Nitrosative Stress Molecules in Multiple Sclerosis: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Data Analysis
3. Results
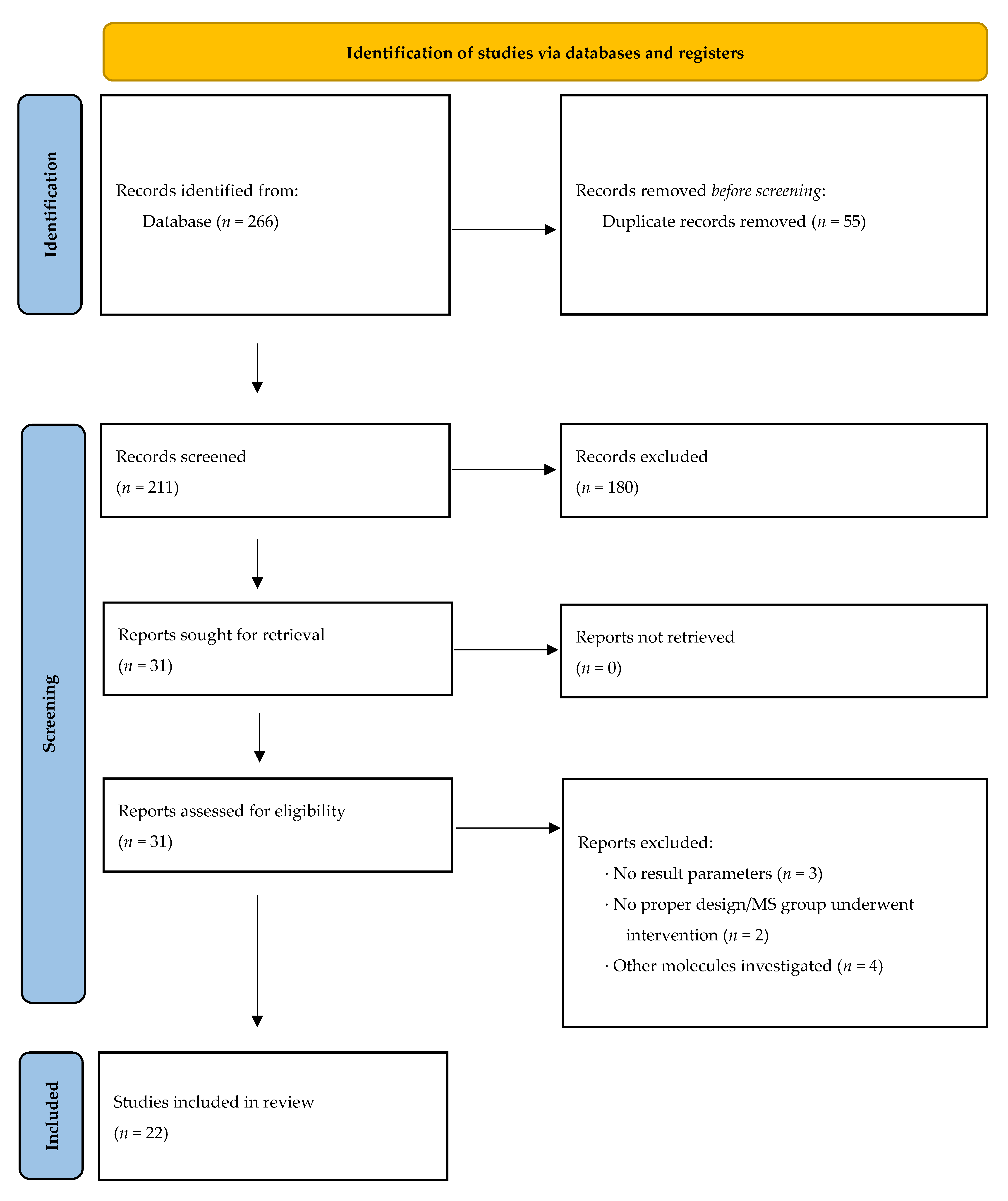
3.1. Inclusion of Studies
3.2. Study and Patient Characteristics
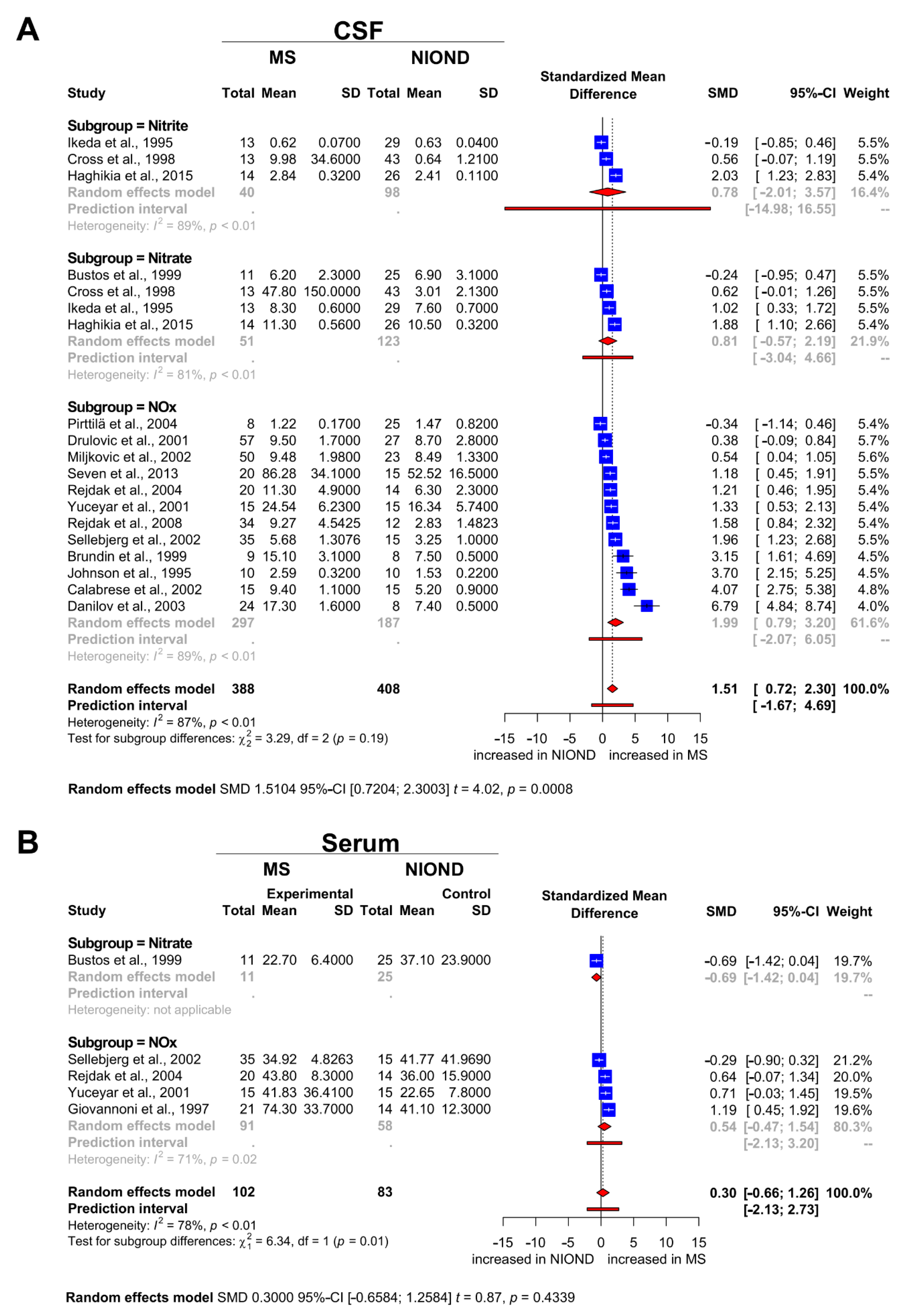
3.3. Nitrosative Stress Molecules in Patients with MS and NIOND
3.4. Nitrosative Stress Molecules in Patients with MS and HC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kremer, D.; Gruchot, J.; Weyers, V.; Oldemeier, L.; Gottle, P.; Healy, L.; Ho Jang, J.; Kang, T.X.Y.; Volsko, C.; Dutta, R.; et al. pHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 15216–15225. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Bar-Or, A.; Li, R. Cellular immunology of relapsing multiple sclerosis: Interactions, checks, and balances. Lancet Neurol. 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Thiel, V.E.; Audus, K.L. Nitric oxide and blood-brain barrier integrity. Antioxid. Redox Signal. 2001, 3, 273–278. [Google Scholar] [CrossRef]
- Zhang, J.; Dawson, V.L.; Dawson, T.M.; Snyder, S.H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science 1994, 263, 687–689. [Google Scholar] [CrossRef]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Calcerrada, P.; Peluffo, G.; Radi, R. Nitric oxide-derived oxidants with a focus on peroxynitrite: Molecular targets, cellular responses and therapeutic implications. Curr. Pharm. Des. 2011, 17, 3905–3932. [Google Scholar] [CrossRef]
- Smith, K.J.; Lassmann, H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002, 1, 232–241. [Google Scholar] [CrossRef]
- Tang, X.; Lan, M.; Zhang, M.; Yao, Z. Effect of nitric oxide to axonal degeneration in multiple sclerosis via downregulating monocarboxylate transporter 1 in oligodendrocytes. Nitric Oxide 2017, 67, 75–80. [Google Scholar] [CrossRef]
- Bizzozero, O.A.; DeJesus, G.; Callahan, K.; Pastuszyn, A. Elevated protein carbonylation in the brain white matter and gray matter of patients with multiple sclerosis. J. Neurosci. Res. 2005, 81, 687–695. [Google Scholar] [CrossRef]
