Primary Cortical Cell Tri-Culture-Based Screening of Neuroinflammatory Response in Toll-like Receptor Activation
Abstract
1. Introduction
2. Methods
2.1. Primary Cortical Culture
2.2. TLR Agonist Treatment
2.3. Immunostaining
2.4. Live/Dead, Apoptosis, and Morphological Analysis
2.5. Statistical Methods
3. Results
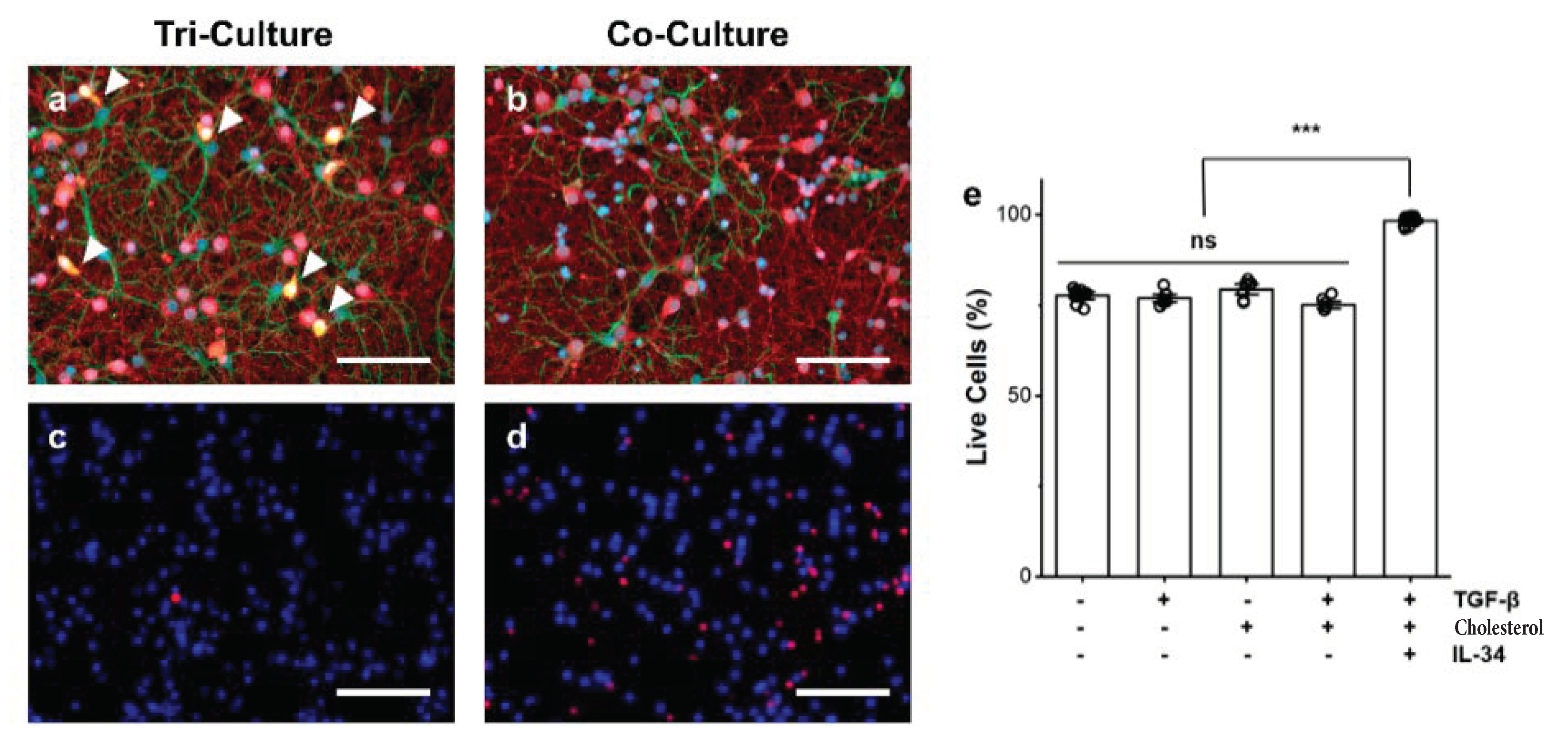
3.1. Influence of Microglia Presence on Cell Death
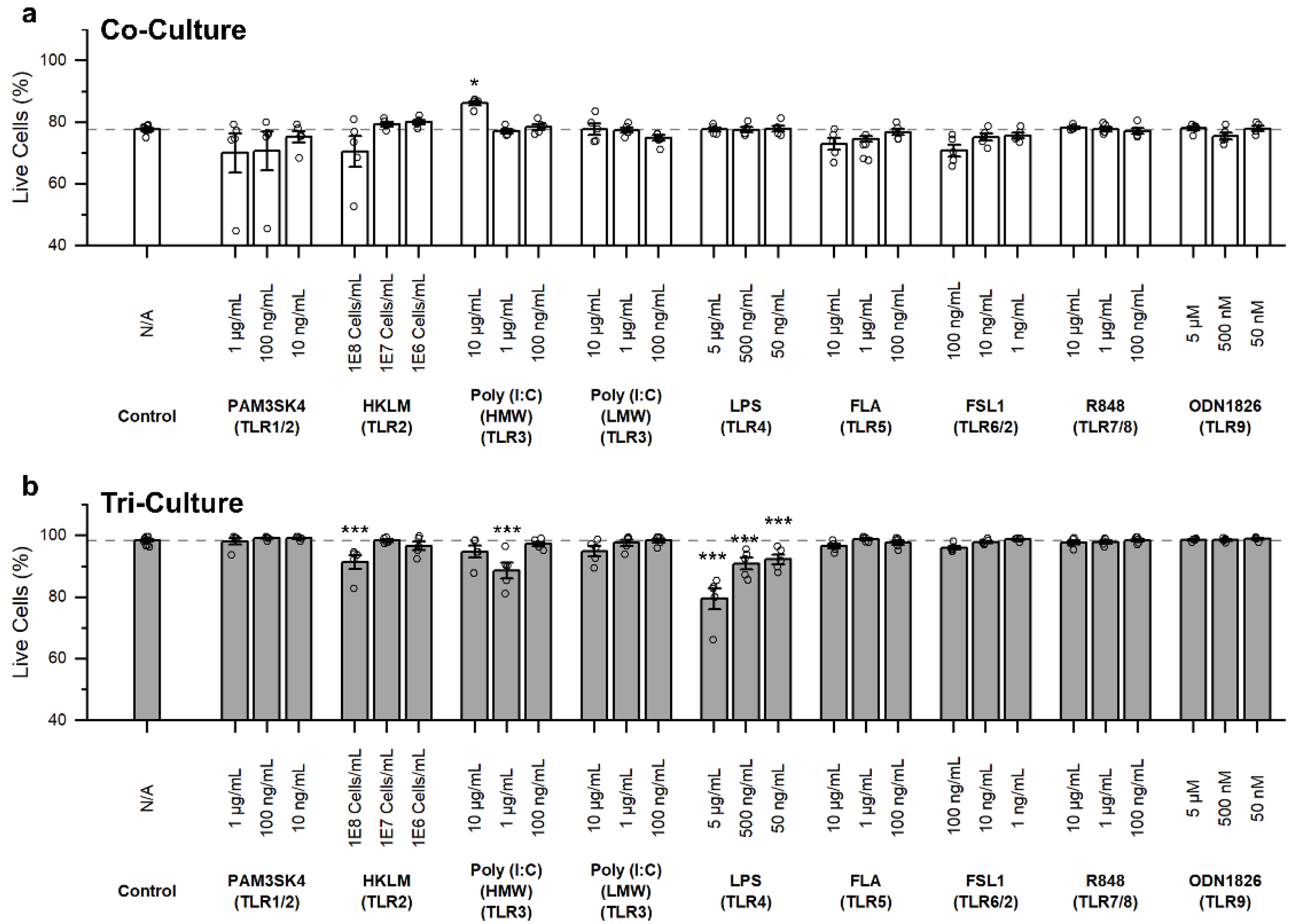
3.2. Influence of TLR Agonists on Cell Death
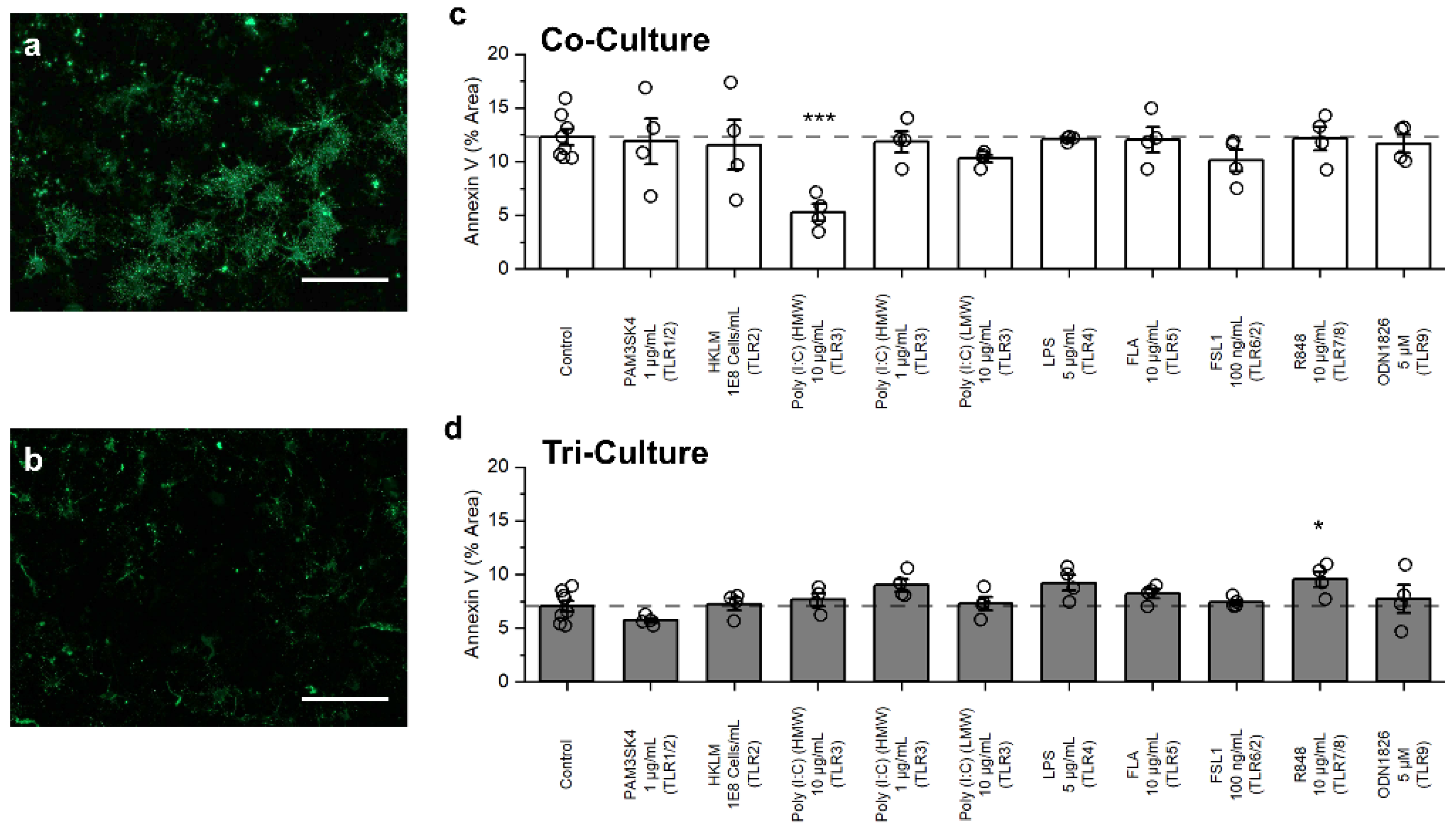
3.3. Influence of TLR Agonists on Apoptosis
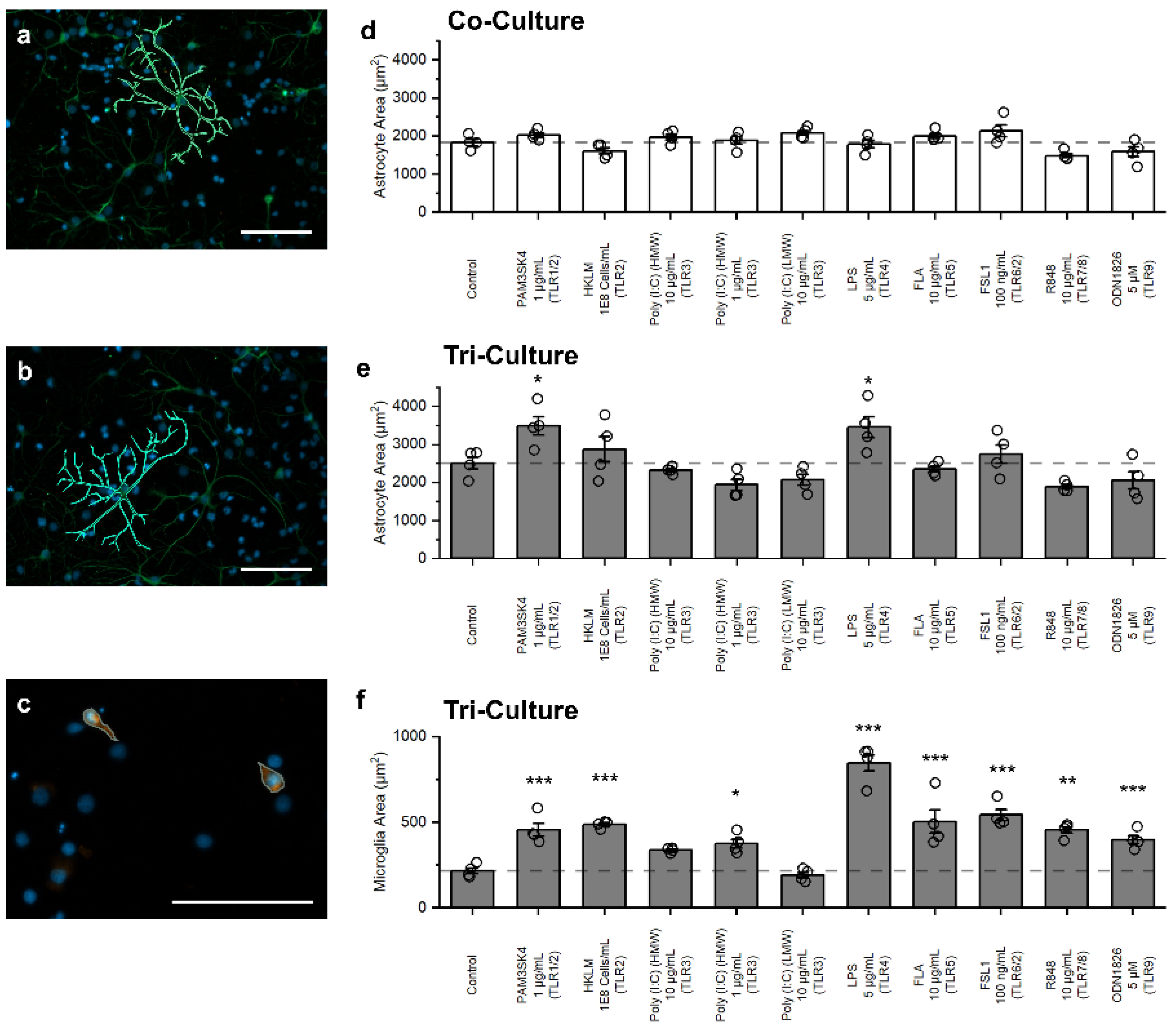
3.4. Influence of TLR Agonists on Glial Cell Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Naegele, M.; Martin, R. The Good and the Bad of Neuroinflammation in Multiple Sclerosis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 122. [Google Scholar]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s Disease: Current Evidence and Future Directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s Disease. Park. Relat. Disord. 2012, 18, 210–212. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of Brain Tissue to Chronically Implanted Neural Electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Miller, S.J. Astrocyte Heterogeneity in the Adult Central Nervous System. Front. Cell. Neurosci. 2018, 12, 401. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2017, 18, 225–242. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia—Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like Receptors and Innate Immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Duncan, D.S.; Miller, S.D. Glial Toll-like Receptor Signaling in Central Nervous System Infection and Autoimmunity. Brain Behav. Immun. 2008, 22, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Le, Y. Toll-like Receptors in Inflammation of the Central Nervous System. Int. Immunopharmacol. 