Ohmori, S.; Nagano, M.; Pan, X.; Ohneda, O.; Khandekar, M.; Yamamoto, M.; Lim, K.-C.; Engel, J.D. Reduced BMP4 abundance in Gata2 hypomorphic mutant mice result in uropathies resembling human CAKUT. Genes Cells 2008, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Bohnenpoll, T.; Kurz, J.; Blank, P.; Airik, R.; Lüdtke, T.H.; Kleppa, M.; Deuper, L.; Kaiser, M.; Mamo, T.M.; et al. Delayed onset of smooth muscle cell differentiation leads to hydroureter formation in mice with conditional loss of the zinc finger transcription factor gene Gata2 in the ureteric mesenchyme. J. Pathol. 2019, 248, 452–463. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Kaygusuz, G.; Wang, L.; Montgomery, K.; Mason, V.; Zhu, S.X.; Marinelli, R.J.; Presti, J.C.; Van De Rijn, M.; Brooks, J.D. Placental S100 (S100P) and GATA3: Markers for Transitional Epithelium and Urothelial Carcinoma Discovered by Complementary DNA Microarray. Am. J. Surg. Pathol. 2007, 31, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Jin, L.; Dai, G.; Yao, Q.; Xiang, H.; Zhang, Y.; Liu, X.; Xue, B. Biological and Clinical Significance of GATA3 Detected from TCGA Database and FFPE Sample in Bladder Cancer Patients. OncoTargets Ther. 2020, 13, 945–958. [Google Scholar] [CrossRef]
- Inoue, S.; Mizushima, T.; Fujita, K.; Meliti, A.; Ide, H.; Yamaguchi, S.; Fushimi, H.; Netto, G.J.; Nonomura, N.; Miyamoto, H. GATA3 immunohistochemistry in urothelial carcinoma of the upper urinary tract as a urothelial marker and a prognosticator. Hum. Pathol. 2017, 64, 83–90. [Google Scholar] [CrossRef]
- Takaku, M.; Grimm, S.A.; Roberts, J.D.; Chrysovergis, K.; Bennett, B.D.; Myers, P.; Perera, L.; Tucker, C.J.; Perou, C.M.; Wade, P.A. GATA3 zinc finger 2 mutations reprogram the breast cancer transcriptional network. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Chou, J.; Provot, S.; Werb, Z. GATA3 in development and cancer differentiation: Cells GATA have it! J. Cell. Physiol. 2009, 222, 42–49. [Google Scholar] [CrossRef]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Williamson, S.R. Clear cell papillary renal cell carcinoma: An update after 15 years. Pathology 2021, 53, 109–119. [Google Scholar] [CrossRef]
- Yu, L.; Moriguchi, T.; Souma, T.; Takai, J.; Satoh, H.; Morito, N.; Engel, J.D.; Yamamoto, M. GATA2 Regulates Body Water Homeostasis through Maintaining Aquaporin 2 Expression in Renal Collecting Ducts. Mol. Cell. Biol. 2014, 34, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Moriguchi, T.; Kaneko, H.; Hayashi, M.; Hasegawa, A.; Nezu, M.; Saya, H.; Yamamoto, M.; Shimizu, R. Reducing Inflammatory Cytokine Production from Renal Collecting Duct Cells by Inhibiting GATA2 Ameliorates Acute Kidney Injury. Mol. Cell. Biol. 2017, 37. [Google Scholar] [CrossRef]
- Chen, P.; Ren, F.; Wang, D.-B. GATA-3 Stimulates Proliferation of Endometriotic Cells. Ginekol. Polska 2019, 90, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Gibbard, E.; Cochrane, D.R.; Pors, J.; Negri, G.L.; Colborne, S.; Cheng, A.S.; Chow, C.; Farnell, D.; Tessier-Cloutier, B.; McAlpine, J.N.; et al. Whole-proteome analysis of mesoneph-ric-derived cancers describes new potential biomarkers. Hum. Pathol. 2020, 108, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Euscher, E.D.; Bassett, R.; Duose, D.Y.; Lan, C.; Wistuba, I.; Ramondetta, L.; Ramalingam, P.; Malpica, A. Mesonephric-like Carcinoma of the Endometrium: A Subset of Endometrial Carcinoma with an Aggressive Behavior. Am. J. Surg. Pathol. 2020, 44, 429–443. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriguchi, T. Development and Carcinogenesis: Roles of GATA Factors in the Sympathoadrenal and Urogenital Systems. Biomedicines 2021, 9, 299. https://doi.org/10.3390/biomedicines9030299
Moriguchi T. Development and Carcinogenesis: Roles of GATA Factors in the Sympathoadrenal and Urogenital Systems. Biomedicines. 2021; 9(3):299. https://doi.org/10.3390/biomedicines9030299
Chicago/Turabian StyleMoriguchi, Takashi. 2021. "Development and Carcinogenesis: Roles of GATA Factors in the Sympathoadrenal and Urogenital Systems" Biomedicines 9, no. 3: 299. https://doi.org/10.3390/biomedicines9030299
APA StyleMoriguchi, T. (2021). Development and Carcinogenesis: Roles of GATA Factors in the Sympathoadrenal and Urogenital Systems. Biomedicines, 9(3), 299. https://doi.org/10.3390/biomedicines9030299




