Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Case-Control Study
2.3. Follow-Up
2.4. Biochemical Assays
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Follow-Up
3.3. Case-Control Study
3.3.1. Intestinal Permeability
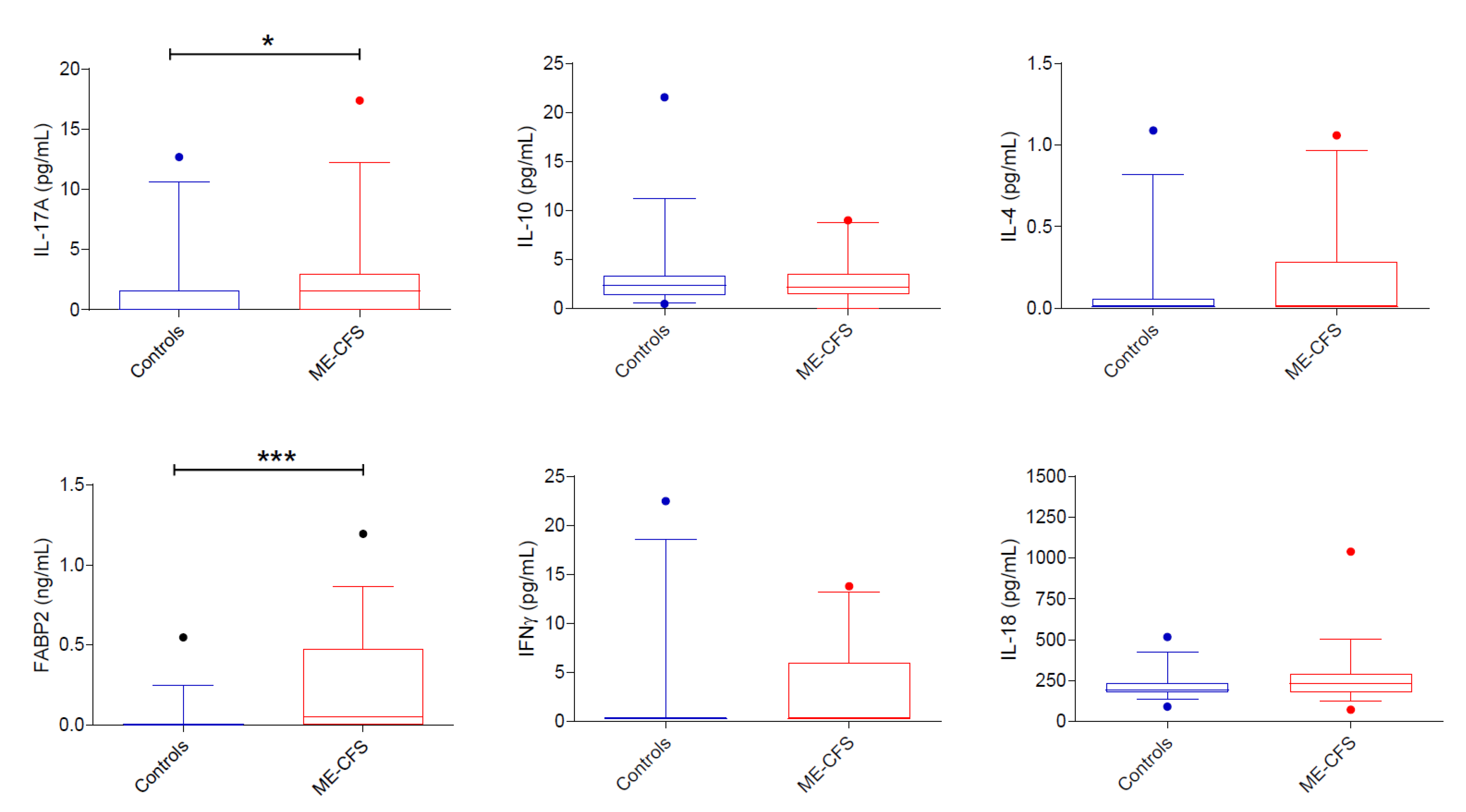
3.3.2. Cytokines
3.3.3. Tryptophan Metabolites
3.4. Comparisons between Post-Infectious and Non-Post-Infectious ME/CFS Patients
4. Discussion
4.1. Study Population
4.2. Intestinal Permeability
4.3. Cytokines
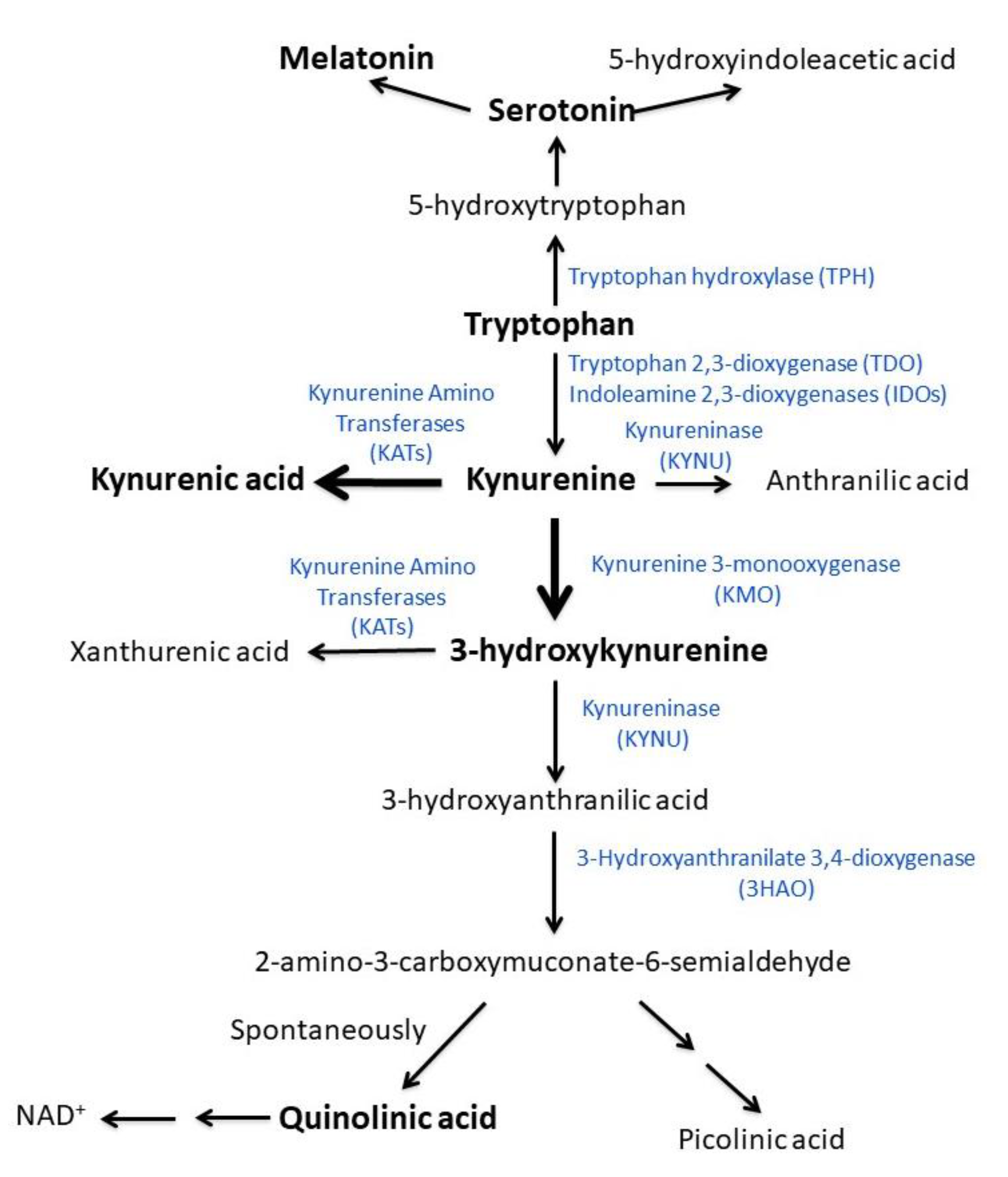
4.4. Tryptophan and Kynurenine Metabolites
4.5. Relevance of the Present Findings for the Explanation of ME/CFS Pathogenesis
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepherd, D.C.; Chaudhuri, D.A. ME/CFS/PVFS: An Exploration of the Key Clinical Issues; ME Association: Gawcott, UK, 2019. [Google Scholar]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front. Neurol. 2018, 9, 1033. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ouchi, Y.; Onoe, H.; Yoshikawa, E.; Tsukada, H.; Takahashi, H.; Iwase, M.; Yamaguti, K.; Kuratsune, H.; Watanabe, Y. Reduction of serotonin transporters of patients with chronic fatigue syndrome. Neuroreport 2004, 15, 2571–2574. [Google Scholar] [CrossRef]
- Noda, M.; Ifuku, M.; Hossain, M.S.; Katafuchi, T. Glial Activation and Expression of the Serotonin Transporter in Chronic Fatigue Syndrome. Front. Psychiatry 2018, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupo, G.F.D.; Rocchetti, G.; Lucini, L.; Lorusso, L.; Manara, E.; Bertelli, M.; Puglisi, E.; Capelli, E. Potential role of microbiome in Chronic Fatigue Syndrome/Myalgic Encephalomyelits (CFS/ME). Sci. Rep. 2021, 11, 7043. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C.; Brown, A.; Strassheim, V.; Elson, J.L.; Newton, J.; Manning, P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE 2017, 12, e0186802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missailidis, D.; Sanislav, O.; Allan, C.Y.; Smith, P.K.; Annesley, S.J.; Fisher, P.R. Dysregulated Provision of Oxidisable Substrates to the Mitochondria in ME/CFS Lymphoblasts. Int. J. Mol. Sci. 2021, 22, 2046. [Google Scholar] [CrossRef]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef] [Green Version]
- Joseph, P.; Arevalo, C.; Oliveira, R.K.F.; Faria-Urbina, M.; Felsenstein, D.; Oaklander, A.L.; Systrom, D.M. Insights From Invasive Cardiopulmonary Exercise Testing of Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Chest 2021, 160, 642–651. [Google Scholar] [CrossRef]