2011, 11, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Toll-like Receptors in the Pathogenesis of Neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Waisman, A.; Liblau, R.S.; Becher, B. Innate and Adaptive Immune Responses in the CNS. Lancet Neurol. 2015, 14, 945–955. [Google Scholar] [CrossRef]
- Bsibsi, M.; Persoon-Deen, C.; Verwer, R.W.H.; Meeuwsen, S.; Ravid, R.; Van Noort, J.M. Toll-like Receptor 3 on Adult Human Astrocytes Triggers Production of Neuroprotective Mediators. Glia 2006, 53, 688–695. [Google Scholar] [CrossRef]
- Jack, C.S.; Arbour, N.; Manusow, J.; Montgrain, V.; Blain, M.; McCrea, E.; Shapiro, A.; Antel, J.P. TLR Signaling Tailors Innate Immune Responses in Human Microglia and Astrocytes. J. Immunol. 2005, 175, 4320–4330. [Google Scholar] [CrossRef]
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A Primary Neural Cell Culture Model to Study Neuron, Astrocyte, and Microglia Interactions in Neuroinflammation. J. Neuroinflamm. 2020, 17, 155. [Google Scholar] [CrossRef]
- Olson, J.K.; Miller, S.D. Microglia Initiate Central Nervous System Innate and Adaptive Immune Responses through Multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.A.R.; Chen, H.; Stamou, M.; Biener, J.; Biener, M.M.; Lein, P.J.; Seker, E. Nanoporous Gold as a Neural Interface Coating: Effects of Topography, Surface Chemistry, and Feature Size. ACS Appl. Mater. Interfaces 2015, 7, 7093–7100. [Google Scholar] [CrossRef] [PubMed]
- Wayman, G.A.; Bose, D.D.; Yang, D.; Lesiak, A.; Bruun, D.; Impey, S.; Ledoux, V.; Pessah, I.N.; Lein, P.J. PCB-95 Modulates the Calcium-Dependent Signaling Pathway Responsible for Activity-Dependent Dendritic Growth. Environ. Health Perspect. 