- Kremer, D.; Schichel, T.; Forster, M.; Tzekova, N.; Bernard, C.; van der Valk, P.; van Horssen, J.; Hartung, H.P.; Perron, H.; Kury, P. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann. Neurol. 2013, 74, 721–732. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Han, J.; Hu, W. Serum levels of Homocysteine, Vitamin B12 and Folate in Patients with Multiple Sclerosis: An Updated Meta-Analysis. Int. J. Med. Sci. 2020, 17, 751–761. [Google Scholar] [CrossRef]
- Trotter, A.; Anstadt, E.; Clark, R.B.; Nichols, F.; Dwivedi, A.; Aung, K.; Cervantes, J.L. The role of phospholipase A2 in multiple Sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2019, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Gui, L.N.; Liu, Y.Y.; Shi, S.; Cheng, Y. Oxidative Stress Marker Aberrations in Multiple Sclerosis: A Meta-Analysis Study. Front. Neurosci. 2020, 14, 823. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sanchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Cross, A.H.; Manning, P.T.; Keeling, R.M.; Schmidt, R.E.; Misko, T.P. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J. Neuroimmunol. 1998, 88, 45–56. [Google Scholar] [CrossRef]
- Haghikia, A.; Kayacelebi, A.A.; Beckmann, B.; Hanff, E.; Gold, R.; Haghikia, A.; Tsikas, D. Serum and cerebrospinal fluid concentrations of homoarginine, arginine, asymmetric and symmetric dimethylarginine, nitrite and nitrate in patients with multiple sclerosis and neuromyelitis optica. Amino Acids 2015, 47, 1837–1845. [Google Scholar] [CrossRef]
- Ikeda, M.; Sato, I.; Matsunaga, T.; Takahashi, M.; Yuasa, T.; Murota, S. Cyclic guanosine monophosphate (cGMP), nitrite and nitrate in the cerebrospinal fluid in meningitis, multiple sclerosis and Guillain-Barre syndrome. Intern. Med. 1995, 34, 734–737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Bustos, F.; Navarro, J.A.; de Andres, C.; Molina, J.A.; Jimenez-Jimenez, F.J.; Orti-Pareja, M.; Gasalla, T.; Tallon-Barranco, A.; Martinez-Salio, A.; Arenas, J. Cerebrospinal fluid nitrate levels in patients with multiple sclerosis. Eur. Neurol. 1999, 41, 44–47. [Google Scholar] [CrossRef]
- Seven, A.; Aslan, M.; Incir, S.; Altintas, A. Evaluation of oxidative and nitrosative stress in relapsing remitting multiple sclerosis: Effect of corticosteroid therapy. Folia Neuropathol. 2013, 51, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Drulovic, J.; Dujmovic, I.; Mesaros, S.; Samardzic, T.; Maksimovic, D.; Stojsavljevic, N.; Levic, Z.; Mostarica Stojokvic, M. Raised cerebrospinal fluid nitrite and nitrate levels in patients with multiple sclerosis: No correlation with disease activity. Mult. Scler. 2001, 7, 19–22. [Google Scholar] [CrossRef]
