Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Colitis
2.3. Fluorocitrate Solution Preparation
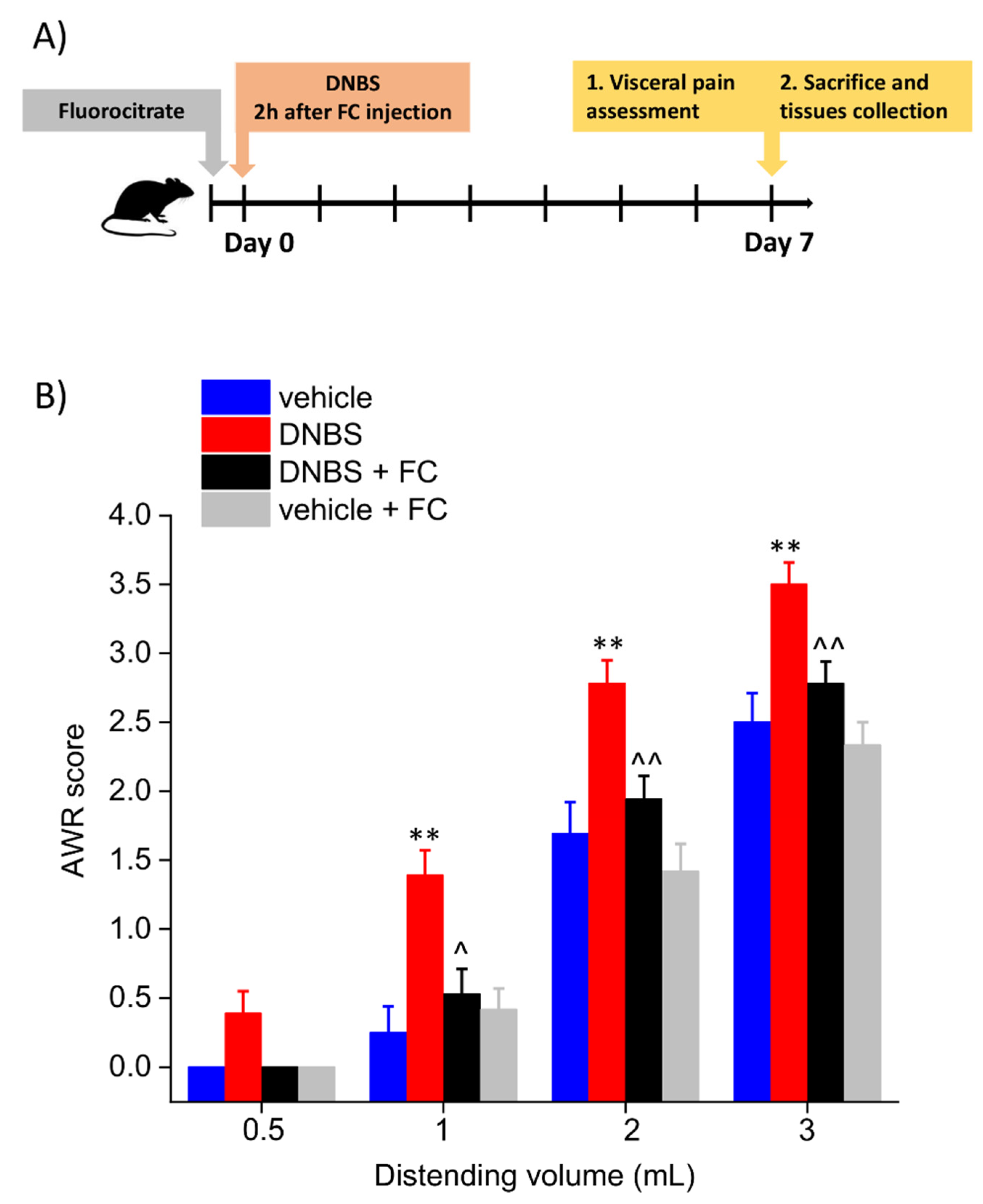
2.4. Assessment of Visceral Sensitivity by Abdominal Withdrawal Reflex to Colorectal Distension
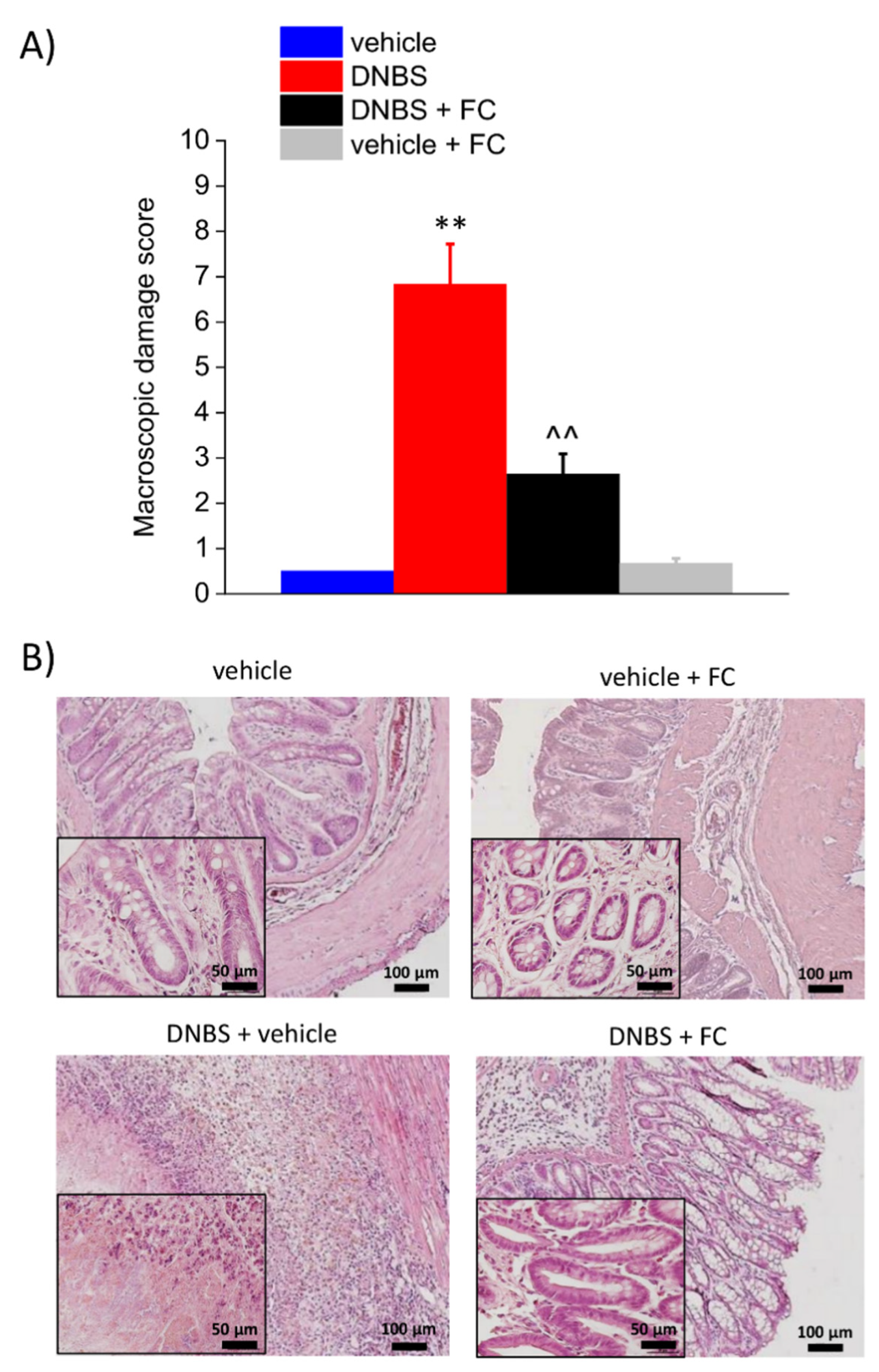
2.5. Histological Analysis of Colon
2.6. Circular Muscle Myenteric Plexus (CMMP) Whole-Mount Preparation
2.7. Immunofluorescence
2.8. Statistics
3. Results
3.1. Enteric Glia Inhibition by FC Prevented the Development of Colitis-Related Visceral Hyperalgesia in Rats
3.2. Enteric Glia Inhibition by FC Reduced the DNBS-Induced Intestinal Damage in Rats
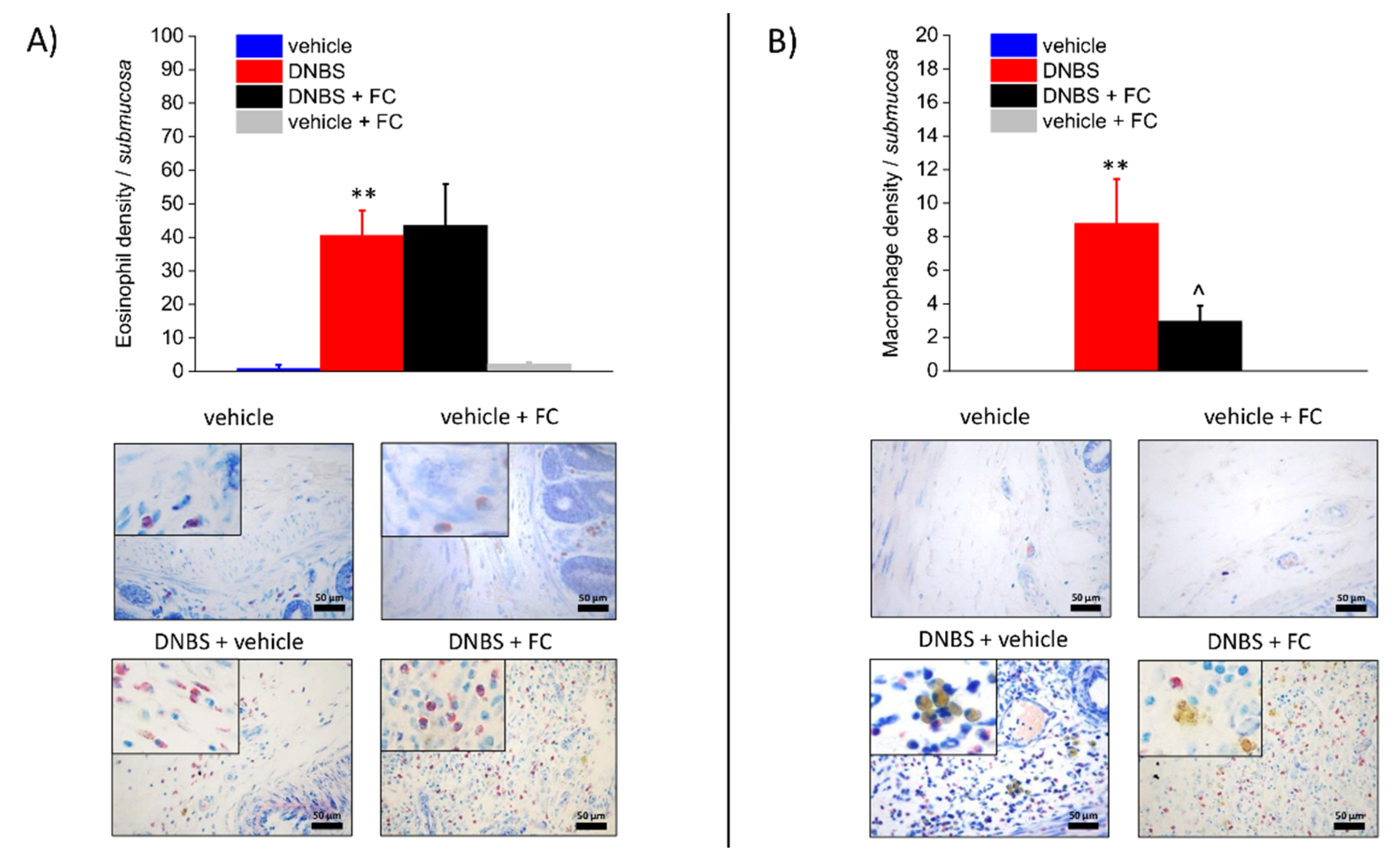
3.3. Enteric Glia Inhibition by FC Reduced MCs Infiltration and Macrophages Activation in the Submucosa of DNBS Treated Rats, but Not Eosinophils Recruitment
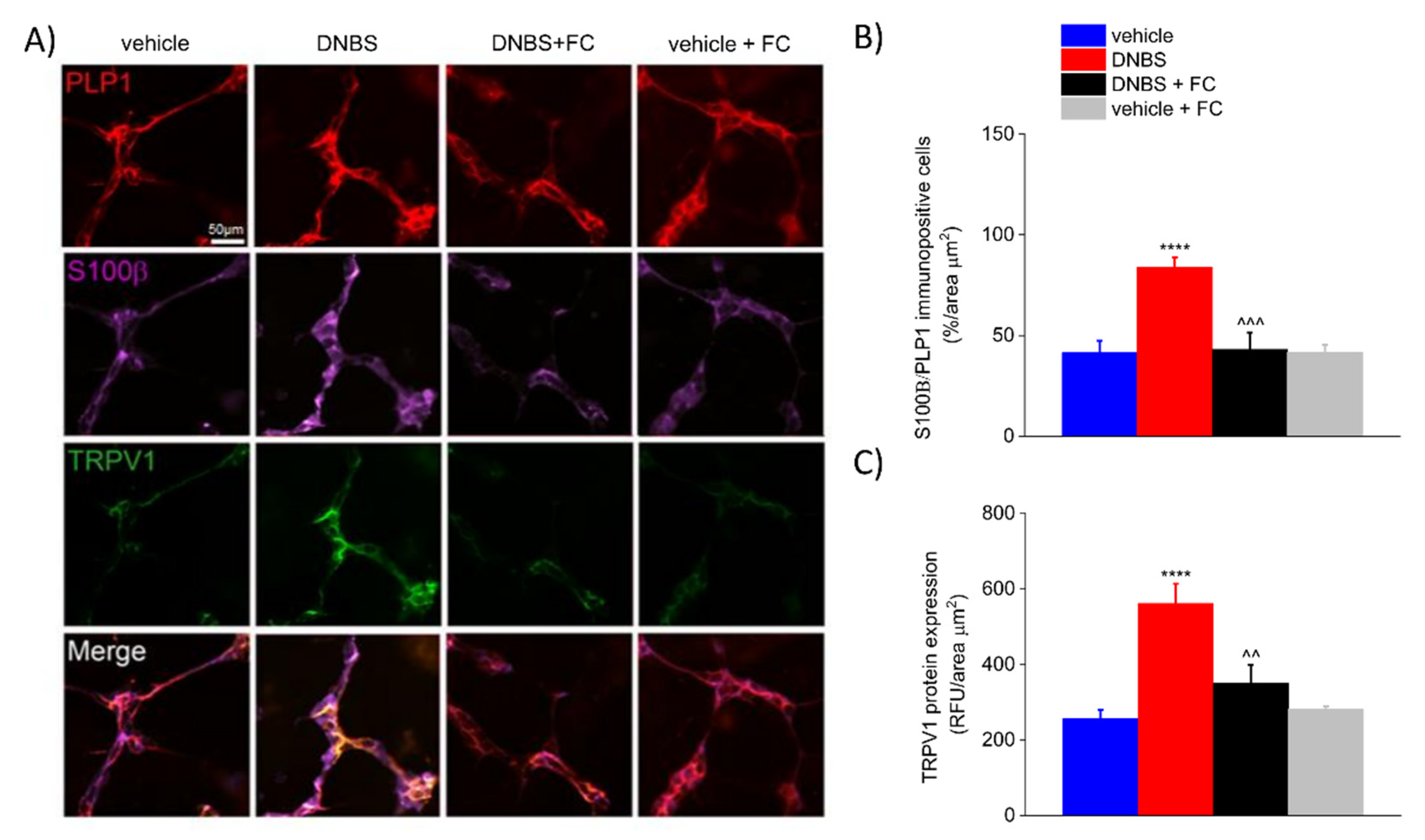
3.4. DNBS Increases the Expression of S100β in the PLP1-Positive Glia and TRPV1 in the Colonic Myenteric Plexus
3.5. DNBS Elicited a S100β Increase in PLP1-Positive Cells and TRPV1 in the DRG
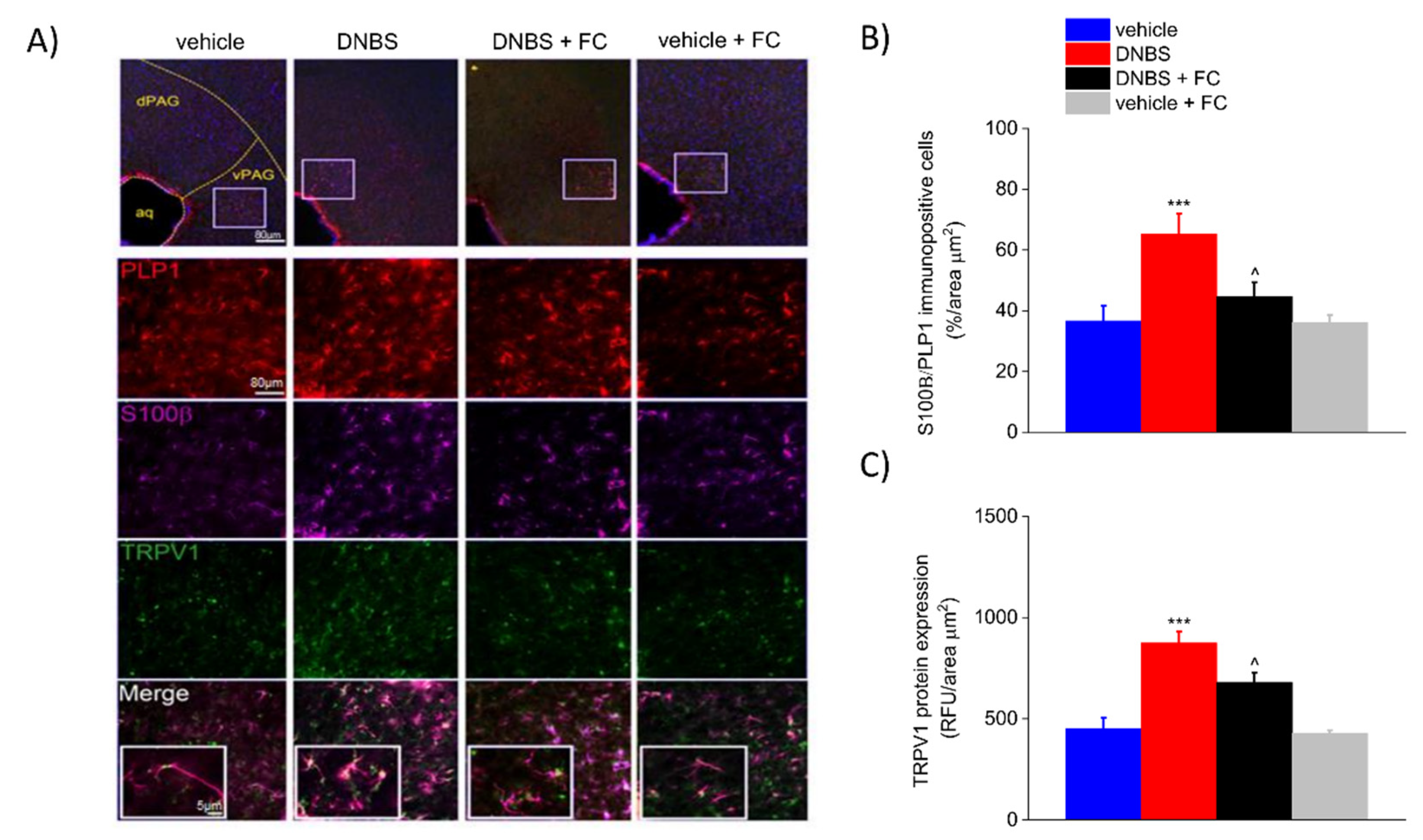
3.6. DNBS Is Associated with Increased Expression of S100β and TRPV1 in the Periaqueductal Grey Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Linden, D.R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Nordstrom, E.; Mannem, A.; Smith, C.; Banerjee, B.; Sengupta, J.N. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 2007, 148, 1021–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapointe, T.K.; Basso, L.; Iftinca, M.C.; Flynn, R.; Chapman, K.; Dietrich, G.; Vergnolle, N.; Altier, C. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G87–G99. [Google Scholar] [CrossRef] [Green Version]
- Dodds, K.N.; Beckett, E.A.; Evans, S.F.; Grace, P.M.; Watkins, L.R.; Hutchinson, M.R. Glial contributions to visceral pain: Implications for disease etiology and the female predominance of persistent pain. Transl. Psychiatry 2016, 6, e888. [Google Scholar] [CrossRef] [Green Version]
