Development of Triiodothyronine Polymeric Nanoparticles for Targeted Delivery in the Cardioprotection against Ischemic Insult
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Analysis
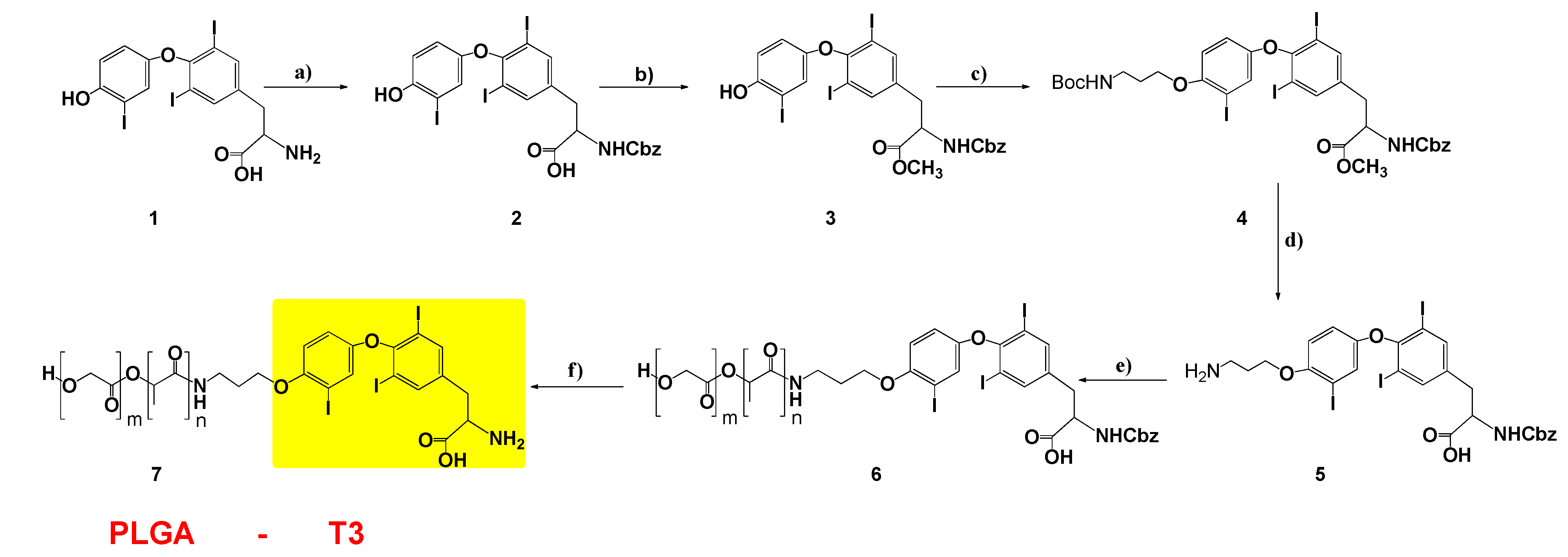
2.2. 2-((benzyloxy)carbonyl)amino)-3-(4-(4-hydroxy-3-iodophenoxy)-3,5 di-iodophenyl) Propanoic Acid (2)
2.3. Methyl2-(benzyloxy)carbonyl)amino)-3-(4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl) Propanoate (3)
2.4. Methyl2-(benzyloxy)carbonyl)amino)-3-(4-(4-(3-(tert-butoxycarbonyl)amino) propoxy)-3-iodophenoxy)-3,5-diiodophenyl) Propanoate (4)
2.5. 3-(4-(4-(3-aminopropoxy)-3-iodophenoxy)-3,5-diiodophenyl)-2 ((benzyloxy)carbonyl)amino) Propanoic Acid (5)
2.6. PLGA-T3-Cbz (6) and PLGA-T3 (7)
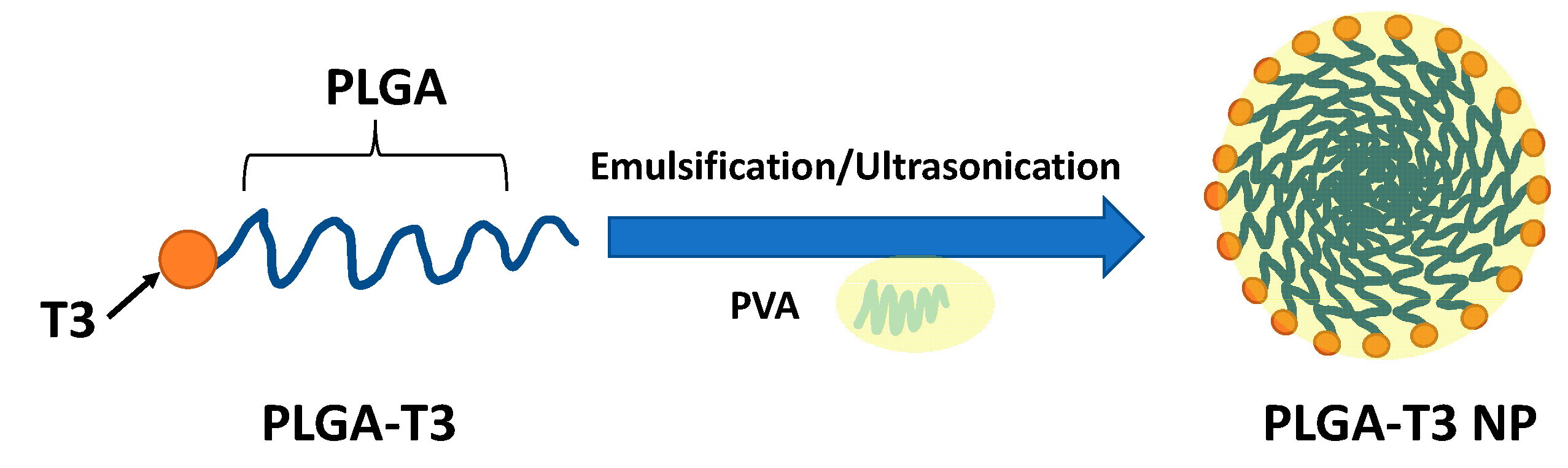
2.7. Synthesis and Characterization of PLGA-T3 NPs
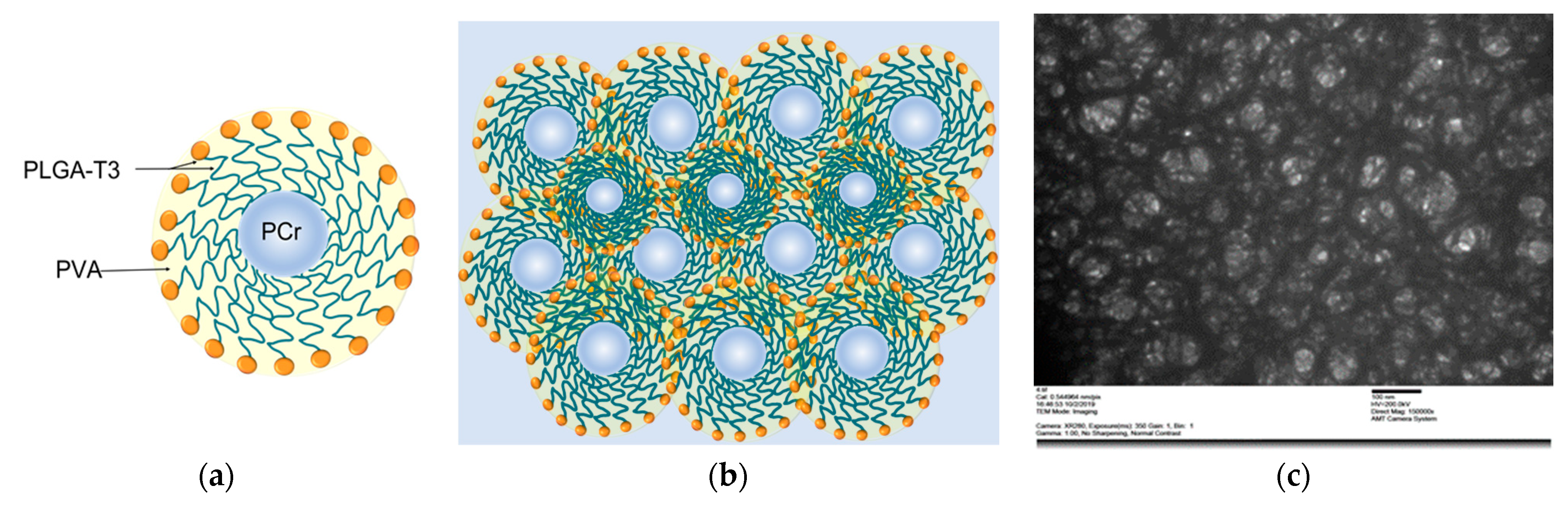
2.8. The Synthesis of PLGA-T3/PCr NPs
2.9. The Characterization of the Prepared PLGA-T3/PCr NPs
2.10. Encapsulation Efficiency (EE) and Loading Ratio (LR)
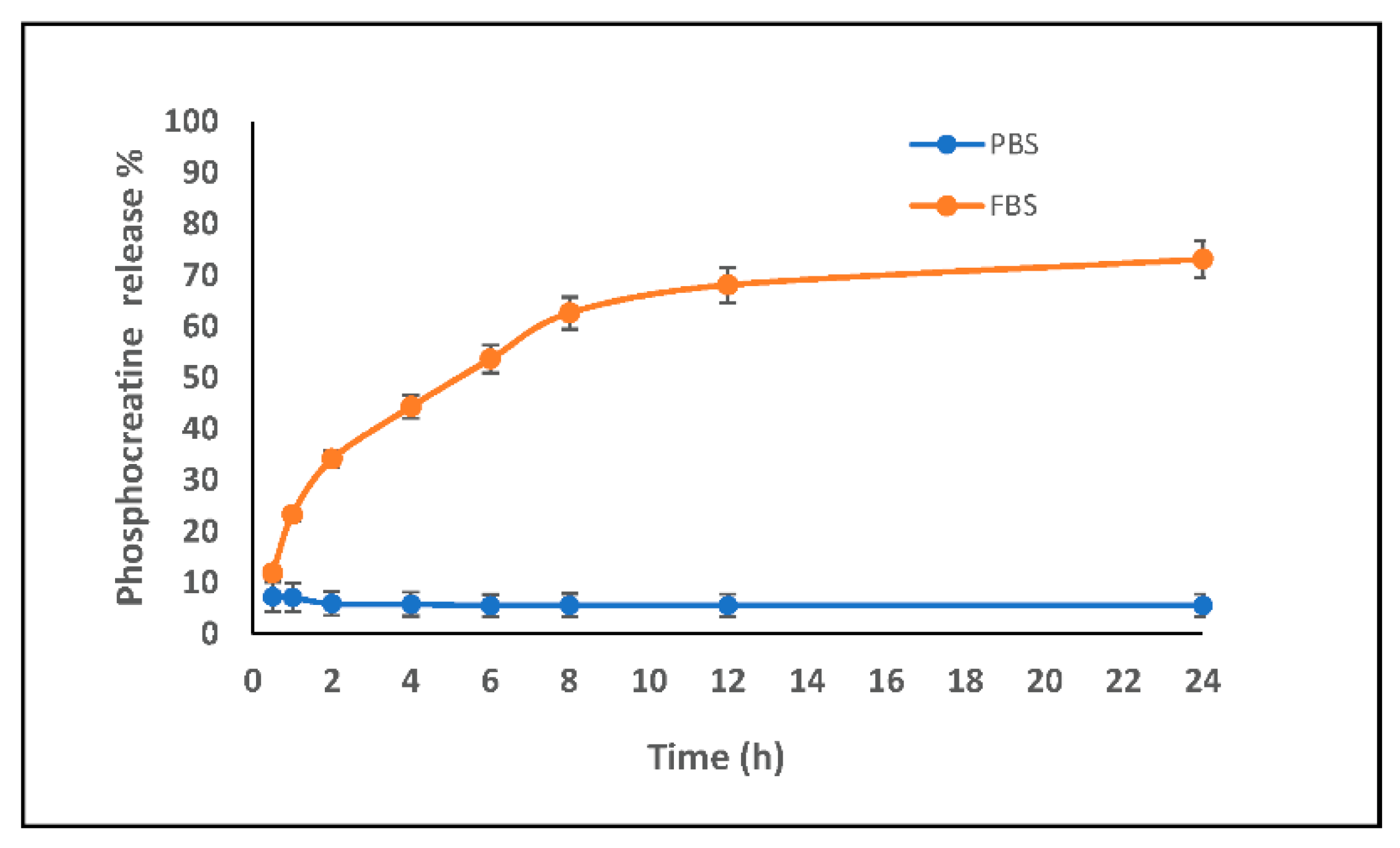
2.11. In Vitro Release Study
2.12. Evaluation of the Cardioprotective Effect of T3 and PLGA-T3/PCr NPs under Hypoxia
2.13. Biodistribution of T3 and PLGA-T3 NPs-Cy7
2.14. Statistical Analysis
3. Results
3.1. Design, Synthesis and Characterization
3.2. Physicochemical Characterization of the Nanoparticles
