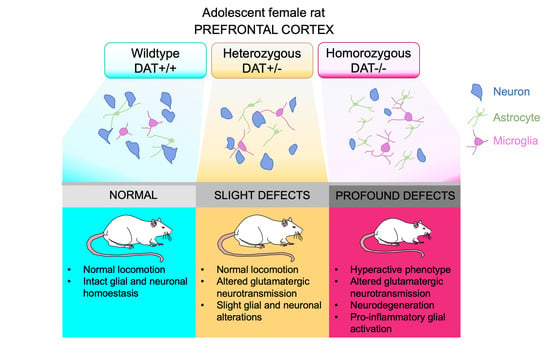
Early Adolescence Prefrontal Cortex Alterations in Female Rats Lacking Dopamine Transporter
Abstract
1. Introduction
2. Methods
2.1. Animals
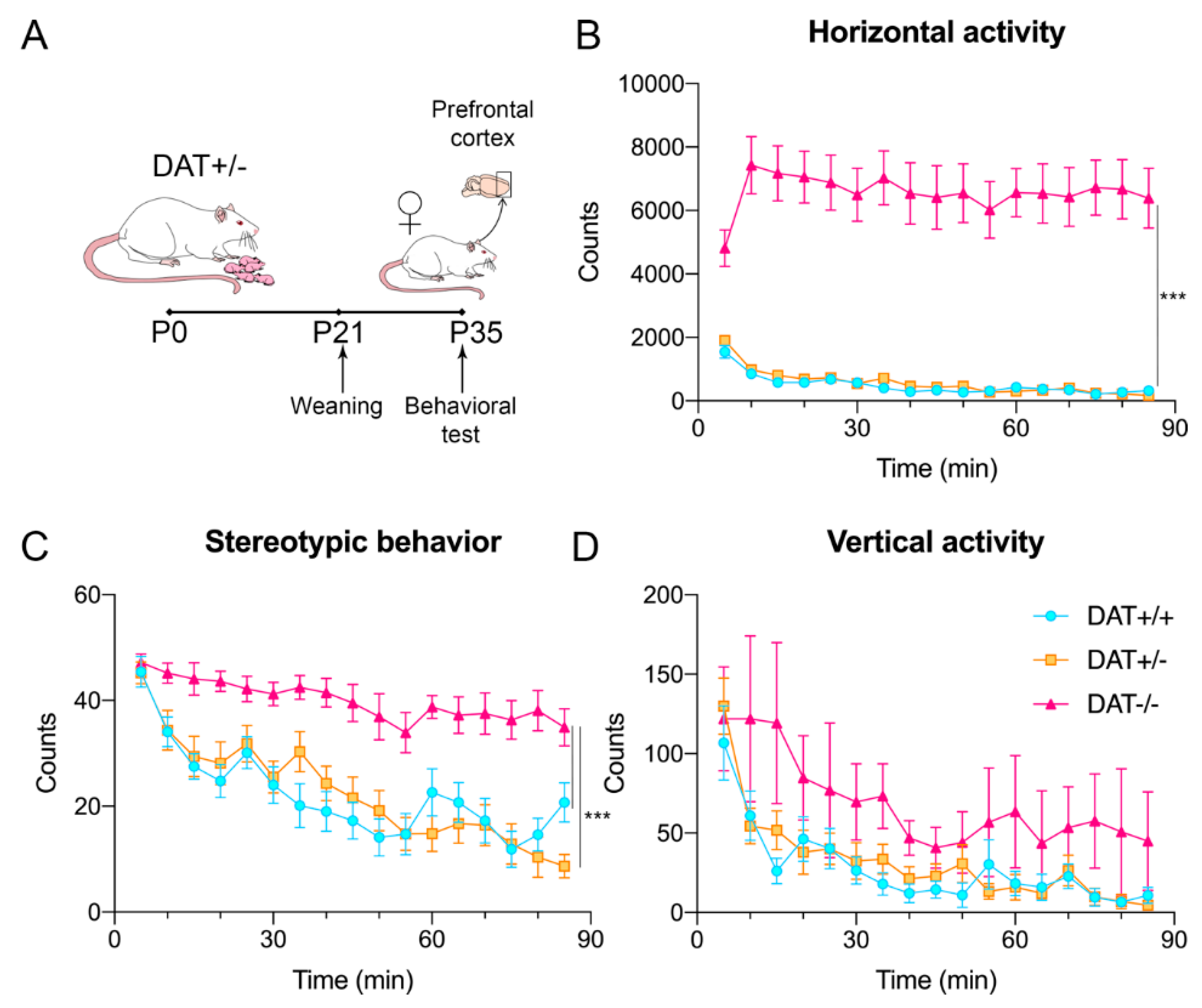
2.2. Locomotor Activity
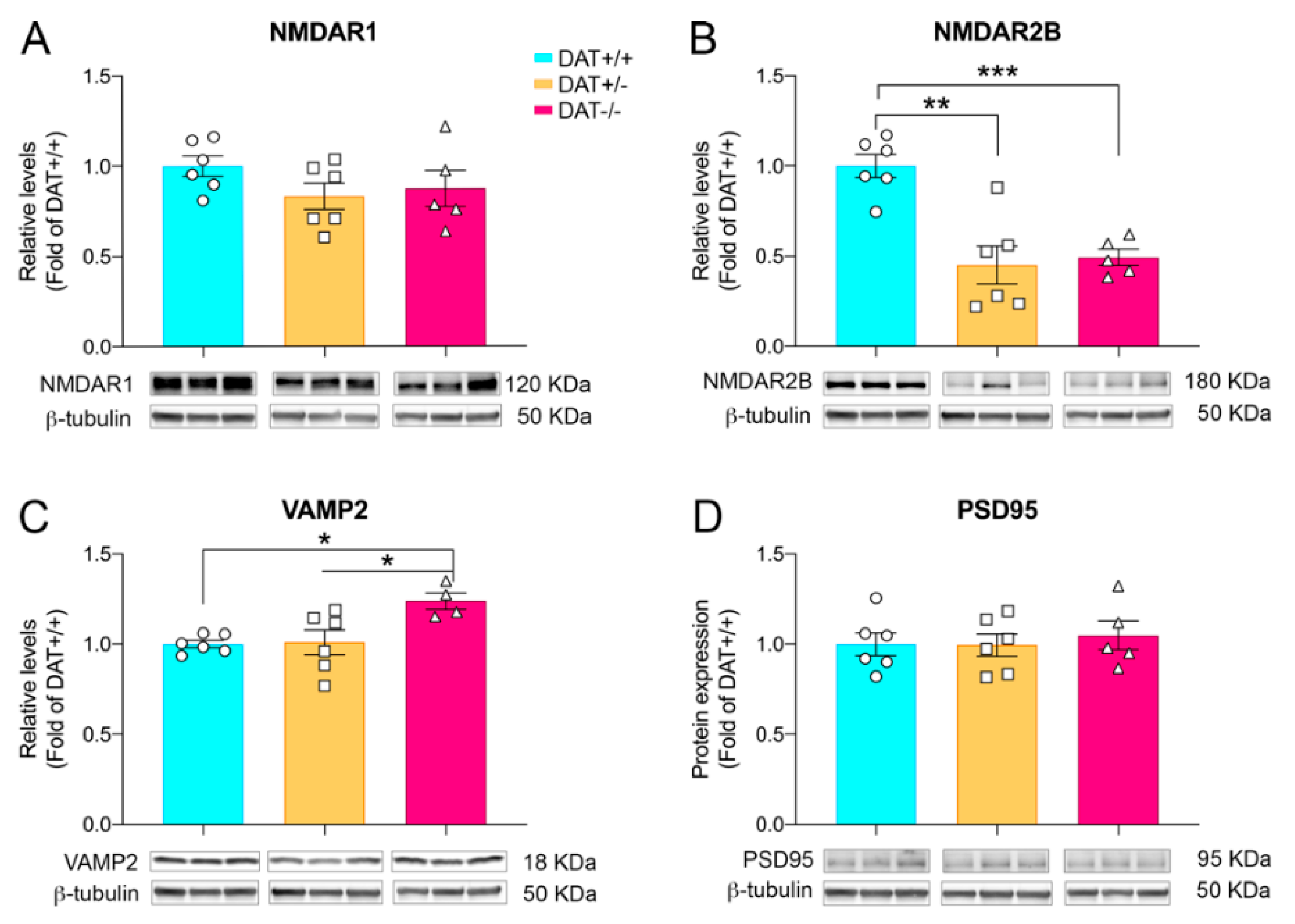
2.3. Western Blotting
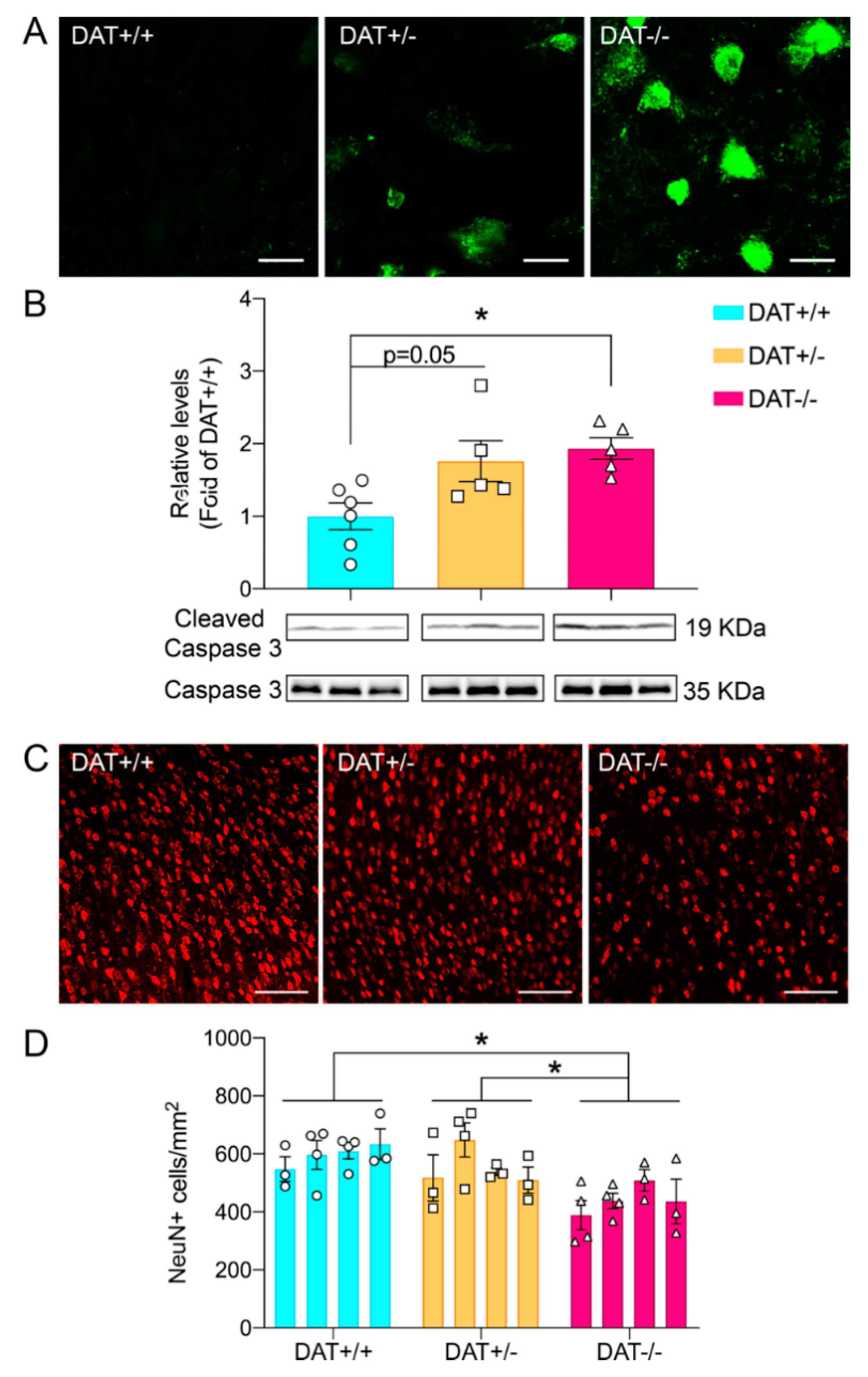
2.4. Tissue Preparation for Histological Assessments
2.5. Image Analyses
2.6. ELISA
2.7. Statistical Analysis
3. Results
3.1. Locomotor Activity of Female Adolescent DAT+/− and DAT−/− Rats
3.2. Glutamatergic and Synaptic Alterations in Female Adolescent DAT+/− and DAT−/− Rats
3.3. DAT−/− Rats Display Neuronal Cell Death in the Prefrontal Cortex
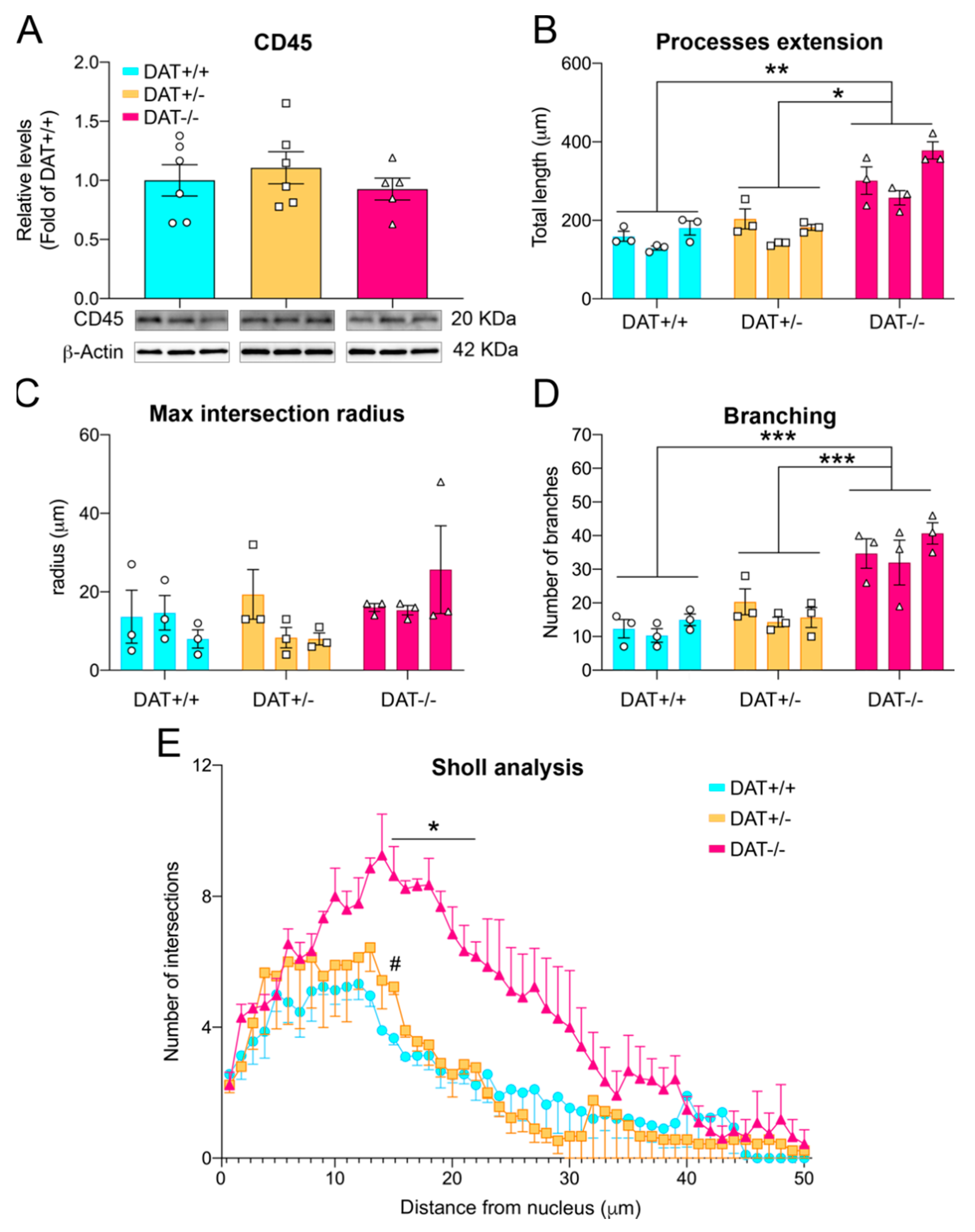
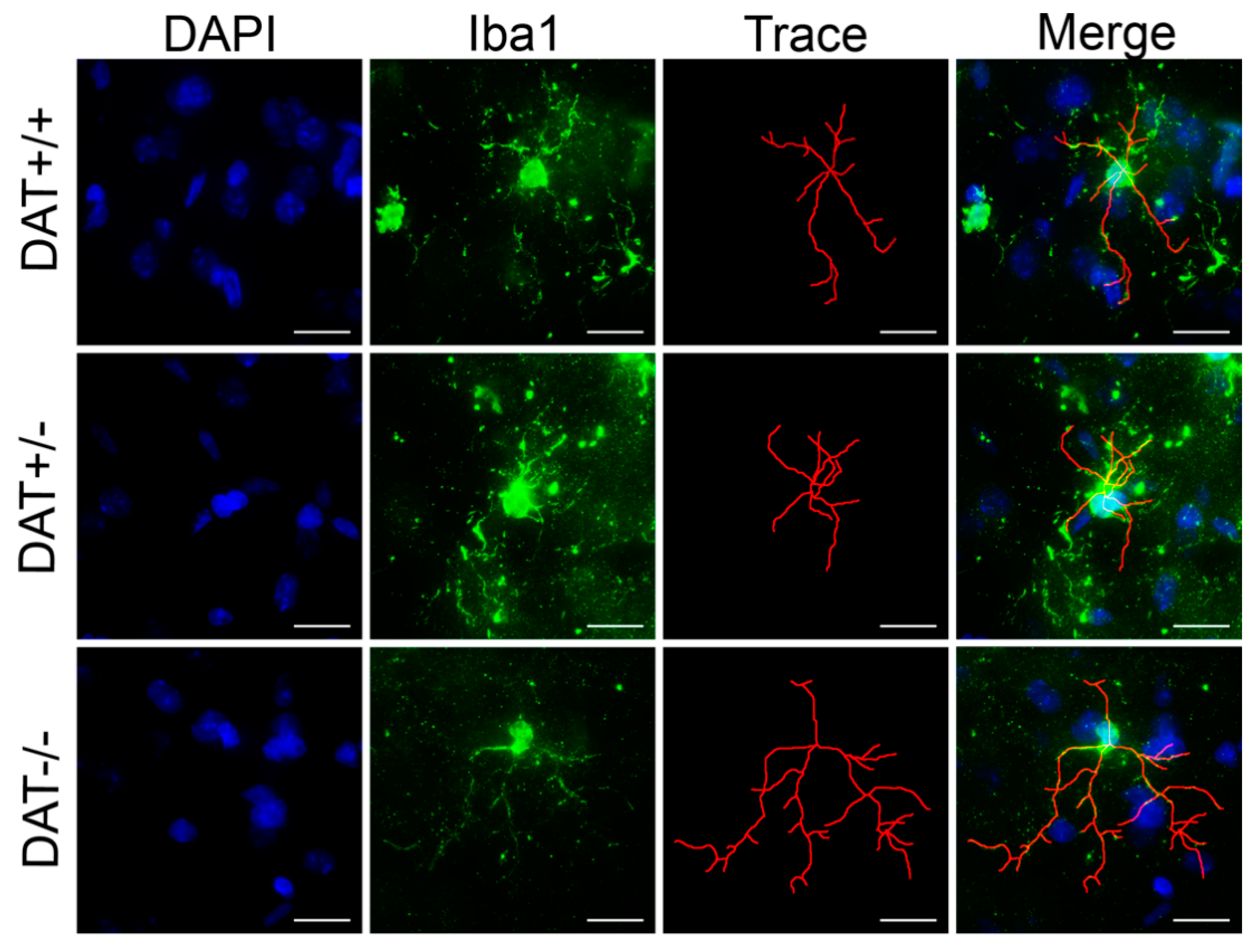
3.4. Pro-Inflammatory Phenotype in the Prefrontal Cortex of DAT−/− Rats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Segman, R.H.; Cooper Kazaz, R.; Macciardu, F.; Goltser, T.; Halfom, Y.; Dobroborski, T.; Shalev, A.Y. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol. Psychiatry 2002, 7, 903–907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drury, S.S.; Brett, Z.H.; Henry, C.; Scheeringa, M. The association of a novel haplotype in the dopamine transporter with preschool age posttraumatic stress disorder. J. Child. Adolesc. Psychopharmacol. 2013, 23, 236–243. [Google Scholar] [CrossRef]
- Lai, T.K.Y.; Su, P.; Zhang, H.; Liu, F. Development of a peptide targeting dopamine transporter to improve ADHD-like deficits. Mol. Brain 2018, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Babinski, D.E.; Pelham, W.E., Jr.; Molina, B.S.G.; Gnagy, E.M.; Waschbusch, D.A.; Yu, J.; Maclean, M.G.; Wymbs, B.T.; Sibley, M.H.; Biswas, A.; et al. Late adolescent and young adult outcomes of girls diagnosed with ADHD in childhood: An exploratory investigation. J. Atten. Disord. 2011, 15, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Javidi, H.; Yadollahie, M. Post-traumatic Stress Disorder. Int. J. Occup. Environ. Med. 2012, 3, 2–9. [Google Scholar]
- Kimerling, R.; Allen, M.C.; Duncan, L.E. Chromosomes to Social Contexts: Sex and Gender Differences in PTSD. Curr. Psychiatry Rep. 2018, 20, 114. [Google Scholar] [CrossRef]