- Shan, Z.Y.; Barnden, L.R.; Kwiatek, R.A.; Bhuta, S.; Hermens, D.F.; Lagopoulos, J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. J. Transl. Med. 2020, 18, 335. [Google Scholar] [CrossRef]
- Cleare, A.J.; Messa, C.; Rabiner, E.A.; Grasby, P.M. Brain 5-HT1A receptor binding in chronic fatigue syndrome measured using positron emission tomography and [11C]WAY-100635. Biol. Psychiatry 2005, 57, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An ¹¹C-(R)-PK11195 PET Study. J. Nucl. Med. 2014, 55, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Morgan, C.J.; Llewelyn, M.B.; Albuquerque, S.R.; Farmer, A. Heterogeneity of serum tryptophan concentration and availability to the brain in patients with the chronic fatigue syndrome. J. Psychopharmacol. 2005, 19, 385–391. [Google Scholar] [CrossRef]
- Georgiades, E.; Behan, W.M.; Kilduff, L.P.; Hadjicharalambous, M.; Mackie, E.E.; Wilson, J.; Ward, S.A.; Pitsiladis, Y.P. Chronic fatigue syndrome: New evidence for a central fatigue disorder. Clin. Sci. 2003, 105, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Branchi, I.; Poggini, S.; Capuron, L.; Benedetti, F.; Poletti, S.; Tamouza, R.; Drexhage, H.A.; Penninx, B.W.; Pariante, C.M. Brain-immune crosstalk in the treatment of major depressive disorder. Eur. Neuropsychopharmacol. 2021, 45, 89–107. [Google Scholar] [CrossRef]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef]
- Hornig, M.; Montoya, J.G.; Klimas, N.G.; Levine, S.; Felsenstein, D.; Bateman, L.; Peterson, D.L.; Gottschalk, C.G.; Schultz, A.F.; Che, X.; et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci. Adv. 2015, 1, e1400121. [Google Scholar] [CrossRef] [Green Version]
- White, P.D. Chronic fatigue syndrome: Is it one discrete syndrome or many? Implications for the “one vs. many” functional somatic syndromes debate. J. Psychosom. Res. 2010, 68, 455–459. [Google Scholar] [CrossRef]