3.3. Loading Percentage and Entrapment Efficiency
3.4. Release Kinetics
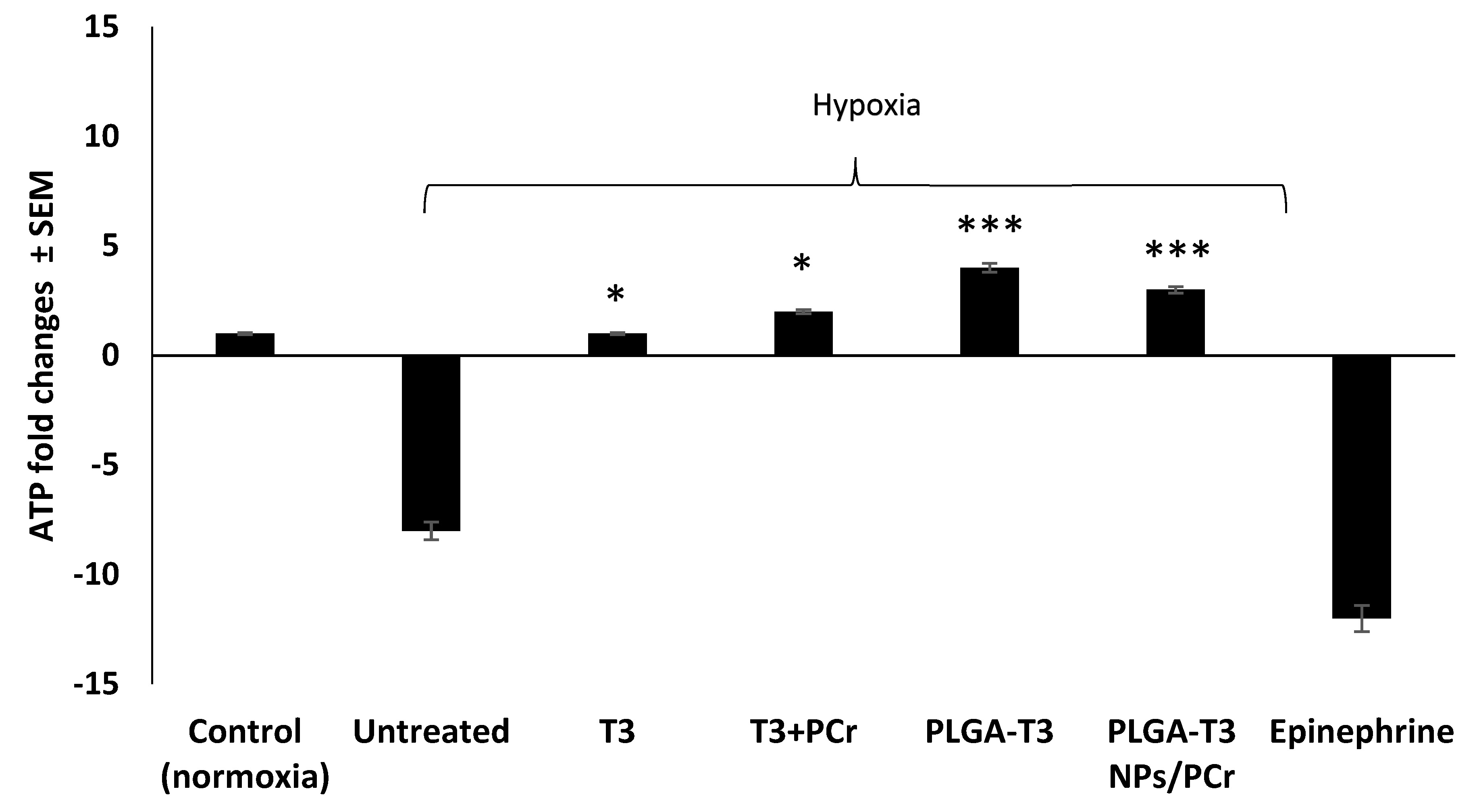
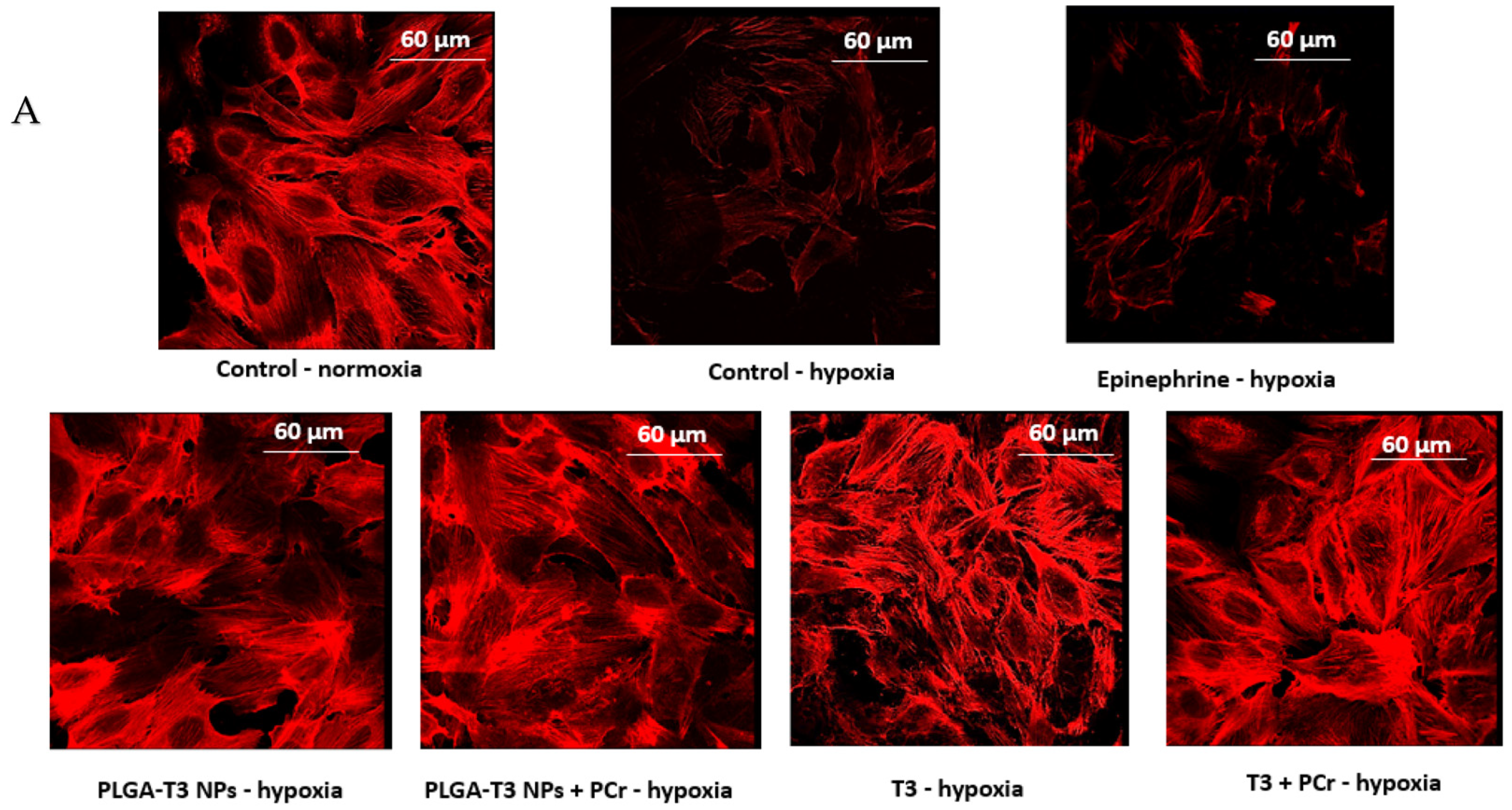
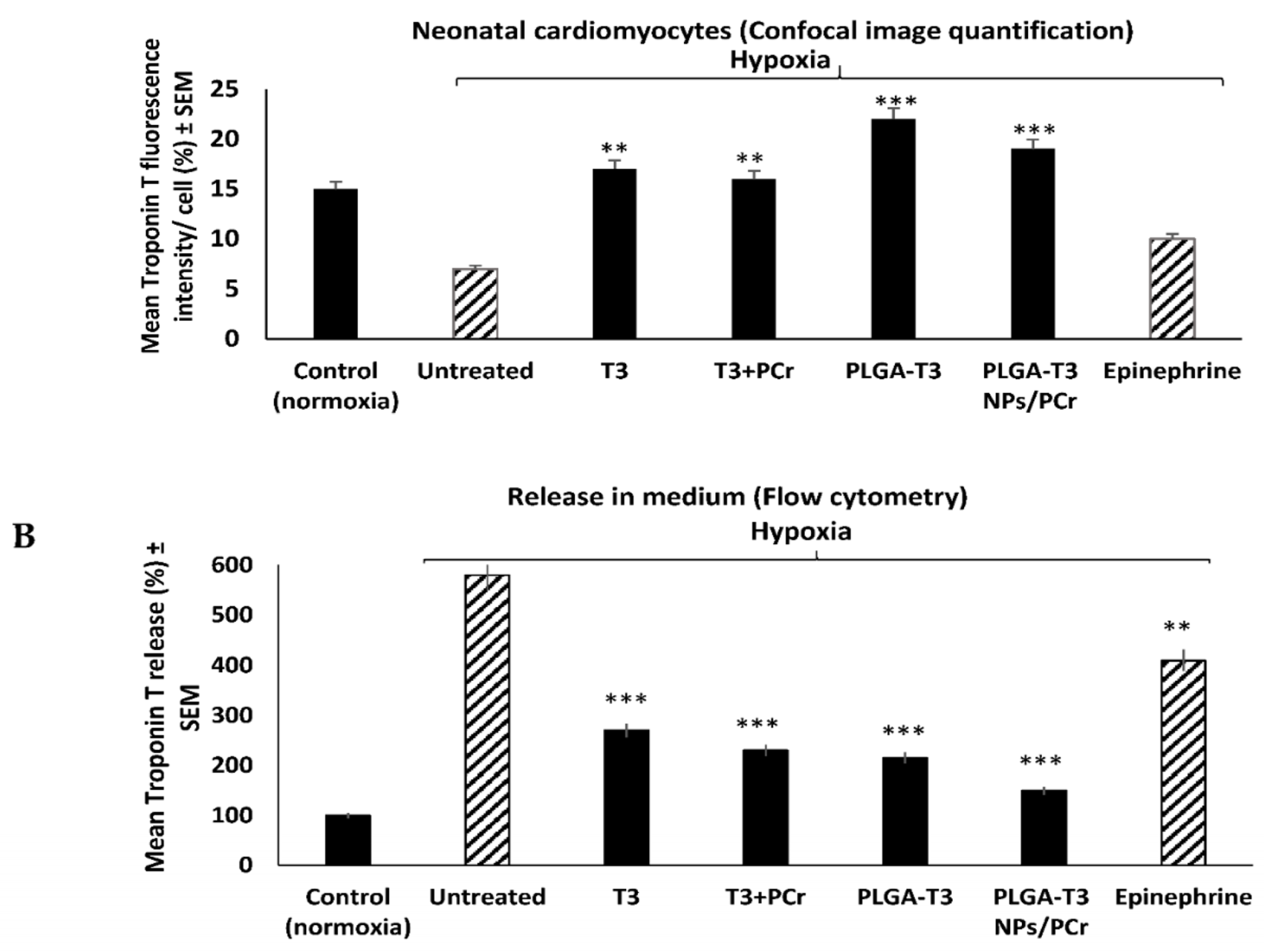
3.5. Cardioprotective Effects of T3 and PLGA-T3/PCr NPs under Hypoxia
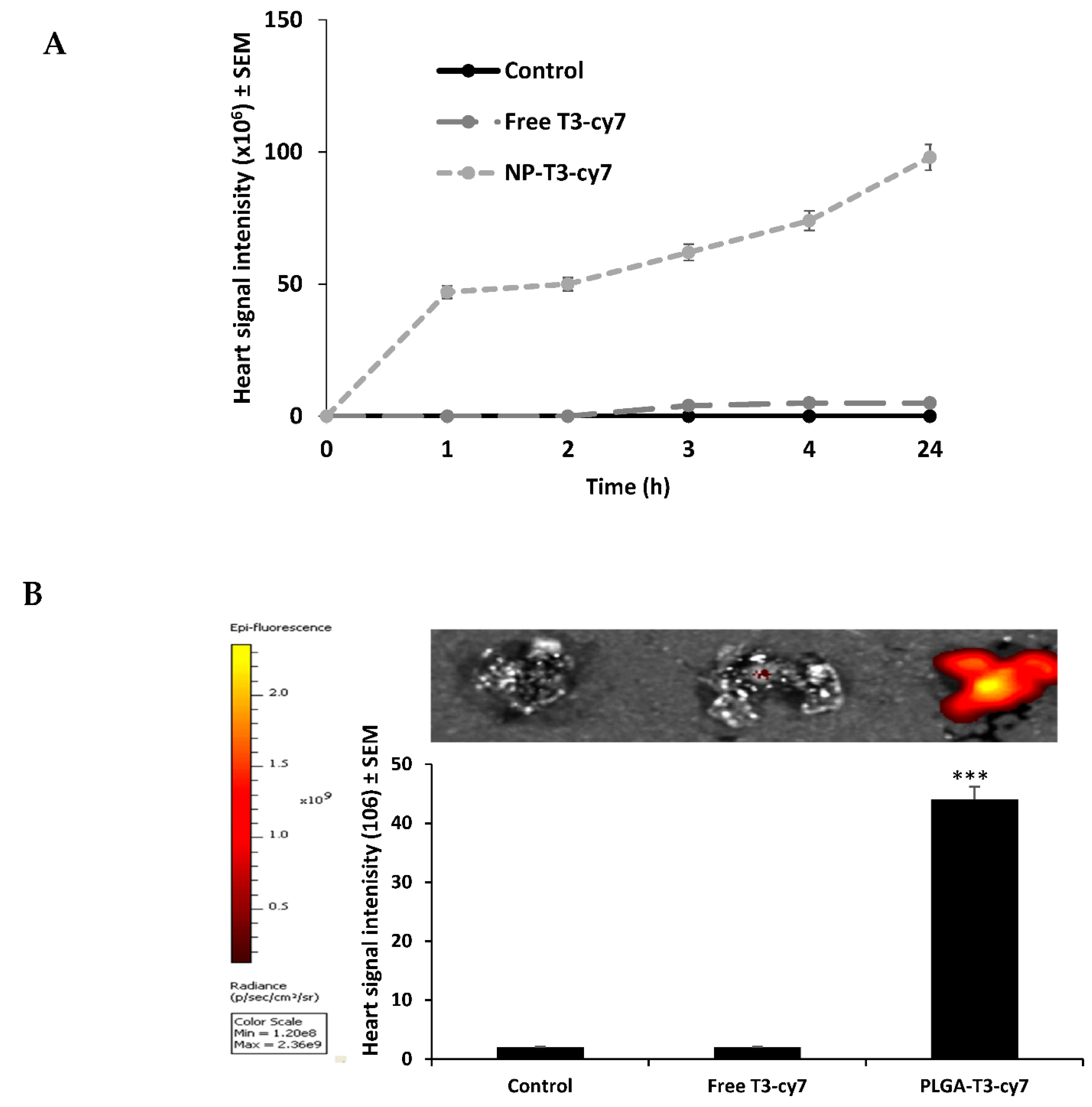
3.6. Evaluation of Free T3 and PLGA-T3 NPs-Cy7 Biodistribution in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantos, C.; Mourouzis, I.; Markakis, K.; Dimopoulos, A.; Xinaris, C.; Kokkinos, A.D.; Panagiotou, M.; Cokkinos, D.V. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. Eur. J. Cardiothorac. Surg. 2007, 32, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dedkov, E.I.; Lee, B., 3rd; Li, Y.; Pun, K.; Gerdes, A.M. Thyroid hormone replacement therapy attenuates atrial remodeling and reduces atrial fibrillation inducibility in a rat myocardial infarction-heart failure model. J. Card Fail. 2014, 20, 1012–1019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoeller, R.T. Neuroendocrine Regulation of Development, Growth and Metabolism–Thyroid. In Handbook of Neuroendocrinology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 259–270. [Google Scholar]
