Surgery for Colorectal Cancer: A Trigger for Liver Metastases Development? New Insights into the Underlying Mechanisms
Abstract
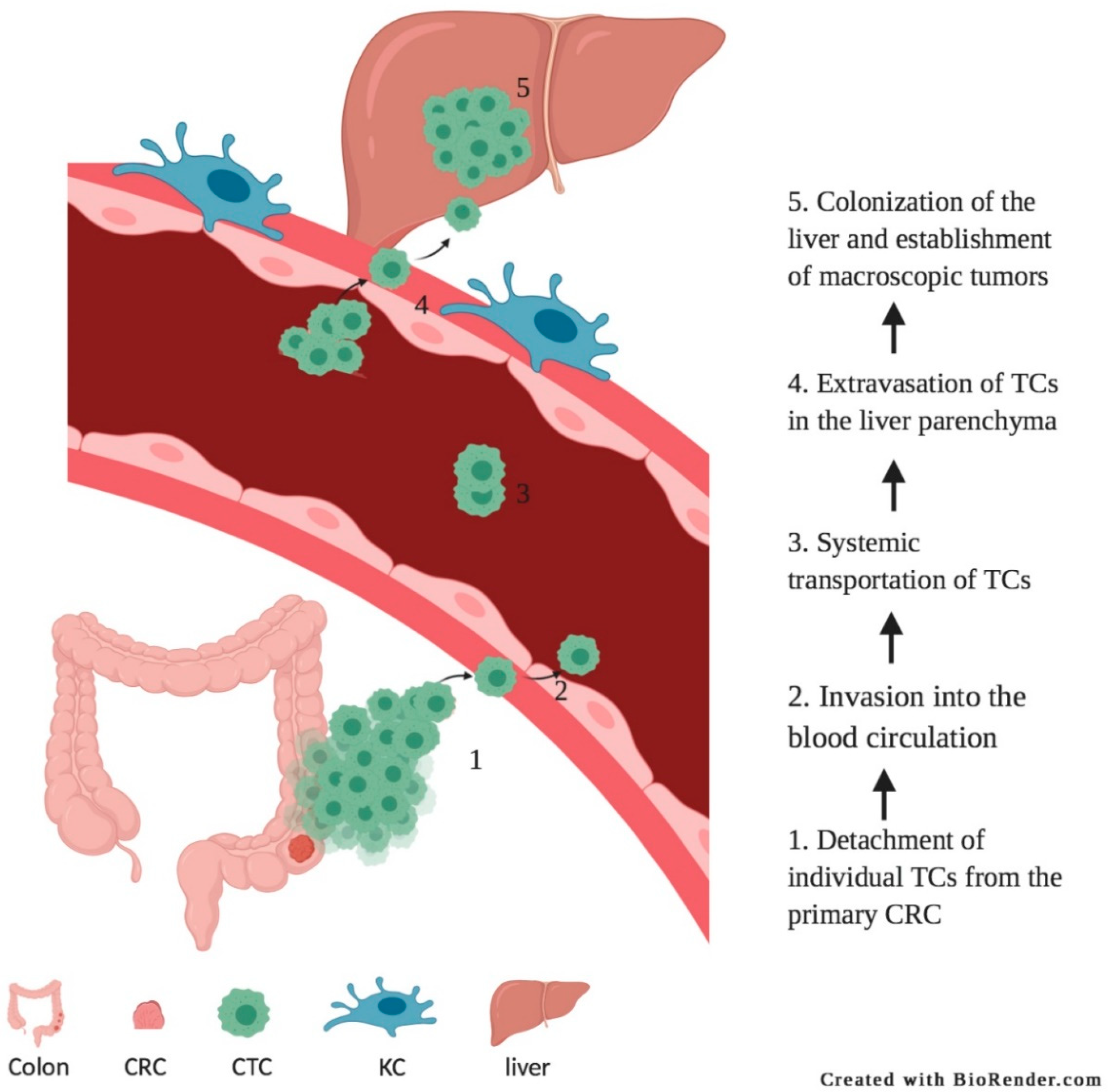
1. Introduction
2. Adhesion of Circulating Tumor Cells
3. Anastomotic Leakage and Bacterial Translocation
4. Surgery Induced Activation of Immune Cells
5. Potential Perioperative Interventions to Prevent Metastases Development
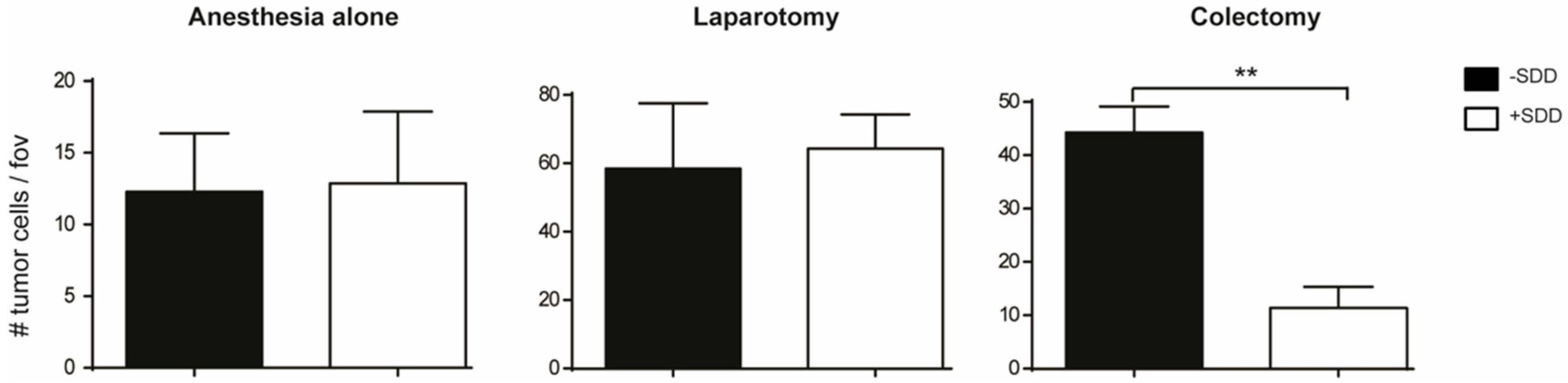
6. Limiting Surgical Trauma?
7. Blocking Tumor Cell Adhesion
8. Reducing Inflammatory Responses
9. Oral Antibiotics for Decontamination of the Digestive Tract
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef]
- Matsuda, T.; Yamashita, K.; Hasegawa, H.; Oshikiri, T.; Hosono, M.; Higashino, N.; Yamamoto, M.; Matsuda, Y.; Kanaji, S.; Nakamura, T.; et al. Recent updates in the surgical treatment of colorectal cancer. Ann. Gastroenterol. Surg. 2018, 2, 129–136. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Dillekås, H.; Straume, O.; Biganzoli, E. Distant metastasis dynamics following subsequent surgeries after primary breast cancer removal. Breast Cancer Res. BCR 2019, 21, 57. [Google Scholar] [CrossRef]
- Hapach, L.A.; Mosier, J.A.; Wang, W.; Reinhart-King, C.A. Engineered models to parse apart the metastatic cascade. NPJ Precis. Oncol. 2019, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Gao, W.; Lu, Y.; Lan, H.; Teng, L.; Cao, F. Mechanisms regulating colorectal cancer cell metastasis into liver (Review). Oncol. Lett. 2012, 3, 11–15. [Google Scholar] [CrossRef][Green Version]
- Weidle, U.H.; Birzele, F.; Krüger, A. Molecular targets and pathways involved in liver metastasis of colorectal cancer. Clin. Exp. Metastasis 2015, 32, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.S.; Chen, J.S.; Shao, H.J.; Wu, J.C.; Lai, J.M.; Lu, S.H.; Hung, T.F.; Chiu, Y.C.; You, J.F.; Hsieh, P.S.; et al. Circulating Tumor Cell Count Correlates with Colorectal Neoplasm Progression and Is a Prognostic Marker for Distant Metastasis in Non-Metastatic Patients. Sci. Rep. 2016, 6, 24517. [Google Scholar] [CrossRef] [PubMed]
- Arrazubi, V.; Mata, E.; Antelo, M.L.; Tarifa, A.; Herrera, J.; Zazpe, C.; Teijeira, L.; Viudez, A.; Suárez, J.; Hernández, I.; et al. Circulating Tumor Cells in Patients Undergoing Resection of Colorectal Cancer Liver Metastases. Clinical Utility for Long-Term Outcome: A Prospective Trial. Ann. Surg. Oncol. 2019, 26, 2805–2811. [Google Scholar] [CrossRef]
- Bork, U.; Grützmann, R.; Rahbari, N.N.; Schölch, S.; Distler, M.; Reissfelder, C.; Koch, M.; Weitz, J. Prognostic relevance of minimal residual disease in colorectal cancer. World J. Gastroenterol. 2014, 20, 10296–10304. [Google Scholar] [CrossRef] [PubMed]
