Acne Syndromes and Mosaicism
Abstract
:1. Introduction
2. Mosaicism
3. Acne
4. Methodology of This Review
5. Acne Mosaicism
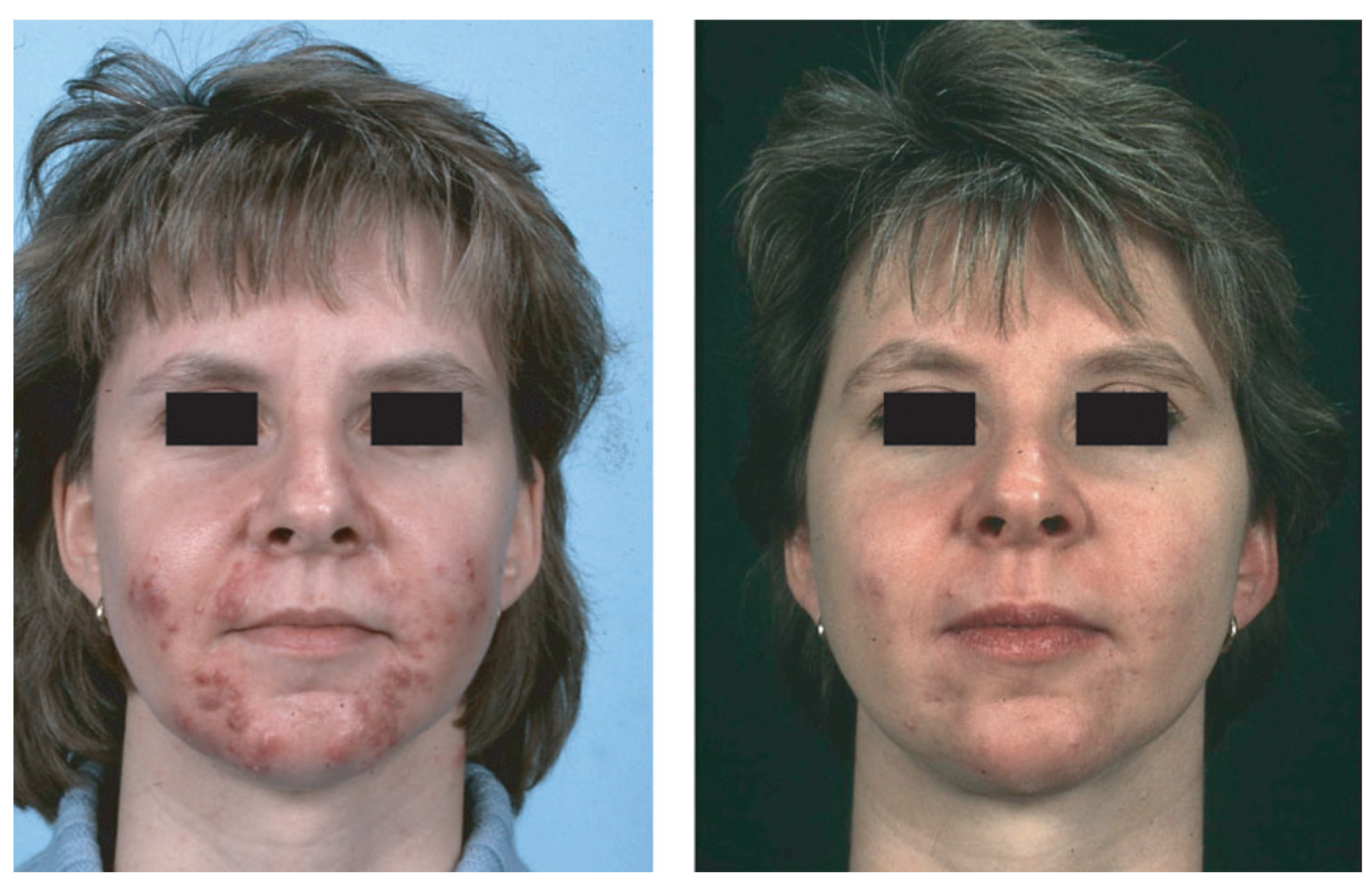
6. Acne-Associated Endocrine/Immunological Syndromes
6.1. CAH
6.2. PCOS
6.3. SAHA Syndrome
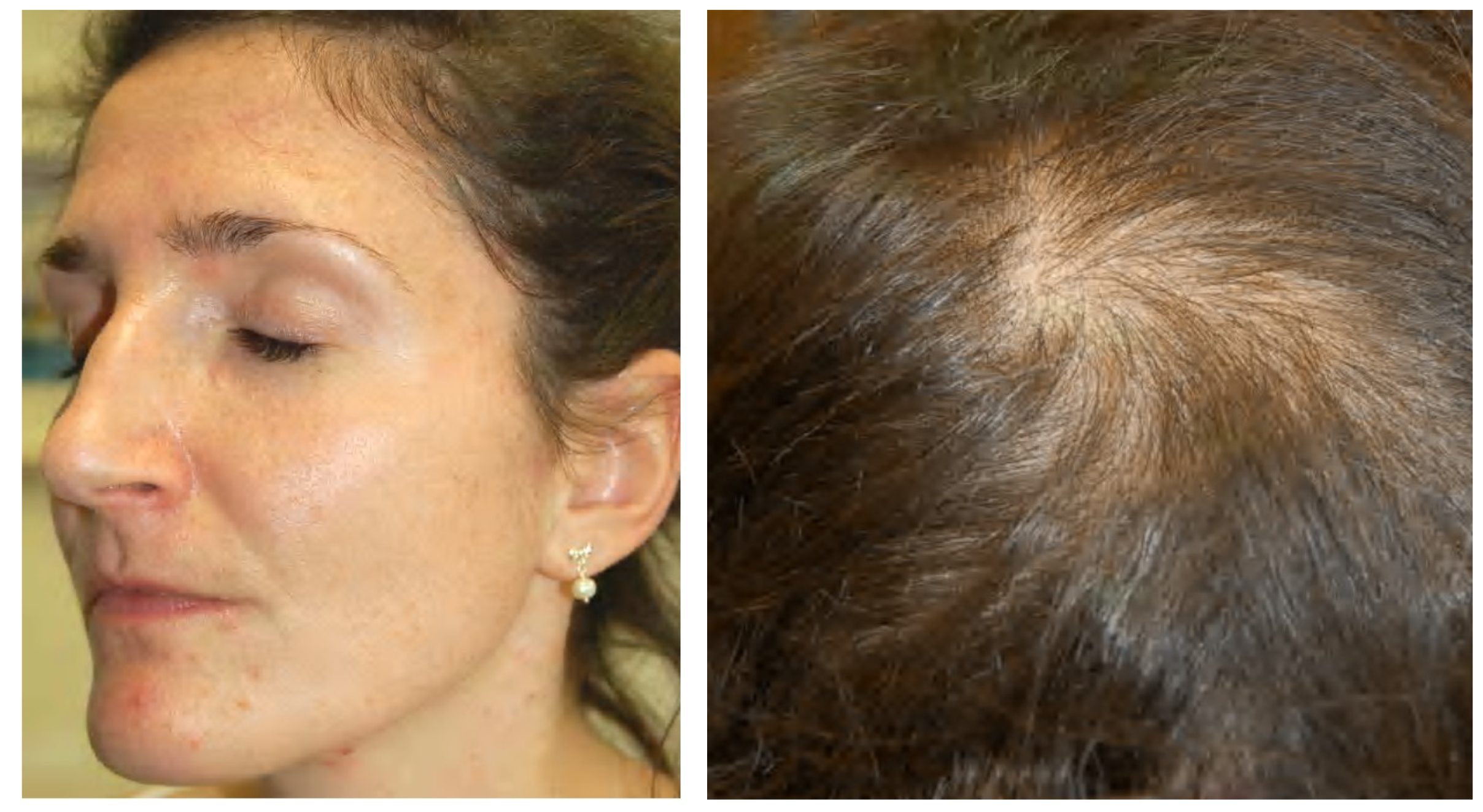
6.4. Primary Ovarian Insufficiency
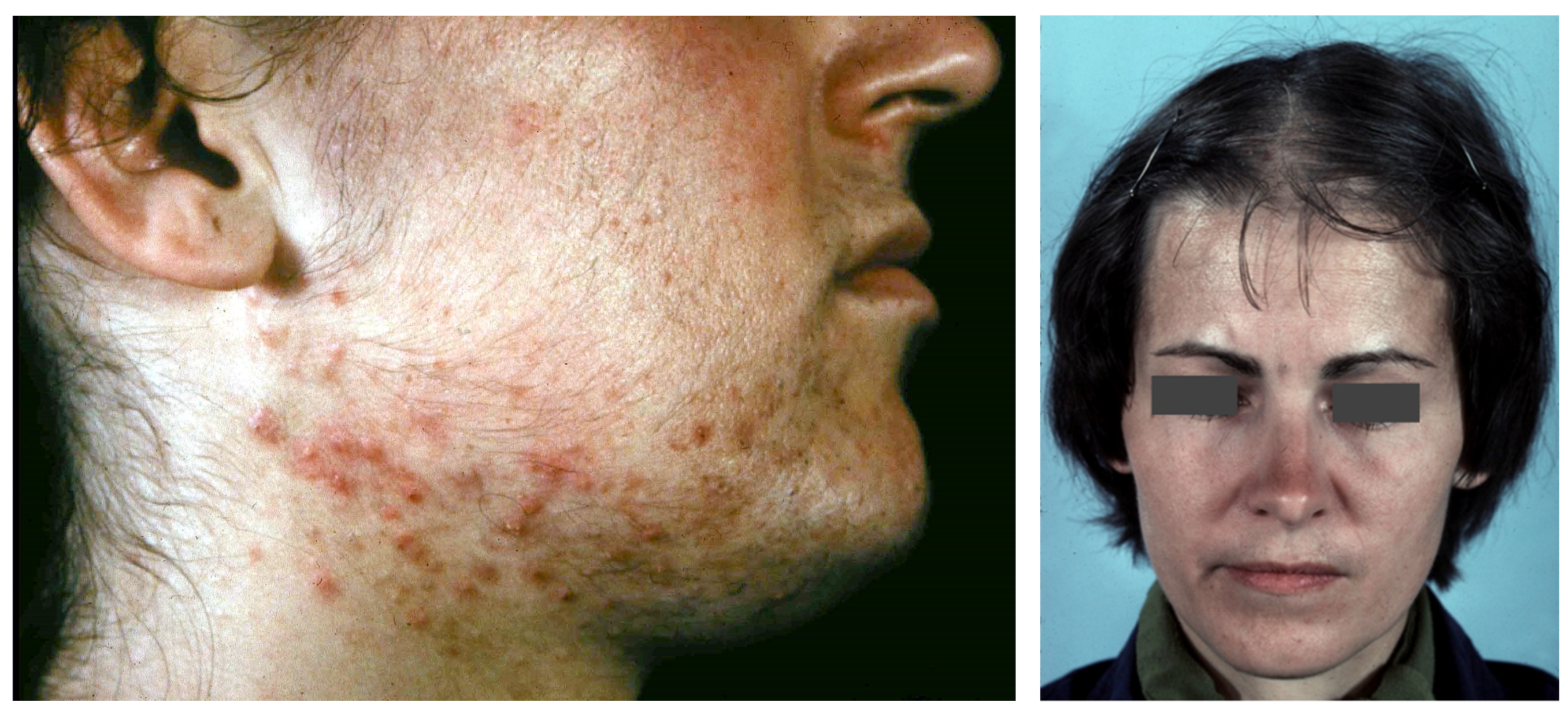
7. Acne in Autoinflammatory Syndromes
7.1. PAPA Syndrome
7.2. PASH Syndrome
7.3. PAPASH Syndrome
7.4. PsAPASH Syndrome
7.5. PsAPSASH Syndrome
7.6. PASS Syndrome
7.7. SAPHO Syndrome
8. Acneiform Eruptions
9. Acneiform Mosaicism
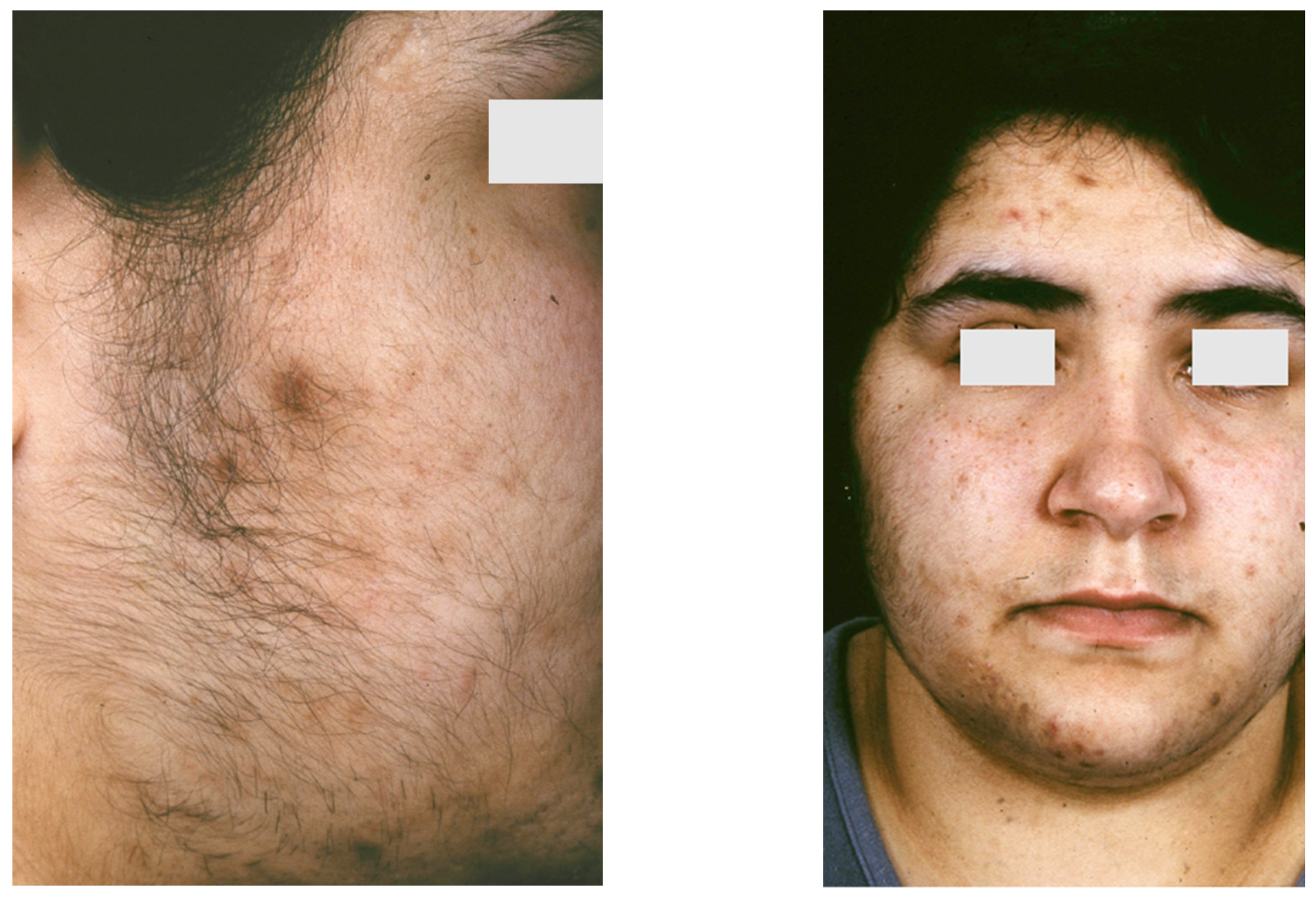
9.1. Apert Syndrome
9.2. Acneiform Unilateral Nevus—Nevus Comedonicus
9.3. Happle-Tinschert Syndrome
9.4. Becker Naevus
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Happle, R. Mosaicism as a Biological Concept. In Mosaicism in Human Skin; Springer: Berlin/Heidelberg, Germany, 2014; pp. 5–12. [Google Scholar]
- Rahbari, R.; Wuster, A.; Lindsay, S.J.; Hardwick, R.J.; Alexandrov, L.B.; Al Turki, S.; Dominiczak, A.; Morris, A.; Porteous, D.; Smith, B.; et al. Timing, rates and spectra of human germline mutation. Nat. Genet. 2015, 48, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Kinsler, V.A. An Introduction to Mosaicism. In Harper’s Textbook of Pediatric Dermatology, 4th ed.; Hoeger, P., Kinsler, V., Yan, A., Harper, J., Oranje, A., Bodemer, C., Larralde, M., Luk, D., Mendiratta, V., Purvis, D., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1229–1236. [Google Scholar]
- Happle, R. Dohi Memorisl Lecture. New aspects of cutaneous mosaicism. J. Dermatol. 2002, 29, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Happle, R. Mosaicism in human skin. Understanding the patterns and mechanisms. Arch. Dermatol. 1993, 129, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Sapp, J.C.; Lindhurst, M.J.; Parker, V.E.; Blumhorst, C.; Darling, T.; Tosi, L.L.; Huson, S.M.; Whitehouse, R.W.; Jakkula, E.; et al. Clinical delineation and natural history of the PIK3CA -related overgrowth spectrum. Am. J. Med. Genet. Part A 2014, 164, 1713–1733. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.C.; Zeng, Z.; Rivière, J.-B.; O’Shaughnessy, R.; Al-Olabi, L.; St.-Onge, J.; Atherton, D.J.; Aubert, H.; Bagazgoitia, L.; Barbarot, S.; et al. Mosaic activating mutations in GNA11 and GNAQ are associated with phakomatosis pigmentovascularis and extensive dermal melanocytosis. J. Investig. Dermatol. 2016, 136, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Kinsler, V.; Boccara, O.; Fraitag, S.; Torrelo, A.; Vabres, P.; Diociaiuti, A. Mosaic abnormalities of the skin: Review and guidelines from the European Reference Network for rare skin diseases. Br. J. Dermatol. 2019, 182, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Kinsler, V.A.; Abu-Amero, S.; Budd, P.; Jackson, I.; Ring, S.M.; Northstone, K.; Atherton, D.J.; Bulstrode, N.; Stanier, P.; Hennekam, R.C.; et al. Germline melanocortin-1-receptor genotype is associated with severity of cutaneous phenotype in congenital melanocytic nevi: A role for MC1R in human fetal development. J. Investig. Dermatol. 2012, 132, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Lindhurst, M.J.; Sapp, J.; Teer, J.K.; Johnston, J.J.; Finn, E.M.; Peters, K.; Turner, J.; Cannons, J.L.; Bick, D.; Blakemore, L.; et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 2011, 365, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Kinsler, V.A.; Krengel, S.; Riviere, J.-B.; Waelchli, R.; Chapusot, C.; Al-Olabi, L.; Faivre, L.; Haenssle, H.A.; Weibel, L.; Jeudy, G.; et al. Next-generation sequencing of nevus spilus–type congenital melanocytic nevus: Exquisite genotype–phenotype correlation in mosaic RASopathies. J. Investig. Dermatol. 2014, 134, 2658–2660. [Google Scholar] [CrossRef] [Green Version]
- Groesser, L.; Herschberger, E.; Ruetten, A.; Ruivenkamp, C.; Lopriore, E.; Zutt, M.; Langmann, T.; Singer, S.; Klingseisen, L.; Schneider-Brachert, W.; et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat. Genet. 2012, 44, 783–787. [Google Scholar] [CrossRef]
- Happle, R. The categories of cutaneous mosaicism: A proposed classification. Am. J. Med. Genet. Part A 2015, 170, 452–459. [Google Scholar] [CrossRef]