- Hoel, F.; Hoel, A.; Pettersen, I.K.; Rekeland, I.G.; Risa, K.; Alme, K.; Sørland, K.; Fosså, A.; Lien, K.; Herder, I.; et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight 2021, 6, e149217. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S. The Doctor’s Guide to Chronic Fatigue Syndrome: Understanding, Treating, and Living with CFIDS; Da Capo Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.P. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Messaoud, A.; Mensi, R.; Douki, W.; Neffati, F.; Najjar, M.F.; Gobbi, G.; Valtorta, F.; Gaha, L.; Comai, S. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J. Biol. Psychiatry 2019, 20, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Nazzari, S.; Molteni, M.; Valtorta, F.; Comai, S.; Frigerio, A. Prenatal IL-6 levels and activation of the tryptophan to kynurenine pathway are associated with depressive but not anxiety symptoms across the perinatal and the post-partum period in a low-risk sample. Brain Behav. Immun. 2020, 89, 175–183. [Google Scholar] [CrossRef]
- Drossman, D.A. The Rome IV Committees editor. History of functional gastrointestinal symptoms and disorders and chronicle of the Rome Foundation. In Rome IV Functional Gastrointestinal Disorders of Gut-Brain Interaction; Drossman, D.A., Chang, L.C., Kellow, W.J., Tack, J., Whitehead, W.E., Eds.; The Rome Foundation: Raleigh, NC, USA, 2016; pp. 549–576. [Google Scholar]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Onset Patterns and Course of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Pediatrics 2019, 7, 12. [Google Scholar] [CrossRef]
- Martín-Martínez, E.; Martín-Martínez, M. Varied Presentation of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and the Needs for Classification and Clinician Education: A Case Series. Clin. Ther. 2019, 41, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.; Birch, C.; Younger, J.; Worthey, E. ME/CFS: Whole genome sequencing uncovers a misclassified case of glycogen storage disease type 13 previously diagnosed as ME/CFS. Mol. Genet. Metab. 2021, 132, S194–S195. [Google Scholar] [CrossRef]
- Goldbach-Mansky, R.; Dailey, N.J.; Canna, S.W.; Gelabert, A.; Jones, J.; Rubin, B.I.; Kim, H.J.; Brewer, C.; Zalewski, C.; Wiggs, E.; et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N. Engl. J. Med. 2006, 355, 581–592. [Google Scholar] [CrossRef] [Green Version]
- De Benedetti, F.; Gattorno, M.; Anton, J.; Ben-Chetrit, E.; Frenkel, J.; Hoffman, H.M.; Koné-Paut, I.; Lachmann, H.J.; Ozen, S.; Simon, A.; et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N. Engl. J. Med. 2018, 378, 1908–1919. [Google Scholar] [CrossRef] [Green Version]