- Bork, U.; Rahbari, N.N.; Schölch, S.; Reissfelder, C.; Kahlert, C.; Büchler, M.W.; Weitz, J.; Koch, M. Circulating tumour cells and outcome in non-metastatic colorectal cancer: A prospective study. Br. J. Cancer 2015, 112, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, M.; Linnemann, U.; Berger, M.R. Detection of disseminated tumour cells in the liver of colorectal cancer patients. Eur. J. Surg. Oncol. 2005, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Takagi, Y.; Aoki, S.; Futamura, M.; Saji, S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann. Surg. 2000, 232, 58–65. [Google Scholar] [CrossRef]
- Martin, O.A.; Anderson, R.L.; Narayan, K.; MacManus, M.P. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat. Rev. Clin. Oncol. 2017, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Seeberg, L.T.; Brunborg, C.; Waage, A.; Hugenschmidt, H.; Renolen, A.; Stav, I.; Bjornbeth, B.A.; Borgen, E.; Naume, B.; Brudvik, K.W.; et al. Survival Impact of Primary Tumor Lymph Node Status and Circulating Tumor Cells in Patients with Colorectal Liver Metastases. Ann. Surg. Oncol. 2017, 24, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Xu, H.; Wang, Z. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015, 15, 202. [Google Scholar] [CrossRef]
- Lu, Y.J.; Wang, P.; Peng, J.; Wang, X.; Zhu, Y.W.; Shen, N. Meta-analysis Reveals the Prognostic Value of Circulating Tumour Cells Detected in the Peripheral Blood in Patients with Non-Metastatic Colorectal Cancer. Sci. Rep. 2017, 7, 905. [Google Scholar] [CrossRef]
- Stasinopoulos, I.; Penet, M.-F.; Krishnamachary, B.; Bhujwalla, Z.M. Molecular and functional imaging of invasion and metastasis: Windows into the metastatic cascade. Cancer Biomark. 2010, 7, 173–188. [Google Scholar] [CrossRef]
- Khatib, A.M.; Auguste, P.; Fallavollita, L.; Wang, N.; Samani, A.; Kontogiannea, M.; Meterissian, S.; Brodt, P. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am. J. Pathol. 2005, 167, 749–759. [Google Scholar] [CrossRef][Green Version]
- McCarty, O.J.T.; Jadhav, S.; Burdick, M.M.; Bell, W.R.; Konstantopoulos, K. Fluid shear regulates the kinetics and molecular mechanisms of activation-dependent platelet binding to colon carcinoma cells. Biophys. J. 2002, 83, 836–848. [Google Scholar] [CrossRef]
- Burdick, M.M.; Konstantopoulos, K. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am. J. Physiol. Cell Physiol. 2004, 287, C539–C547. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Männel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, H.M.; Verheul, H.M.; D’Amato, R.J.; Folkman, J. Involvement of platelets in tumour angiogenesis? Lancet 1998, 352, 1775–1777. [Google Scholar] [CrossRef]
- Gout, S.; Tremblay, P.L.; Huot, J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin. Exp. Metastasis 2008, 25, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Borsig, L. Selectins as mediators of lung metastasis. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2010, 3, 97–105. [Google Scholar] [CrossRef]