- Riccardi, V.M. Von Recklinghausen neurofibromatosis. N. Engl. J. Med. 1981, 305, 1617–1627. [Google Scholar] [CrossRef]
- Toro, J.R.; Nickerson, M.L.; Wei, M.-H.; Warren, M.B.; Glenn, G.M.; Turner, M.L.; Stewart, L.; Duray, P.; Tourre, O.; Sharma, N.; et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am. J. Hum. Genet. 2003, 73, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Green, A.; Smith, M.; Yates, J.R. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat. Genet. 1994, 6, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, K.H.; Lee, M.M.; Scotto, J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch. Dermatol. 1987, 123, 241–250. [Google Scholar] [CrossRef]
- Chin, L.; Garraway, L.A.; Fisher, D.E. Malignant melanoma: Genetics and therapeutics in the genomic era. Genes Dev. 2006, 20, 2149–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gailani, M.R.; Leffell, D.J.; Ziegler, A.; Gross, E.G.; Brash, D.E.; Bale, A.E. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J. Natl. Cancer Inst. 1996, 88, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [Green Version]
- Dupin, E.; Sommer, L. Neural crest progenitors and stem cells: From early development to adulthood. Dev. Biol. 2012, 366, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Selleck, M.A.; Scherson, T.Y.; Bronner, M. Origins of neural crest cell diversity. Dev. Biol. 1993, 159, 1–11. [Google Scholar] [CrossRef]
- Molho-Pessach, V.; Schaffer, J.V. Blaschko lines and other patterns of cutaneous mosaicism. Clin. Dermatol. 2011, 29, 205–225. [Google Scholar] [CrossRef]
- Itin, P.; Burger, B. Mosaik-Erscheinungen von monogenen Hauterkrankungen. J. Dtsch. Dermatol. Ges. 2009, 7, 744–748. [Google Scholar] [CrossRef]
- Hwang, L.Y.; Lee, J.B.; Richard, G.; Uitto, J.J.; Hsu, S. Type 1 segmental manifestation of Hailey-Hailey disease. J. Am. Acad. Dermatol. 2003, 49, 712–714. [Google Scholar] [CrossRef]
- Nazzaro, V.; Ermacora, E.; Santucci, B.; Caputo, R. Epidermolytic hyperkeratosis: Generalized form in children from parents with systematized linear form. Br. J. Dermatol. 1990, 122, 417–422. [Google Scholar] [CrossRef]
- Tinschert, S.; Naumann, I.; Stegmann, E.; Buske, A.; Kaufmann, D.; Thiel, G.; Jenne, D. Segmental neurofibromatosis is caused by somatic mutation of the neurofibromatosis type 1 (NF1) gene. Eur. J. Hum. Genet. 2000, 8, 455–459. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne as a chronic systemic disease. Clin. Dermatol. 2013, 32, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.P.; Zouboulis, C.C. Not all acne is acne vulgaris. Dtsch. Aerzteblatt Online 2014, 111, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouboulis, C.C. Endocrinology and immunology of acne: Two sides of the same coin. Exp. Dermatol. 2020, 29, 840–859. [Google Scholar] [CrossRef]
- Lovászi, M.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermatoendocrinol. 2017, 9, e1375636. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.C.; Dellavalle, R.; Garner, S. Acne vulgaris. Lancet 2011, 379, 361–372. [Google Scholar] [CrossRef]
- Tuchayi, S.M.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Prim. 2015, 1, 1–20. [Google Scholar] [CrossRef]
- Schäfer, T.; Kahl, C.; Rzany, B. Epidemiologie der Akne. J. Dtsch. Dermatol. Ges. 2010, 8, S4–S6. [Google Scholar] [CrossRef]
- Schaefer, I.; Rustenbach, S.; Zimmer, L.; Augustin, M. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology 2008, 217, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Schafer, T.; Nienhaus, A.; Vieluf, D.; Berger, J.; Ring, J. Epidemiology of acne in the general population: The risk of smoking. Br. J. Dermatol. 2001, 145, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.N.; Harper, J.C.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef]
- Ghodsi, S.Z.; Orawa, H.; Zouboulis, C.C. Prevalence, severity, and severity risk factors of acne in high school pupils: A community-based study. J. Investig. Dermatol. 2009, 129, 2136–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaenglein, A.L. Acne vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef]