2012, 120, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Daggumati, P.; Kurtulus, O.; Chapman, C.A.R.; Dimlioglu, D.; Seker, E. Microfabrication of Nanoporous Gold Patterns for Cell-Material Interaction Studies. J. Vis. Exp. 2013, 77, e50678. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Coch, C.; Peters, C.; Hartmann, G.; Wesch, D.; Kabelitz, D. Monocyte-Dependent Co-Stimulation of Cytokine Induction in Human Γδ T Cells by TLR8 RNA Ligands. Sci. Rep. 2021, 11, 15231. [Google Scholar] [CrossRef]
- Udayan, S.; Buttó, L.F.; Rossini, V.; Velmurugan, J.; Martinez-Lopez, M.; Sancho, D.; Melgar, S.; O’Toole, P.W.; Nally, K. Macrophage Cytokine Responses to Commensal Gram-Positive Lactobacillus Salivarius Strains Are TLR2-Independent and Myd88-Dependent. Sci. Rep. 2021, 11, 5896. [Google Scholar] [CrossRef]
- Grubman, A.; Choo, X.Y.; Chew, G.; Ouyang, J.F.; Sun, G.; Croft, N.P.; Rossello, F.J.; Simmons, R.; Buckberry, S.; Landin, D.V.; et al. Transcriptional Signature in Microglia Associated with Aβ Plaque Phagocytosis. Nat. Commun. 2021, 12, 3015. [Google Scholar] [CrossRef]
- Affram, K.O.; Mitchell, K.; Symes, A.J. Microglial Activation Results in Inhibition of TGF-β-Regulated Gene Expression. J. Mol. Neurosci. 2017, 63, 308–319. [Google Scholar] [CrossRef]
- Niu, T.; De Rosny, C.; Chautard, S.; Rey, A.; Patoli, D.; Groslambert, M.; Cosson, C.; Lagrange, B.; Zhang, Z.; Visvikis, O.; et al. NLRP3 Phosphorylation in Its LRR Domain Critically Regulates Inflammasome Assembly. Nat. Commun. 2021, 12, 5862. [Google Scholar] [CrossRef]
- Allacher, P.; Baumgartner, C.K.; Pordes, A.G.; Ahmad, R.U.; Schwarz, H.P.; Reipert, B.M. Stimulation and Inhibition of FVIII-Specific Memory B-Cell Responses by CpG-B (ODN 1826), a Ligand for Toll-like Receptor 9. Blood 2011, 117, 259–267. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, J.J.; Olabarria, M.; Chvatal, A.; Verkhratsky, A. Astroglia in Dementia and Alzheimer’s Disease. Cell Death Differ. 