- Eichbaum, C.; Meyer, A.S.; Wang, N.; Bischofs, E.; Steinborn, A.; Bruckner, T.; Brodt, P.; Sohn, C.; Eichbaum, M.H. Breast cancer cell-derived cytokines, macrophages and cell adhesion: Implications for metastasis. Anticancer Res. 2011, 31, 3219–3227. [Google Scholar]
- Veenhof, A.A.; Vlug, M.S.; van der Pas, M.H.; Sietses, C.; van der Peet, D.L.; de Lange-de Klerk, E.S.; Bonjer, H.J.; Bemelman, W.A.; Cuesta, M.A. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: A randomized trial. Ann. Surg. 2012, 255, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Sturm, J.W.; Magdeburg, R.; Berger, K.; Petruch, B.; Samel, S.; Bonninghoff, R.; Keese, M.; Hafner, M.; Post, S. Influence of TNFA on the formation of liver metastases in a syngenic mouse model. Int. J. Cancer 2003, 107, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ten Kate, M.; Hofland, L.J.; van Grevenstein, W.M.; van Koetsveld, P.V.; Jeekel, J.; van Eijck, C.H. Influence of proinflammatory cytokines on the adhesion of human colon carcinoma cells to lung microvascular endothelium. Int. J. Cancer 2004, 112, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Ziprin, P.; Ridgway, P.F.; Pfistermuller, K.L.; Peck, D.H.; Darzi, A.W. ICAM-1 mediated tumor-mesothelial cell adhesion is modulated by IL-6 and TNF-alpha: A potential mechanism by which surgical trauma increases peritoneal metastases. Cell Commun. Adhes. 2003, 10, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Pietila, M.; Ivaska, J. The complexity of integrins in cancer and new scopes for therapeutic targeting. Br. J. Cancer 2016, 115, 1017–1023. [Google Scholar] [CrossRef]
- Raab-Westphal, S.; Marshall, J.F.; Goodman, S.L. Integrins as Therapeutic Targets: Successes and Cancers. Cancers 2017, 9, 110. [Google Scholar] [CrossRef]
- Munshi, H.G.; Stack, M.S. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006, 25, 45–56. [Google Scholar] [CrossRef]
- Cox, D.; Brennan, M.; Moran, N. Integrins as therapeutic targets: Lessons and opportunities. Nat. Rev. Drug Discov. 2010, 9, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Van der Bij, G.J.; Oosterling, S.J.; Beelen, R.H.; Meijer, S.; Coffey, J.C.; van Egmond, M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann. Surg. 2009, 249, 727–734. [Google Scholar] [CrossRef]
- Van Grevenstein, W.M.; Aalbers, A.G.; Ten Raa, S.; Sluiter, W.; Hofland, L.J.; Jeekel, H.; van Eijck, C.H. Surgery-derived reactive oxygen species produced by polymorphonuclear leukocytes promote tumor recurrence: Studies in an in vitro model. J. Surg. Res. 2007, 140, 115–120. [Google Scholar] [CrossRef]
