Streptozotocin-Induced Diabetes in a Mouse Model (BALB/c) Is Not an Effective Model for Research on Transplantation Procedures in the Treatment of Type 1 Diabetes
Abstract
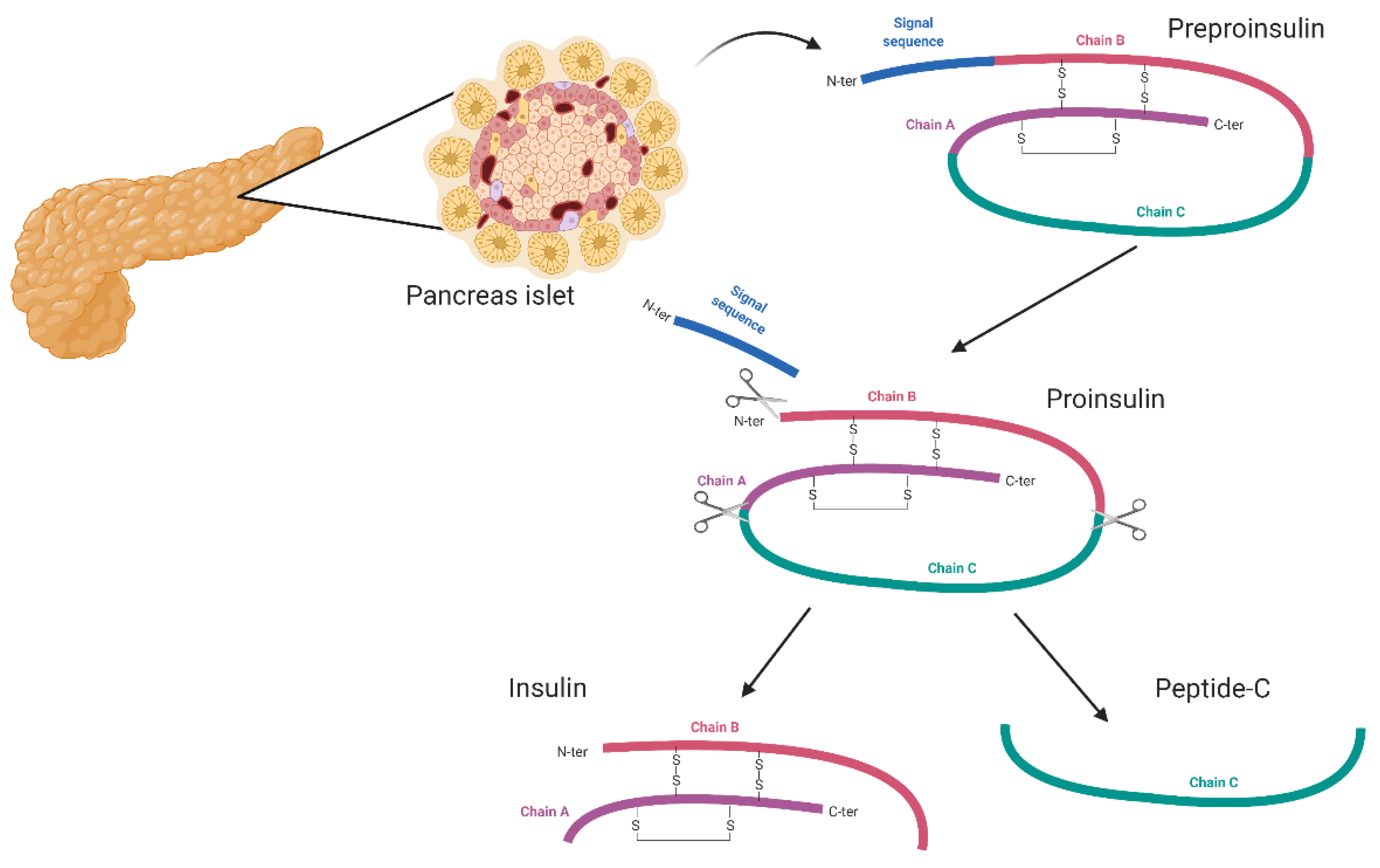
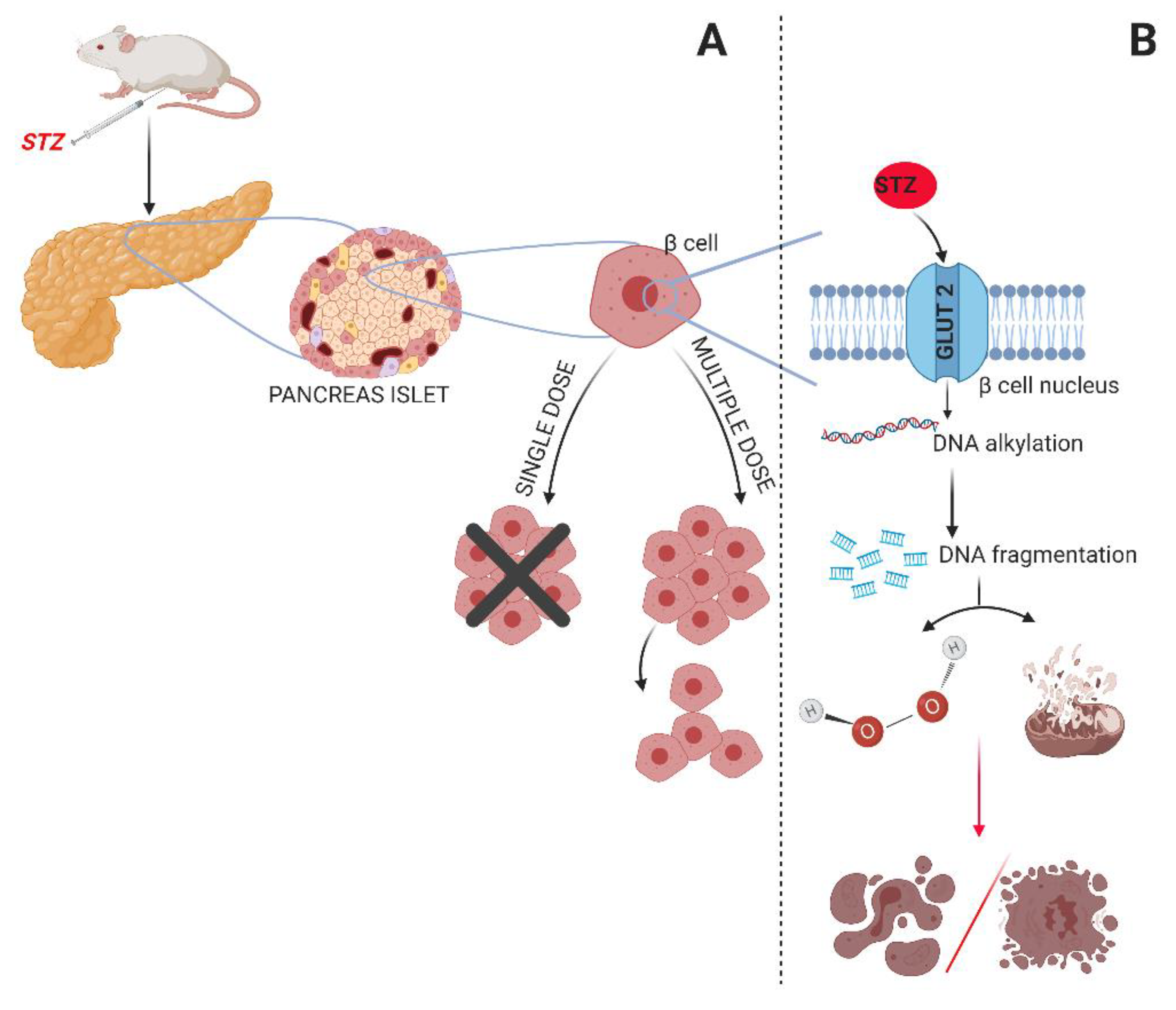
1. Introduction
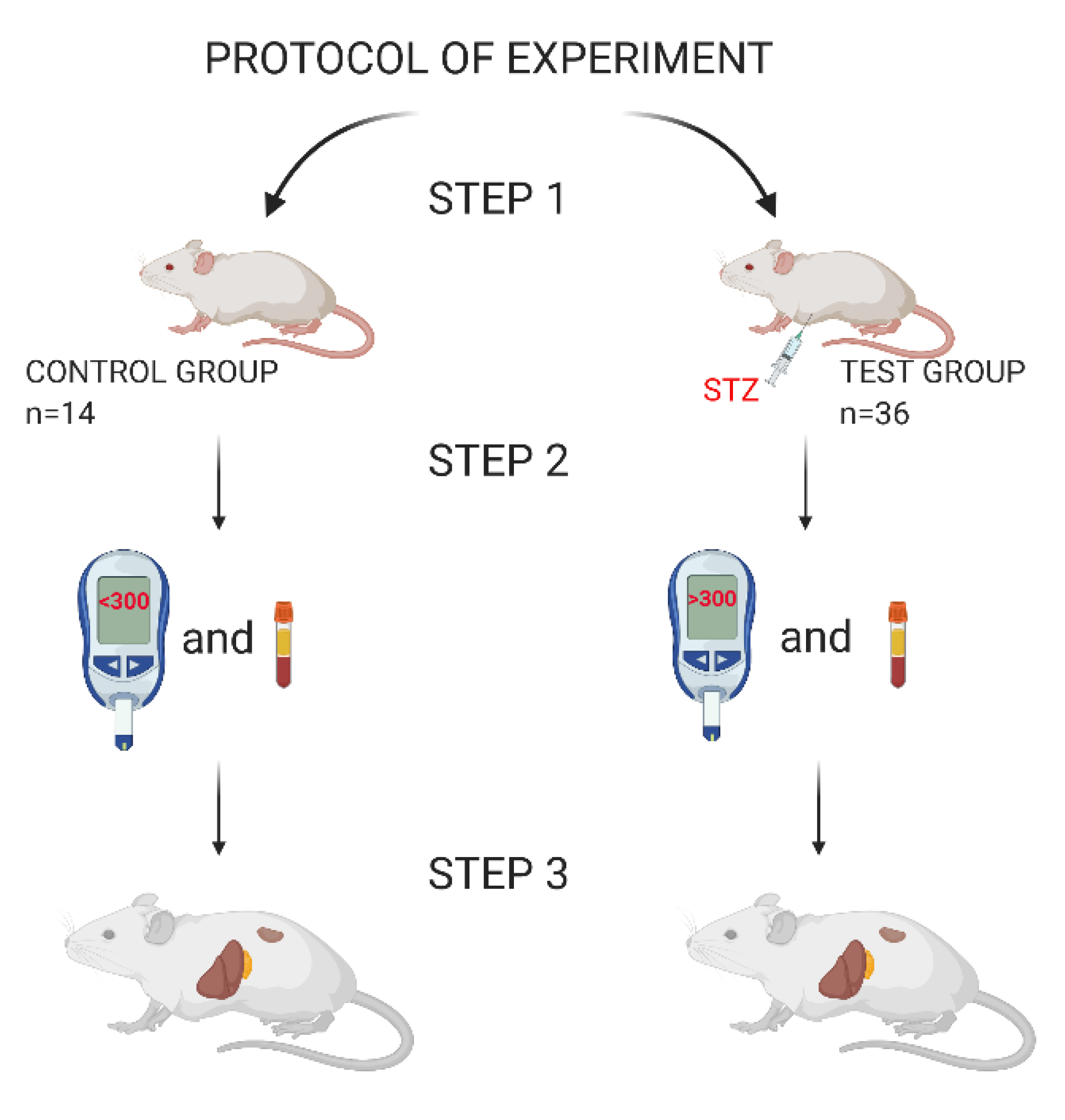