2009, 16, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Shin, Y.; Sivathanu, V.; Campisi, M.; Kamm, R.D. In Vitro Microfluidic Models for Neurodegenerative Disorders. Adv. Heal. Mater. 2018, 7, 1700489. [Google Scholar] [CrossRef]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In Vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef]
- Garden, G.A.; La Spada, A.R. Intercellular (Mis)Communication in Neurodegenerative Disease. Neuron 2012, 73, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Nandi, S.; Mehler, M.F.; Stanley, E.R. Emerging Roles for CSF-1 Receptor and Its Ligands in the Nervous System. Trends Neurosci. 2016, 39, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.R.P.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-Stimulating Factor 1 Receptor Signaling Is Necessary for Microglia Viability, Unmasking a Microglia Progenitor Cell in the Adult Brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef]
- Erblich, B.; Zhu, L.; Etgen, A.M.; Dobrenis, K.; Pollard, J.W. Absence of Colony Stimulation Factor-1 Receptor Results in Loss of Microglia, Disrupted Brain Development and Olfactory Deficits. PLoS ONE 2011, 6, e26317. [Google Scholar] [CrossRef]
- Chapman, C.A.R.; Goshi, N.; Seker, E. Multifunctional Neural Interfaces for Closed-Loop Control of Neural Activity. Adv. Funct. Mater. 2017, 28, 1703523. [Google Scholar] [CrossRef]
- Beynon, S.B.; Walker, F.R. Microglial Activation in the Injured and Healthy Brain: What Are We Really Talking about? Practical and Theoretical Issues Associated with the Measurement of Changes in Microglial Morphology. Neuroscience 2012, 225, 162–171. [Google Scholar] [CrossRef]
