The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease
Abstract
1. Introduction
2. Insulin Resistance as Trigger Event for NAFLD Onset, Progression, and Clinical Course
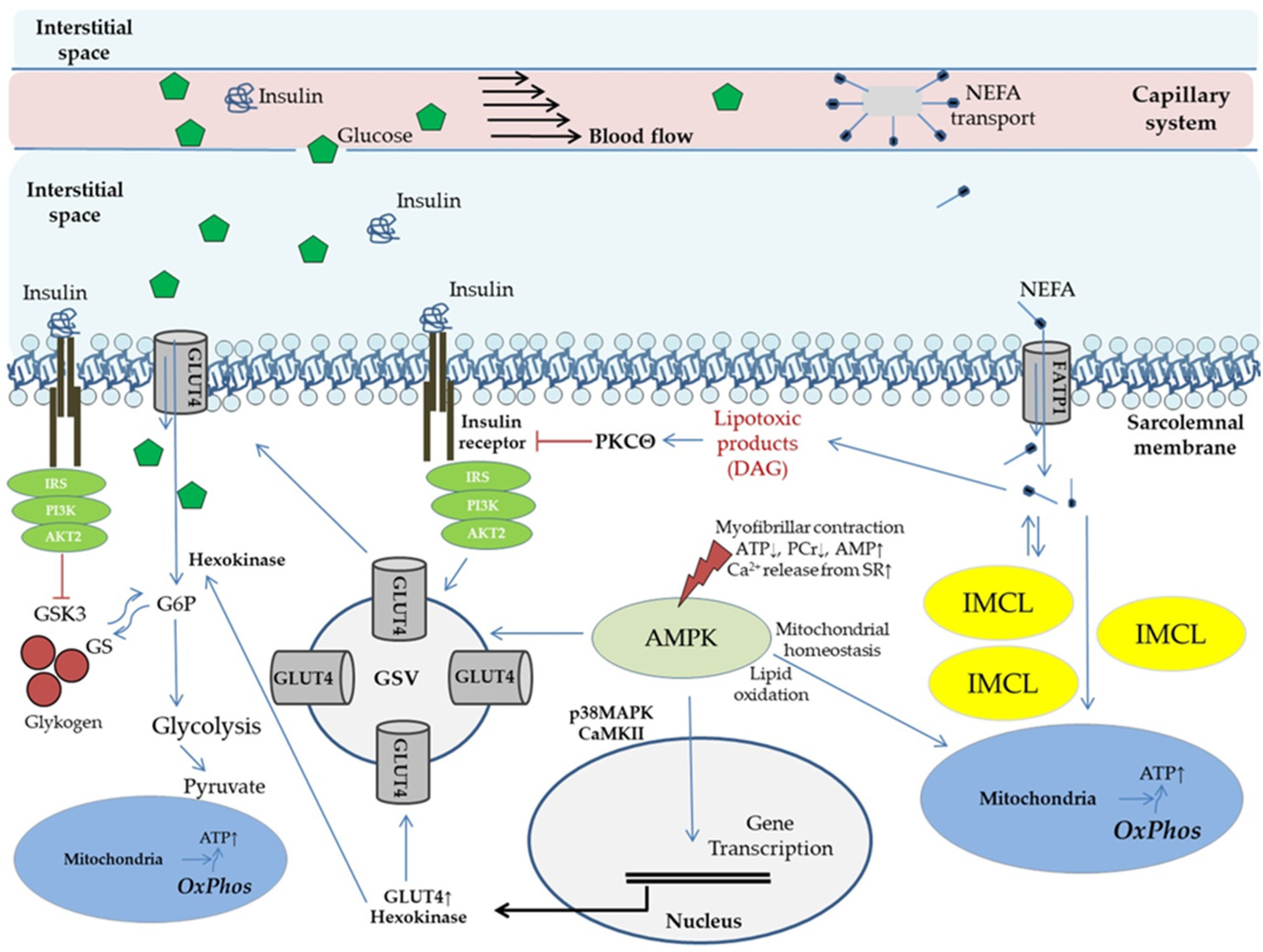
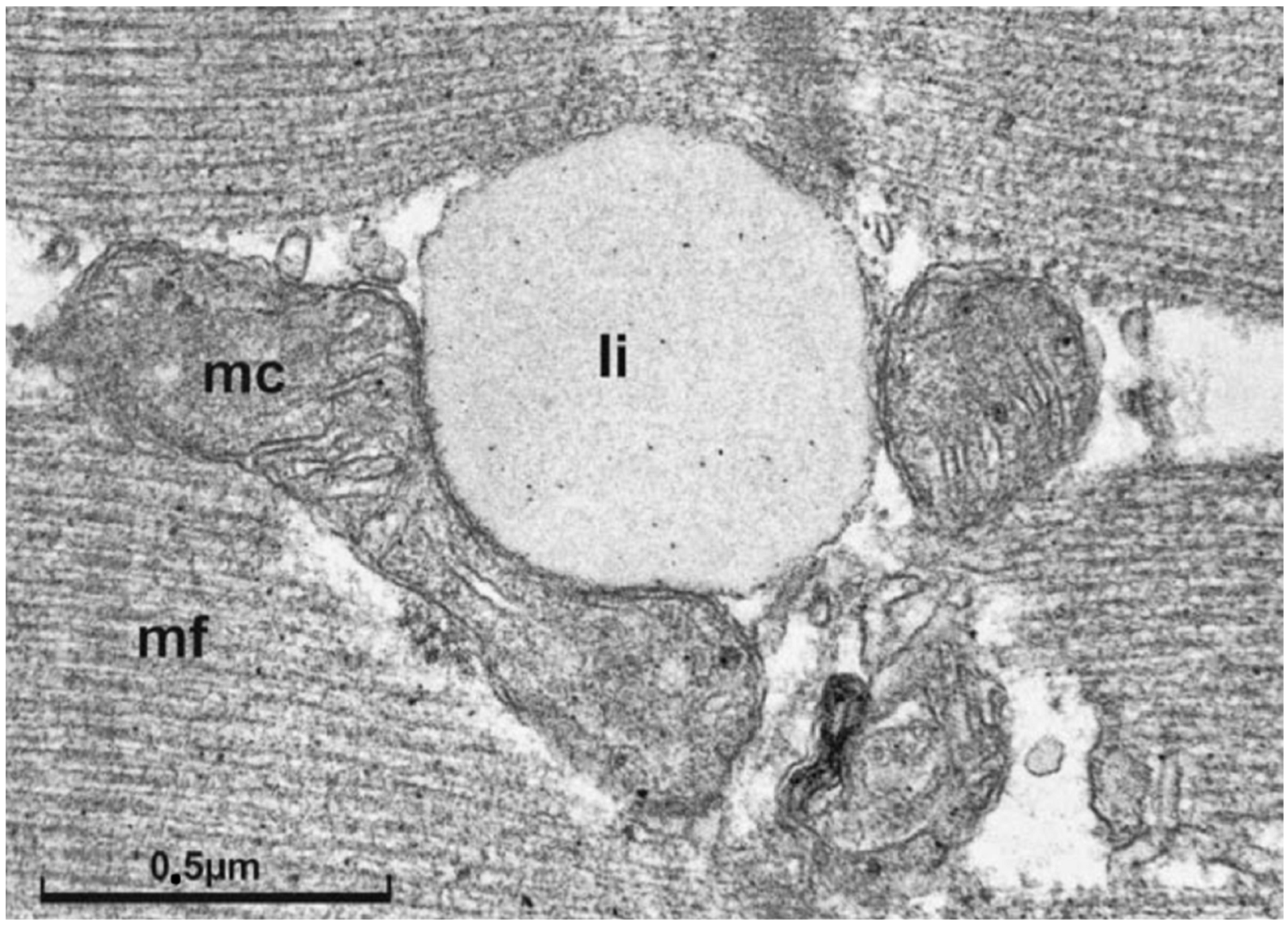
3. The Concept of Metabolic Flexibility: Molecular Mechanisms of Physical Activity on Glucose Metabolism and Insulin Signaling in Skeletal Muscle
4. Data on Lifestyle Interventions under Conditions of Insulin Resistance and NAFLD/MAFLD
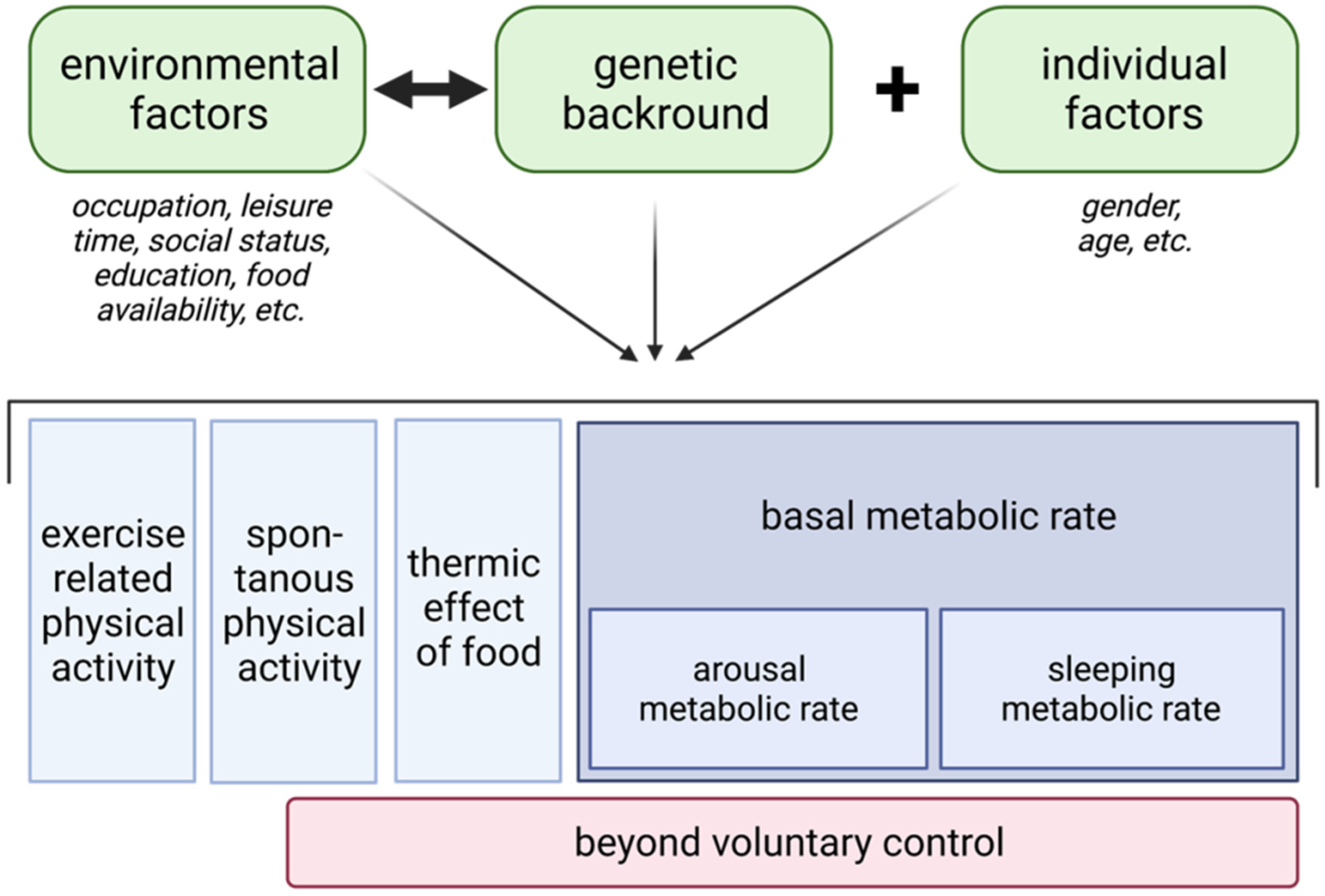
5. The Concept of Non-Exercise Activity Thermogenesis
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Hannah, W.N.; Harrison, S.A. Noninvasive imaging methods to determine severity of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2016, 64, 2234–2243. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. 1), 81–84. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Stamatakis, E.; Gale, J.; Bauman, A.; Ekelund, U.; Hamer, M.; Ding, D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019, 73, 2062–2072. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Eriksson, K.F.; Lindgärde, F. No excess 12-year mortality in men with impaired glucose tolerance who participated in the Malmö Preventive Trial with diet and exercise. Diabetologia 1998, 41, 1010–1016. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Molitch, M.E.; Fujimoto, W.; Hamman, R.F.; Knowler, W.C. The diabetes prevention program and its global implications. J. Am. Soc. Nephrol. 2003, 14, S103–S107. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; van Rooyen, D.; Gan, L.; Chitturi, S. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver 2012, 6, 149–171. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef]
- Söderberg, C.; Stål, P.; Askling, J.; Glaumann, H.; Lindberg, G.; Marmur, J.; Hultcrantz, R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010, 51, 595–602. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef]
- Bertot, L.C.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-A.; Lee, H.C.; Choe, J.; Kim, M.-J.; Lee, M.J.; Chang, H.-S.; Bae, I.Y.; Kim, H.-K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018, 68, 140–146. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Lu, H.; Liu, H.; Hu, F.; Zou, L.; Luo, S.; Sun, L. Independent Association between Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2013, 2013, 124958. [Google Scholar] [CrossRef] [PubMed]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, K.J.; Yoo, M.E.; Kim, G.; Yoon, H.-J.; Jo, K.; Youn, J.-C.; Yun, M.; Park, J.Y.; Shim, C.Y.; et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J. Hepatol. 2018, 68, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef]
- Yeung, M.-W.; Wong, G.L.-H.; Choi, K.C.; Luk, A.O.-Y.; Kwok, R.; Shu, S.S.-T.; Chan, A.W.-H.; Lau, E.S.H.; Ma, R.C.W.; Chan, H.L.-Y.; et al. Advanced liver fibrosis but not steatosis is independently associated with albuminuria in Chinese patients with type 2 diabetes. J. Hepatol. 2018, 68, 147–156. [Google Scholar] [CrossRef]
- Buckley, A.J.; Thomas, E.L.; Lessan, N.; Trovato, F.M.; Trovato, G.M.; Taylor-Robinson, S.D. Non-alcoholic fatty liver disease: Relationship with cardiovascular risk markers and clinical endpoints. Diabetes Res. Clin. Pract. 2018, 144, 144–152. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Q.; Greten, T.F. Nonalcoholic fatty liver disease promotes hepatocellular carcinoma through direct and indirect effects on hepatocytes. FEBS J. 2018, 285, 752–762. [Google Scholar] [CrossRef]