- Brundin, L.; Morcos, E.; Olsson, T.; Wiklund, N.P.; Andersson, M. Increased intrathecal nitric oxide formation in multiple sclerosis; cerebrospinal fluid nitrite as activity marker. Eur. J. Neurol. 1999, 6, 585–590. [Google Scholar] [CrossRef]
- Johnson, A.W.; Land, J.M.; Thompson, E.J.; Bolanos, J.P.; Clark, J.B.; Heales, S.J. Evidence for increased nitric oxide production in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1995, 58, 107. [Google Scholar] [CrossRef] [PubMed]
- Yuceyar, N.; Taskiran, D.; Sagduyu, A. Serum and cerebrospinal fluid nitrite and nitrate levels in relapsing-remitting and secondary progressive multiple sclerosis patients. Clin. Neurol. Neurosurg. 2001, 103, 206–211. [Google Scholar] [CrossRef]
- Rejdak, K.; Eikelenboom, M.J.; Petzold, A.; Thompson, E.J.; Stelmasiak, Z.; Lazeron, R.H.; Barkhof, F.; Polman, C.H.; Uitdehaag, B.M.; Giovannoni, G. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology 2004, 63, 1439–1445. [Google Scholar] [CrossRef]
- Rejdak, K.; Petzold, A.; Stelmasiak, Z.; Giovannoni, G. Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult. Scler. 2008, 14, 59–66. [Google Scholar] [CrossRef]
- Pirttila, T.; Vanhatalo, S.; Turpeinen, U.; Riikonen, R. Cerebrospinal fluid insulin-like growth factor-1, insulin growth factor binding protein-2 or nitric oxide are not increased in MS or ALS. Acta Neurol. Scand. 2004, 109, 337–341. [Google Scholar] [CrossRef]
- Danilov, A.I.; Andersson, M.; Bavand, N.; Wiklund, N.P.; Olsson, T.; Brundin, L. Nitric oxide metabolite determinations reveal continuous inflammation in multiple sclerosis. J. Neuroimmunol. 2003, 136, 112–118. [Google Scholar] [CrossRef]
- Miljkovic, D.; Drulovic, J.; Trajkovic, V.; Mesaros, S.; Dujmovic, I.; Maksimovic, D.; Samardzic, T.; Stojsavljevic, N.; Levic, Z.; Mostarica Stojkovic, M. Nitric oxide metabolites and interleukin-6 in cerebrospinal fluid from multiple sclerosis patients. Eur. J. Neurol. 2002, 9, 413–418. [Google Scholar] [CrossRef]
- Giovannoni, G.; Heales, S.J.; Silver, N.C.; O’Riordan, J.; Miller, R.F.; Land, J.M.; Clark, J.B.; Thompson, E.J. Raised serum nitrate and nitrite levels in patients with multiple sclerosis. J. Neurol. Sci. 1997, 145, 77–81. [Google Scholar] [CrossRef]