- Abd-El-Basset, E.; Fedoroff, S. Effect of Bacterial Wall Lipopolysaccharide (LPS) on Morphology, Motility, and Cytoskeletal Organization of Microglia in Cultures. J. Neurosci. Res. 1995, 41, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Brantefjord, M.; Hansson, E.; Ronnback, L. Lipopolysaccharide Increases Microglial GLT-1 Expression and Glutamate Uptake Capacity in Vitro by a Mechanism Dependent on TNF-α. Glia 2005, 51, 111–120. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, M.; Wang, X.; Li, J.; Wang, Y.; Ye, L.; Dai, M.; Zhou, L.; Persidsky, Y.; Ho, W. TLR3 Activation Efficiency by High or Low Molecular Mass Poly I:C. Innate Immun. 2013, 19, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Carty, M.; Bowie, A.G. Evaluating the Role of Toll-like Receptors in Diseases of the Central Nervous System. Biochem. Pharmacol. 2011, 81, 825–837. [Google Scholar] [CrossRef]
- Aravalli, R.N.; Peterson, P.K.; Lokensgard, J.R. Toll-like Receptors in Defense and Damage of the Central Nervous System. J. Neuroimmune Pharmacol. 2007, 2, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, M.; Hinkelmann, L.; Kuhrt, L.D.; Efe, I.E.; Kumbol, V.; Buonfiglioli, A.; Krüger, C.; Jordan, P.; Fulde, M.; Noda, M.; et al. Activation of Toll-like Receptor 5 in Microglia Modulates Their Function and Triggers Neuronal Injury. Acta Neuropathol. Commun. 2020, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773.e8. [Google Scholar] [CrossRef]
- Butchi, N.B.; Woods, T.; Du, M.; Morgan, T.W.; Peterson, K.E. TLR7 and TLR9 Trigger Distinct Neuroinflammatory Responses in the CNS. Am. J. Pathol. 2011, 179, 783–794. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Kielian, T. Toll-like Receptors in Central Nervous System Glial Inflammation and Homeostasis. J. Neurosci. Res. 2006, 83, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Farhat, K.; Riekenberg, S.; Heine, H.; Debarry, J.; Lang, R.; Mages, J.; Buwitt-Beckmann, U.; Röschmann, K.; Jung, G.; Wiesmüller, K.