- Huber, Y.; Labenz, C.; Michel, M.; Wörns, M.-A.; Galle, P.R.; Kostev, K.; Schattenberg, J.M. Tumor Incidence in Patients with Non-Alcoholic Fatty Liver Disease. Dtsch. Ärzteblatt Int. 2020, 117, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, S.; Nakano, D.; Kawaguchi, T.; Eslam, M.; Ouchi, A.; Nagata, T.; Kuroki, H.; Kawata, H.; Abe, H.; Nouno, R.; et al. Non-Obese MAFLD Is Associated with Colorectal Adenoma in Health Check Examinees: A Multicenter Retrospective Study. Int. J. Mol. Sci. 2021, 22, 5462. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, J.; Gomatos, I.P.; Tiniakos, D.G.; Memos, N.; Boutsikou, M.; Garatzioti, A.; Archimandritis, A.; Betrosian, A. Liver histology in ICU patients dying from sepsis: A clinico-pathological study. World J. Gastroenterol. 2008, 14, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Tabbaa, A.; Albeldawi, M.; Alkhouri, N. Liver transplantation for nonalcoholic fatty liver disease: New challenges and new opportunities. World J. Gastroenterol. 2014, 20, 5320–5330. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Riaz, D.R.; Shi, G.; Liu, C.; Dai, Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 394–402. [Google Scholar] [CrossRef]
- Hoppe, S.; von Loeffelholz, C.; Lock, J.F.; Doecke, S.; Sinn, B.V.; Rieger, A.; Malinowski, M.; Pfeiffer, A.F.H.; Neuhaus, P.; Stockmann, M. Nonalcoholic steatohepatits and liver steatosis modify partial hepatectomy recovery. J. Investig. Surg. 2015, 28, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Henry, L.; Garg, R.; Kalwaney, S.; Saab, S.; Younossi, Z. Risk of de novo post-transplant type 2 diabetes in patients undergoing liver transplant for non-alcoholic steatohepatitis. BMC Gastroenterol. 2015, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Otgonsuren, M.; de Avila, L.; Sayiner, M.; Rafiq, N.; Younossi, Z.M. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine 2018, 97, e0214. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, O.; von Loeffelholz, C.; Diab, M.; Kiessling, S.; Doenst, T.; Bauer, M.; Sponholz, C. Association between high dose catecholamine support and liver dysfunction following cardiac surgery. J. Card. Surg. 2020, 35, 1228–1236. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Paik, J.M.; Golabi, P.; Biswas, R.; Alqahtani, S.; Venkatesan, C.; Younossi, Z.M. Nonalcoholic Fatty Liver Disease and Alcoholic Liver Disease are Major Drivers of Liver Mortality in the United States. Hepatol. Commun. 2020, 4, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed]
- von Loeffelholz, C.; Coldewey, S.M.; Birkenfeld, A.L. A Narrative Review on the Role of AMPK on De Novo Lipogenesis in Non-Alcoholic Fatty Liver Disease: Evidence from Human Studies. Cells 2021, 10, 1822. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Barb, D.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Suman, A.; Weber, M.H.; Budd, J.T.; Lupi, M.E.; Cusi, K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017, 65, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.T.; de Laet, C.; Franco, O.H.; Peeters, A.; Mackenbach, J.; Nusselder, W.J. Physical activity and life expectancy with and without diabetes: Life table analysis of the Framingham Heart Study. Diabetes Care 2006, 29, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C.; White, R.D. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 1433–1438. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef]
- Golabi, P.; Gerber, L.; Paik, J.M.; Deshpande, R.; de Avila, L.; Younossi, Z.M. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100171. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J. Gastroenterol. 2021, 56, 951–963. [Google Scholar] [CrossRef]
- Kelley, D.E. Skeletal muscle fat oxidation: Timing and flexibility are everything. J. Clin. Investig. 2005, 115, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, E.; Saris, W.H.M.; Blaak, E.E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: Effects of lifestyle. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Simoneau, J.A.; Thaete, F.L.; Kelley, D.E. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J. Clin. Investig. 1995, 95, 1846–1853. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef]
- Petersen, K.F.; Shulman, G.I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 11G–18G. [Google Scholar] [CrossRef]