- Razvi, S. Novel uses of thyroid hormones in cardiovascular conditions. Endocrine 2019, 66, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Davis, P.J.; Leonard, J.L.; Lin, H.Y.; Leinung, M.; Mousa, S.A. Molecular Basis of Nongenomic Actions of Thyroid Hormone. Vitam. Horm. 2018, 106, 67–96. [Google Scholar] [CrossRef]
- Fang, L.; Xu, Z.; Lu, J.; Hong, L.; Qiao, S.; Liu, L.; An, J. Cardioprotective effects of triiodothyronine supplementation against ischemia reperfusion injury by preserving calcium cycling proteins in isolated rat hearts. Exp. Ther. Med. 2019, 18, 4935–4941. [Google Scholar] [CrossRef]
- Forini, F.; Kusmic, C.; Nicolini, G.; Mariani, L.; Zucchi, R.; Matteucci, M.; Iervasi, G.; Pitto, L. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 2014, 155, 4581–4590. [Google Scholar] [CrossRef]
- Forini, F.; Nicolini, G.; Iervasi, G. Mitochondria as key targets of cardioprotection in cardiac ischemic disease: Role of thyroid hormone triiodothyronine. Int. J. Mol. Sci. 2015, 16, 6312–6336. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef]
- Pingitore, A.; Iervasi, G.; Forini, F. Role of the Thyroid System in the Dynamic Complex Network of Cardioprotection. Eur. Cardiol. 2016, 11, 36–42. [Google Scholar] [CrossRef]
- Sudha, T.; Bharali, D.J.; Yalcin, M.; Darwish, N.H.; Coskun, M.D.; Keating, K.A.; Lin, H.Y.; Davis, P.J.; Mousa, S.A. Targeted delivery of cisplatin to tumor xenografts via the nanoparticle component of nano-diamino-tetrac. Nanomedicine 2017, 12, 195–205. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Guvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Landoni, G.; Zangrillo, A.; Lomivorotov, V.V.; Likhvantsev, V.; Ma, J.; De Simone, F.; Fominskiy, E. Cardiac protection with phosphocreatine: A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 637–646. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef]
- Li, T.; Wang, N.; Zhao, M. Neuroprotective effect of phosphocreatine on focal cerebral ischemia-reperfusion injury. J. Biomed. Biotechnol. 2012, 2012, 168756. [Google Scholar] [CrossRef][Green Version]
- Strumia, E.; Pelliccia, F.; D’Ambrosio, G. Creatine phosphate: Pharmacological and clinical perspectives. Adv. Ther. 2012, 29, 99–123. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Preparation of poly(DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int. J. Pharm. 1999, 187, 143–152. [Google Scholar] [CrossRef]
- Sudha, T.; Bharali, D.J.; Yalcin, M.; Darwish, N.H.; Debreli Coskun, M.; Keating, K.A.; Lin, H.Y.; Davis, P.J.; Mousa, S.A. Targeted delivery of paclitaxel and doxorubicin to cancer xenografts via the nanoparticle of nano-diamino-tetrac. Int. J. Nanomed. 2017, 12, 1305–1315. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, C.; Ding, X.; Yuan, D.; Chen, K.; Gao, B.; Chen, Y.; Sun, A. Simultaneous determination of adenine nucleotides, creatine phosphate and creatine in rat liver by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 66, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Ehler, E.; Moore-Morris, T.; Lange, S. Isolation and culture of neonatal mouse cardiomyocytes. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Yalcin, M.; Davis, P.J.; Mousa, S.A. Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: A nanomedicine approach to treat drug-resistant breast cancer. Nanomedicine 2013, 8, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Gomberg-Maitland, M.; Frishman, W.H. Thyroid hormone and cardiovascular disease. Am. Heart J. 1998, 135, 187–196. [Google Scholar] [CrossRef]
- He, Z.; Sun, Y.; Cao, J.; Duan, Y. Degradation behavior and biosafety studies of the mPEG-PLGA-PLL copolymer. Phys. Chem. Chem. Phys. 2016, 18, 11986–11999. [Google Scholar] [CrossRef] [PubMed]
- Cody, V.; Davis, P.J.; Davis, F.B. Molecular modeling of the thyroid hormone interactions with αvβ3 integrin. Steroids 2007, 72, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Sallam, A.; Darwish, N. Cardiovascular Protective Effect of Thyroxine and Nano-Thyroxine Against Ischemic Insults. Circulation 2019, 140, A133. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Morton, A.R. Chapter 8—Exercise Physiology. In Pediatric Respiratory Medicine, 2nd ed.; Taussig, L.M., Landau, L.I., Eds.; Mosby: Philadelphia, PA, USA, 2008; pp. 89–99. [Google Scholar]