2. Materials and Methods
2.1. Materials
2.2. Elisa Kit
2.3. Glucose Measurement
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
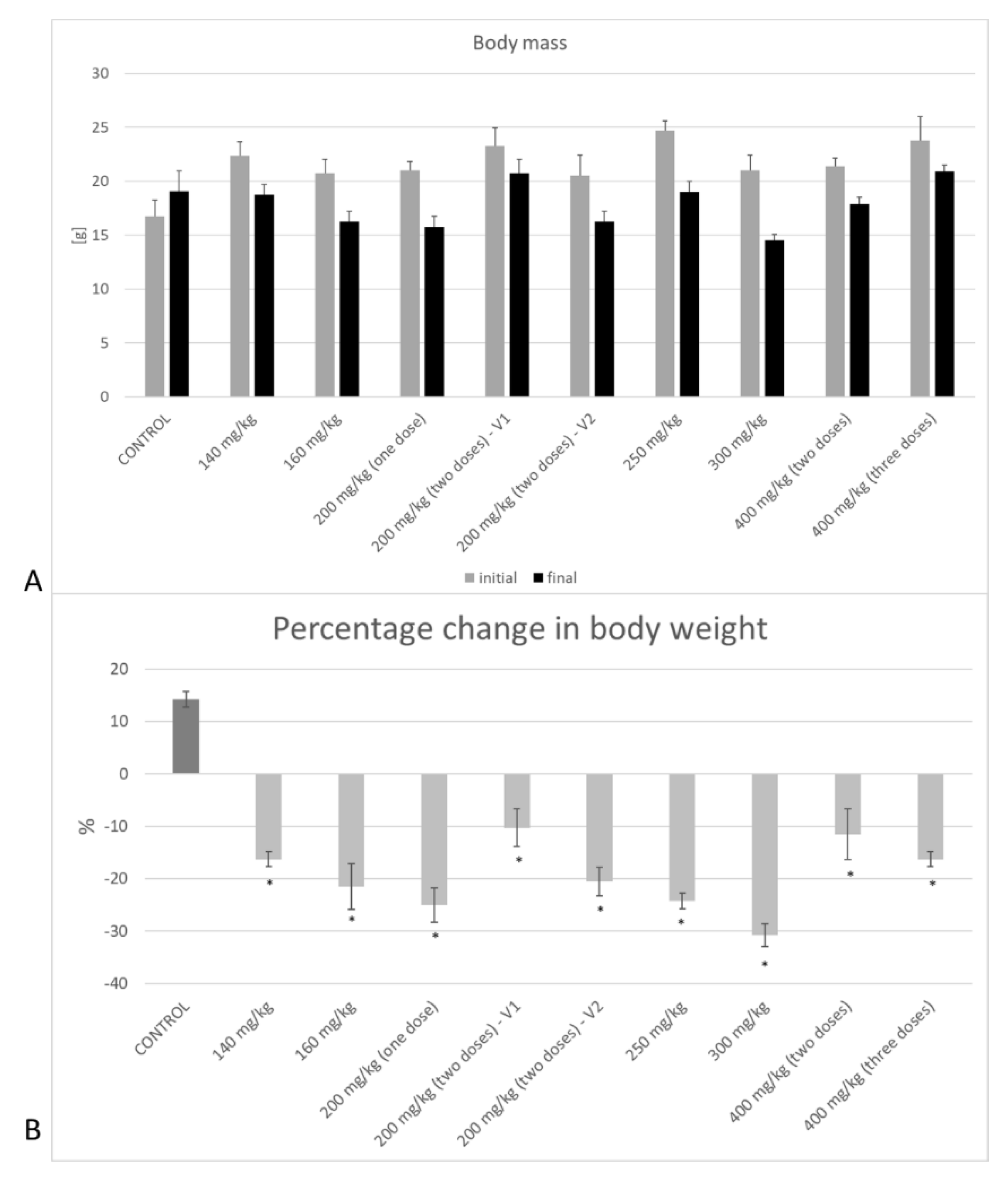
3.1. Body Weight Control
3.2. Assessment of Glucose Concentration
3.3. Assessment of Serum C-Peptide Level
3.4. STZ-Induced Diabetic Mice Showed Impairments in Pancreatic Islets, Glomeruli, and the Liver
3.4.1. Islets Analysis
3.4.2. Kidney and Liver Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- International Diabetes Federation—Facts & Figures. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 10 November 2020).