- Bayless, D.W.; Perez, M.C.; Daniel, J.M. Comparison of the validity of the use of the spontaneously hypertensive rat as a model of attention deficit hyperactivity disorder in males and females. Behav. Brain Res. 2015, 286, 85–92. [Google Scholar] [CrossRef]
- Nunes, F.; Pochmann, D.; Almeida, A.S.; Marques, D.M.; Porciuncula, L.O. Differential Behavioral and Biochemical Responses to Caffeine in Male and Female Rats from a Validated Model of Attention Deficit and Hyperactivity Disorder. Mol. Neurobiol. 2018, 55, 8486–8498. [Google Scholar] [CrossRef]
- Illiano, P.; Bigford, G.E.; Gainetdinov, R.R.; Pardo, M. Rats Lacking Dopamine Transporter Display Increased Vulnerability and Aberrant Autonomic Response to Acute Stress. Biomolecules 2020, 10, 842. [Google Scholar] [CrossRef]
- Adinolfi, A.; Zelli, S.; Leo, D.; Carbone, C.; Mus, L.; Illiano, P.; Alleva, E.; Gainetdinov, R.R.; Adriani, W. Behavioral characterization of DAT-KO rats and evidence of asocial-like phenotypes in DAT-HET rats: The potential involvement of norepinephrine system. Behav. Brain Res. 2019, 359, 516–527. [Google Scholar] [CrossRef]
- Cinque, S.; Zoratto, F.; Poleggi, A.; Leo, D.; Cerniglia, L.; Cimino, S.; Tambelli, R.; Alleva, E.; Gainetdinov, R.R.; Laviola, G.; et al. Behavioral Phenotyping of Dopamine Transporter Knockout Rats: Compulsive Traits, Motor Stereotypies, and Anhedonia. Front. Psychiatry 2018, 9, 43. [Google Scholar] [CrossRef]
- Adinolfi, A.; Carbone, C.; Leo, D.; Gainetdinov, R.R.; Laviola, G.; Adriani, W. Novelty-related behavior of young and adult dopamine transporter knockout rats: Implication for cognitive and emotional phenotypic patterns. Genes Brain Behav. 2018, 17, e12463. [Google Scholar] [CrossRef]
- Petrelli, F.; Dallérac, D.; Pucci, L.; Calì, C.; Zehnder, T.; Sultan, S.; Lecca, S.; Chicca, A.; Ivanov, A.; Asensio, C.S.; et al. Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Mol. Psychiatry 2020, 25, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: A rational bridge between genetics and the symptoms of mental illness. Cereb. Cortex 2007, 17 (Suppl. 1), i6–i15. [Google Scholar] [CrossRef] [PubMed]