- Jafri, M.S.; Dudycha, S.J.; O’Rourke, B. Cardiac energy metabolism: Models of cellular respiration. Annu. Rev. Biomed. Eng. 2001, 3, 57–81. [Google Scholar] [CrossRef]
- Abd-Elfattah, A.S.; Tuchy, G.E.; Jessen, M.E.; Salter, D.R.; Goldstein, J.P.; Brunsting, L.A., III; Wechsler, A.S. Hot shot induction and reperfusion with a specific blocker of the es-ENT1 nucleoside transporter before and after hypothermic cardioplegia abolishes myocardial stunning in acutely ischemic hearts despite metabolic derangement: Hot shot drug delivery before hypothermic cardioplegia. J. Thorac. Cardiovasc. Surg. 2013, 146, 961–970.e963. [Google Scholar]
- Davis, P.J.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Non Genomic Actions of Thyroid Hormones in Cancer. Front. Endocrinol. 2019, 10, 847. [Google Scholar] [CrossRef]
- Lei, J.; Nowbar, S.; Mariash, C.N.; Ingbar, D.H. Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L762–772. [Google Scholar] [CrossRef]
- Davis, P.J.; Davis, F.B.; Cody, V. Membrane receptors mediating thyroid hormone action. Trends Endocrinol. Metab. 2005, 16, 429–435. [Google Scholar] [CrossRef]
- van der Meel, R.; Vehmeijer, L.J.; Kok, R.J.; Storm, G.; van Gaal, E.V. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv. Drug Deliv. Rev. 2013, 65, 1284–1298. [Google Scholar] [CrossRef]