- Karciauskiene, J.; Valiukeviciene, S.; Gollnick, H.; Stang, A. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 733–740. [Google Scholar] [CrossRef]
- Bataille, V.; Snieder, H.; MacGregor, A.; Sasieni, P.; Spector, T. The influence of genetics and environmental factors in the pathogenesis of acne: A twin study of acne in women. J. Investig. Dermatol. 2002, 119, 1317–1322. [Google Scholar] [CrossRef] [Green Version]
- Plewig, G.; Melnik, B.; Chen, W. Acne Epidemiology and Genetics. In Plewig and Kligman’s Acne and Rosacea; Springer: Cham, Switzerland, 2019; pp. 35–44. [Google Scholar]
- Thiboutot, D.; Gollnick, H.; Bettoli, V.; Dréno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A.Y.; et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne Group. J. Am. Acad. Dermatol. 2009, 60, S1–S50. [Google Scholar] [CrossRef]
- Kim, J.; Ochoa, M.-T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistowska, M.; Meier-Schiesser, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, R.; Kealey, T. The effects of inflammatory cytokines on the isolated human sebaceous infundibulum. J. Investig. Dermatol. 1998, 110, 410–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnik, B.; Schmitz, G. FGFR2-Signaltransduktionswege in der Pathogenese der Akne. J. Dtsch. Dermatol. Ges. 2008, 6, 721–728. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 527–532. [Google Scholar] [CrossRef]
- Ottaviani, M.; Camera, E.; Picardo, M. Lipid mediators in acne. Mediat. Inflamm. 2010, 2010, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M. The skin barrier as an innate immune element. Semin. Immunopathol. 2007, 29, 3–14. [Google Scholar] [CrossRef]
- Elias, P.M. Stratum corneum defensive functions: An integrated view. J. Investig. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Kao, J.; Ahn, S.K.; Feingold, K.R.; Elias, P.M.; Jain, M. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Investig. Dermatol. 2001, 117, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Nagy, I.; Pivarcsi, A.; Koreck, A.; Széll, M.; Urbán, E.; Kemény, L. Distinct strains of Propionibacterium acnes induce selective human β-defensin-2 and interleukin-8 expression in human keratinocytes through Toll-like receptors. J. Investig. Dermatol. 2005, 124, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Cunliffe, W.; Shuster, S. Pathogenesis of acne. Lancet 1969, 293, 685–687. [Google Scholar] [CrossRef]
- Ayer, J.; Burrows, N. Acne: More than skin deep. Postgrad. Med. J. 2006, 82, 500–506. [Google Scholar] [CrossRef]
- Lukaviciute, L.; Navickas, P.; Grigaitiene, J.; Ganceviciene, R.; Zouboulis, C.C. Quality of life, anxiety prevalence, depression symptomatology and suicidal ideation among acne patients in Lithuania. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1900–1906. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Zouboulis, C.C.; Aragonés, L.T.; Jemec, G.B.; Manolache, L.; Tzellos, T.; Sampogna, F.; Evers, A.; Dessinioti, C.; Marron, S.E.; et al. Quality of life measurement in acne. Position paper of the European Academy of Dermatology and Venereology Task Forces on Quality of Life and Patient Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2017, 32, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, E.; Manfredini, M.; Petrini, N.; Farnetani, F.; Chester, J.; Bennardo, L.; Schipani, G.; Tamburi, F.; Sannino, M.; Cannarozzo, G.; et al. Daylight photodynamic therapy with 5-aminolevulinic acid 5% gel for the treatment of mild-to-moderate inflammatory acne. Ital. J. Dermatol. Venerol. 2019, 156, 46–50. [Google Scholar] [CrossRef]
- Rivera, A.E. Acne scarring: A review and current treatment modalities. J. Am. Acad. Dermatol. 2008, 59, 659–676. [Google Scholar] [CrossRef]
- Cannarozzo, G.; Silvestri, M.; Tamburi, F.; Sicilia, C.; Del Duca, E.; Scali, E.; Bennardo, L.; Nisticò, S.P. A new 675-nm laser device in the treatment of acne scars: An observational study. Lasers Med. Sci. 2020, 36, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.E.; Shalita, A.R. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J. Cutan. Med. Surg. 1998, 3, 9–15. [Google Scholar] [CrossRef]