- Hanani, M.; Caspi, A.; Belzer, V. Peripheral inflammation augments gap junction-mediated coupling among satellite glial cells in mouse sympathetic ganglia. Neuron. Glia Biol. 2010, 6, 85–89. [Google Scholar] [CrossRef]
- Hanani, M. Role of satellite glial cells in gastrointestinal pain. Front. Cell. Neurosci. 2015, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Gulbransen, B.D.; Sharkey, K.A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 625–632. [Google Scholar] [CrossRef]
- Brown, I.A.; McClain, J.L.; Watson, R.E.; Patel, B.A.; Gulbransen, B.D. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Delvalle, N.M.; Dharshika, C.; Morales-Soto, W.; Fried, D.E.; Gaudette, L.; Gulbransen, B.D. Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 321–344. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef]
- Costa, D.V.; Bon-Frauches, A.C.; Silva, A.M.; Lima-Júnior, R.C.; Martins, C.S.; Leitão, R.F.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L. 5-fluorouracil induces enteric neuron death and glial activation during intestinal mucositis via a S100B-RAGE-NFκB-dependent pathway. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Cirillo, C.; Sarnelli, G.; De Filippis, D.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; Petruzzelli, R.; Grosso, M.; Izzo, P.; et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 2007, 133, 918–925. [Google Scholar] [CrossRef]
- Von Boyen, G.; Steinkamp, M. The role of enteric glia in gut inflammation. Neuron. Glia Biol. 2010, 6, 231–236. [Google Scholar] [CrossRef]