- Gul, N.; Bogels, M.; Grewal, S.; van der Meer, A.J.; Rojas, L.B.; Fluitsma, D.M.; van den Tol, M.P.; Hoeben, K.A.; van Marle, J.; de Vries, H.E.; et al. Surgery-induced reactive oxygen species enhance colon carcinoma cell binding by disrupting the liver endothelial cell lining. Gut 2011, 60, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Oosterling, S.J.; van der Bij, G.J.; Bogels, M.; ten Raa, S.; Post, J.A.; Meijer, G.A.; Beelen, R.H.; van Egmond, M. Anti-beta1 integrin antibody reduces surgery-induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Ann. Surg. 2008, 247, 85–94. [Google Scholar] [CrossRef]
- van der Bij, G.J.; Oosterling, S.J.; Bogels, M.; Bhoelan, F.; Fluitsma, D.M.; Beelen, R.H.; Meijer, S.; van Egmond, M. Blocking alpha2 integrins on rat CC531s colon carcinoma cells prevents operation-induced augmentation of liver metastases outgrowth. Hepatology 2008, 47, 532–543. [Google Scholar] [CrossRef]
- Choi, H.K.; Law, W.L.; Ho, J.W. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: Analysis of risk factors. Dis. Colon Rectum 2006, 49, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Frasson, M.; Flor-Lorente, B.; Rodriguez, J.L.; Granero-Castro, P.; Hervas, D.; Alvarez Rico, M.A.; Brao, M.J.; Sanchez Gonzalez, J.M.; Garcia-Granero, E. Risk Factors for Anastomotic Leak After Colon Resection for Cancer: Multivariate Analysis and Nomogram From a Multicentric, Prospective, National Study With 3193 Patients. Ann. Surg. 2015, 262, 321–330. [Google Scholar] [CrossRef]
- Van Rooijen, S.J.; Jongen, A.C.; Wu, Z.-Q.; Ji, J.-F.; Slooter, G.D.; Roumen, R.M.; Bouvy, N.D. Definition of colorectal anastomotic leakage: A consensus survey among Dutch and Chinese colorectal surgeons. World J. Gastroenterol. 2017, 23, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Belt, E.J.; Stockmann, H.B.; Abis, G.S.; de Boer, J.M.; de Lange-de Klerk, E.S.; van Egmond, M.; Meijer, G.A.; Oosterling, S.J. Peri-operative bowel perforation in early stage colon cancer is associated with an adverse oncological outcome. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2012, 16, 2260–2266. [Google Scholar] [CrossRef]
- Kube, R.; Mroczkowski, P.; Granowski, D.; Benedix, F.; Sahm, M.; Schmidt, U.; Gastinger, I.; Lippert, H. Anastomotic leakage after colon cancer surgery: A predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur. J. Surg. Oncol. 2010, 36, 120–124. [Google Scholar] [CrossRef]
- Law, W.L.; Choi, H.K.; Lee, Y.M.; Ho, J.W.; Seto, C.L. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J. Gastrointest. Surg. 2007, 11, 8–15. [Google Scholar] [CrossRef]
- Goto, S.; Hasegawa, S.; Hida, K.; Uozumi, R.; Kanemitsu, Y.; Watanabe, T.; Sugihara, K.; Sakai, Y. Multicenter analysis of impact of anastomotic leakage on long-term oncologic outcomes after curative resection of colon cancer. Surgery 2017, 162, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.T.; Wibe, A.; Norstein, J.; Haffner, J.; Wiig, J.N. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis. 2005, 7, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Espin, E.; Ciga, M.A.; Pera, M.; Ortiz, H. Oncological outcome following anastomotic leak in rectal surgery. Br. J. Surg. 2015, 102, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, A.; Mirnezami, R.; Chandrakumaran, K.; Sasapu, K.; Sagar, P.; Finan, P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: Systematic review and meta-analysis. Ann. Surg. 2011, 253, 890–899. [Google Scholar] [CrossRef]
- Lu, Z.R.; Rajendran, N.; Lynch, A.C.; Heriot, A.G.; Warrier, S.K. Anastomotic Leaks After Restorative Resections for Rectal Cancer Compromise Cancer Outcomes and Survival. Dis. Colon Rectum 2016, 59, 236–244. [Google Scholar] [CrossRef]
- Ha, G.W.; Kim, J.H.; Lee, M.R. Oncologic Impact of Anastomotic Leakage Following Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 3289–3299. [Google Scholar] [CrossRef] [PubMed]
- Furnée, E.J.B.; Aukema, T.S.; Oosterling, S.J.; Borstlap, W.A.A.; Bemelman, W.A.; Tanis, P.J.; Dutch Snapshot Research, G. Influence of Conversion and Anastomotic Leakage on Survival in Rectal Cancer Surgery; Retrospective Cross-sectional Study. J. Gastrointest. Surg. 2019, 23, 2007–2018. [Google Scholar] [CrossRef]
- Schietroma, M.; Pessia, B.; Carlei, F.; Cecilia, E.M.; De Santis, G.; Amicucci, G. Laparoscopic versus open colorectal surgery for colon cancer: The effect of surgical trauma on the bacterial translocation. A prospective randomized study. Am. J. Surg. 2015, 210, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Grewal, S.; Korthouwer, R.; Bögels, M.; Braster, R.; Heemskerk, N.; Budding, A.E.; Pouw, S.M.; van Horssen, J.; Ankersmit, M.; Meijerink, J.; et al. Spillage of bacterial products during colon surgery increases the risk of liver metastases development in a rat colon carcinoma model. OncoImmunology 2018. [Google Scholar] [CrossRef]
- Chin, K.F.; Kallam, R.; O’Boyle, C.; MacFie, J. Bacterial translocation may influence the long-term survival in colorectal cancer patients. Dis. Colon Rectum 2007, 50, 323–330. [Google Scholar] [CrossRef]
- Koratzanis, G.; Giamarellos-Bourboulis, E.J.; Papalambros, E.; Giamarellou, H. Bacterial translocation following intrabdominal surgery. Any influence of antimicrobial prophylaxis? Int. J. Antimicrob. Agents 2002, 20, 457–460. [Google Scholar] [CrossRef]
- Reddy, B.S.; MacFie, J.; Gatt, M.; Macfarlane-Smith, L.; Bitzopoulou, K.; Snelling, A.M. Commensal bacteria do translocate across the intestinal barrier in surgical patients. Clin. Nutr. 2007, 26, 208–215. [Google Scholar] [CrossRef]
- Buttenschoen, K.; Buttenschoen, D.C.; Berger, D.; Vasilescu, C.; Schafheutle, S.; Goeltenboth, B.; Seidelmann, M.; Beger, H.G. Endotoxemia and acute-phase proteins in major abdominal surgery. Am. J. Surg. 2001, 181, 36–43. [Google Scholar] [CrossRef]