- Pang, Y.; He, C.; Liu, Y.; Wang, K.; Xiao, T.; Wang, Y.; Zhu, H.; Wei, B.; Zhao, N.; Jiang, Y.; et al. Combination of short CAG and GGN repeats in theandrogen receptorgene is associated with acne risk in North East China. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1445–1451. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, H.; Cheng, B.; Tang, W.; Dong, Y.; Xiao, C.; He, L. Relationship between the CAG repeat polymorphism in the androgen receptor gene and acne in the Han ethnic group. Dermatology 2009, 218, 302–306. [Google Scholar] [CrossRef]
- Munro, C.S.; Wilkie, A. Epidermal mosaicism producing localised acne: Somatic mutation in FGFR2. Lancet 1998, 352, 704–705. [Google Scholar] [CrossRef]
- Ostlere, L.S.; Rumsby, G.; Holownia, P.; Jacobs, H.S.; Rustin, M.H.A.; Honour, J.W. Carrier status for steroid 21-hydroxylase deficiency is only one factor in the variable phenotype of acne. Clin. Endocrinol. 1998, 48, 209–215. [Google Scholar] [CrossRef]
- He, L.; Yang, Z.; Yu, H.; Cheng, B.; Tang, W.; Dong, Y.; Xiao, C. The Relationship between CYP17 –34T/C polymorphism and acne in Chinese subjects revealed by sequencing. Dermatology 2006, 212, 338–342. [Google Scholar] [CrossRef]
- Paraskevaidis, A.; Drakoulis, N.; Roots, I.; Orfanos, C.; Zouboulis, C.C. Polymorphisms in the human cytochrome P-450 1A1 gene (CYP1A1) as a factor for developing acne. Dermatology 1998, 196, 171–175. [Google Scholar] [CrossRef]
- Herane, M.I.; Ando, I. Acne in infancy and acne genetics. Dermatology 2003, 206, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Tax, G.; Kis, K.; Szegedi, K.D.; Diószegi, C.; Koreck, A.; Teodorescu-Brinzeu, D.G.; Széll, M.; Kemény, L. Interleukin-1A +4845(G> T) polymorphism is a factor predisposing to acne vulgaris. Tissue Antigens 2010, 76, 411–415. [Google Scholar] [CrossRef]
- Tian, L.-M.; Xie, H.-F.; Yang, T.; Hu, Y.-H.; Li, J.; Wang, W.-Z. Association study of tumor necrosis factor receptor type 2 M196R and Toll-like receptor 2 Arg753Gln polymorphisms with acne vulgaris in a Chinese Han ethnic group. Dermatology 2010, 221, 276–284. [Google Scholar] [CrossRef]
- Karaca, N.; Ozturk, G.; Gerceker, B.; Turkmen, M.; Berdeli, A. TLR2 and TLR4 gene polymorphisms in Turkish vitiligo patients. J. Eur. Acad. Dermatol. Venereol. 2012, 27, e85–e90. [Google Scholar] [CrossRef] [PubMed]
- Szabó, K.; Tax, G.; Teodorescu-Brinzeu, D.; Koreck, A.; Kemény, L. TNFα gene polymorphisms in the pathogenesis of acne vulgaris. Arch. Dermatol. Res. 2010, 303, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bonano, V.I.; Oltean, S.; Brazas, R.M.; Garcia-Blanco, M.A. Imaging the alternative silencing of FGFR2 exon IIIb in vivo. RNA 2006, 12, 2073–2079. [Google Scholar] [CrossRef] [Green Version]
- Oldridge, M.; Zackai, E.H.; McDonald-McGinn, D.M.; Iseki, S.; Morriss-Kay, G.M.; Twigg, S.; Johnson, D.; Wall, S.A.; Jiang, W.; Theda, C.; et al. De novo Alu-element insertions in FGFR2 identify a distinct pathological basis for Apert syndrome. Am. J. Hum. Genet. 1999, 64, 446–461. [Google Scholar] [CrossRef] [Green Version]
- Housley, R.M.; Morris, C.F.; Boyle, W.; Ring, B.; Biltz, R.; E Tarpley, J.; Aukerman, S.L.; Devine, P.L.; Whitehead, R.H.; Pierce, G.F. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J. Clin. Investig. 1994, 94, 1764–1777. [Google Scholar] [CrossRef]
- Orr-Urtreger, A.; Bedford, M.T.; Burakova, T.; Arman, E.; Zimmer, Y.; Yayon, A.; Givol, D.; Lonai, P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev. Biol. 1993, 158, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation: Normalization of the nu/nu follicular differentiation defect amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar]
- Katoh, M. FGFR2 abnormalities underlie a spectrum of bone, skin, and cancer pathologies. J. Investig. Dermatol. 2009, 129, 1861–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, L.M.; Fretzin, D.; Pruzansky, S. Pilosebaceous abnormalities in Apert’s syndrome. Arch. Dermatol. 1970, 102, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Atherton, D.J.; Rebello, T.; Wells, R.S. Apert’s Syndrome with severe acne vulgaris. Proc. R. Soc. Med. 1976, 69, 517–518. [Google Scholar] [CrossRef] [Green Version]