- Capoccia, E.; Cirillo, C.; Gigli, S.; Pesce, M.; D’Alessandro, A.; Cuomo, R.; Sarnelli, G.; Steardo, L.; Esposito, G. Enteric glia: A new player in inflammatory bowel diseases. Int. J. Immunopathol. Pharmacol. 2015, 28, 443–451. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, E.; Parisio, C.; Branca, J.J.; Segnani, C.; Ippolito, C.; Pellegrini, C.; Antonioli, L.; Fornai, M.; Micheli, L.; Pacini, A. Deepening the mechanisms of visceral pain persistence: An evaluation of the gut-spinal cord relationship. Cells 2020, 9, 1772. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Pacini, A.; Micheli, L.; Tani, A.; Zanardelli, M.; Ghelardini, C. Glial role in oxaliplatin-induced neuropathic pain. Exp. Neurol. 2014, 261, 22–33. [Google Scholar] [CrossRef]
- Paulsen, R.E.; Contestabile, A.; Villani, L.; Fonnum, F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: The use of fluorocitrate. J. Neurochem. 1987, 48, 1377–1385. [Google Scholar] [CrossRef]
- Wang, P.; Du, C.; Chen, F.-X.; Li, C.-Q.; Yu, Y.-B.; Han, T.; Akhtar, S.; Zuo, X.-L.; Tan, X.-D.; Li, Y.-Q. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Nasser, Y.; Fernandez, E.; Keenan, C.M.; Ho, W.; Oland, L.D.; Tibbles, L.A.; Schemann, M.; MacNaughton, W.K.; Rühl, A.; Sharkey, K.A. Role of enteric glia in intestinal physiology: Effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver. Physiol. 2006, 291, G912–G927. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Patel, B.A.; Keenan, C.M.; Dicay, M.; Chapman, K.; McCafferty, D.M.; Savidge, T.C.; Beck, P.L.; MacNaughton, W.K.; Sharkey, K.A. Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 2015, 149, 445–455.e3. [Google Scholar] [CrossRef] [Green Version]
- Fonnum, F.; Johnsen, A.; Hassel, B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 1997, 21, 106–113. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, C.; Tang, Y.; Chen, A.Q.; Liu, C.Y.; Lu, D.L. ZD 7288, an HCN channel blocker, attenuates chronic visceral pain in irritable bowel syndrome-like rats. World J. Gastroenterol. 2014, 20, 2091–2097. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Colucci, R.; Ghisu, N.; Da Settimo, F.; Natale, G.; Kastsiuchenka, O.; Duranti, E.; Virdis, A.; Vassalle, C.; et al. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J. Pharmacol. Exp. Ther. 2007, 322, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Esquerre, N.; Basso, L.; Dubuquoy, C.; Djouina, M.; Chappard, D.; Blanpied, C.; Desreumaux, P.; Vergnolle, N.; Vignal, C.; Body-Malapel, M. Aluminum ingestion promotes colorectal hypersensitivity in rodents. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.Y.; Lorentz, T.G.; Poon, C.S. Xanthogranulomatous inflammation of the slgmold colon: A case report. Aust. N. Z. J. Surg. 1996, 66, 643–644. [Google Scholar] [CrossRef]
- Zou, X.; Cao, J.; Yao, Y.; Liu, W.; Chen, L. Endoscopic findings and clinicopathologic characteristics of ischemic colitis: A report of 85 cases. Dig. Dis. Sci. 2009, 54, 2009–2015. [Google Scholar] [CrossRef]
- Sukhorukov, V.N.; Khotina, V.A.; Bagheri Ekta, M.; Ivanova, E.A.; Sobenin, I.A.; Orekhov, A.N. Endoplasmic reticulum stress in macrophages: The vicious circle of lipid accumulation and pro-inflammatory response. Biomedicines 2020, 8, 210. [Google Scholar] [CrossRef]
- Van Landeghem, L.; Mahé, M.M.; Teusan, R.; Léger, J.; Guisle, I.; Houlgatte, R.; Neunlist, M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: Impact on intestinal epithelial barrier functions. BMC Genom. 2009, 10, 507. [Google Scholar] [CrossRef] [Green Version]
- Boesmans, W.; Lasrado, R.; Vanden Berghe, P.; Pachnis, V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015, 63, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turco, F.; Sarnelli, G.; Cirillo, C.; Palumbo, I.; De Giorgi, F.; D’Alessandro, A.; Cammarota, M.; Giuliano, M.; Cuomo, R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2014, 63, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Baydas, G.; Nedzvetskii, V.S.; Tuzcu, M.; Yasar, A.; Kirichenko, S.V. Increase of glial fibrillary acidic protein and S-100B in hippocampus and cortex of diabetic rats: Effects of vitamin E. Eur. J. Pharmacol. 2003, 462, 67–71. [Google Scholar] [CrossRef]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef]