- Sibley, M.H.; Ortiz, M.; Graziano, P.; Dick, A.; Estrada, E. Metacognitive and motivation deficits, exposure to trauma, and high parental demands characterize adolescents with late-onset ADHD. Eur. Child. Adolesc. Psychiatry 2020, 29, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Leggio, G.M.; Drago, F.; Salomone, S. Therapeutic Challenges of Post-traumatic Stress Disorder: Focus on the Dopaminergic System. Front. Pharmacol. 2019, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Engert, V.; Pruessner, J.C. Dopaminergic and noradrenergic contributions to functionality in ADHD: The role of methylphenidate. Curr. Neuropharmacol. 2008, 6, 322–328. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef]
- Peteri, U.K.; Niukkanen, M.; Castren, M.L. Astrocytes in Neuropathologies Affecting the Frontal Cortex. Front. Cell Neurosci. 2019, 13, 44. [Google Scholar] [CrossRef]
- Van Zundert, B.; Yoshii, A.; Constantine-Paton, M. Receptor compartmentalization and trafficking at glutamate synapses: A developmental proposal. Trends Neurosci. 2004, 27, 428–437. [Google Scholar] [CrossRef]
- Willing, J.; Juraska, J.M. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience 2015, 301, 268–275. [Google Scholar] [CrossRef]
- Drzewiecki, C.M.; Willing, J.; Juraska, J.M. Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse 2016, 70, 361–368. [Google Scholar] [CrossRef]
- Carlsson, M.; Carlsson, A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990, 13, 272–276. [Google Scholar] [CrossRef]
- Svensson, A.; Carlsson, M.L.; Carlsson, A. Interaction between glutamatergic and dopaminergic tone in the nucleus accumbens of mice: Evidence for a dual glutamatergic function with respect to psychomotor control. J. Neural. Transm. Gen. Sect. 1992, 88, 235–240. [Google Scholar] [CrossRef]
- Bellini, S.; Fleming, K.E.; De, M.; McCauley, J.P.; Petroccione, M.A.; D’Brant, L.Y.; Tkachenko, A.; Kwon, S.; Jones, L.A.; Scimemi, A.; et al. Neuronal Glutamate Transporters Control Dopaminergic Signaling and Compulsive Behaviors. J. Neurosci. 2018, 38, 937–961. [Google Scholar] [CrossRef]
- Li, C.T.; Yang, K.C.; Lin, W.C. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence From Clinical Neuroimaging Studies. Front. Psychiatry 2018, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Anderson, R.L.; Billingslea, E.N.; Carlson, G.C.; Roberts, T.P.; Siegel, S.J. Mice with reduced NMDA receptor expression: More consistent with autism than schizophrenia? Genes Brain Behav. 