- Ng, J.M.; Azuma, K.; Kelley, C.; Pencek, R.; Radikova, Z.; Laymon, C.; Price, J.; Goodpaster, B.H.; Kelley, D.E. PET imaging reveals distinctive roles for different regional adipose tissue depots in systemic glucose metabolism in nonobese humans. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1134–E1141. [Google Scholar] [CrossRef]
- Haddad, F.; Adams, G.R. Selected contribution: Acute cellular and molecular responses to resistance exercise. J. Appl. Physiol. 2002, 93, 394–403. [Google Scholar] [CrossRef]
- Henriksen, E.J. Invited review: Effects of acute exercise and exercise training on insulin resistance. J. Appl. Physiol. 2002, 93, 788–796. [Google Scholar] [CrossRef]
- Zierath, J.R. Invited review: Exercise training-induced changes in insulin signaling in skeletal muscle. J. Appl. Physiol. 2002, 93, 773–781. [Google Scholar] [CrossRef]
- Sakamoto, K.; Goodyear, L.J. Invited review: Intracellular signaling in contracting skeletal muscle. J. Appl. Physiol. 2002, 93, 369–383. [Google Scholar] [CrossRef]
- Roden, M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News Physiol. Sci. 2004, 19, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Exercise-induced increase in muscle insulin sensitivity. J. Appl. Physiol. 2005, 99, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, B.D.; Turner, N.; Cooney, G.J.; Kraegen, E.W. Insulin resistance and fuel homeostasis: The role of AMP-activated protein kinase. Acta Physiol. 2009, 196, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Nielsen, S.; Guo, Z.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Roden, M. Mechanisms of Disease: Hepatic steatosis in type 2 diabetes—Pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; McLaughlin, T.; Lamendola, C.; Reaven, G.M. The relationship between glucose disposal in response to physiological hyperinsulinemia and basal glucose and free fatty acid concentrations in healthy volunteers. J. Clin. Endocrinol. Metab. 2000, 85, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Dubé, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.S.; et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef]
- Coen, P.M.; Menshikova, E.V.; Distefano, G.; Zheng, D.; Tanner, C.J.; Standley, R.A.; Helbling, N.L.; Dubis, G.S.; Ritov, V.B.; Xie, H.; et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes 2015, 64, 3737–3750. [Google Scholar] [CrossRef]
- Chee, C.; Shannon, C.E.; Burns, A.; Selby, A.L.; Wilkinson, D.; Smith, K.; Greenhaff, P.L.; Stephens, F.B. Relative Contribution of Intramyocellular Lipid to Whole-Body Fat Oxidation Is Reduced with Age but Subsarcolemmal Lipid Accumulation and Insulin Resistance Are Only Associated with Overweight Individuals. Diabetes 2016, 65, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Choi, C.S.; Birkenfeld, A.L.; Alves, T.C.; Jornayvaz, F.R.; Jurczak, M.J.; Zhang, D.; Woo, D.K.; Shadel, G.S.; Ladiges, W.; et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010, 12, 668–674. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Dufour, S.; Befroy, D.; Frattini, J.; Shatzkes, N.; Neschen, S.; White, M.F.; Bilz, S.; Sono, S.; et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Investig. 2005, 115, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Cleland, P.J.; Appleby, G.J.; Rattigan, S.; Clark, M.G. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J. Biol. Chem. 1989, 264, 17704–17711. [Google Scholar] [CrossRef]
- Ishizuka, T.; Cooper, D.R.; Hernandez, H.; Buckley, D.; Standaert, M.; Farese, R.V. Effects of insulin on diacylglycerol-protein kinase C signaling in rat diaphragm and soleus muscles and relationship to glucose transport. Diabetes 1990, 39, 181–190. [Google Scholar] [CrossRef]