- Nogueira, L.T.; Costa, D.V.; Gomes, A.S.; Martins, C.S.; Silva, A.M.; Coelho-Aguiar, J.M.; Castelucci, P.; Lima-Júnior, R.C.; Leitão, R.F.; Moura-Neto, V.; et al. The involvement of mast cells in the irinotecan-induced enteric neurons loss and reactive gliosis. J. Neuroinflammation 2017, 14, 79. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. S1), S10–S28. [Google Scholar] [CrossRef]
- Cairns, B.E.; Arendt-Nielsen, L.; Sacerdote, P. Perspectives in pain research 2014: Neuroinflammation and glial cell activation: The cause of transition from acute to chronic pain? Scand. J. Pain 2015, 6, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 2011, 62, 381–396. [Google Scholar] [CrossRef] [Green Version]
- Cervero, F.; Laird, J.M. Understanding the signaling and transmission of visceral nociceptive events. J. Neurobiol. 2004, 61, 45–54. [Google Scholar] [CrossRef]
- Regueiro, M.; Greer, J.B.; Szigethy, E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology 2017, 152, 430–439.e4. [Google Scholar] [CrossRef] [Green Version]
- Raoof, R.; Willemen, H.; Eijkelkamp, N. Divergent roles of immune cells and their mediators in pain. Rheumatology 2018, 57, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Albert-Bayo, M.; Paracuellos, I.; González-Castro, A.M.; Rodríguez-Urrutia, A.; Rodríguez-Lagunas, M.J.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Intestinal mucosal mast cells: Key modulators of barrier function and homeostasis. Cells 2019, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Mawe, G.M. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J. Clin. Investig. 2015, 125, 949–955. [Google Scholar] [CrossRef] [Green Version]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [Green Version]