- Rocha, I.R.C.; Perez-Reyes, E.; Chacur, M. Effect of photobiomodulation on mitochondrial dynamics in peripheral nervous system in streptozotocin-induced type 1 diabetes in rats. Photochem. Photobiol. Sci. 2021, 20, 293–301. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Yosten, G.L.C.; Maric-Bilkan, C.; Luppi, P.; Wahren, J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E955. [Google Scholar] [CrossRef]
- Leighton, E.; Sainsbury, C.A.; Jones, G.C. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Larsson, C. C-peptide: New findings and therapeutic possibilities. Diabetes Res. Clin. Pract. 2015, 107, 309–319. [Google Scholar] [CrossRef]
- Faber, O.K.; Rubenstein, A.H.; Rossing, N.; Faber, K.; Hagen, C.; Binder, C.; Blix, P.M.; Kuzuya, H.; Horwitz, D.L. Kinetics of human connecting peptide in normal and diabetic subjects. Kinetics of Human Connecting Peptide in Normal and Diabetic Subjects. J. Clin. Investig. 1978, 62, 197–203. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Tronier, B.; Bülow, J.B. Kinetics of circulating endogenous insulin, C-peptide, and proinsulin in fasting nondiabetic man. Metabolism 1987, 36, 463–468. [Google Scholar] [CrossRef]
- Melles, E.; Jörnvall, H.; Tryggvason, S.; Gemzell Danielsson, K.; Ekberg, K.; Tryggvason, K.; Wahren, J.; Bergman, T. Degradation of proinsulin C-peptide in kidney and placenta extracts by a specific endoprotease activity. Cell. Mol. Life Sci. 2004, 61, 2979–2982. [Google Scholar] [CrossRef]
- Bansal, R.; Ahmad, N.; Kidwai, J.R. Alloxan-glucose interaction: Effect on incorporation of14C-leucine into pancreatic islets of rat. Acta Diabetol. Lat. 1980, 17, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L.; Janecek, J.L.; Kittredge, J.A.; Hering, B.J.; Schuurman, H.J. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comp. Med. 2011, 61, 356–360. [Google Scholar] [PubMed]
- Nahdi, A.M.T.A.; John, A.; Raza, H. Elucidation of Molecular Mechanisms of Streptozotocin-Induced Oxidative Stress, Apoptosis, and Mitochondrial Dysfunction in Rin-5F Pancreatic β-Cells. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, Y.; Wei, L.; Jin, X.; Zeng, L.; Ren, Y.; Zhang, J.; Wang, L.; Li, H.; Lu, Y.; et al. Treatment and risk factor analysis of hypoglycemia in diabetic rhesus monkeys. Exp. Biol. Med. 2011, 236, 212–218. [Google Scholar] [CrossRef]
- Islam, M.S.; Loots, D.T. Experimental rodent models of type 2 diabetes: A review. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Karunanayake, E.H.; Baker, J.R.J.; Christian, R.A.; Hearse, D.J.; Mellows, G. Autoradiographic study of the distribution and cellular uptake of (14C)—Streptozotocin in the rat. Diabetologia 1976, 12, 123–128. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, S.P.; Woodley, S.E.; Patton, N.J.; Wilson, G.L. Mechanisms of Nitrosourea-Induced β-Cell Damage: Alterations in DNA. Diabetes 1986, 35, 866–872. [Google Scholar] [CrossRef]
- Murata, M.; Takahashi, A.; Saito, I.; Kawanishi, S. Site-specific DNA methylation and apoptosis: Induction by diabetogenic streptozotocin. Biochem. Pharmacol. 1999, 57, 881–887. [Google Scholar] [CrossRef]
- Yamamoto, H.; Uchigata, Y.; Okamoto, H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP–ribose) synthetase in pancreatic islets. Nature 1981, 294, 284–286. [Google Scholar] [CrossRef]
- Sandler, S.; Swenne, I. Streptozotocin, but not alloxan, induces DNA repair synthesis in mouse pancreatic islets in vitro. Diabetologia 1983, 25, 444–447. [Google Scholar] [CrossRef]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Nukatsuka, M.; Yoshimura, Y.; Nishida, M.; Kawada, J. Importance of the concentration of ATP in rat pancreatic β cells in the mechanism of streptozotocin-induced cytotoxicity. J. Endocrinol. 1990, 127, 161–165. [Google Scholar] [CrossRef]