- You, J.-S.; Lincoln, H.C.; Kim, C.-R.; Frey, J.W.; Goodman, C.A.; Zhong, X.-P.; Hornberger, T.A. The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J. Biol. Chem. 2014, 289, 1551–1563. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Roden, M. Mitochondrial fitness and insulin sensitivity in humans. Diabetologia 2008, 51, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R.; Lundby, C.; Qvortrup, K.; Sahlin, K. Mitochondrial plasticity with exercise training and extreme environments. Exerc. Sport Sci. Rev. 2014, 42, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Steenberg, D.E.; Jørgensen, N.B.; Birk, J.B.; Sjøberg, K.A.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F.P. Exercise training reduces the insulin-sensitizing effect of a single bout of exercise in human skeletal muscle. J. Physiol. 2019, 597, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Lundby, A.-K.M.; Jacobs, R.A.; Gehrig, S.; de Leur, J.; Hauser, M.; Bonne, T.C.; Flück, D.; Dandanell, S.; Kirk, N.; Kaech, A.; et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol. 2018, 222, e12905. [Google Scholar] [CrossRef] [PubMed]
- Essén-Gustavsson, B.; Tesch, P.A. Glycogen and triglyceride utilization in relation to muscle metabolic characteristics in men performing heavy-resistance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 5–10. [Google Scholar] [CrossRef]
- Koopman, R.; Manders, R.J.F.; Jonkers, R.A.M.; Hul, G.B.J.; Kuipers, H.; van Loon, L.J.C. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur. J. Appl. Physiol. 2006, 96, 525–534. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.C.; Goodpaster, B.H. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflug. Arch. 2006, 451, 606–616. [Google Scholar] [CrossRef]
- Hoppeler, H.; Howald, H.; Conley, K.; Lindstedt, S.L.; Claassen, H.; Vock, P.; Weibel, E.R. Endurance training in humans: Aerobic capacity and structure of skeletal muscle. J. Appl. Physiol. 1985, 59, 320–327. [Google Scholar] [CrossRef]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef]
- Bogardus, C.; Lillioja, S.; Stone, K.; Mott, D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J. Clin. Investig. 1984, 73, 1185–1190. [Google Scholar] [CrossRef]
- Ivy, J.L. Muscle glycogen synthesis before and after exercise. Sports Med. 1991, 11, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Acheson, K.J.; Schutz, Y.; Bessard, T.; Anantharaman, K.; Flatt, J.P.; Jéquier, E. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am. J. Clin. Nutr. 1988, 48, 240–247. [Google Scholar] [CrossRef]
- Ivy, J.L. Regulation of muscle glycogen repletion, muscle protein synthesis and repair following exercise. J. Sports Sci. Med. 2004, 3, 131–138. [Google Scholar] [PubMed]
- Stephens, F.B.; Tsintzas, K. Metabolic and molecular changes associated with the increased skeletal muscle insulin action 24–48 h after exercise in young and old humans. Biochem. Soc. Trans. 2018, 46, 111–118. [Google Scholar] [CrossRef]
- de Bock, K.; Richter, E.A.; Russell, A.P.; Eijnde, B.O.; Derave, W.; Ramaekers, M.; Koninckx, E.; Léger, B.; Verhaeghe, J.; Hespel, P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J. Physiol. 2005, 564, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Gaster, M.; Vach, W.; Beck-Nielsen, H.; Schrøder, H.D. GLUT4 expression at the plasma membrane is related to fibre volume in human skeletal muscle fibres. APMIS 2002, 110, 611–619. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Del Aguila, L.F. Insulin signalling, exercise and cellular integrity. Biochem. Soc. Trans. 2003, 31, 1281–1285. [Google Scholar] [CrossRef]
- King, D.S.; Feltmeyer, T.L.; Baldus, P.J.; Sharp, R.L.; Nespor, J. Effects of eccentric exercise on insulin secretion and action in humans. J. Appl. Physiol. 1993, 75, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970.e6. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, N.J.; Parker, B.L.; Chaudhuri, R.; Fisher-Wellman, K.H.; Kleinert, M.; Humphrey, S.J.; Yang, P.; Holliday, M.; Trefely, S.; Fazakerley, D.J.; et al. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015, 22, 922–935. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Newcomer, B.R.; Hunter, G.R. Influence of endurance running and recovery diet on intramyocellular lipid content in women: A 1H NMR study. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E95–E106. [Google Scholar] [CrossRef]