2012, 11, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.; Lane, H.Y.; Tsai, G.E. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr. Pharm. Des. 2014, 20, 5180–5185. [Google Scholar] [CrossRef]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Mariano, S.; Pardo, M.; Bucchetri, C.; Illiano, P.; Adinolfi, A.; Russo, S.L.M.L.; Alleva, E.; Carbone, C.; Adriani, W. Own or dam’s genotype? Classical colony breeding may bias spontaneous and stress-challenged activity in DAT-mutant rats. Dev. Psychobiol. 2020, 62, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates-The New Coronal Set; Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.; Martins, M.; Corretia, J.S.; Sardinha, V.M.; Guerra-Gomes, S.; Das Neves, S.P.; Marques, F.; Sousa, N.; Oliveira, J.F. Employing an open-source tool to assess astrocyte tridimensional structure. Brain Struct. Funct. 2017, 222, 1989–1999. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjöström, P.J.; Van Meyel, D.J. Neuronal morphometry directly from bitmap images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, A.; Shi, M.Y.; Yan, Z. Disrupted Glutamatergic Transmission in Prefrontal Cortex Contributes to Behavioral Abnormality in an Animal Model of ADHD. Neuropsychopharmacology 2017, 42, 2096–2104. [Google Scholar] [CrossRef]
- Cyr, M.; Beaulieu, J.; Laakso, A.; Sotnikova, T.D.; Yao, W.-D.; Bohn, L.M.; Gainetdinov, R.R.; Calron, M.G. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc. Natl. Acad. Sci. USA 2003, 100, 11035–11040. [Google Scholar] [CrossRef] [PubMed]
- Ehara, A.; Ueda, S. Application of Fluoro-Jade C in acute and chronic neurodegeneration models: Utilities and staining differences. Acta Histochem. Cytochem. 2009, 42, 171–179. [Google Scholar] [CrossRef]
- Jarskog, L.F.; Gilmoret, J.H.; Glantz, L.A.; Gable, K.L.; German, T.T.; Tong, R.I.; Lieberman, J.A. Caspase-3 activation in rat frontal cortex following treatment with typical and atypical antipsychotics. Neuropsychopharmacology 2007, 32, 95–102. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Bijlard, M.; Klundetr, B.; De Jonge, J.C.; Nomden, A.; Tyagi, S.; De Vries, H.; Hoekstra, D.; Baron, W. Transcriptional expression of myelin basic protein in oligodendrocytes depends on functional syntaxin 4: A potential correlation with autocrine signaling. Mol. Cell Biol. 2015, 35, 675–687. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zheng, Y.; Luo, Y.; Du, Y.; Zhao, Y.; Guan, J.; Zhang, X.; Fu, J. TREM-2-p38 MAPK signaling regulates neuroinflammation during chronic cerebral hypoperfusion combined with diabetes mellitus. J. Neuroinflamm. 2020, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Barcia, C.; Ros, C.M.; Annese, V.; Gomez, A.; Ros-Bernal, F.; Aguado-Llera, D.; Martinez-Pagan, M.E.; De Pablos, V.; Fernandez-Villalba, E.; Herrero, M.T. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2012, 3, e379. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Mohammad, D.; Trepanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Jones, S.R.; Caron, M.G. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry 1999, 46, 303–311. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Wetsel, W.C.; Jones, S.R.; Levin, E.D.; Jaber, M.; Caron, M.G. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 1999, 283, 397–401. [Google Scholar] [CrossRef]
- Illiano, P.; Bass, C.E.; Fichera, L.; Mus, L.; Budygin, E.A.; Sotnikova, T.D.; Leo, D.; Espinoza, S.; Gainetdinov, R.R. Recombinant Adeno-Associated Virus-mediated rescue of function in a mouse model of Dopamine Transporter Deficiency Syndrome. Sci. Rep. 2017, 7, 46280. [Google Scholar] [CrossRef] [PubMed]
- Mereu, M.; Contarini, G.; Buonaguro, E.F.; Latte, G.; Managò, F.; Iasevoli, F.; De Bartolomeis, A.; Papaleo, F. Dopamine transporter (DAT) genetic hypofunction in mice produces alterations consistent with ADHD but not schizophrenia or bipolar disorder. Neuropharmacology 2017, 121, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, T.D.; Efimova, E.V.; Gainetdinov, R.R. Enhanced Dopamine Transmission and Hyperactivity in the Dopamine Transporter Heterozygous Mice Lacking the D3 Dopamine Receptor. Int. J. Mol. Sci. 2020, 21, 8216. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Chen, A.; Li, Y.; Xing, X.; Lu, H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019, 51, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Mohn, A.R.; Bohn, L.M.; Caron, M.G. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc. Natl. Acad. Sci. USA 2001, 98, 11047–11054. [Google Scholar] [CrossRef]