- Buttenschoen, K.; Schneider, M.E.; Utz, K.; Kornmann, M.; Beger, H.G.; Buttenschoen, D.C. Effect of major abdominal surgery on endotoxin release and expression of Toll-like receptors 2/4. Langenbeck’s Arch. Surg. Dtsch. Ges. Chir. 2009, 394, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Schietroma, M.; Carlei, F.; Cappelli, S.; Amicucci, G. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann. Surg. 2006, 243, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Anderson, A.L.; Devereaux, M.W.; Fillon, S.A.; Harris, J.K.; Lovell, M.A.; Finegold, M.J.; Sokol, R.J. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology 2012, 55, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Robertson, A.E.; Stoletov, K.; Leith, S.J.; Chin, C.A.; Chien, A.E.; Hague, M.N.; Ablack, A.; Carmine-Simmen, K.; McPherson, V.A.; et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014, 8, 1558–1570. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Cavnar, M.J.; Turcotte, S.; Katz, S.C.; Kuk, D.; Gonen, M.; Shia, J.; Allen, P.J.; Balachandran, V.P.; D’Angelica, M.I.; Kingham, T.P.; et al. Tumor-Associated Macrophage Infiltration in Colorectal Cancer Liver Metastases is Associated With Better Outcome. Ann. Surg. Oncol. 2017, 24, 1835–1842. [Google Scholar] [CrossRef]
- Tanis, E.; Julie, C.; Emile, J.F.; Mauer, M.; Nordlinger, B.; Aust, D.; Roth, A.; Lutz, M.P.; Gruenberger, T.; Wrba, F.; et al. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur. J. Cancer 2015, 51, 2708–2717. [Google Scholar] [CrossRef]
- Meshcheryakova, A.; Tamandl, D.; Bajna, E.; Stift, J.; Mittlboeck, M.; Svoboda, M.; Heiden, D.; Stremitzer, S.; Jensen-Jarolim, E.; Grunberger, T.; et al. B cells and ectopic follicular structures: Novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PLoS ONE 2014, 9, e99008. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, M.; Peng, R.; Liu, J.; Wang, F.; Li, Y.; Zhao, Q.; Liu, J. The prognostic and clinicopathological value of tumor-associated macrophages in patients with colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2020, 35, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Norton, S.E.; Dunn, E.T.; McCall, J.L.; Munro, F.; Kemp, R.A. Gut macrophage phenotype is dependent on the tumor microenvironment in colorectal cancer. Clin. Transl. Immunol. 2016, 5, e76. [Google Scholar] [CrossRef] [PubMed]
- Edin, S.; Wikberg, M.L.; Oldenborg, P.A.; Palmqvist, R. Macrophages: Good guys in colorectal cancer. Oncoimmunology 2013, 2, e23038. [Google Scholar] [CrossRef]
- Matsubara, D.; Arita, T.; Nakanishi, M.; Kuriu, Y.; Murayama, Y.; Kudou, M.; Konishi, H.; Komatsu, S.; Shiozaki, A.; Otsuji, E. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 602–613. [Google Scholar] [CrossRef]
- Kuraishy, A.; Karin, M.; Grivennikov, S.I. Tumor promotion via injury- and death-induced inflammation. Immunity 2011, 35, 467–477. [Google Scholar] [CrossRef]