- Van Dyke, K.; Ghareeb, E.; Van Dyke, M.; Sosa, A.; Hoeldtke, R.D.; Van Thiel, D.H. Luminescence experiments involved in the mechanism of streptozotocin diabetes and cataract formation. Luminescence 2008, 23, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Friederich, M.; Hansell, P.; Palm, F. Diabetes; 2009, undefined Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr. Diabetes Rev. 2009, 5, 120–144. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Silva, A.M.; Fortuna, A.; García, M.L.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Strategies for In Vivo Administration: Part-II. J. Clin. Med. 2019, 8, 1332. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 1–7. [Google Scholar] [CrossRef]
- Hayashi, K.; Kojima, R.; Ito, M. Strain differences in the diabetogenic activity of streptozotocin in mice. Biol. Pharm. Bull. 2006, 29, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.J.; Lee, D.D.; Tao, J.; Wilson, R.A.; Park, S.Y.; Bell, G.I.; Chong, A.S. Glycemic control promotes pancreatic beta-cell regeneration in streptozotocin-induced diabetic mice. PLoS ONE 2010, 5, e8749. [Google Scholar] [CrossRef]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab. Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Brouwers, B.; Pruniau, V.P.E.G.; Cauwelier, E.J.G.; Schuit, F.; Lerut, E.; Ectors, N.; Declercq, J.; Creemers, J.W.M. Phlorizin pretreatment reduces acute renal toxicity in a mouse model for diabetic nephropathy. J. Biol. Chem. 2013, 42, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Brosius, F.; The University of Michigan Medical Center. High.-Dose Streptozotocin Induction Protocol (Mouse); The University of Michigan Medical Center: Ann Arbor, MI, USA, 2015. [Google Scholar]
- Wang, Z.; Dohle, C.; Friemann, J.; Green, B.S.; Gleichmann, H. Prevention of high- and low-dose STZ-induced diabetes with D-glucose and 5-thio-D-glucose. Diabetes 1993, 42, 420–428. [Google Scholar] [CrossRef]
- Like, A.A.; Rossini, A.A. Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science 1976, 193, 415–417. [Google Scholar] [CrossRef]
- Beattie, G.; Lannom, R.; Lipsick, J.; Kaplan, N.O.; Osler, A.G. Streptozotocin-induced diabetes in athymic and conventional BALB/c mice. Diabetes 1980, 29, 146–150. [Google Scholar] [CrossRef]
- Weide, L.G.; Lacy, P.E. Low-dose streptozocin-induced autoimmune diabetes in islet transplantation model. Diabetes 1991, 40, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Huang, T.; Yang, L.; Xu, L.; Ma, L. Human umbilical cord Wharton jelly cells promote extra-pancreatic insulin formation and repair of renal damage in STZ-induced diabetic mice. Cell Commun. Signal. 2017, 15, 43. [Google Scholar] [CrossRef]
- Robinson, M.M.; Dasari, S.; Karakelides, H.; Bergen, H.R.; Nair, K.S. Release of skeletal muscle peptide fragments identifies individual proteins degraded during insulin deprivation in type 1 diabetic humans and mice. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E628–E637. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Sunkari, V.G.; Kaber, G.; Hasbun, S.; Lam, D.N.; Speake, C.; Sanda, S.; McLaughlin, T.L.; Wight, T.N.; Long, S.R.; et al. Hyaluronan levels are increased systemically in human type 2 but not type 1 diabetes independently of glycemic control. Matrix Biol. 2019, 80, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Safley, S.A.; Kenyon, N.S.; Berman, D.M.; Barber, G.F.; Willman, M.; Duncanson, S.; Iwakoshi, N.; Holdcraft, R.; Gazda, L.; Thompson, P.; et al. Microencapsulated adult porcine islets transplanted intraperitoneally in streptozotocin-diabetic non-human primates. Xenotransplantation 2018, 25, e12450. [Google Scholar] [CrossRef]
- Sainchez-Andres, J.V.; Gomis, A.; Valdeolmillos, M. The electrical activity of mouse pancreatic fl-cells recorded in vivo shows glucose-dependent oscillations. J. Physiol. 1995, 486, 223–228. [Google Scholar] [CrossRef]