- Hansen, F.H.; Skjørringe, T.; Yasmeen, S.; Arends, N.V.; Sahai, M.A.; Erreger, K.; Andreassen, T.F.; Holy, M.; Hamilton, P.J.; Neergheen, V.; et al. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J. Clin. Investig. 2014, 124, 3107–3120. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, T.A.; Schork, N.J.; Eskin, E.; Kelsoe, J.R. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol. Psychiatry 2006, 11, 125–133, 115. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.A.; Li, Y.; Zhen, J.; Meyer, E.; Hai, N.; Christen, H.J.; Hoffmann, G.F.; Jardine, P.; von Moers, A.; Mordekar, S.R.; et al. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: An observational cohort and experimental study. Lancet Neurol. 2011, 10, 54–62. [Google Scholar] [CrossRef]
- Ng, J.; Zhen, J.; Meyer, E.; Erreger, K.; Li, Y.; Kakar, N.; Ahmad, J.; Thiele, H.; Kubisch, C.; Rider, N.L.; et al. Dopamine transporter deficiency syndrome: Phenotypic spectrum from infancy to adulthood. Brain 2014, 137, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Blot, K.; Tzavara, E.; Nosten-Bertrand, M.; Giros, B.; Otalni, S. Inhibition of dopamine transporter activity impairs synaptic depression in rat prefrontal cortex through over-stimulation of D1 receptors. Cereb Cortex 2014, 24, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Ann. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Sagheddu, C.; Pintori, N.; Kalaba, P.; Dragačetvić, V.; Piras, G.; Aradska, J.; Simola, N.; De Luca, M.A.; Lubec, G.; Pistis, M. Neurophysiological and Neurochemical Effects of the Putative Cognitive Enhancer (S)-CE-123 on Mesocorticolimbic Dopamine System. Biomolecules 2020, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Lohani, S.; Martig, A.K.; Deisseroth, K.; Witten, I.B.; Moghaddam, B. Dopamine Modulation of Prefrontal Cortex Activity Is Manifold and Operates at Multiple Temporal and Spatial Scales. Cell Rep. 2019, 27, 99–114. [Google Scholar] [CrossRef]
- Sanna, F.; Bratzu, J.; Serra, M.P.; Leo, D.; Quartu, M.; Boi, M.; Espinoza, S.; Gainetdinov, R.R.; Melis, M.R.; Argiolas, A. Altered Sexual Behavior in Dopamine Transporter (DAT) Knockout Male Rats: A Behavioral, Neurochemical and Intracerebral Microdialysis Study. Front. Behav. Neurosci. 2020, 14, 58. [Google Scholar] [CrossRef]