- Kimura, F.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Miyazaki, M. Immunosuppression following surgical and traumatic injury. Surg. Today 2010, 40, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-M.; Lee, Y.-W.; Huang, Y.-J.; Wei, P.-L. Comparison of clinical outcomes between laparoscopic and open surgery for left-sided colon cancer: A nationwide population-based study. Sci. Rep. 2020, 10, 75. [Google Scholar] [CrossRef]
- Lacy, A.M.; Garcia-Valdecasas, J.C.; Delgado, S.; Castells, A.; Taura, P.; Pique, J.M.; Visa, J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet 2002, 359, 2224–2229. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; Cuesta, M.A.; van der Pas, M.H.; de Lange-de Klerk, E.S.; Lacy, A.M.; Bemelman, W.A.; Andersson, J.; Angenete, E.; et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N. Engl. J. Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef]
- Jayne, D.G.; Guillou, P.J.; Thorpe, H.; Quirke, P.; Copeland, J.; Smith, A.M.; Heath, R.M.; Brown, J.M. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J. Clin. Oncol. 2007, 25, 3061–3068. [Google Scholar] [CrossRef]
- Buunen, M.; Veldkamp, R.; Hop, W.C.; Kuhry, E.; Jeekel, J.; Haglind, E.; Pahlman, L.; Cuesta, M.A.; Msika, S.; Morino, M.; et al. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.G.; Thorpe, H.C.; Copeland, J.; Quirke, P.; Brown, J.M.; Guillou, P.J. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br. J. Surg. 2010, 97, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.M.; Fallavollita, L.; Wancewicz, E.V.; Monia, B.P.; Brodt, P. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 2002, 62, 5393–5398. [Google Scholar]
- Vidal-Vanaclocha, F.; Fantuzzi, G.; Mendoza, L.; Fuentes, A.M.; Anasagasti, M.J.; Martin, J.; Carrascal, T.; Walsh, P.; Reznikov, L.L.; Kim, S.H.; et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc. Natl. Acad. Sci. USA 2000, 97, 734–739. [Google Scholar] [CrossRef]
- Grose, R.; Hutter, C.; Bloch, W.; Thorey, I.; Watt, F.M.; Fassler, R.; Brakebusch, C.; Werner, S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 2002, 129, 2303–2315. [Google Scholar] [PubMed]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Ramphal, W.; Boeding, J.R.E.; Gobardhan, P.D.; Rutten, H.J.T.; de Winter, L.; Crolla, R.; Schreinemakers, J.M.J. Oncologic outcome and recurrence rate following anastomotic leakage after curative resection for colorectal cancer. Surg. Oncol. 2018, 27, 730–736. [Google Scholar] [CrossRef]
- Noh, G.T.; Ann, Y.S.; Cheong, C.; Han, J.; Cho, M.S.; Hur, H.; Min, B.S.; Lee, K.Y.; Kim, N.K. Impact of anastomotic leakage on long-term oncologic outcome and its related factors in rectal cancer. Medicine 2016, 95, e4367. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef]