- Yadlapati, S.; Efthimiou, P. Impact of IL-1 inhibition on fatigue associated with autoinflammatory syndromes. Mod. Rheumatol. 2016, 26, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Van Bon, B.W.; Mefford, H.C.; Menten, B.; Koolen, D.A.; Sharp, A.J.; Nillesen, W.M.; Innis, J.W.; de Ravel, T.J.; Mercer, C.L.; Fichera, M.; et al. Further delineation of the 15q13 microdeletion and duplication syndromes: A clinical spectrum varying from non-pathogenic to a severe outcome. J. Med. Genet. 2009, 46, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, K.; Reardon, C. The cholinergic anti-inflammatory pathway revisited. Neurogastroenterol. Motil. 2018, 30, e13288. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Chavan, S.S.; Pavlov, V.A. Cholinergic Control of Inflammation, Metabolic Dysfunction, and Cognitive Impairment in Obesity-Associated Disorders: Mechanisms and Novel Therapeutic Opportunities. Front. Neurosci. 2019, 13, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benfante, R.; Antonini, R.A.; De Pizzol, M.; Gotti, C.; Clementi, F.; Locati, M.; Fornasari, D. Expression of the α7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J. Neuroimmunol. 2011, 230, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, V.; Abumrad, N.A. Enterocyte Fatty Acid Handling Proteins and Chylomicron Formation. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1087–1107. [Google Scholar]
- Nagy-Szakal, D.; Barupal, D.K.; Lee, B.; Che, X.; Williams, B.L.; Kahn, E.J.R.; Ukaigwe, J.E.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci. Rep. 2018, 8, 10056. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model. PLoS ONE 2016, 11, e0161635. [Google Scholar] [CrossRef]
- Corbitt, M.; Eaton-Fitch, N.; Staines, D.; Cabanas, H.; Marshall-Gradisnik, S. A systematic review of cytokines in chronic fatigue syndrome/myalgic encephalomyelitis/systemic exertion intolerance disease (CFS/ME/SEID). BMC Neurol. 2019, 19, 207. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [Green Version]
- Pucino, V.; Certo, M.; Bulusu, V.; Cucchi, D.; Goldmann, K.; Pontarini, E.; Haas, R.; Smith, J.; Headland, S.E.; Blighe, K.; et al. Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4+ T Cell Metabolic Rewiring. Cell Metab. 2019, 30, 1055–1074.e8. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Che, X.; Briese, T.; Allicock, O.; Yates, R.A.; Cheng, A.; Ranjan, A.; March, D.; Hornig, M.; Komaroff, A.L.; et al. Deficient butyrate-producing capacity in the gut microbiome of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome patients is associated with fatigue symptoms. medRxiv 2021. [Google Scholar] [CrossRef]