- Vidal, P.M.; Pacheco, R. The Cross-Talk between the Dopaminergic and the Immune System Involved in Schizophrenia. Front. Pharmacol. 2020, 11, 394. [Google Scholar] [CrossRef]
- Montoya, A.; Elgueta, D.; Campos, J.; Chovar, O.; Falcón, P.; Matus, S.; Alfaro, I.; Bono, M.R.; Pacheco, R. Dopamine receptor D3 signalling in astrocytes promotes neuroinflammation. J. Neuroinflamm. 2019, 16, 258. [Google Scholar] [CrossRef]
- Xia, Q.P.; Cheng, Z.Y.; He, L. The modulatory role of dopamine receptors in brain neuroinflammation. Int. Immunopharmacol. 2019, 76, 105908. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.J.; Redish, A.D. Complex neural codes in rat prelimbic cortex are stable across days on a spatial decision task. Front. Behav. Neurosci. 2014, 8, 120. [Google Scholar] [CrossRef]
- Juarez, B.; Han, M.H. Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure. Neuropsychopharmacology 2016, 41, 2424–2446. [Google Scholar] [CrossRef]
- Petralia, R.S. Distribution of extrasynaptic NMDA receptors on neurons. Sci. World J. 2012, 267120. [Google Scholar] [CrossRef]
- Monaco, S.A.; Gulchina, Y.; Gao, W.J. NR2B subunit in the prefrontal cortex: A double-edged sword for working memory function and psychiatric disorders. Neurosci. Biobehav. Rev. 2015, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.L.; Wei, L.C.; Shi, M.; Wang, Y.Q.; Cao, R.; Chen, L.W. Fluoro-Jade C can specifically stain the degenerative neurons in the substantia nigra of the 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine-treated C57BL/6 mice. Brain Res. 2007, 1150, 55–61. [Google Scholar] [CrossRef]
- Falcicchia, C.; Tozzi, F.; Arancio, O.; Watterson, D.M.; Origlia, N. Involvement of p38 MAPK in Synaptic Function and Dysfunction. Int. J. Mol. Sci. 2020, 21, 5624. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Takagi, R.; Saika, K.; Matsui, M.; Matsushita, S. Dopamine regulates cytokine secretion during innate and adaptive immune responses. Int. Immunol. 2018, 30, 591–606. [Google Scholar] [CrossRef]