- Premilovac, D.; Gasperini, R.J.; Sawyer, S.; West, A.; Keske, M.A.; Taylor, B.V.; Foa, L. A New Method for Targeted and Sustained Induction of Type 2 Diabetes in Rodents. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Jiang, T.; Yang, S.S.; Yang, Y. Pioglitazone ameliorates intracerebral insulin resistance and tau-protein hyperphosphorylation in rats with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2013, 121, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.B.H.; Vinayagam, K.S.; Moorthy, B.T.; Palanivelu, S.; Panchanatham, S. Anti-inflammatory and anti-hyperlipidemic effect of Semecarpus anacardium in a High fat diet: STZ-induced Type 2 diabetic rat model. Inflammopharmacology 2012, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.M.; Abdel-Aziz, A.; Gabr, M.; Hemmaid, K.Z. Myocardial and metabolic dysfunction in type 2 diabetic rats: Impact of ghrelin. Can. J. Physiol. Pharmacol. 2011, 90, 99–111. [Google Scholar] [CrossRef]
- Danda, R.S.; Habiba, N.M.; Rincon-Choles, H.; Bhandari, B.K.; Barnes, J.L.; Abboud, H.E.; Pergola, P.E. Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int. 2005, 68, 2562–2571. [Google Scholar] [CrossRef]
- Wang, L.; Shang, Q.; Guo, W.; Wu, X.; Wu, L.; Wu, L.; Chen, T. Evaluation of the hypoglycemic effect of probiotics via directly consuming glucose in intestines of STZ-induced diabetic mice and glucose water-induced diabetic mice. J. Funct. Foods 2020, 64, 103614. [Google Scholar] [CrossRef]
- Ibuki, F.K.; Bergamaschi, C.T.; da Silva Pedrosa, M.; Nogueira, F.N. Effect of vitamin C and E on oxidative stress and antioxidant system in the salivary glands of STZ-induced diabetic rats. Arch. Oral Biol. 2020, 116, 104765. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.A.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Hu, W.; Hara, H.; Dai, Y.; Cai, Z.; Mou, L. Induction of diabetes in cynomolgus monkey with one shot of analytical grade streptozotocin. Anim. Model. Exp. Med. 2020, 3, 79–86. [Google Scholar] [CrossRef]
- Jin, X.; Zeng, L.; He, S.; Chen, Y.; Tian, B.; Mai, G.; Yang, G.; Wei, L.; Zhang, Y.; Li, H.; et al. Comparison of single high-dose streptozotocin with partial pancreatectomy combined with low-dose streptozotocin for diabetes induction in rhesus monkeys. Exp. Biol. Med. 2010, 235, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Fousteri, G.; Ippolito, E.; Ahmed, R.; Hamad, A.R.A. Beta-cell specific autoantibodies: Are they just an indicator of type 1 diabetes? Curr. Diabetes Rev. 2017, 13, 322. [Google Scholar] [CrossRef]
- Mcevoy, R.C.; Thomas, N.M.; Hellerstrtim, C.; Ginsberg-Fellner, F.; Moran, T.M. Multiple low-dose streptozotocin-induced diabetes in the mouse: Further evidence for involvement of an anti-B cell cytotoxic cellular auto-immune response. Diabetologia 1987, 30, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Bottazzo, G.F.; Dean, B.M.; McNally, J.M.; MacKay, E.H.; Swift, P.G.F.; Gamble, D.R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N. Engl. J. Med. 1985, 313, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, U.; Falkenberg, F.W.; Kolb, H. Genetic control of low-dose streptozotocin-induced autoimmune diabetes in mice. J. Immunol. 1983, 130, 1719–1722. [Google Scholar]
- Kiesel, U.; Kolb, H. Low-dose streptozotocin-induced autoimmune diabetes is under the genetic control of the major histocompatibility complex in mice. Diabetologia 1982, 23, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Drew, L. How stem cells could fix type 1 diabetes. Nature 2021, 595, S64–S66. [Google Scholar] [CrossRef]
- Cantarelli, E.; Citro, A.; Marzorati, S.; Melzi, R.; Scavini, M.; Piemonti, L. Murine animal models for preclinical islet transplantation: No model fits all (research purposes). Islets 2013, 5, 79. [Google Scholar] [CrossRef]