- Zafiriou, E.; Daponte, A.I.; Siokas, V.; Tsigalou, C.; Dardiotis, E.; Bogdanos, D.P. Depression and Obesity in Patients With Psoriasis and Psoriatic Arthritis: Is IL-17-Mediated Immune Dysregulation the Connecting Link? Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, A.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Sekine, H.; Mimura, J.; Oshima, M.; Okawa, H.; Kanno, J.; Igarashi, K.; Gonzalez, F.J.; Ikuta, T.; Kawajiri, K.; Fujii-Kuriyama, Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol. Cell. Biol. 2009, 29, 6391–6400. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, L.E.; Leopold, M.C.; Huang, X.; Atwood, C.S.; Saunders, A.J.; Hartshorn, M.; Lim, J.T.; Faget, K.Y.; Muffat, J.A.; Scarpa, R.C.; et al. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote alpha-crystallin cross-linking by metal ion reduction. Biochemistry 2000, 39, 7266–7275. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Jeon, S.W. Neuroinflammation and the Immune-Kynurenine Pathway in Anxiety Disorders. Curr. Neuropharmacol. 2018, 16, 574–582. [Google Scholar] [CrossRef]
- Groven, N.; Reitan, S.K.; Fors, E.A.; Guzey, I.C. Kynurenine metabolites and ratios differ between Chronic Fatigue Syndrome, Fibromyalgia, and healthy controls. Psychoneuroendocrinology 2021, 131, 105287. [Google Scholar] [CrossRef]
- Carl, G.F.; Hoffman, W.H.; Blankenship, P.R.; Litaker, M.S.; Hoffman, M.G.; Mabe, P.A. Diabetic ketoacidosis depletes plasma tryptophan. Endocr. Res. 2002, 28, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.H.; Whelan, S.A.; Lee, N. Tryptophan, kynurenine pathway, and diabetic ketoacidosis in type 1 diabetes. PLoS ONE 2021, 16, e0254116. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, E.; Kleffmann, T.; Edgar, C.; de Lange, M.; Vallings, R.; Tate, W. A SWATH-MS analysis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J. Transl. Med. 2020, 18, 365. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Błońska, A.; Kaczka, A.; Chojnacki, J.; Stępień, A.; Gąsiorowska, A. Evaluation of serotonin and dopamine secretion and metabolism in patients with irritable bowel syndrome. Pol. Arch. Intern. Med. 2018, 128, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manocha, M.; Khan, W.I. Serotonin and GI Disorders: An Update on Clinical and Experimental Studies. Clin. Transl. Gastroenterol. 2012, 3, e13. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef] [PubMed]