-H.; et al. Heterodimerization of TLR2 with TLR1 or TLR6 Expands the Ligand Spectrum but Does Not Lead to Differential Signaling. J. Leukoc. Biol. 2008, 83, 692–701. [Google Scholar] [CrossRef]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra Are Key Regulators of the Inflammatory Response to RNA Vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef]
- Teti, E.; Golenbock, D.T.; Sundan, A.; Trude Flo, T.H.; Halaas, Ø.; Lien, E.; Ryan, L. Human Toll-Like Receptor 2 Mediates. J. Immunol. Ref. 2022, 164, 2064–2069. [Google Scholar] [CrossRef]
- McKernan, D.P.; Gaszner, G.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Altered Peripheral Toll-like Receptor Responses in the Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2011, 33, 1045–1052. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The Role of Interleukin-6 Signaling in Nervous Tissue. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Town, T.; Jeng, D.; Alexopoulou, L.; Tan, J.; Flavell, R.A. Microglia Recognize Double-Stranded RNA via TLR3. J. Immunol. 2006, 176, 3804–3812. [Google Scholar] [CrossRef]
- Zuiderwijk-Sick, E.A.; van der Putten, C.; Bsibsi, M.; Deuzing, I.P.; de Boer, W.; Persoon-Deen, C.; Kondova, I.; Boven, L.A.; van Noort, J.M.; ’t Hart, B.A.; et al. Differentiation of Primary Adult Microglia Alters Their Response to TLR8-Mediated Activation but Not Their Capacity as APC. Glia 2007, 55, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Lannes, N.; Summerfield, A.; Filgueira, L. Regulation of Inflammation in Japanese Encephalitis. J. Neuroinflamm. 2017, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Chiu, I.; Wang, Y.; Sloane, J.A.; Lü, J.; Kosaras, B.; Sidman, R.L.; Volpe, J.J.; Vartanian, T. Toll-like Receptor 8 Functions as a Negative Regulator of Neurite Outgrowth and Inducer of Neuronal Apoptosis. J. Cell Biol. 2006, 175, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, K.; Derkow, K.; Dembny, P.; Krüger, C.; Schott, E.; Lehnardt, S. The Impact of Single and Pairwise Toll-like Receptor Activation on Neuroinflammation and Neurodegeneration. J. Neuroinflamm. 2014, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