- Idaszek, J.; Volpi, M.; Paradiso, A.; Nguyen Quoc, M.; Górecka, Ż.; Klak, M.; Tymicki, G.; Berman, A.; Wierzbicki, M.; Jaworski, S.; et al. Alginate-based tissue-specific bioinks for multi-material 3D-bioprinting of pancreatic islets and blood vessels: A step towards vascularized pancreas grafts. Bioprinting 2021, 24, e00163. [Google Scholar] [CrossRef]
- Dorman, H.; Meschino, W.S.; Allanson, J.; Blaine, S.M.; Cremin, C.; Gibbons, C.A.; Honeywell, C.; Permaul, J.; Carroll, J.C. Type 2 Diabetes; Methods in Molecular Biology; Stocker, C., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 560, ISBN 978-1-934115-15-2. [Google Scholar]
- Shpakov, A.O.; Granstrem, O.K. C-peptide physiological effects. Ross. Fiziol. Zh. Im. I. M. Sechenova 2013, 99, 196–211. [Google Scholar]
- Poteryaeva, O.N.; Usynin, I.F. Molecular mechanisms of action and physiological effects of the proinsulin C-peptide (a systematic review). Biomed. Khim. 2020, 66, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Poteryaeva, O.N.; Usynin, I.F. Molecular Mechanisms of Action and Physiological Effects of the Proinsulin C-Peptide (a Systematic Review). Biochem. Suppl. Ser. B Biomed. Chem. 2021, 15, 27–39. [Google Scholar] [CrossRef]
- Abdollahi, M.; Hosseini, A. Streptozotocin. Encycl. Toxicol. Third Ed. 2014, 4, 402–404. [Google Scholar] [CrossRef]
- Uil, M.; Scantlebery, A.M.L.; Butter, L.M.; Larsen, P.W.B.; De Boer, O.J.; Leemans, J.C.; Florquin, S.; Roelofs, J.J.T.H. Combining streptozotocin and unilateral nephrectomy is an effective method for inducing experimental diabetic nephropathy in the “resistant” C57Bl/6J mouse strain. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Naeem-ul-Hassan Naqvi, S.; Ahmed, M.; Kaimkhani, Z.A. Altered Kidney Morphology and Enzymes in Streptozotocin Induced Diabetic Rats. Int. J. Morphol. 2009, 27, 783–790. [Google Scholar] [CrossRef][Green Version]
- Samnegård, B.; Jacobson, S.H.; Jaremko, G.; Johansson, B.L.; Ekberg, K.; Isaksson, B.; Eriksson, L.; Wahren, J.; Sjöquist, M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol. Dial. Transplant. 2005, 20, 532–538. [Google Scholar] [CrossRef]
- Hills, C.E.; Al-Rasheed, N.; Al-Rasheed, N.; Willars, G.B.; Brunskill, N.J. C-peptide reverses TGF-β1-induced changes in renal proximal tubular cells: Implications for treatment of diabetic nephropathy. Am. J. Physiol.-Ren. Physiol. 2009, 296, 614–621. [Google Scholar] [CrossRef]
- Wahren, J. C-peptide and the pathophysiology of microvascular complications of diabetes. J. Intern. Med. 2017, 281, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Motyl, K.; McCabe, L.R. Streptozotocin, type i diabetes severity and bone. Biol. Proced. Online 2009, 11, 296–315. [Google Scholar] [CrossRef]
- Coe, L.M.; Zhang, J.; McCabe, L.R. Both spontaneous Ins2+/- and streptozotocin-induced type I diabetes cause bone loss in young mice. J. Cell. Physiol. 2013, 228, 689–695. [Google Scholar] [CrossRef]
- Botolin, S.; Faugere, M.C.; Malluche, H.; Orth, M.; Meyer, R.; McCabe, L.R. Increased bone adiposity and peroxisomal proliferator-activated receptor-γ2 expression in type I diabetic mice. Endocrinology 2005, 146, 3622–3631. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.R. Understanding the pathology and mechanisms of type I diabetic bone loss. J. Cell. Biochem. 2007, 102, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, J.L.; Bunn, R.C.; Liu, L.; Wahl, E.C.; Coleman, H.N.; Cockrell, G.E.; Perrien, D.S.; Lumpkin, C.K.; Thrailkill, K.M. Runt-related transcription factor 2 (RUNX2) and RUNX2-related osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology 2008, 149, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Koulmanda, M.; Qipo, A.; Auchincloss, H.; Smith, R.N. Effects of streptozotocin on autoimmune diabetes in NOD mice. Clin. Exp. Immunol. 2003, 134, 210–216. [Google Scholar] [CrossRef]