| Variable | All (n = 40) |
|---|---|
| Age, years a | 32 (23–46) |
| Males | 14 (35%) |
| Disease duration, years a,b | 6 (3–12) |
| Symptom start: | |
| Slow (months) | 18/40 (45%) |
| Fast (weeks) | 22/40 (55%) |
| ME/CFS, fatigue, or fibromyalgia in the family | 13/38 d (34%) |
| Immune diseases in the family | 17/38 d (44%) |
| Symptom score (0–6): a | |
| Fatigue | 6 (5–6) |
| PEM | 5 (5–6) |
| Unrefreshing sleep | 4 (4–5) |
| Pain | 4 (3–5) |
| Cognitive anomalies | 5 (4–5) |
| OI/POTS | 4 (4–5) |
| GI abnormalities | 4 (3–4) |
| Flu-like symptoms | 4 (3–5) |
| Bell scale score (0–100%) a,c | 30 (25–50) |
| Bowel habit: a | |
| Normal | 23/38 d (61%) |
| Styptic | 10/38 d (26%) |
| Diarrheic | 5/38 d (13%) |
| Abdominal pain—IBS e | 23/40 (58%) |
| Variable | Number of Patients (%) | Notes |
|---|---|---|
| Disease start | ||
| After a neoplasia | 2 (5) | Meningioma, Hodgkin lymphoma |
| After a vaccine | 1 (2) | |
| Symptoms, signs, and laboratory data | ||
| Skin hyperlaxity | 4 (10) | |
| Livedo reticularis | 2 (5) | |
| Dry eyes/mouth | 2 (5) | |
| Oral aphthae | 2 (5) | |
| Paresthesias | 3 (7) | |
| Jerks | 2 (5) | |
| Hypersomnia | 2 (5) | |
| Bouts of fever, flu-like symptoms, increase in CRP and serum amyloid A | 2 (5) | Response to colchicine, canakinumab |
| Muscle biopsy anomalies | 1 (2) | Thickened basal membrane of small vessels, glycogen accumulation within myocytes |
| Serum cytokines above normal * | ||
| IL-1α | 2 (5) | |
| IL-2 | 9 (22) | |
| IL-6 | 2 (5) | |
| IL-8 | 1 (2) | |
| TNF-α | 7 (17) | |
| TGF-β | 13 (32) | |
| Genetic abnormalities | ||
| CASQ1 gene mutation | 1 (2) | N227I, heterozygous. Muscle biopsy: thickened vessel basal membrane, fibrils of unknown nature around myocytes. |
| 15q13.3 duplication (340 BP) | 1 (2) | |
| Ehlers–Danlos syndrome | 1 (2) | |
| Myotonia congenita | 1 (2) |
| Follow-Up, Months a | 41 (32–47) |
|---|---|
| Symptom evolution during follow up: | |
| Unchanged | 8/40 (20%) |
| Worsened | 21/40 (52%) |
| Improved | 11/40 (28%) |
| Prevailing symptoms during follow-up: | |
| Fatigue | 20 (50%) |