- Zderic, T.W.; Davidson, C.J.; Schenk, S.; Byerley, L.O.; Coyle, E.F. High-fat diet elevates resting intramuscular triglyceride concentration and whole-body lipolysis during exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E217–E225. [Google Scholar] [CrossRef]
- Hu, G.; Eriksson, J.; Barengo, N.C.; Lakka, T.A.; Valle, T.T.; Nissinen, A.; Jousilahti, P.; Tuomilehto, J. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation 2004, 110, 666–673. [Google Scholar] [CrossRef]
- Lindström, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003, 26, 3230–3236. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Roumen, C.; Corpeleijn, E.; Feskens, E.J.M.; Mensink, M.; Saris, W.H.M.; Blaak, E.E. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: The SLIM study. Diabet. Med. 2008, 25, 597–605. [Google Scholar] [CrossRef]
- den Boer, A.T.; Herraets, I.J.T.; Stegen, J.; Roumen, C.; Corpeleijn, E.; Schaper, N.C.; Feskens, E.; Blaak, E.E. Prevention of the metabolic syndrome in IGT subjects in a lifestyle intervention: Results from the SLIM study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1147–1153. [Google Scholar] [CrossRef]
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.; Jakicic, J.; Blackburn, G.; Bloomquist, P.; Bray, G.; Clark, J.; Coday, M.; Curtis, J.; Egan, C.; Evans, M.; et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Wasserman, D.H.; Castaneda-Sceppa, C. Physical activity/exercise and type 2 diabetes. Diabetes Care 2004, 27, 2518–2539. [Google Scholar] [CrossRef]
- Davies, K.A.B.; Sprung, V.S.; Norman, J.A.; Thompson, A.; Mitchell, K.L.; Halford, J.C.G.; Harrold, J.A.; Wilding, J.P.H.; Kemp, G.J.; Cuthbertson, D.J. Short-term decreased physical activity with increased sedentary behaviour causes metabolic derangements and altered body composition: Effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia 2018, 61, 1282–1294. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, J.A.; Gray, L.J.; Bodicoat, D.H.; Willis, S.A.; Stensel, D.J.; Nimmo, M.A.; Aithal, G.P.; King, J.A. The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1446–1459. [Google Scholar] [CrossRef]
- Ueno, T.; Sugawara, H.; Sujaku, K.; Hashimoto, O.; Tsuji, R.; Tamaki, S.; Torimura, T.; Inuzuka, S.; Sata, M.; Tanikawa, K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J. Hepatol. 1997, 27, 103–107. [Google Scholar] [CrossRef]
- Slentz, C.A.; Bateman, L.A.; Willis, L.H.; Shields, A.T.; Tanner, C.J.; Piner, L.W.; Hawk, V.H.; Muehlbauer, M.J.; Samsa, G.P.; Nelson, R.C.; et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1033–E1039. [Google Scholar] [CrossRef]
- Fealy, C.E.; Haus, J.M.; Solomon, T.P.J.; Pagadala, M.; Flask, C.A.; McCullough, A.J.; Kirwan, J.P. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. J. Appl. Physiol. 2012, 113, 1–6. [Google Scholar] [CrossRef]
- Bacchi, E.; Negri, C.; Targher, G.; Faccioli, N.; Lanza, M.; Zoppini, G.; Zanolin, E.; Schena, F.; Bonora, E.; Moghetti, P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013, 58, 1287–1295. [Google Scholar] [CrossRef]
- Haus, J.M.; Solomon, T.P.J.; Kelly, K.R.; Fealy, C.E.; Kullman, E.L.; Scelsi, A.R.; Lu, L.; Pagadala, M.R.; McCullough, A.J.; Flask, C.A.; et al. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2013, 98, E1181–E1188. [Google Scholar] [CrossRef]
- Khaoshbaten, M.; Gholami, N.; Sokhtehzari, S.; Monazami, A.H.; Nejad, M.R. The effect of an aerobic exercise on serum level of liver enzymes and liver echogenicity in patients with non alcoholic fatty liver disease. Gastroenterol. Hepatol. Bed Bench 2013, 6, S112–S116. [Google Scholar]