- Hugues, S.; Mougneau, E.; Ferlin, W.; Jeske, D.; Hofman, P.; Homann, D.; Beaudoin, L.; Schrike, C.; Von Herrath, M.; Lehuen, A.; et al. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic β cells. Immunity 2002, 16, 169–181. [Google Scholar] [CrossRef]
- Yin, D.; Tao, J.; Lee, D.D.; Shen, J.; Hara, M.; Lopez, J.; Kuznetsov, A.; Philipson, L.H.; Chong, A.S. Recovery of islet β-cell function in streptozotocin-induced diabetic mice: An indirect role for the spleen. Diabetes 2006, 55, 3256–3263. [Google Scholar] [CrossRef]
- Nicoletti, F.; Di Marco, R.; Papaccio, G.; Conget, I.; Gomis, R.; Bernardini, R.; Sims, J.E.; Shoenfeld, Y.; Bendtzen, K. Essential pathogenic role of endogenous IL-18 in murine diabetes induced by multiple low doses of streptozotocin. Prevention of hyperglycemia and insulitis by a recombinant IL-18-binding protein: Fc construct. Eur. J. Immunol. 2003, 33, 2278–2286. [Google Scholar] [CrossRef]
- Sun, N.; Yang, G.; Zhao, H.; Savelkoul, H.F.J.; An, L. Multidose streptozotocin induction of diabetes in BALB/c mice induces a dominant oxidative macrophage and a conversion of TH1 to T H2 phenotypes during disease progression. Mediators Inflamm. 2005, 2005, 202–209. [Google Scholar] [CrossRef]
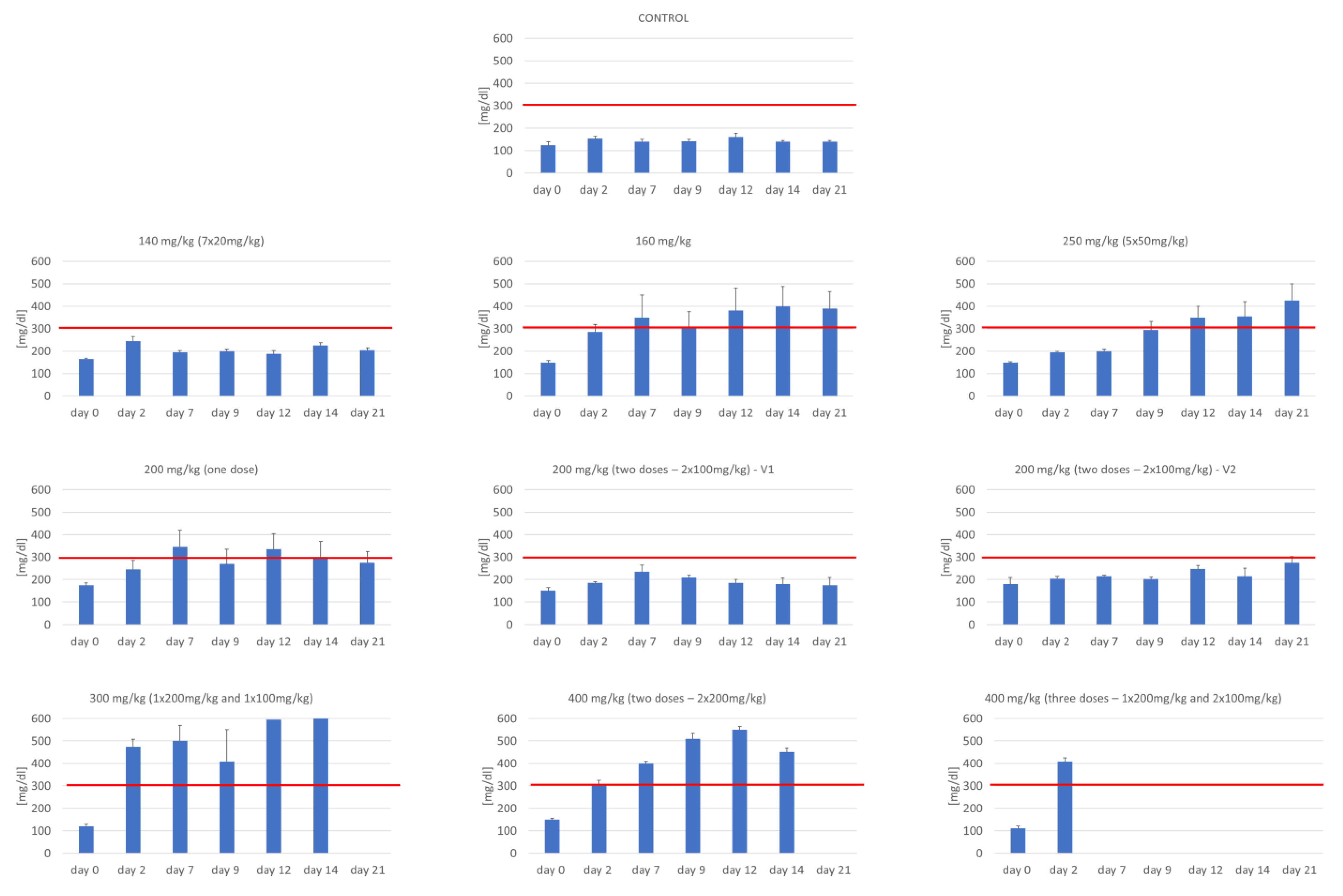
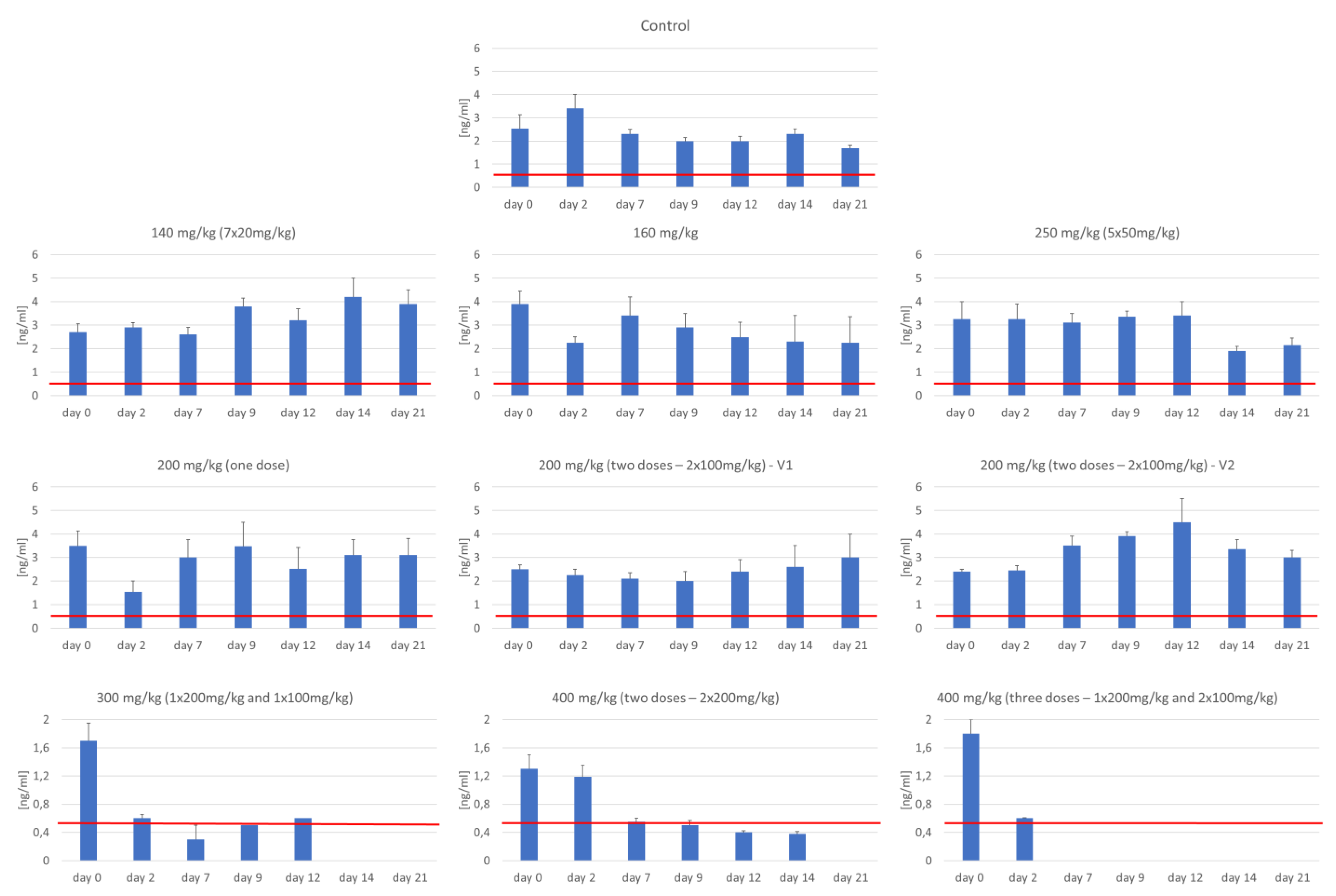



| No of Group | Group | Number (n) | Total Dose of STZ (mg/kg) | Dosage—Experiment Day (mg/kg b.w.) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||
| 1 | 400 mg/kg (three doses) | 4 | 400 | 200 | 100 | 100 | - | - | - | - |
| 2 | 400 mg/kg (two doses) | 4 | 400 | 200 | - | - | - | - | - | 200 |
| 3 | 300 mg/kg | 4 | 300 | 200 | 100 | - | - | - | - | - |
| 4 | 250 mg/kg | 4 | 250 | 50 | 50 | 50 | 50 | 50 | - | - |
| 5 | 200 mg/kg (one dose) | 4 | 200 | 200 | - | - | - | - | - | - |
| 6 | 200 mg/kg (two doses)–V1 | 4 | 200 | 100 | 100 | - | - | - | - | - |
| 7 | 200 mg/kg (two doses)–V2 | 4 | 200 | 100 | 100 | - | - | - | - | - |
| 8 | 160 mg/kg | 4 | 160 | 160 | - | - | - | - | - | - |
| 9 | 140 mg/kg | 4 | 140 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wszola, M.; Klak, M.; Kosowska, A.; Tymicki, G.; Berman, A.; Adamiok-Ostrowska, A.; Olkowska-Truchanowicz, J.; Uhrynowska-Tyszkiewicz, I.; Kaminski, A. Streptozotocin-Induced Diabetes in a Mouse Model (BALB/c) Is Not an Effective Model for Research on Transplantation Procedures in the Treatment of Type 1 Diabetes. Biomedicines 2021, 9, 1790. https://doi.org/10.3390/biomedicines9121790
Wszola M, Klak M, Kosowska A, Tymicki G, Berman A, Adamiok-Ostrowska A, Olkowska-Truchanowicz J, Uhrynowska-Tyszkiewicz I, Kaminski A. Streptozotocin-Induced Diabetes in a Mouse Model (BALB/c) Is Not an Effective Model for Research on Transplantation Procedures in the Treatment of Type 1 Diabetes. Biomedicines. 2021; 9(12):1790. https://doi.org/10.3390/biomedicines9121790
Chicago/Turabian StyleWszola, Michal, Marta Klak, Anna Kosowska, Grzegorz Tymicki, Andrzej Berman, Anna Adamiok-Ostrowska, Joanna Olkowska-Truchanowicz, Izabela Uhrynowska-Tyszkiewicz, and Artur Kaminski. 2021. "Streptozotocin-Induced Diabetes in a Mouse Model (BALB/c) Is Not an Effective Model for Research on Transplantation Procedures in the Treatment of Type 1 Diabetes" Biomedicines 9, no. 12: 1790. https://doi.org/10.3390/biomedicines9121790
APA StyleWszola, M., Klak, M., Kosowska, A., Tymicki, G., Berman, A., Adamiok-Ostrowska, A., Olkowska-Truchanowicz, J., Uhrynowska-Tyszkiewicz, I., & Kaminski, A. (2021). Streptozotocin-Induced Diabetes in a Mouse Model (BALB/c) Is Not an Effective Model for Research on Transplantation Procedures in the Treatment of Type 1 Diabetes. Biomedicines, 9(12), 1790. https://doi.org/10.3390/biomedicines9121790