- Traina, G. Mast cells in gut and brain and their potential role as an emerging therapeutic target for neural diseases. Front. Cell. Neurosci. 2019, 13, 345. [Google Scholar] [CrossRef]
- McClain, J.L.; Mazzotta, E.A.; Maradiaga, N.; Duque-Wilckens, N.; Grants, I.; Robison, A.J.; Christofi, F.L.; Moeser, A.J.; Gulbransen, B.D. Histamine-dependent interactions between mast cells, glia, and neurons are altered following early-life adversity in mice and humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G655–G668. [Google Scholar] [CrossRef]
- Moloney, R.D.; O’Leary, O.F.; Felice, D.; Bettler, B.; Dinan, T.G.; Cryan, J.F. Early-life stress induces visceral hypersensitivity in mice. Neurosci. Lett. 2012, 512, 99–102. [Google Scholar] [CrossRef]
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.A.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 2020, 32, 108100. [Google Scholar] [CrossRef]
- Kermarrec, L.; Durand, T.; Neunlist, M.; Naveilhan, P.; Neveu, I. Enteric glial cells have specific immunosuppressive properties. J. Neuroimmunol. 2016, 295–296, 79–83. [Google Scholar] [CrossRef]
- Langness, S.; Kojima, M.; Coimbra, R.; Eliceiri, B.P.; Costantini, T.W. Enteric glia cells are critical to limiting the intestinal inflammatory response after injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G274–G282. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Morales-Soto, W.; Gulbransen, B.D. Enteric glia: A new player in abdominal pain. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126, 264–272. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Cali, G.; D’armiento, F.P.; Rocco, A.; Nardone, G. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209–e112. [Google Scholar] [CrossRef]