- Calabrese, V.; Scapagnini, G.; Ravagna, A.; Bella, R.; Foresti, R.; Bates, T.E.; Giuffrida Stella, A.M.; Pennisi, G. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J. Neurosci. Res. 2002, 70, 580–587. [Google Scholar] [CrossRef]
- Sellebjerg, F.; Giovannoni, G.; Hand, A.; Madsen, H.O.; Jensen, C.V.; Garred, P. Cerebrospinal fluid levels of nitric oxide metabolites predict response to methylprednisolone treatment in multiple sclerosis and optic neuritis. J. Neuroimmunol. 2002, 125, 198–203. [Google Scholar] [CrossRef]
- Svenningsson, A.; Petersson, A.S.; Andersen, O.; Hansson, G.K. Nitric oxide metabolites in CSF of patients with MS are related to clinical disease course. Neurology 1999, 53, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, K.; Petzold, A.; Kocki, T.; Kurzepa, J.; Grieb, P.; Turski, W.A.; Stelmasiak, Z. Astrocytic activation in relation to inflammatory markers during clinical exacerbation of relapsing-remitting multiple sclerosis. J. Neural Transm. 2007, 114, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Acar, G.; Idiman, F.; Idiman, E.; Kirkali, G.; Cakmakci, H.; Ozakbas, S. Nitric oxide as an activity marker in multiple sclerosis. J. Neurol. 2003, 250, 588–592. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Kallaur, A.P.; Reiche, E.M.V.; Kaimen-Maciel, D.R.; Panis, C.; Lozovoy, M.A.B.; Morimoto, H.K.; Maes, M.; Dichi, I.; Simao, A.N.C. Albumin and Protein Oxidation are Predictors that Differentiate Relapsing-Remitting from Progressive Clinical Forms of Multiple Sclerosis. Mol. Neurobiol. 2017, 54, 2961–2968. [Google Scholar] [CrossRef]
- Obradovic, D.; Andjelic, T.; Ninkovic, M.; Dejanovic, B.; Kotur-Stevuljevic, J. Superoxide dismutase (SOD), advanced oxidation protein products (AOPP), and disease-modifying treatment are related to better relapse recovery after corticosteroid treatment in multiple sclerosis. Neurol. Sci. 2020, 42, 3241–3247. [Google Scholar] [CrossRef]
- Giovannoni, G.; Heales, S.J.; Land, J.M.; Thompson, E.J. The potential role of nitric oxide in multiple sclerosis. Mult. Scler. 1998, 4, 212–216. [Google Scholar] [CrossRef] [PubMed]