- Neeman, E.; Zmora, O.; Ben-Eliyahu, S. A new approach to reducing postsurgical cancer recurrence: Perioperative targeting of catecholamines and prostaglandins. Clin. Cancer Res. 2012, 18, 4895–4902. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Hyoju, S.K.; Weigerinck, M.; Gilbert, J.A. The gut microbiome and the mechanism of surgical infection. Br. J. Surg. 2017, 104, e14–e23. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Gessler, B.; Eriksson, O.; Angenete, E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int. J. Colorectal Dis. 2017, 32, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.; Gronnier, C.; Duhamel, A.; Mabrut, J.Y.; Bail, J.P.; Carrere, N.; Lefevre, J.H.; Brigand, C.; Vaillant, J.C.; Adham, M.; et al. The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann. Surg. 2015, 262, 972–980. [Google Scholar] [CrossRef]
- Zandstra, D.F.; Van Saene, H.K. Selective decontamination of the digestive tract as infection prevention in the critically ill. A level 1 evidence-based strategy. Minerva Anestesiol. 2011, 77, 212–219. [Google Scholar]
- Farran, L.; Llop, J.; Sans, M.; Kreisler, E.; Miro, M.; Galan, M.; Rafecas, A. Efficacy of enteral decontamination in the prevention of anastomotic dehiscence and pulmonary infection in esophagogastric surgery. Dis. Esophagus 2008, 21, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.; Dijksman, L.M.; Sondermeijer, B.M.; Oudemans-van Straaten, H.M.; De Wit, L.T.; Gerhards, M.F. Perioperative selective decontamination of the digestive tract (SDD) in elective colorectal surgery. J. Gastrointest. Surg. 2009, 13, 1839–1844. [Google Scholar] [CrossRef]
- Roos, D.; Dijksman, L.M.; Oudemans-van Straaten, H.M.; de Wit, L.T.; Gouma, D.J.; Gerhards, M.F. Randomized clinical trial of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Br. J. Surg. 2011, 98, 1365–1372. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grewal, S.; Oosterling, S.J.; van Egmond, M. Surgery for Colorectal Cancer: A Trigger for Liver Metastases Development? New Insights into the Underlying Mechanisms. Biomedicines 2021, 9, 177. https://doi.org/10.3390/biomedicines9020177
Grewal S, Oosterling SJ, van Egmond M. Surgery for Colorectal Cancer: A Trigger for Liver Metastases Development? New Insights into the Underlying Mechanisms. Biomedicines. 2021; 9(2):177. https://doi.org/10.3390/biomedicines9020177
Chicago/Turabian StyleGrewal, Simran, Steven J. Oosterling, and Marjolein van Egmond. 2021. "Surgery for Colorectal Cancer: A Trigger for Liver Metastases Development? New Insights into the Underlying Mechanisms" Biomedicines 9, no. 2: 177. https://doi.org/10.3390/biomedicines9020177
APA StyleGrewal, S., Oosterling, S. J., & van Egmond, M. (2021). Surgery for Colorectal Cancer: A Trigger for Liver Metastases Development? New Insights into the Underlying Mechanisms. Biomedicines, 9(2), 177. https://doi.org/10.3390/biomedicines9020177