- Bianchi, R.; Adami, C.; Giambanco, I.; Donato, R. S100B binding to RAGE in microglia stimulates COX-2 expression. J. Leukoc. Biol. 2007, 81, 108–118. [Google Scholar] [CrossRef]
- Villarreal, A.; Aviles Reyes, R.X.; Angelo, M.F.; Reines, A.G.; Ramos, A.J. S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-κB signaling. J. Neurochem. 2011, 117, 321–332. [Google Scholar] [CrossRef]
- Lam, A.G.; Koppal, T.; Akama, K.T.; Guo, L.; Craft, J.M.; Samy, B.; Schavocky, J.P.; Watterson, D.M.; Van Eldik, L.J. Mechanism of glial activation by S100B: Involvement of the transcription factor NFkappaB. Neurobiol. Aging 2001, 22, 765–772. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Walters, J.R.; Anand, P.; Ghosh, S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008, 57, 923–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Nishiyama, M.; Iizuka, S.; Suzuki, S.; Suzuki, N.; Aiso, S.; Nakahara, J. Transient receptor potential vanilloid 1-immunoreactive signals in murine enteric glial cells. World J. Gastroenterol. 2016, 22, 9752–9764. [Google Scholar] [CrossRef] [PubMed]
- Clairembault, T.; Leclair-Visonneau, L.; Neunlist, M.; Derkinderen, P. Enteric glial cells: New players in Parkinson’s disease? Mov. Disord. 2015, 30, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Capoccia, E.; Gigli, S.; Pesce, M.; Bruzzese, E.; D’Alessandro, A.; Cirillo, C.; Di Cerbo, A.; Cuomo, R.; Seguella, L. HIV-1 Tat-induced diarrhea evokes an enteric glia-dependent neuroinflammatory response in the central nervous system. Sci. Rep. 2017, 7, 7735. [Google Scholar] [CrossRef] [Green Version]
- Seguella, L.; Capuano, R.; Sarnelli, G.; Esposito, G. Play in advance against neurodegeneration: Exploring enteric glial cells in gut-brain axis during neurodegenerative diseases. Expert Rev. Clin. Pharmacol. 2019, 12, 555–564. [Google Scholar] [CrossRef]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. Elife 2017, 6, e25887. [Google Scholar] [CrossRef]