| Study | Year | Specimen | MS Patients | NIOND Patients | Assay | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (m/f) | Mean Age in Yrs (±SD) | C Mean (Nitrite) (±SD) | C Mean (Nitrate) (±SD) | C Mean (NOx) (±SD) | N (m/f) | Mean Age in Yrs (±SD) | C Mean (Nitrite) (±SD) | C Mean (Nitrate) (±SD) | C Mean (NOx) (±SD) | ||||
| Cross et al. [19] | 1998 | CSF | 13 (n/a) | n/a | 9.98 (34.6) | 47.8 (150) | n/a | 43 (n/a) | n/a | 0.64 (1.21) | 3.01 (2.13) | n/a | Fluorometric assay (DAN) |
| Haghikia et al. [20] | 2015 | CSF | 14 (6/8) | 45 (9.6) | 2.84 (0.32) | 11.3 (0.56) | n/a | 26 (12/14) | 56.27 (15.8) | 2.41 (0.11) | 10.5 (0.32) | n/a | GC/MS |
| Ikeda et al. [21] | 1995 | CSF | 13 (n/a) | 34.6 (4.1) | 0.62 (0.07) | 8.3 (0.6) | n/a | 29 (n/a) | 54.0 (3.8) | 0.63 (0.04) | 7.6 (0.7) | n/a | Spectrophotometric assay (Griess) |
| de Bustos et al. [22] | 1999 | CSF | 11 (5/6) | 36.0 (11.3) | n/a | 6.2 (2.3) | n/a | 25 (10/15) | 35.4 (10.7) | n/a | 6.9 (3.1) | n/a | Semiautomated assay (Griess) |
| serum | n/a | 22.7 (6.4) | n/a | n/a | 37.1 (23.9) | n/a | |||||||
| Seven et al. [23] | 2013 | CSF | 20 (7/13) | 31.0 (9.6) | n/a | n/a | 86.28 (34.1) | 15 (6/9) | 28.33 (5.31) | n/a | n/a | 52.52 (16.5) | Fluorometric assay (Sulphanilamide) |
| Drulovic et al. [24] | 2001 | CSF | 57 (n/a) | n/a | n/a | n/a | 9.5 (1.7) | 27 (n/a) | n/a | n/a | n/a | 8.7 (2.8) | Spectrophotometric assay (Griess) |
| Brundin et al. [25] | 1999 | CSF | 9 (n/a) | 43.1 (15.0) | 9.3 (2.8) b | 9.5 (1.7) b | 15.1 (3.1) b | 8 (2/6) | 45.0 (17.0) | 2.3 (0.5) b | 5.2 (0.5) b | 7.5 (0.5) b | Capillary electrophoresis |
| Johnson et al. [26] | 1995 | CSF | 10 (n/a) | n/a | n/a | n/a | 2.59 (0.32) b | 10 (n/a) | n/a | n/a | n/a | 1.53 (0.22) b | Spectrophotometric assay (Griess) |
| Yuceyar et al. [27] | 2001 | CSF | 15 (2/13) | 29.93 (n/a) | 4.85 (3.35) | 19.64 (5.59) | 24.54 (6.23) | 15 (5/10) | 43.2 (19.7) | 2.61 (1.77) | 13.72 (5.17) | 16.34 (5.74) | Spectrophotometric assay (Griess) |
| serum | 5.84 (2.86) | 35.98 (35.04) | 41.83 (36.41) | 2.89 (3.31) | 19.75 (6.62) | 22.65 (7.8) | |||||||
| Rejdak et al. [28] | 2004 | CSF | 20 (n/a) | n/a | n/a | n/a | 11.3 (4.9) | 14 (6/8) | 45 (23–74) a | n/a | n/a | 6.3 (2.3) | Spectrophotometric assay (Griess) |
| serum | n/a | n/a | 43.8 (8.3) | n/a | n/a | 36.0 (15.9) | |||||||