- Yoshimura, E.; Kumahara, H.; Tobina, T.; Matsuda, T.; Ayabe, M.; Kiyonaga, A.; Anzai, K.; Higaki, Y.; Tanaka, H. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J. Obes. 2014, 2014, 197216. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shida, T.; Yamagishi, K.; Tanaka, K.; So, R.; Tsujimoto, T.; Shoda, J. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: A retrospective study. Hepatology 2015, 61, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Shamsoddini, A.; Sobhani, V.; Chehreh, M.E.G.; Alavian, S.M.; Zaree, A. Effect of Aerobic and Resistance Exercise Training on Liver Enzymes and Hepatic Fat in Iranian Men with Nonalcoholic Fatty Liver Disease. Hepat. Mon. 2015, 15, e31434. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Abe, K.; Usami, K.; Imaizumi, H.; Hayashi, M.; Okai, K.; Kanno, Y.; Tanji, N.; Watanabe, H.; Ohira, H. Simple Resistance Exercise helps Patients with Non-alcoholic Fatty Liver Disease. Int. J. Sports Med. 2015, 36, 848–852. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, P.; Kuljiratitikal, K.; Aksornchanya, O.; Chaiyasoot, K.; Bandidniyamanon, W.; Charatcharoenwitthaya, N. Moderate-Intensity Aerobic vs. Resistance Exercise and Dietary Modification in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. Clin. Transl. Gastroenterol. 2021, 12, e00316. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Sachinwalla, T.; Walton, D.W.; Smith, K.; Armstrong, A.; Thompson, M.W.; George, J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009, 50, 1105–1112. [Google Scholar] [CrossRef]
- Hallsworth, K.; Fattakhova, G.; Hollingsworth, K.G.; Thoma, C.; Moore, S.; Taylor, R.; Day, C.P.; Trenell, M.I. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011, 60, 1278–1283. [Google Scholar] [CrossRef]
- Lee, S.; Bacha, F.; Hannon, T.; Kuk, J.L.; Boesch, C.; Arslanian, S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: A randomized, controlled trial. Diabetes 2012, 61, 2787–2795. [Google Scholar] [CrossRef]
- Sullivan, S.; Kirk, E.P.; Mittendorfer, B.; Patterson, B.W.; Klein, S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 2012, 55, 1738–1745. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Buch, A.; Yeshua, H.; Vaisman, N.; Webb, M.; Harari, G.; Kis, O.; Fliss-Isakov, N.; Izkhakov, E.; Halpern, Z.; et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J. Gastroenterol. 2014, 20, 4382–4392. [Google Scholar] [CrossRef]
- Hallsworth, K.; Thoma, C.; Hollingsworth, K.G.; Cassidy, S.; Anstee, Q.M.; Day, C.P.; Trenell, M.I. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Sci. 2015, 129, 1097–1105. [Google Scholar] [CrossRef]
- Keating, S.E.; Hackett, D.A.; Parker, H.M.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Baker, M.K.; Chuter, V.H.; Caterson, I.D.; George, J.; et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015, 63, 174–182. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Shojaee-Moradie, F.; Sprung, V.S.; Jones, H.; Pugh, C.J.A.; Richardson, P.; Kemp, G.J.; Barrett, M.; Jackson, N.C.; Thomas, E.L.; et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin. Sci. 2016, 130, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; He, J.; Pan, L.-L.; Ma, Z.-M.; Han, C.-K.; Chen, C.-S.; Chen, Z.; Han, H.-W.; Chen, S.; Sun, Q.; et al. Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Thoma, C.; Hallsworth, K.; Cassidy, S.; Hardy, T.; Burt, A.D.; Tiniakos, D.; Hollingsworth, K.G.; Taylor, R.; Day, C.P.; et al. Exercise Reduces Liver Lipids and Visceral Adiposity in Patients with Nonalcoholic Steatohepatitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2017, 15, 96–102.e3. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef] [PubMed]
- von Loeffelholz, C.; Jahreis, G. Einfluss von Widerstandstraining auf Parameter des Glukosestoffwechsels bei Gesunden, Typ-2-Diabetikern und Individuen mit Anzeichen einer Insulinresistenz. Aktuelle Ernährungsmedizin 2005, 30, 261–272. [Google Scholar] [CrossRef]
- Pugh, C.J.A.; Sprung, V.S.; Jones, H.; Richardson, P.; Shojaee-Moradie, F.; Umpleby, A.M.; Green, D.J.; Cable, N.T.; Trenell, M.I.; Kemp, G.J.; et al. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int. J. Obes. 2016, 40, 1927–1930. [Google Scholar] [CrossRef]