| PEM | 7 (17%) |
| Unrefreshing sleep | 2 (5%) |
| Pain | 6 (15%) |
| Cognitive anomalies | 12 (30%) |
| OI/POTS | 1 (2%) |
| Abdominal pain/IBS b | 1 (2%) |
| Flu-like | 1 (2%) |
| Variable | ME/CFS Did Not Start after an Infection (n = 21) | ME/CFS Started after an Infection (n = 19) | Statistics |
|---|---|---|---|
| Age, years a | 30 (20–44) | 35 (28–48) | U = 261.5, p = 0.093 |
| Males | 8 (38%) | 6 (32%) | χ2 = 0.186, p = 0.66 |
| Disease duration, years a,b | 8 (3–13) | 5 (3–11) | U = 169.5, p = 0.59 |
| Symptom start: | χ2 = 23.089, p < 0.0001 | ||
| Slow (months) | 17/21 (81%) | 1/19 (5%) | |
| Fast (weeks) | 4/21 (19%) | 18/19 (95%) | |
| ME/CFS, fatigue or fibromyalgia in the family | 7/19 d (37%) | 6/19 (31%) | χ2 = 0.000, 0.99 |
| Immune diseases in the family | 9/19 d (47%) | 8/19 (42%) | χ2 = 0.010, 0.92 |
| Symptom score (0–6): a | |||
| Fatigue | 6 (5–6) | 6 (5–6) | |
| PEM | 5 (5–6) | 5 (5–6) | U = 193.0, p = 0.84 U = 211.0 |
| Unrefreshing sleep | 5 (4–5) | 4 (4–5) | p = 0.74 U = 214.0, |
| Pain | 4 (3–5) | 4 (3–6) | p = 0.69 U = 212.0, |
| Cognitive anomalies | 5 (4–5) | 5 (4–5) | p = 0.73 U = 179.0 |
| OI/POTS | 4 (4–5) | 4 (4–5) | p = 0.55 U = 249.0, |
| GI abnormalities | 4 (0–4) | 4 (3–5) | p = 0.17 U = 250.0, |
| Flu-like symptoms | 4 (3–4) | 5 (3–5) | p = 0.16 U = 242.0, p = 0.24 |
| Bell scale score (0–100%) a,c | 30 (22–41) | 43 (30–50) | U = 236.0, p = 0.02 |
| Bowel habit: a | χ2 = 5.353, p = 0.07 | ||
| Normal d | 11/20 (55%) | 12/18 (67%) | |
| Styptic d | 8/20 (40%) | 2/18 (11%) | |
| Diarrheic d | 1/20 (5%) | 4/18 (22%) | |
| Abdominal pain—IBS e | 13/21 (62%) | 10/19 (53%) | χ2 = 0.351, p = 0.55 |
| ME/CFS Did Not Start after an Infection (n = 21) | ME/CFS Started after an Infection (n = 19) | Statistics | |
|---|---|---|---|
| Cytokines | |||
| IL-17A (pg/mL) | 1.6 (0.2–5.3) | 1.6 (0.0–2.8) | F1,36 = 0.073, p = 0.789, ηp2 = 0.002 |
| IL-10 (pg/mL) | 2.2 (1.7–4.2) | 2.0 (1.3–2.7) | F1,36 = 1.311, p = 0.260, ηp2 = 0.035 |
| IL-4 (pg/mL) | 0.0 (0.0–0.5) | 0.0 (0.0–0.3) | F1,36 = 0.503, p = 0.485, ηp2 = 0.021 |
| IFN-γ (pg/mL) | 0.3 (0.3–8.2) | 0.3 (0.3–4.5) | F1,36 = 0.205, p = 0.654 ηp2 = 0.006 |
| IL-18 (pg/mL) | 255.3 (195.9–303.4) | 196.9 (155.6–250.1) | F1,36 = 4.521, p = 0.068, ηp2 = 0.091 |
| FABP-2 (ng/mL) | 0.0 (0.0–0.5) | 0.13 (0.0–0.5) | F1,34 = 0.492 p = 0.488, ηp2 = 0.015 |