| Rejdak et al. [29] | 2008 | CSF | 34 (9/25) | 31.0 (20–52) a | n/a | n/a | 8.5 (2.5–21.5) a | 12 (3/9) | 29 (22–50) a | n/a | n/a | 2.5 (0.9–7.1) a | Spectrophotometric assay (Griess) |
| Pirrtilä et al. [30] | 2004 | CSF | 8 (1/7) | 28.9 (8.9) | n/a | n/a | 1.22 (0.17) | 25 (7/18) | 47.5 (12.9) | n/a | n/a | 1.47 (0.82) | Spectrophotometric assay (Griess) |
| Danilov et al. [31] | 2003 | CSF | 24 (6/18) | 43.5 (19–60) a | 7.7 (1.1) b | 9.6 (0.7) b | 17.3 (1.6) b | 8 (2/6) | 44.7 (26–66) a | n/a | 5.4 (0.3) b | 7.4 (0.5) b | Capillary electrophoresis |
| Miljkovic et al. [32] | 2002 | CSF | 50 (13/37) | 35.1 (10.6) | n/a | n/a | 9.48 (1.98) | 23 (n/a) | n/a | n/a | n/a | 8.49 (1.33) | Spectrophotometric assay (Griess) |
| Giovannoni et al. [33] | 1997 | serum | 21 (5/16) | 40.6 (10.7) | n/a | n/a | 74.3 (33.7) | 14 (7/7) | 47.8 (17.8) | n/a | n/a | 41.1 (12.3) | Spectrophotometric assay (Griess) |
| Calabrese et al. [34] | 2002 | CSF | 15 (3/12) | 31.0 (7.3) | n/a | n/a | 9.4 (1.1) b | 15 (2/13) | 32.4 (11.0) | n/a | n/a | 5.2 (0.9) b | Spectrophotometric assay (Griess) |
| Sellebjerg et al. [35] | 2002 | CSF | 35 (5/30) | 38 (32.0–43.0) c | n/a | n/a | 5.5 (3.6–9.1) c | 15 (4/11) | 45 (45.0–60.0) c | n/a | n/a | 3.3 (1.4–4.9) c | Spectrophotometric assay (Griess) |
| serum | n/a | n/a | 34.3 (27.0–47.3) c | n/a | n/a | 40.7 (36.5–51.1) c | |||||||
| Study | Year | Specimen | MS Patients | HC | Assay | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (m/f) | Mean Age in Yrs (±SD) | C Mean (Nitrite) (±SD) | C Mean (Nitrate) (±SD) | C Mean (NOx) (±SD) | N (m/f) | Mean Age in Yrs (±SD) | C Mean (Nitrite) (±SD) | C Mean (Nitrate) (±SD) | C Mean (NOx) (±SD) | ||||
| Svenningsson et al. [36] | 1999 | CSF | 12 (n/a) | n/a | 0.63 (0.06) b | 8.8 (1.1) b | n/a | 15 (n/a) | n/a | 0.38 (0.02) b | 7.5 (1.0) b | n/a | GC–MS |
| Rejdak et al. [37] | 2007 | CSF | 20 (6/14) | 28 (21–46) a | n/a | n/a | 9.1 (2.5–21.5) a | 10 (4/6) | 29 (20–40) a | n/a | n/a | 2.2 (0.9–7.1) a | Spectrophotometric assay (Griess) |
| Acar et al. [38] | 2003 | CSF | 24 (9/15) | 30.2 (8.3) | n/a | n/a | 11.16 (8.6) | 18 (8/10) | 32.0 (2.34) | n/a | n/a | 4.32 (1.63) | Spectrophotometric assay (Griess) |
| serum | n/a | n/a | 12.89 (7.62) | n/a | n/a | 7.42 (2.81) | |||||||