- von Loeffelholz, C.; Birkenfeld, A. Endotext: The Role of Non-Exercise Activity Thermogenesis in Human Obesity; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., Kaltsas, G., Koch, C., Kopp, P., Korbonits, M., Kovacs, C.S., Kuohung, W., Laferrère, B., Levy, M., McGee, E.A., McLachlan, R., Morley, J.E., New, M., Purnell, J., Sahay, R., Singer, F., Sperling, M.A., Stratakis, C.A., Trence, D.L., Wilson, D.P., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2018.
- Dasgupta, K.; Chan, C.; Da Costa, D.; Pilote, L.; de Civita, M.; Ross, N.; Strachan, I.; Sigal, R.; Joseph, L. Walking behaviour and glycemic control in type 2 diabetes: Seasonal and gender differences—Study design and methods. Cardiovasc. Diabetol. 2007, 6, 1. [Google Scholar] [CrossRef]
- Hawley, J.A.; Gibala, M.J. Exercise intensity and insulin sensitivity: How low can you go? Diabetologia 2009, 52, 1709–1713. [Google Scholar] [CrossRef][Green Version]
- Chung, N.; Park, M.-Y.; Kim, J.; Park, H.-Y.; Hwang, H.; Lee, C.-H.; Han, J.-S.; So, J.; Park, J.; Lim, K. Non-exercise activity thermogenesis (NEAT): A component of total daily energy expenditure. J. Exerc. Nutr. Biochem. 2018, 22, 23–30. [Google Scholar] [CrossRef]
- Silva, A.M.; Júdice, P.B.; Carraça, E.V.; King, N.; Teixeira, P.J.; Sardinha, L.B. What is the effect of diet and/or exercise interventions on behavioural compensation in non-exercise physical activity and related energy expenditure of free-living adults? A systematic review. Br. J. Nutr. 2018, 119, 1327–1345. [Google Scholar] [CrossRef]
- Alahmadi, M.A.; Hills, A.P.; King, N.A.; Byrne, N.M. Exercise intensity influences nonexercise activity thermogenesis in overweight and obese adults. Med. Sci. Sports Exerc. 2011, 43, 624–631. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Chen, C.-H.; Wu, M.-H.; Ding, Y.-F. Nonexercise Activity Thermogenesis-Induced Energy Shortage Improves Postprandial Lipemia and Fat Oxidation. Life 2020, 10, 166. [Google Scholar] [CrossRef] [PubMed]
| Author | Design | Intervention and Methods | Outcomes | Drop Out |
|---|---|---|---|---|
| [128] | Randomized, placebo controlled n = 23 sedentary NAFLD/MAFLD patients | 1 month supervised aerobic cycling exercise vs. stretching (placebo) IR (HOMA-IR), dietary record monitoring, visceral adipose volume, liver fat (1H-MR spectroscopy) | Significant reduction of liver fat and visceral adipose volume (intervention group) under conditions of unaltered dietary habits No effects on IR No effects on body weight | Drop out/excluded from analysis: n = 4 (17%) |
| [129] | Randomized, controlled n = 21 NAFLD/MAFLD patients | Partially supervised resistance exercise (2 months) vs. control Glucose control/IR (fsOGTT-AUC, HOMA-IR), liver lipids and abdominal fat (1H-MR spectroscopy), body weight | Significant reduction of liver fat (intervention group) Improved glycemic control and IR (intervention group) No effect on body weight and body fat | Drop out/excluded from analysis: n = 2 (9%) |
| [130] | Randomized, controlled n = 45 obese adolescent males | Supervised aerobic vs. resistance exercise vs. control (3 months) Insulin sensitivity (HE and HH clamp), liver fat (1H-MR spectroscopy, in subgroups), abdominal fat and body fat (whole-body magnetic resonance imaging) | Body weight stabilization (both intervention groups) compared to controls (weight gain) Significant reduction of liver fat and visceral adipose volume (intervention groups) Improved insulin sensitivity (resistance exercise group) | Drop out/excluded from analysis: n = 3 (7%) |
| [131] | Randomized, controlled, n = 33 NAFLD/MAFLD Patients | Partially supervised aerobic exercise vs. control (4 months) NAFLD/MAFLD-related Lipoprotein kinetics (tracer methods), body composition (DEXA), liver fat (1H-MR spectroscopy) | Significant reduction of liver fat (intervention group) No effect on body weight and body fat No effect on lipoprotein kinetics | Drop out/excluded from analysis: n = 15 (45%) |