- Higgins, R.; Pink, A.; Hunger, R.; Yawalkar, N.; Navarini, A.A. Generalized comedones, acne, and hidradenitis suppurativa in a patient with an FGFR2 missense mutation. Front. Med. 2017, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.; Slaney, S.F.; Oldridge, M.; Poole, M.D.; Ashworth, G.J.; Hockley, A.D.; Hayward, R.D.; David, D.J.; Pulleyn, L.J.; Rutland, P.; et al. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat. Genet. 1995, 9, 165–172. [Google Scholar] [CrossRef]
- Goriely, A.; McVean, G.A.T.; Röjmyr, M.; Ingemarsson, B.; Wilkie, A.O.M. Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science 2003, 301, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, O.A.; Zhang, F.; Eliseenkova, A.V.; Itoh, N.; Linhardt, R.J.; Mohammadi, M. Biochemical analysis of pathogenic ligand-dependent FGFR2 mutations suggests distinct pathophysiological mechanisms for craniofacial and limb abnormalities. Hum. Mol. Genet. 2004, 13, 2313–2324. [Google Scholar] [CrossRef] [Green Version]
- Moloney, D.M.; Slaney, S.R.; Oldridge, M.; Wall, S.A.; Sahlin, P.; Stenman, G.; Wilkie, A.O. Exclusive paternal origin of new mutations in Apert syndrome. Nat. Genet. 1996, 13, 48–53. [Google Scholar] [CrossRef]
- Yu, K.; Herr, A.; Waksman, G.; Ornitz, D.M. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc. Natl. Acad. Sci. USA 2000, 97, 14536–14541. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Obermayer-Pietsch, B.; Hong, J.-B.; Melnik, B.C.; Yamasaki, O.; Dessinioti, C.; Ju, Q.; I Liakou, A.; Al-Khuzaei, S.; Katsambas, A.; et al. Acne-associated syndromes: Models for better understanding of acne pathogenesis. J. Eur. Acad. Dermatol. Venereol. 2010, 25, 637–646. [Google Scholar] [CrossRef]
- Melnik, B. Role of FGFR2-signaling in the pathogenesis of acne. Dermatoendocrinol. 2009, 1, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Z.; Schüller, A.C.; Suhling, K.; Tregidgo, C.; Ladbury, J.E. Extracellular point mutations in FGFR2 elicit unexpected changes in intracellular signalling. Biochem. J. 2008, 413, 37–49. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G.; Zouboulis, C.C. Anti-acne agents attenuate FGFR2 signal transduction in acne. J. Investig. Dermatol. 2009, 129, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Gonzalez, M.; Porter, R. Pathways to inflammation: Acne pathophysiology. Eur. J. Dermatol. EJD 2011, 21, 323–333. [Google Scholar] [CrossRef]
- Benjamin, L.T.; Trowers, A.B.; Schachner, L.A. Successful acne management in Apert syndrome twins. Pediatr. Dermatol. 2005, 22, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, M.; Puig, L.; Alomar, A. Acne treatment with isotretinoin in a patient with Apert syndrome. Eur. J. Dermatol. 2011, 21, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Orfanos, C.E.; Adler, Y.D.; Zouboulis, C.C. The SAHA syndrome. Horm. Res. 2000, 54, 251–258. [Google Scholar] [CrossRef]
- Trakakis, E.; Basios, G.; Trompoukis, P.; Labos, G.; Grammatikakis, I.; Kassanos, D. An update to 21-hydroxylase deficient congenital adrenal hyperplasia. Gynecol. Endocrinol. 2009, 26, 63–71. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Degitz, K. Androgen action on human skin—From basic research to clinical significance. Exp. Dermatol. 2004, 13, 5–10. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Piquero-Martín, J. Update and future of systemic acne treatment. Dermatology 2003, 206, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Recabarren, S.; Gonzalez, F.J.; Diamanti-Kandarakis, E.; Cheang, K.I.; Duleba, A.J.; Legro, R.S.; Homburg, R.; Pasquali, R.; Lobo, R.A.; et al. Emerging concepts about prenatal genesis, aberrant metabolism and treatment paradigms in polycystic ovary syndrome. Endocrine 2012, 42, 526–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuan, S.S.; Chang, R.J. Polycystic ovary syndrome and acne. Skin Ther. Lett. 2010, 15, 1–4. [Google Scholar]
- Zouboulis, C.C.; Dessinioti, C. The SAHA Syndrome. In Pathogenesis and Treatment of Acne and Rosacea; Zouboulis, C.C., Kat-sambas, A.D., Kligman, A.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Adler, Y.D.; Zouboulis, C.C.; Geilen, C.C.; Orfanos, C.E. Eine seltene Variante des SAHA Syndroms. Z. Hautkr. 2001, 76, 327–328. [Google Scholar]