- Iyer, A.K.; Singh, A.; Ganta, S.; Amiji, M.M. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1784–1802. [Google Scholar] [CrossRef]
- Kottke, M.; Wallimann, T.; Brdiczka, D. Dual electron microscopic localization of mitochondrial creatine kinase in brain mitochondria. Biochem. Med. Metab. Biol. 1994, 51, 105–117. [Google Scholar] [CrossRef]
- Gaddi, A.; Galuppo, P.; Yang, J. Creatine phosphate administration in cell energy impairment conditions: A summary of past and present research. Heart Lung Circ. 2017, 26, 1026–1035. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Sun, M.; Tang, H.-Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. L-Thyroxine vs. 3, 5, 3′-triiodo-L-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol.-Cell Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef]
- Lin, H.Y.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Cao, J.H.; Zhou, M.; Davis, P.J. Molecular basis for certain neuroprotective effects of thyroid hormone. Front. Mol. Neurosci. 2011, 4, 29. [Google Scholar] [CrossRef]
| Nanoformulation | Z-Average Size (d.nm) | Polydisperse Index |
|---|---|---|
| Void NPs | 254 | 0.116 |
| PLGA-T3 NPs | 175 | 0.19 |
| PLGA-T3/PCr NPs | 176 | 0.194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakus, O.O.; Darwish, N.H.E.; Sudha, T.; Salaheldin, T.A.; Fujioka, K.; Dickinson, P.C.T.; Weil, B.; Mousa, S.A. Development of Triiodothyronine Polymeric Nanoparticles for Targeted Delivery in the Cardioprotection against Ischemic Insult. Biomedicines 2021, 9, 1713. https://doi.org/10.3390/biomedicines9111713
Karakus OO, Darwish NHE, Sudha T, Salaheldin TA, Fujioka K, Dickinson PCT, Weil B, Mousa SA. Development of Triiodothyronine Polymeric Nanoparticles for Targeted Delivery in the Cardioprotection against Ischemic Insult. Biomedicines. 2021; 9(11):1713. https://doi.org/10.3390/biomedicines9111713
Chicago/Turabian StyleKarakus, Ozlem Ozen, Noureldien H. E. Darwish, Thangirala Sudha, Taher A. Salaheldin, Kazutoshi Fujioka, Peter C. Taylor Dickinson, Brian Weil, and Shaker A. Mousa. 2021. "Development of Triiodothyronine Polymeric Nanoparticles for Targeted Delivery in the Cardioprotection against Ischemic Insult" Biomedicines 9, no. 11: 1713. https://doi.org/10.3390/biomedicines9111713
APA StyleKarakus, O. O., Darwish, N. H. E., Sudha, T., Salaheldin, T. A., Fujioka, K., Dickinson, P. C. T., Weil, B., & Mousa, S. A. (2021). Development of Triiodothyronine Polymeric Nanoparticles for Targeted Delivery in the Cardioprotection against Ischemic Insult. Biomedicines, 9(11), 1713. https://doi.org/10.3390/biomedicines9111713