- Miller, A.H. Beyond depression: The expanding role of inflammation in psychiatric disorders. World Psychiatry 2020, 19, 108–109. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Graeber, M.B. Changing face of microglia. Science 2010, 330, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Benusa, S.D.; George, N.M.; Sword, B.A.; DeVries, G.H.; Dupree, J.L. Acute neuroinflammation induces AIS structural plasticity in a NOX2-dependent manner. J. Neuroinflamm. 2017, 14, 116. [Google Scholar] [CrossRef]
- World Health Organization. Women’s Mental Health: An Evidence Based Review. 2000. Available online: https://www.who.int/mental_health/publications/women_mh_evidence_review/en/ (accessed on 15 November 2020).
- Bolea-Alamanac, B.; Bailey, S.J.; Lovick, T.A.; Scheele, D.; Valentino, R. Female psychopharmacology matters! Towards a sex-specific psychopharmacology. J. Psychopharmacol. 2018, 32, 125–133. [Google Scholar] [CrossRef]
- Almeida Montes, L.G.; Hernandez Garcia, A.O.; Ricardo-Garcell, J. ADHD prevalence in adult outpatients with nonpsychotic psychiatric illnesses. J. Atten. Disord. 2007, 11, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tolin, D.F.; Foa, E.B. Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychol. Bull. 2006, 132, 959–992. [Google Scholar] [CrossRef]
- Choi, N.G.; DiNitto, D.M.; Marti, C.N.; Choi, B.Y. Association of adverse childhood experiences with lifetime mental and substance use disorders among men and women aged 50+ years. Int. Psychogeriatr. 2017, 29, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illiano, P.; Leo, D.; Gainetdinov, R.R.; Pardo, M. Early Adolescence Prefrontal Cortex Alterations in Female Rats Lacking Dopamine Transporter. Biomedicines 2021, 9, 157. https://doi.org/10.3390/biomedicines9020157
Illiano P, Leo D, Gainetdinov RR, Pardo M. Early Adolescence Prefrontal Cortex Alterations in Female Rats Lacking Dopamine Transporter. Biomedicines. 2021; 9(2):157. https://doi.org/10.3390/biomedicines9020157
Chicago/Turabian StyleIlliano, Placido, Damiana Leo, Raul R. Gainetdinov, and Marta Pardo. 2021. "Early Adolescence Prefrontal Cortex Alterations in Female Rats Lacking Dopamine Transporter" Biomedicines 9, no. 2: 157. https://doi.org/10.3390/biomedicines9020157
APA StyleIlliano, P., Leo, D., Gainetdinov, R. R., & Pardo, M. (2021). Early Adolescence Prefrontal Cortex Alterations in Female Rats Lacking Dopamine Transporter. Biomedicines, 9(2), 157. https://doi.org/10.3390/biomedicines9020157