- Youn, S.W. The role of facial sebum secretion in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, A.; Chabbert-Buffet, N.; Salenave, S.; Kerlan, V.; Galand-Portier, M.-B. Anti-androgen treatments. Ann. Endocrinol. (Paris) 2010, 71, 19–24. [Google Scholar] [CrossRef]
- Jin, M.; Yu, Y.; Huang, H. An update on primary ovarian insufficiency. Sci. China Life Sci. 2012, 55, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Zouboulis, C.C.; Achenbach, A.; Makrantonaki, E. Acne tarda and male-pattern baldness unmasking primary ovarian insufficiency: A case and review. Dermatology 2014, 229, 51–54. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. The skin as a mirror of the aging process in the human organism—State of the art and results of the aging research in the German National Genome Research Network 2 (NGFN-2). Exp. Gerontol. 2007, 42, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Nikolakis, G.; Kaleta, K.P.; Vaiopoulos, A.G.; Wolter, K.; Baroud, S.; Wojas-Pelc, A.; Zouboulis, C.C. Phenotypes and pathophysiology of syndromic hidradenitis suppurativa: Different faces of the same disease? A systematic review. Dermatology 2020, 237, 673–697. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Ishak, R.S.; Colombo, A.; Caroli, F.; Crosti, C. Pyoderma Gangrenosum, Acne and suppurative hidradenitis syndrome following bowel bypass surgery. Dermatology 2012, 225, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Cugno, M.; Trevisan, V.; Fanoni, D.; Venegoni, L.; Berti, E.; Crosti, C. Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin. Exp. Immunol. 2010, 162, 100–107. [Google Scholar] [CrossRef]
- Marzano, A.V.; Fanoni, D.; Antiga, E.; Quaglino, P.; Caproni, M.; Crosti, C.; Meroni, P.L.; Cugno, M. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin. Exp. Immunol. 2014, 178, 48–56. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Aletras, A.J.; Glass, E.; Tsogas, P.; Dionyssopoulos, A.; Adjaye, J.; Fimmel, S.; Gouvousis, P.; Herwig, R.; Lehrach, H.; et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J. Investig. Dermatol. 2005, 125, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Demidowich, A.P.; Freeman, A.F.; Kuhns, D.B.; Aksentijevich, I.; Gallin, J.I.; Turner, M.L.; Kastner, D.L.; Holland, S.M. Brief report: Genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis Rheum. 2011, 64, 2022–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoham, N.G.; Centola, M.; Mansfield, E.; Hull, K.M.; Wood, G.; Wise, C.; Kastner, D.L. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 13501–13506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzzese, V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: Efficacy of anti-tumor necrosis factor α therapy. J. Clin. Rheumatol. 2012, 18, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Ceccherini, I.; Gattorno, M.; Fanoni, D.; Caroli, F.; Rusmini, M.; Grossi, A.; de Simone, C.; Borghi, M.O.; Meroni, P.L.; et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine 2014, 93, e187. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.; Borghi, A.; Meroni, P.L.; Cugno, M. Pyoderma gangrenosum and its syndromic forms: Evidence for a link with autoinflammation. Br. J. Dermatol. 2016, 175, 882–891. [Google Scholar] [CrossRef] [Green Version]
- Staub, J.; Pfannschmidt, N.; Strohal, R.; Braun-Falco, M.; Lohse, P.; Goerdt, S.; Leverkus, M. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 2243–2247. [Google Scholar] [CrossRef]
- Marzano, A.V.; Trevisan, V.; Gattorno, M.; Ceccherini, I.; De Simone, C.; Crosti, C. Pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa (PAPASH): A new autoinflammatory syndrome associated with a novel mutation of the PSTPIP1 gene. JAMA Dermatol. 2013, 149, 762–764. [Google Scholar] [CrossRef]