| Kynurenine pathway | |||
| Tryptophan (μg/mL) | 9.36 (8.26–12.01) | 10.68 (9.56–11.99) | F1,37 = 1.640, p = 0.208, ηp2 = 0.042 |
| Kynurenine (ng/mL) | 413.4 (339.2–539.6) | 347.6 (260.1–397.9) | F1,37 = 5.410, p = 0.026, ηp2 = 0.128 |
| 3-hydroxykynurenine (ng/mL) | 12.3 (3.3–41.7) | 17.5 (5.9–39.6) | F1,37 = 1.102, p = 0.301, ηp2 = 0.029 |
| Kynurenic acid (ng/mL) | 16.6 (11.0–24.1) | 14.2 (9.1–35.8) | F1,37 = 0.216, p = 0.645, ηp2 = 0.006 |
| Quinolinic acid (ng/mL) | 59.6 (40.9–102.6) | 67.2 (59.6–111.6) | F1,37 = 0.298, p = 0.588, ηp2 = 0.008 |
| Kynurenine/tryptophan ratio ∗ 1000 | 44.1 (32.9–57.9) | 33.2 (24.8–38.8) | F1,37 = 6.525, p = 0.015, ηp2 = 0.150 |
| 3-hydroxykynurenine/kynurenine ratio ∗ 1000 | 42.4 (7.8–97.9) | 80.6 (19.0–107.7) | F1,37 = 0.499, p = 0.485, ηp2 = 0.013 |
| Kynurenic acid/kynurenine ratio | 39.6 (26.5–59.7) | 50.6 (27.6–84.1) | F1,37 = 0.313, p = 0.579, ηp2 = 0.008 |
| Kynurenic acid/quinolinic acid ratio | 0.2 (0.1–0.4) | 0.2 (0.1–0.3) | F1,37 = 0.131, p = 0.719, ηp2 = 0.004 |
| Serotonin pathway | |||
| Serotonin (ng/mL) | 301.4 (218.3–382.4) | 258.8 (180.4–377.4) | F1,37 = 1.261, p = 0.269, ηp2 = 0.033 |
| Melatonin (pg/mL) | 9.8 (6.3–20.2) | 10.2 (8.1–13.7) | F1,37 = 0.076, p = 0.784, ηp2 = 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U.; et al. Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2021, 9, 1724. https://doi.org/10.3390/biomedicines9111724
Simonato M, Dall’Acqua S, Zilli C, Sut S, Tenconi R, Gallo N, Sfriso P, Sartori L, Cavallin F, Fiocco U, et al. Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines. 2021; 9(11):1724. https://doi.org/10.3390/biomedicines9111724
Chicago/Turabian StyleSimonato, Manuela, Stefano Dall’Acqua, Caterina Zilli, Stefania Sut, Romano Tenconi, Nicoletta Gallo, Paolo Sfriso, Leonardo Sartori, Francesco Cavallin, Ugo Fiocco, and et al. 2021. "Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome" Biomedicines 9, no. 11: 1724. https://doi.org/10.3390/biomedicines9111724
APA StyleSimonato, M., Dall’Acqua, S., Zilli, C., Sut, S., Tenconi, R., Gallo, N., Sfriso, P., Sartori, L., Cavallin, F., Fiocco, U., Cogo, P., Agostinis, P., Aldovini, A., Bruttomesso, D., Marcolongo, R., Comai, S., & Baritussio, A. (2021). Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines, 9(11), 1724. https://doi.org/10.3390/biomedicines9111724