| [132] | Randomized, controlled, n = 82 NAFLD/MAFLD Patients | 3 months of partially Supervised resistance Exercise vs. placebo (stretching) Body composition (DEXA), dietary record monitoring, liver steatosis (HRI) | Significant reduction of liver fat, body fat and trunc fat mass (intervention group) under conditions of unaltered dietary habits | Drop out/excluded from analysis: n = 18 (22%) |
| [133] | Randomized, controlled n = 29 NAFLD/MAFLD patients | Partially supervised high intensity interval cycling (3 months) vs. control Glucose control/IR (fsOGTT-AUC, HOMA-IR), body composition (air displacement plethysmography), liver fat (1H-MR spectroscopy) | Significant reduction of liver fat (intervention group) Improved 2-h glucose, no effect on IR Body fat and body weight reduction (intervention group) | Drop out/excluded from analysis: n = 6 (21%) |
| [134] | Randomized, placebo controlled n = 48 sedentary NAFLD/MAFLD patients | 2 months supervised aerobic cycling exercise (subgroups with varying volume and intensity) vs. stretching/self massage/ fitness ball (placebo) Dietary monitoring, visceral adipose volume (magnetic resonance imaging), liver fat (1H-MR spectroscopy) | Significant reduction of liver fat and visceral adipose volume (intervention group) under conditions of unaltered dietary habits | Drop out: n = 0 (0%) |
| [135] | Randomized, controlled n = 69 NAFLD/MAFLD patients | Supervised aerobic exercise (4 months) vs. counselling (control) Peripheral insulin sensitivity, dietary monitoring, hepatic glucose production (HE clamp in a subgroup), abdominal fat (magnetic resonance imaging), liver fat (1H-MR spectroscopy) | Significant reduction of liver fat (supervised exercise) (p = 0.05) under conditions of unaltered dietary habits Improved glycemic control and peripheral insulin sensitivity (supervised exercise) Body weight and abdominal fat mass reduction (supervised exercise) | Drop out/excluded from analysis: n = 19 (28%) |
| [136] | Randomized, controlled n = 220 NAFLD/MAFLD patients | 6 months of vigorous- moderate exercise (jogging and brisk walking) vs. 12 months of moderate exercise (brisk walking) vs. control (no exercise) Liver fat (1H-MR spectroscopy after 6 and 12 month), body weight, waist circumference, body fat | Significant reduction of liver fat after 6 and 12 months (in both exercise groups) Reduced body fat (vigorous- moderate exercise group after 6 and 12 months) Reduced waist circumference (both exercise groups after 12 months) Reduced body weight (both exercise groups after 12 months) | Drop out/excluded from analysis: n = 9 after 6 months (4%), n = 14 after 12 months (6%) |
| [137] | Randomized, controlled, n = 26 sedentary NASH patients | Supervised combined aerobic and resistance exercise (3 months) vs. standard care (control) Body composition (air displacement plethysmography), glycemic control/IR (fsOGTT-AUC, HbA1c, HOMA-IR), circulating markers of liver fibrosis, liver fat (1H-MR spectroscopy) | Significant reduction of liver fat and visceral adipose tissue (intervention group) No effects on glycemic control or IR No effects on body composition No effects on circulatory markers of fibrosis | Drop out/excluded from analysis: n = 2 (8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Loeffelholz, C.; Roth, J.; Coldewey, S.M.; Birkenfeld, A.L. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines 2021, 9, 1853. https://doi.org/10.3390/biomedicines9121853
von Loeffelholz C, Roth J, Coldewey SM, Birkenfeld AL. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines. 2021; 9(12):1853. https://doi.org/10.3390/biomedicines9121853
Chicago/Turabian Stylevon Loeffelholz, Christian, Johannes Roth, Sina M. Coldewey, and Andreas L. Birkenfeld. 2021. "The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease" Biomedicines 9, no. 12: 1853. https://doi.org/10.3390/biomedicines9121853
APA Stylevon Loeffelholz, C., Roth, J., Coldewey, S. M., & Birkenfeld, A. L. (2021). The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines, 9(12), 1853. https://doi.org/10.3390/biomedicines9121853