- Gottlieb, J.; Madrange, M.; Gardair, C.; Sbidian, E.; Frazier, A.; Wolkenstein, P.; Hickman, G.; Schneider, P.; Baudry, C.; Claudepierre, P.; et al. PAPASH, PsAPASH and PASS autoinflammatory syndromes: Phenotypic heterogeneity, common biological signature and response to immunosuppressive regimens. Br. J. Dermatol. 2019, 181, 866–869. [Google Scholar] [CrossRef]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- Saraceno, R.; Babino, G.; Chiricozzi, A.; Zangrilli, A.; Chimenti, S. PsAPASH: A new syndrome associated with hidradenitis suppurativa with response to tumor necrosis factor inhibition. J. Am. Acad. Dermatol. 2015, 72, e42–e44. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, G.; Kreibich, K.; Vaiopoulos, A.; Kaleta, K.; Talas, J.; Becker, M.; Zouboulis, C.C. Case report: PsAPSASH syndrome: An alternative phenotype of syndromic hidradenitis suppurativa treated with the IL-17A inhibitor secukinumab. F1000Research 2021, 10, 381. [Google Scholar] [CrossRef]
- Leuenberger, M.; Berner, J.; Di Lucca, J.; Fischer, L.; Kaparos, N.; Conrad, C.; Hohl, D.; So, A.; Gilliet, M. PASS syndrome: An IL-1-driven autoinflammatory disease. Dermatology 2016, 232, 254–258. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Borchers, A.; Selmi, C.; Naguwa, S.M.; Cheema, G.; Gershwin, M.E. The SAPHO syndrome. Semin. Arthritis Rheum. 2012, 42, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Suei, Y.; Taguchi, A.; Tanimoto, K. Diffuse sclerosing osteomyelitis of the mandible: Its characteristics and possible relationship to synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. J. Oral Maxillofac. Surg. 1996, 54, 1194–1199. [Google Scholar] [CrossRef]
- Kubo, Y.; Ito, K.; Fujiwara, Y.; Yoshida, T.; Kusumoto, M. Case report: SAPHO syndrome mimicking bone metastases during treatment with pembrolizumab for non-small cell lung cancer. Front. Med. 2021, 8, 1136. [Google Scholar] [CrossRef] [PubMed]
- Hayem, G.; Bouchaud-Chabot, A.; Benali, K.; Roux, S.; Palazzo, E.; Silbermann-Hoffman, O.; Kahn, M.-F.; Meyer, O. SAPHO syndrome: A long-term follow-up study of 120 cases. Semin. Arthritis Rheum. 1999, 29, 159–171. [Google Scholar] [CrossRef]
- Colina, M.; Govoni, M.; Orzincolo, C.; Trotta, F. Clinical and radiologic evolution of synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: A single center study of a cohort of 71 subjects. Arthritis Rheum. 2009, 61, 813–821. [Google Scholar] [CrossRef]
- Wendling, D.; Prati, C.; Aubin, F. Anakinra treatment of SAPHO syndrome: Short-term results of an open study. Ann. Rheum. Dis. 2012, 71, 1098–1100. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Gerschman, T.; Sebire, N.; Woo, P.; Pilkington, C.A.; Brogan, P.A. Biologic therapy in refractory chronic non-bacterial osteomyelitis of childhood. Rheumatology 2010, 49, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Plewig, G.; Jansen, T. Acneiform dermatoses. Dermatology 1998, 196, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Zeichner, J.A. Acneiform Eruptions in Dermatology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Dessinioti, C.; Antoniou, C.; Katsambas, A. Acneiform eruptions. Clin. Dermatol. 2014, 32, 24–34. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Elek, C.; Susánszky, E. Birth prevalence study of the apert syndrome. Am. J. Med. Genet. 1992, 42, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-B.; Prucha, H.; Melnik, B.; Ziai, M.; Ring, J.; Chen, W. Seltene Akne-assoziierte Syndrome und deren Bedeutung für das Verständnis der Pathogenese der Akne. Hautarzt 2013, 64, 274–279. [Google Scholar] [CrossRef]
- Apert, E. Del’acrocephalosyndactylie. Bull. Soc. Med. 1906, 23, 1310–1330. [Google Scholar]
- Freiman, A.; Tessler, O.; Barankin, B. Apert syndrome. Int. J. Dermatol. 2006, 45, 1341–1343. [Google Scholar] [CrossRef]
- Cohen, M.C.M.; Kreiborg, S. Cutaneous manifestations of Apert syndrome. Am. J. Med. Genet. 1995, 58, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Margolis, S.; Siegel, I.M.; Choy, A.; Breinin, G.M. Oculocutaneous albinism associated with Apert’s syndrome. Am. J. Ophthalmol. 1977, 84, 830–839. [Google Scholar] [CrossRef]
- McNaughton, P.Z.; Rodman, O.G. Apert’s syndrome. Cutis 1980, 25, 538–540. [Google Scholar] [PubMed]
- Kofmann, S. Ein Fall von seltener Localisation und Verbreitung von Comedonen. Arch. Dermatol. Res. 1895, 32, 177–178. [Google Scholar] [CrossRef] [Green Version]
- Torchia, D.; Schachner, L.A.; Izakovic, J. Segmental acne versus mosaic conditions with acne lesions. Dermatology 2012, 224, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.; Paduraru, L.; Eusebiu, G.V.; Stratakis, C.; Zouboulis, C.C. Extensive unilateral nevus comedonicus without genetic abnormality. Dermatol. Online J. 2016, 22. [Google Scholar] [CrossRef]