| Primary Antibodies (Antigen) | Host | Dilution | Source | Cat#/RRID |
| Tryptase | Rabbit | 1:200 | Genetex | GTX32931 |
| PLP1 | Mouse | 1:100 | Invitrogen—Thermofisher scientific | MA1-80034 |
| TRPV1 | Rabbit | 1:100 | Bioss | bs-1931R |
| S100β | Goat | 1:100 | Neuromics | GT15160 |
| Secondary antibodies | Host | Dilution | Source | Cat#/RRID |
| Anti-Rabbit IgG (H + L) Secondary antibody [FITC] | Donkey | 1:1000 | Novusbio | NB120-6798 |
| Anti-Mouse IgG (H-+ L) Secondary antibody [Texas Red] | Goat | 1:500 | Novusbio | NB120-6787 |
| Anti-Goat IgG (H-+-L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 647 | Donkey | 1:500 | Invitrogen—Thermofisher Scientific | A32849 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, E.; Seguella, L.; Vincenzi, M.; Parisio, C.; Micheli, L.; Toti, A.; Corpetti, C.; Del Re, A.; Squillace, S.; Maftei, D.; et al. Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines 2021, 9, 1671. https://doi.org/10.3390/biomedicines9111671
Lucarini E, Seguella L, Vincenzi M, Parisio C, Micheli L, Toti A, Corpetti C, Del Re A, Squillace S, Maftei D, et al. Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines. 2021; 9(11):1671. https://doi.org/10.3390/biomedicines9111671
Chicago/Turabian StyleLucarini, Elena, Luisa Seguella, Martina Vincenzi, Carmen Parisio, Laura Micheli, Alessandra Toti, Chiara Corpetti, Alessandro Del Re, Silvia Squillace, Daniela Maftei, and et al. 2021. "Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats" Biomedicines 9, no. 11: 1671. https://doi.org/10.3390/biomedicines9111671
APA StyleLucarini, E., Seguella, L., Vincenzi, M., Parisio, C., Micheli, L., Toti, A., Corpetti, C., Del Re, A., Squillace, S., Maftei, D., Lattanzi, R., Ghelardini, C., Di Cesare Mannelli, L., & Esposito, G. (2021). Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines, 9(11), 1671. https://doi.org/10.3390/biomedicines9111671