| Toll-like Receptor Activation | Co-Culture | Tri-Culture |
|---|---|---|
| TLR1/2 | No Change | Increased Astrocyte Area Increased Microglia Area |
| TLR2 | No Change | Increased Cell Death Increased Microglia Area |
| TLR3 | Reduced Cell Death Reduced Apoptosis | Increased Cell Death 1 Increased Microglia Area 1 |
| TLR4 | No Change | Increased Cell Death Increased Astrocyte Area Increased Microglia Area |
| TLR5 | No Change | Increased Microglia Area |
| TLR6/2 | No Change | Increased Microglia Area |
| TLR7/8 | No Change | Increased Apoptosis Increased Microglia Area |
| TLR9 | No Change | Increased Microglia Area |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goshi, N.; Kim, H.; Seker, E. Primary Cortical Cell Tri-Culture-Based Screening of Neuroinflammatory Response in Toll-like Receptor Activation. Biomedicines 2022, 10, 2122. https://doi.org/10.3390/biomedicines10092122
Goshi N, Kim H, Seker E. Primary Cortical Cell Tri-Culture-Based Screening of Neuroinflammatory Response in Toll-like Receptor Activation. Biomedicines. 2022; 10(9):2122. https://doi.org/10.3390/biomedicines10092122
Chicago/Turabian StyleGoshi, Noah, Hyehyun Kim, and Erkin Seker. 2022. "Primary Cortical Cell Tri-Culture-Based Screening of Neuroinflammatory Response in Toll-like Receptor Activation" Biomedicines 10, no. 9: 2122. https://doi.org/10.3390/biomedicines10092122
APA StyleGoshi, N., Kim, H., & Seker, E. (2022). Primary Cortical Cell Tri-Culture-Based Screening of Neuroinflammatory Response in Toll-like Receptor Activation. Biomedicines, 10(9), 2122. https://doi.org/10.3390/biomedicines10092122