- Engber, P.B. The nevus comedonicus syndrome: A case report with emphasis on associated internal manifestations. Int. J. Dermatol. 1978, 17, 745–749. [Google Scholar] [CrossRef]
- Kurokawa, I.; Nakai, Y.; Nishimura, K.; Hakamada, A.; Isoda, K.-I.; Yamanaka, K.-I.; Mizutani, H.; Tsubura, A. Cytokeratin and filaggrin expression in nevus comedonicus. J. Cutan. Pathol. 2007, 34, 338–341. [Google Scholar] [CrossRef]
- Downs, A.M.R.; Condon, C.A.; Tan, A.C. Isotretinoin therapy for antibiotic-refractory acne in Apert’s syndrome. Clin. Exp. Dermatol. 1999, 24, 461–463. [Google Scholar] [CrossRef]
- Melnik, B.; Vakilzadeh, F.; Aslanidis, C.; Schmitz, G. Unilateral segmental acneiform naevus: A model disorder towards understanding fibroblast growth factor receptor 2 function in acne? Br. J. Dermatol. 2008, 158, 1397–1399. [Google Scholar] [CrossRef]
- Itin, P.H. Happle-Tinschert syndrome. Dermatology 2008, 218, 221–225. [Google Scholar] [CrossRef]
- Burck, U.; Held, K.R. Unilateral skin lesions, cataracts, optic glioma and retardation—A variant of the epidermal nevus syndrome? Dermatology 1982, 165, 208–214. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Graziene, V.; Böhm, M.; Zouboulis, C.C. Increased in situ expression of melanocortin-1 receptor in sebaceous glands of lesional skin of patients with acne vulgaris. Exp. Dermatol. 2007, 16, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, E.; Durusoy, C.; Ozbilim, G.; Karpuzoglu, G.; Yilmaz, E. Nevus comedonicus syndrome: A case associated with multiple basal cell carcinomas and a rudimentary toe. Int. J. Dermatol. 2005, 44, 499–501. [Google Scholar] [CrossRef]
- Dasegowda, S.B.; Basavaraj, G.; Nischal, K.; Swaroop, M.; Umashankar, N.; Swamy, S.S. Becker′s nevus syndrome. Indian J. Dermatol. 2014, 59, 421. [Google Scholar] [CrossRef] [PubMed]
- Losee, J.E.; Serletti, J.M.; Pennino, R.P. Epidermal nevus syndrome: A review and case report. Ann. Plast. Surg. 1999, 43, 211–214. [Google Scholar] [PubMed]
- Danarti, R.; König, A.; Salhi, A.; Bittar, M.; Happle, R. Becker’s nevus syndrome revisited. J. Am. Acad. Dermatol. 2004, 51, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Atherton, D.J.; Moss, C. Naevi and Other Developmental Defects. In Rook’s Textbook of Dermatology, 7th ed.; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Blackwell: Malden, MA, USA, 2004; pp. 569–682. [Google Scholar]
- Person, J.R.; Longcope, C. Becker’s nevus: An androgen-mediated hyperplasia with increased androgen receptors. J. Am. Acad. Dermatol. 1984, 10, 235–238. [Google Scholar] [CrossRef]
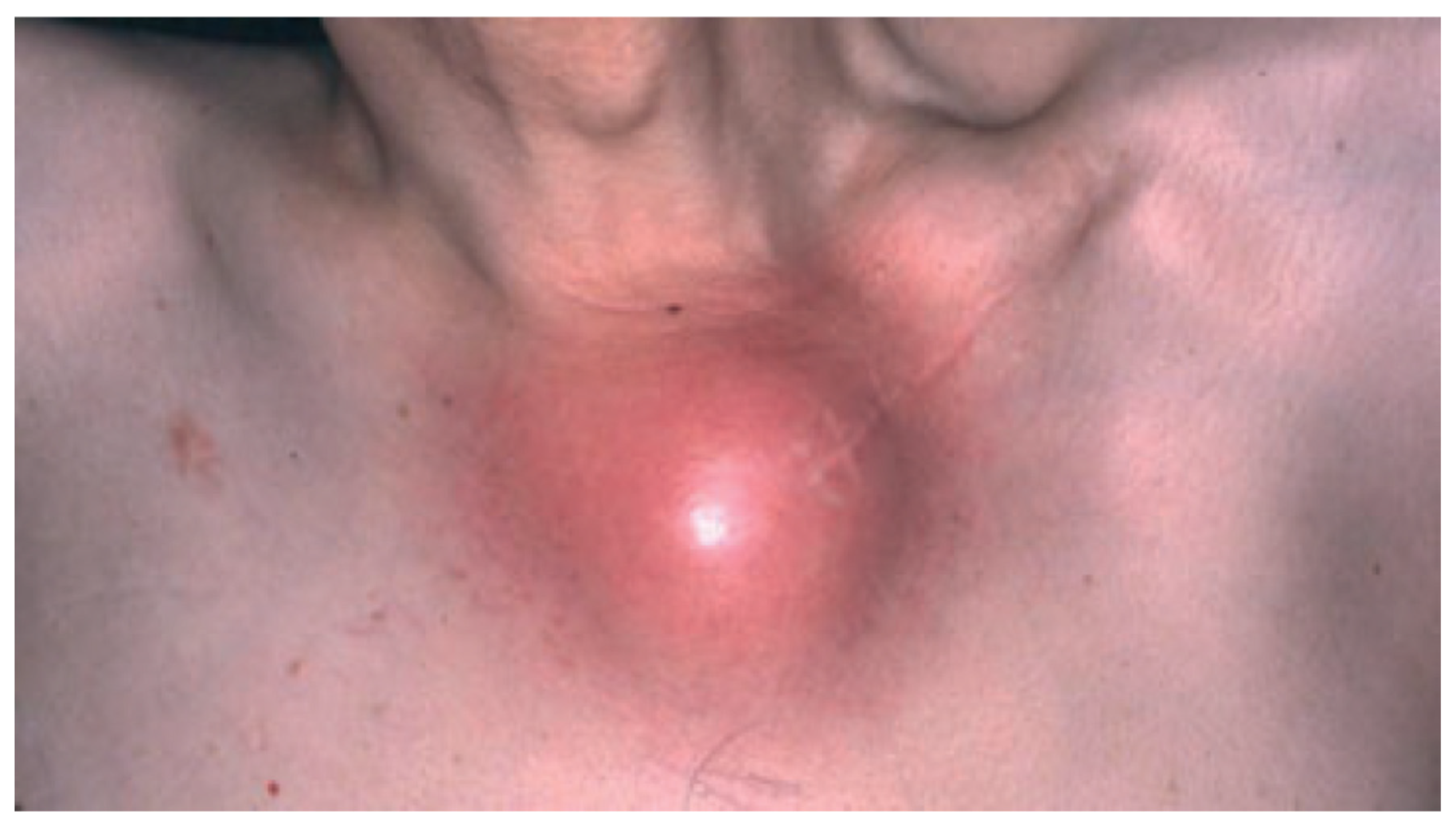
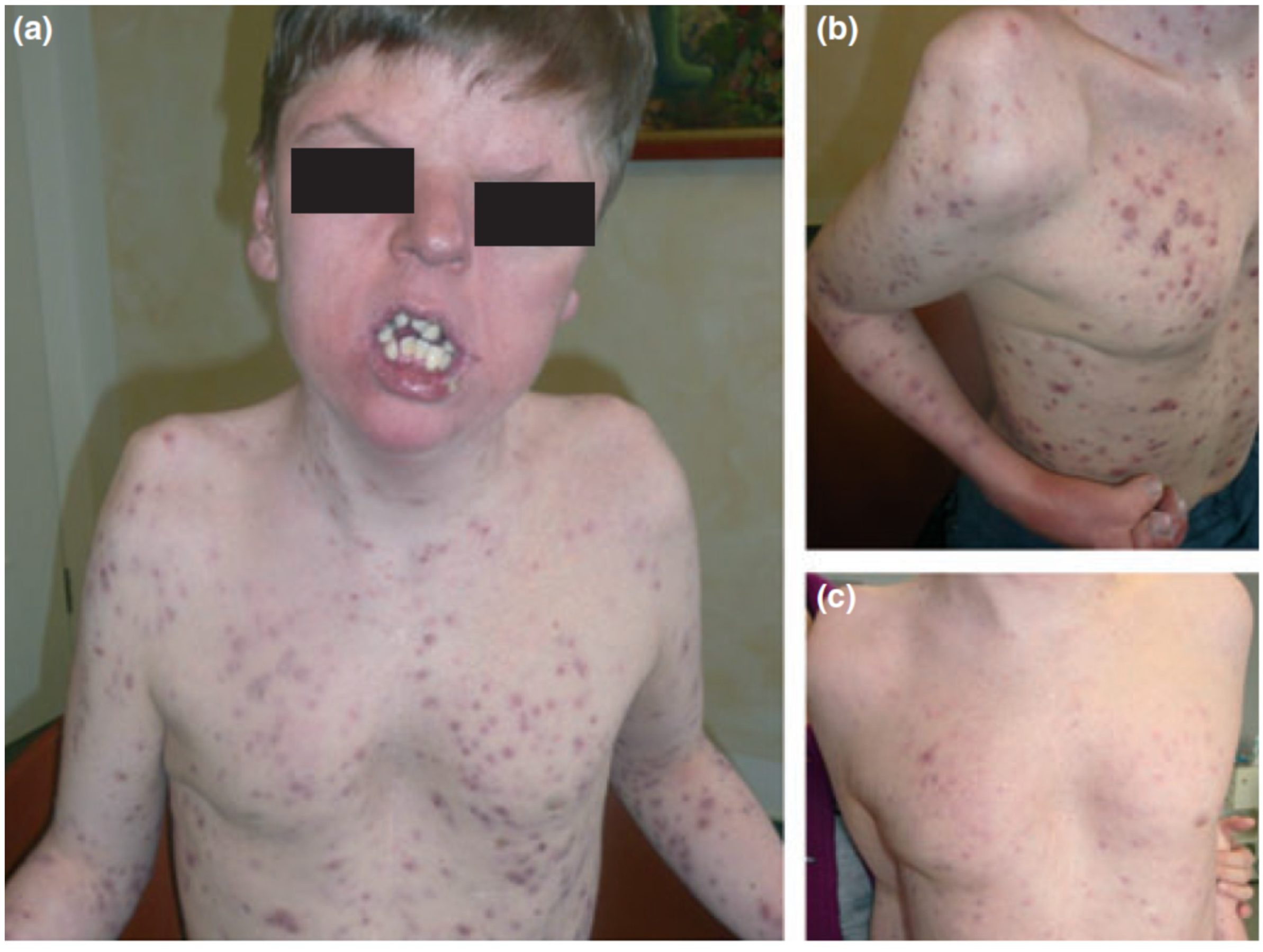
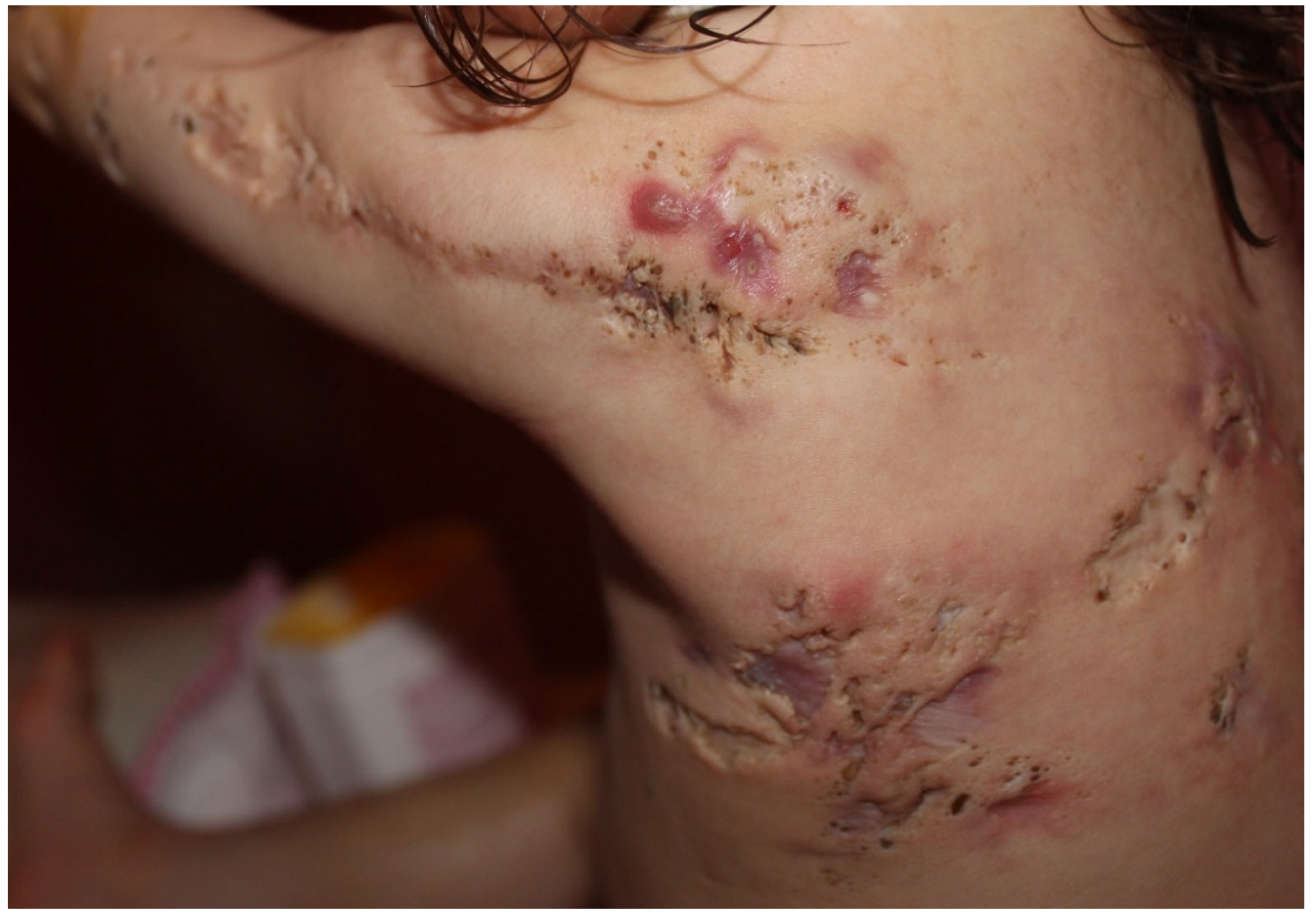
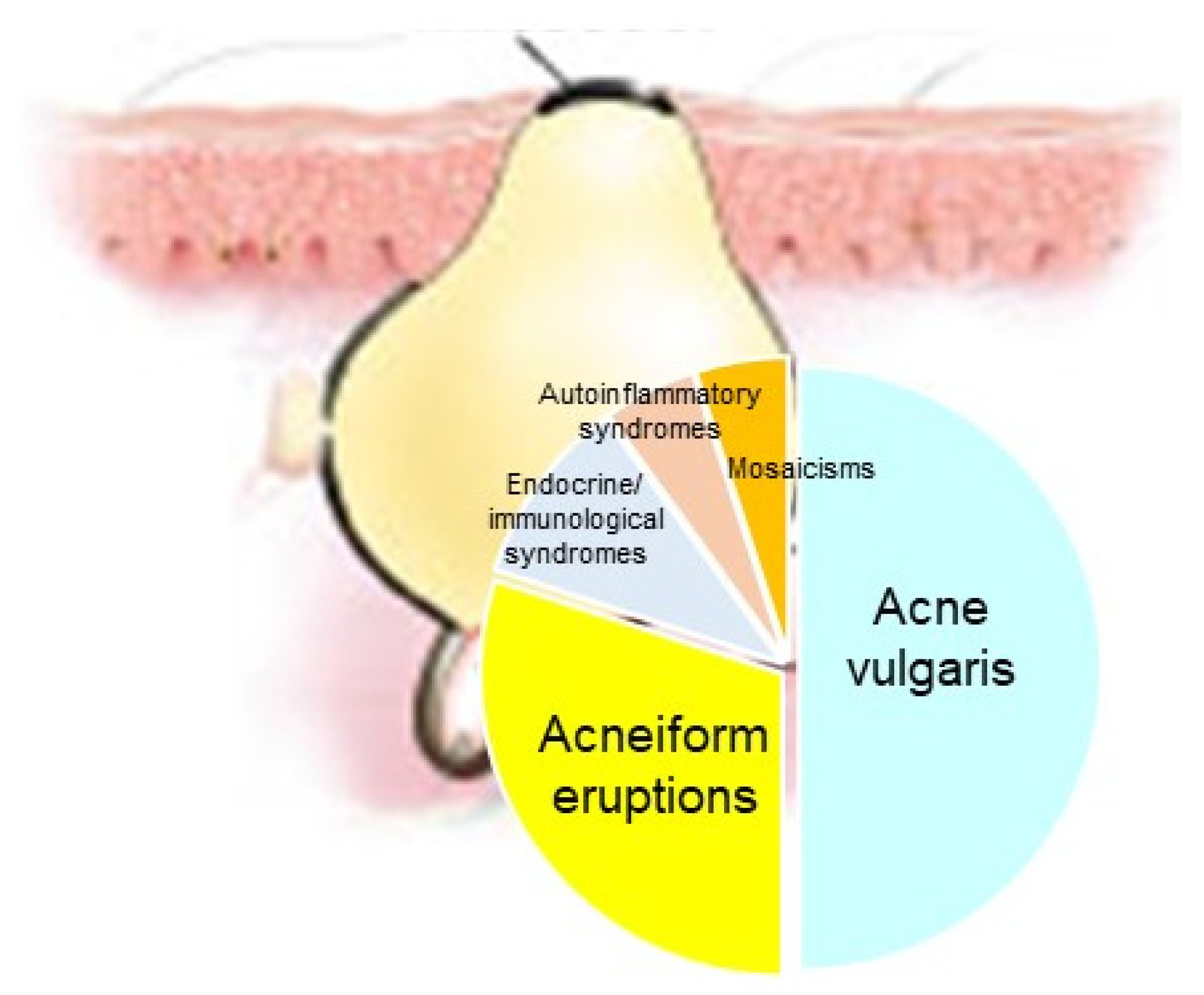
| Endocrinologic Syndromes | Autoinflammatory Syndromes |
|---|---|
|
|
| Syndrome Variant | Characteristics |
|---|---|
| • Familial | Ethnic hyperandrogenism: common in southern Europe and Arab countries, usually no alteration of peripheral hormonal levels |
| • Ovarian | Most common cause of hyperandrogenism, often PCOS |
| • Adrenal | Anatomical or only functional adrenal hyperplasia |
| • Hyperprolactinemic | Clinical manifestations similar with those of adrenal SAHA, increased serum prolactin |
| • HAIRAN syndrome | Hyperandrogenism, insulin resistance, acanthosis nigricans, variant of SAHA syndrome with polyendocrinopathy |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroud, S.; Wu, J.; Zouboulis, C.C. Acne Syndromes and Mosaicism. Biomedicines 2021, 9, 1735. https://doi.org/10.3390/biomedicines9111735
Baroud S, Wu J, Zouboulis CC. Acne Syndromes and Mosaicism. Biomedicines. 2021; 9(11):1735. https://doi.org/10.3390/biomedicines9111735
Chicago/Turabian StyleBaroud, Sumer, Jim Wu, and Christos C. Zouboulis. 2021. "Acne Syndromes and Mosaicism" Biomedicines 9, no. 11: 1735. https://doi.org/10.3390/biomedicines9111735
APA StyleBaroud, S., Wu, J., & Zouboulis, C. C. (2021). Acne Syndromes and Mosaicism. Biomedicines, 9(11), 1735. https://doi.org/10.3390/biomedicines9111735