| Danilov et al. [31] | 2003 | CSF | 24 (6/18) | 43.5 (19–60) a | 7.7 (1.1) b | 9.6 (0.7) b | 17.3 (1.6) b | 11 (3/8) | 40.0 (27–58) a | 1.9 (0.4) b | 4.4 (0.3) b | 6.2 (0.6) b | Capillary electrophoresis |
| Oliveira et al. [39] | 2017 | serum | 175 (n/a) | n/a | n/a | n/a | 21.68 (31.54) | 249 (72/177) | 36.7 (10.9) | n/a | n/a | 45.99 (26.01) | Spectrophotometric assay (Griess) |
| Seven et al. [23] | 2013 | serum | 20 (7/13) | 31.0 (9.6) | n/a | n/a | 86.28 (34.1) | 15 (5/10) | 30.2 (5.51) | n/a | n/a | 76.61 (21.68) | Fluorometric assay (Sulphanilamide) |
| Yuceyar et al. [27] | 2001 | serum | 15 (2/13) | 29.93 (n/a) | 5.84 (2.86) | 35.98 (35.04) | 41.83 (36.41) | 18 (8/10) | 33.12 (5.5) | 2.1 (3.3) | 17.2 (4.1) | 19.3 (3.7) | Spectrophotometric assay (Griess) |
| Obradovic et al. [40] | 2020 | serum | 59 (24/35) | 40.0 (10.2) | n/a | n/a | 4.5 (1.5) | 88 (36/52) | 38.9 (9.0) | n/a | n/a | 2.6 (0.9) | Semiautomated assay (Griess) |
| Giovannoni et al. [33] | 1997 | serum | 21 (5/16) | 40.6 (10.7) | n/a | n/a | 74.3 (33.7) | 22 (11/11) | 33.8 (7.4) | n/a | n/a | 32.8 (12.2) | Spectrophotometric assay (Griess) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Förster, M.; Nelke, C.; Räuber, S.; Lassmann, H.; Ruck, T.; Sormani, M.P.; Signori, A.; Hartung, H.-P.; Küry, P.; Meuth, S.G.; et al. Nitrosative Stress Molecules in Multiple Sclerosis: A Meta-Analysis. Biomedicines 2021, 9, 1899. https://doi.org/10.3390/biomedicines9121899
Förster M, Nelke C, Räuber S, Lassmann H, Ruck T, Sormani MP, Signori A, Hartung H-P, Küry P, Meuth SG, et al. Nitrosative Stress Molecules in Multiple Sclerosis: A Meta-Analysis. Biomedicines. 2021; 9(12):1899. https://doi.org/10.3390/biomedicines9121899
Chicago/Turabian StyleFörster, Moritz, Christopher Nelke, Saskia Räuber, Hans Lassmann, Tobias Ruck, Maria Pia Sormani, Alessio Signori, Hans-Peter Hartung, Patrick Küry, Sven G. Meuth, and et al. 2021. "Nitrosative Stress Molecules in Multiple Sclerosis: A Meta-Analysis" Biomedicines 9, no. 12: 1899. https://doi.org/10.3390/biomedicines9121899
APA StyleFörster, M., Nelke, C., Räuber, S., Lassmann, H., Ruck, T., Sormani, M. P., Signori, A., Hartung, H.-P., Küry, P., Meuth, S. G., & Kremer, D. (2021). Nitrosative Stress Molecules in Multiple Sclerosis: A Meta-Analysis. Biomedicines, 9(12), 1899. https://doi.org/10.3390/biomedicines9121899









