Vitamin-Containing Antioxidant Formulation Reduces Carcinogen-Induced DNA Damage through ATR/Chk1 Signaling in Bronchial Epithelial Cells In Vitro
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals, Kits, and Antibodies
2.2. Cell Culture
2.3. Cell Viability by MTS Assay
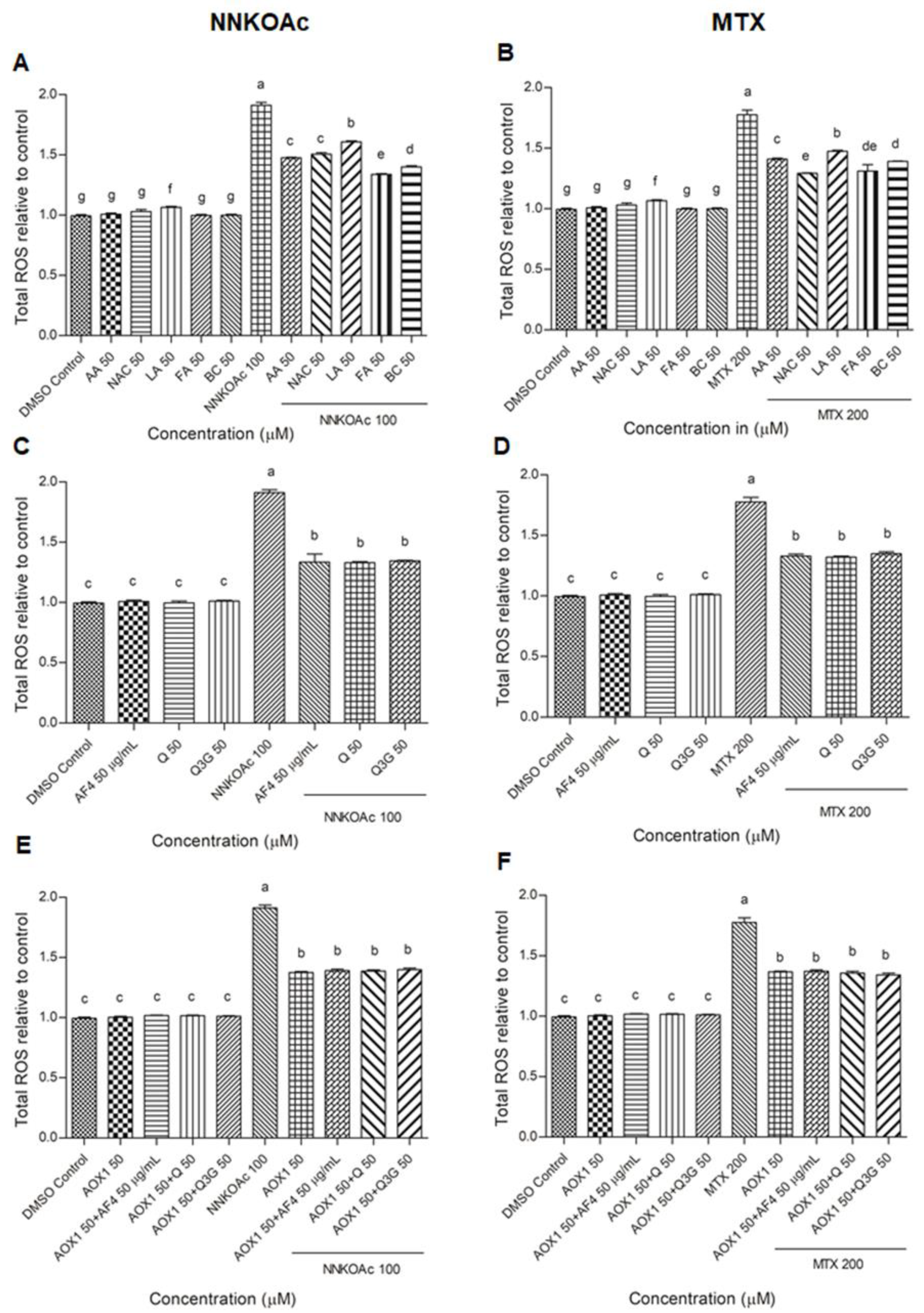
2.4. Measurement of Intracellular ROS
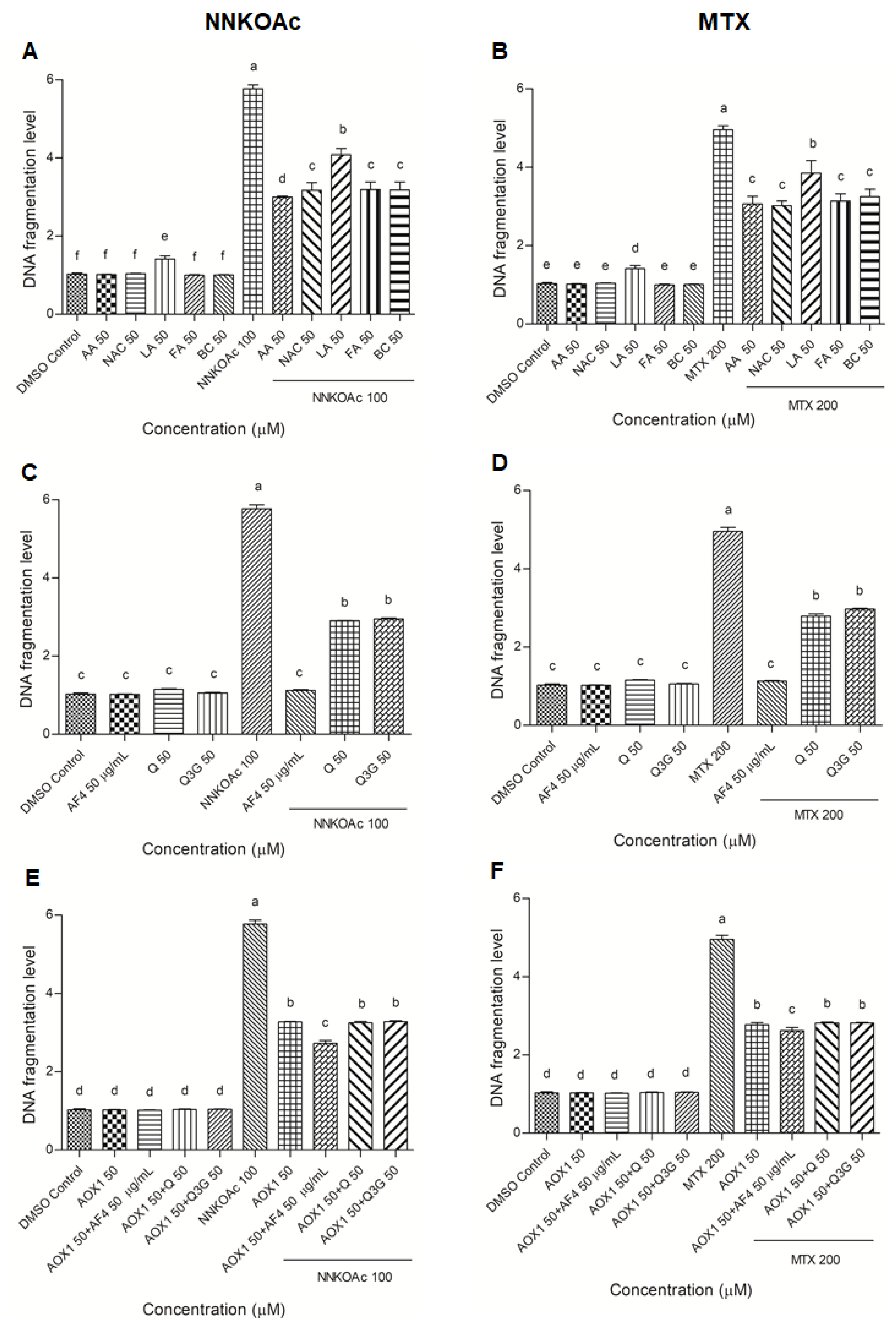
2.5. DNA Fragmentation Analysis
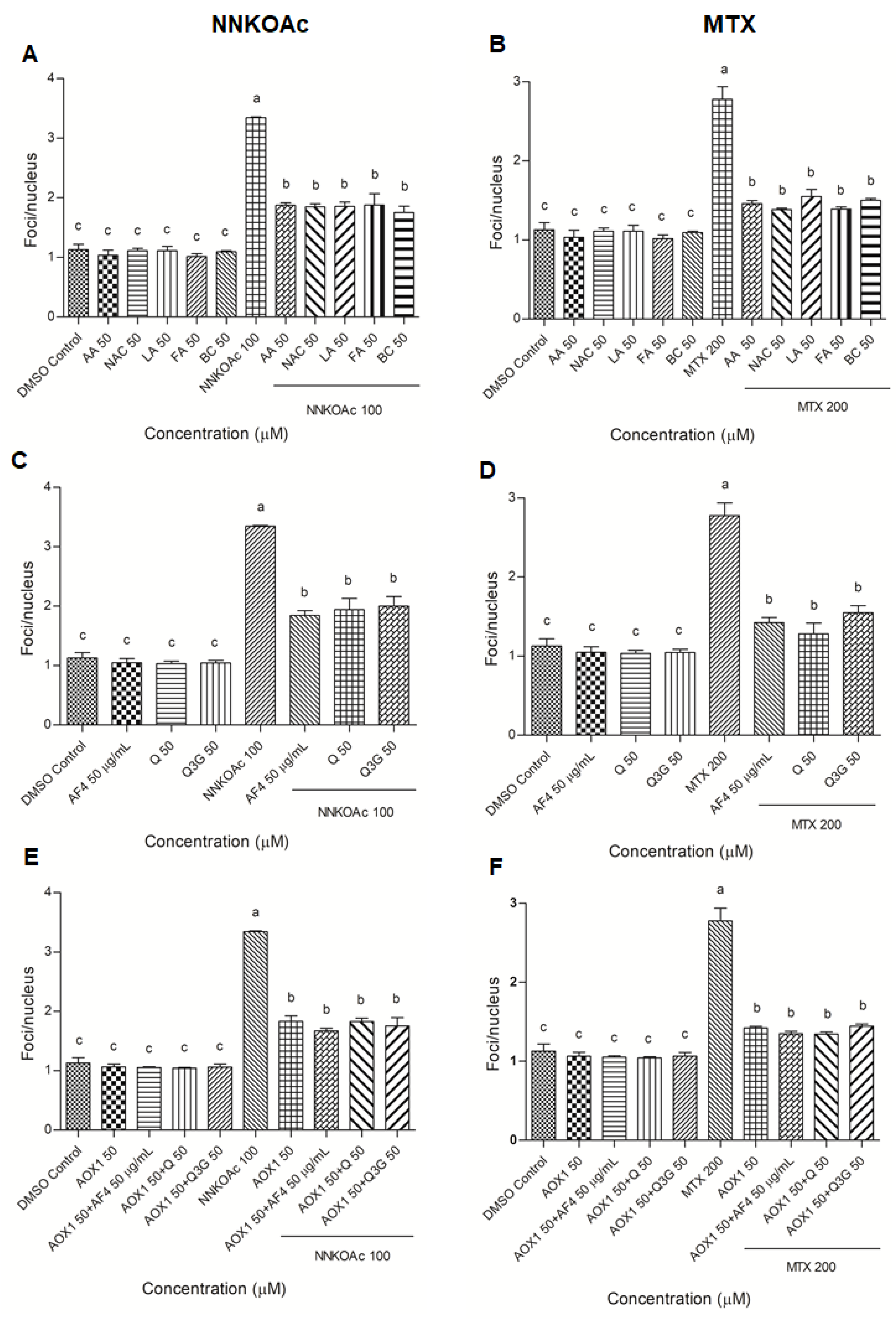
2.6. γ-H2AX Immunofluorescence Assay
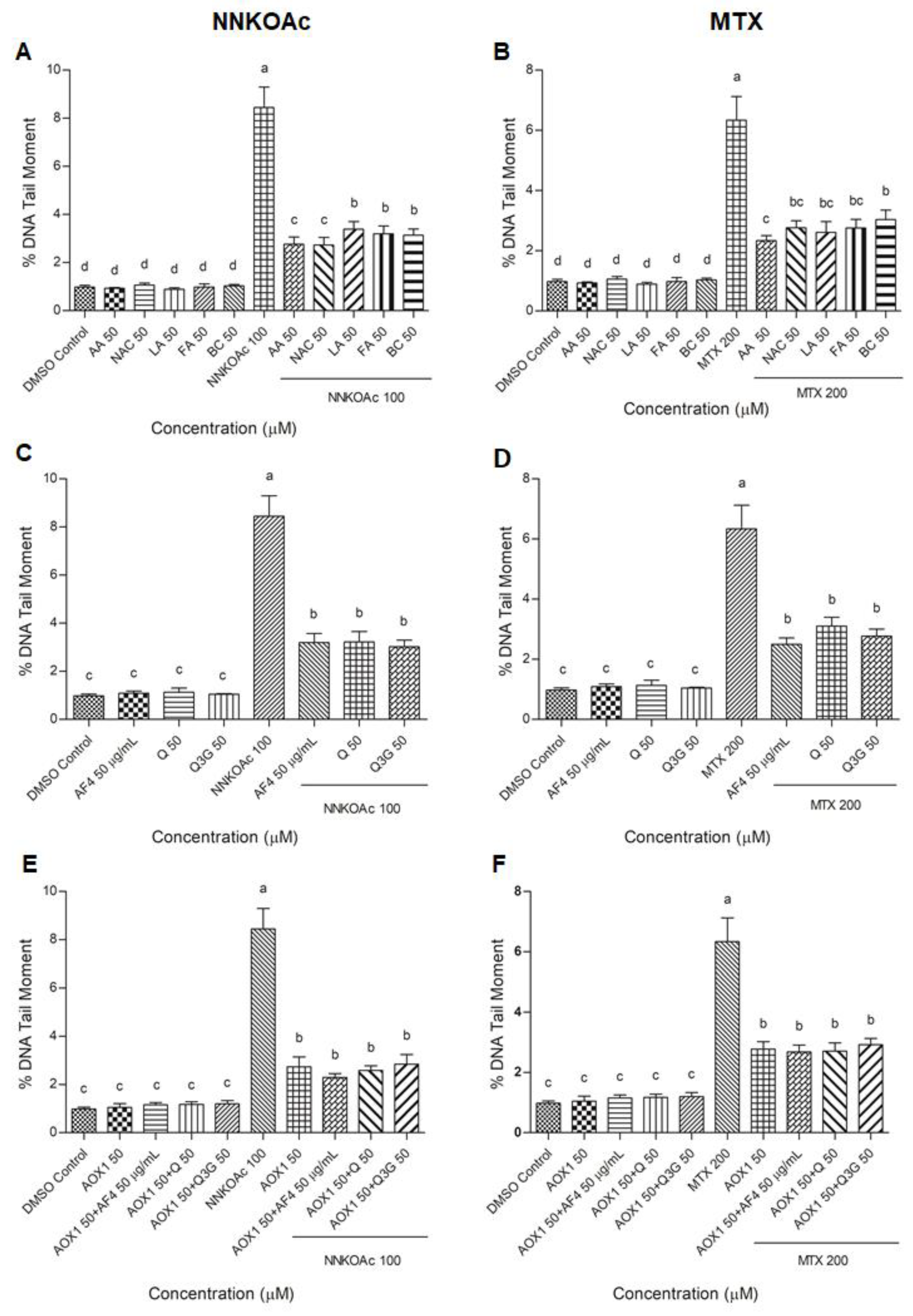
2.7. Comet Assay
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Cell Viability Owing to Vitamins, AF4, Q, and Q3G Treatement
3.2. Reduction of Intracellular ROS Levels by AOX1 Alone or with the Combination of AF4, Q, or Q3G
3.3. Protection from Carcinogen-Induced DNA Fragmentation in BEAS-2B Cells by AOX1 Alone and with the Combination of AF4, Q, or Q3G
3.4. Pre-Exposure to Antioxidants Reduces Carcinogen-Induced DNA Damage Indicator, Phospho-Histone Variant γ-H2AX
3.5. Antioxidants Reduce Carcinogen-Induced DNA Damage Measured by Comet Assay in BEAS-2B Cells
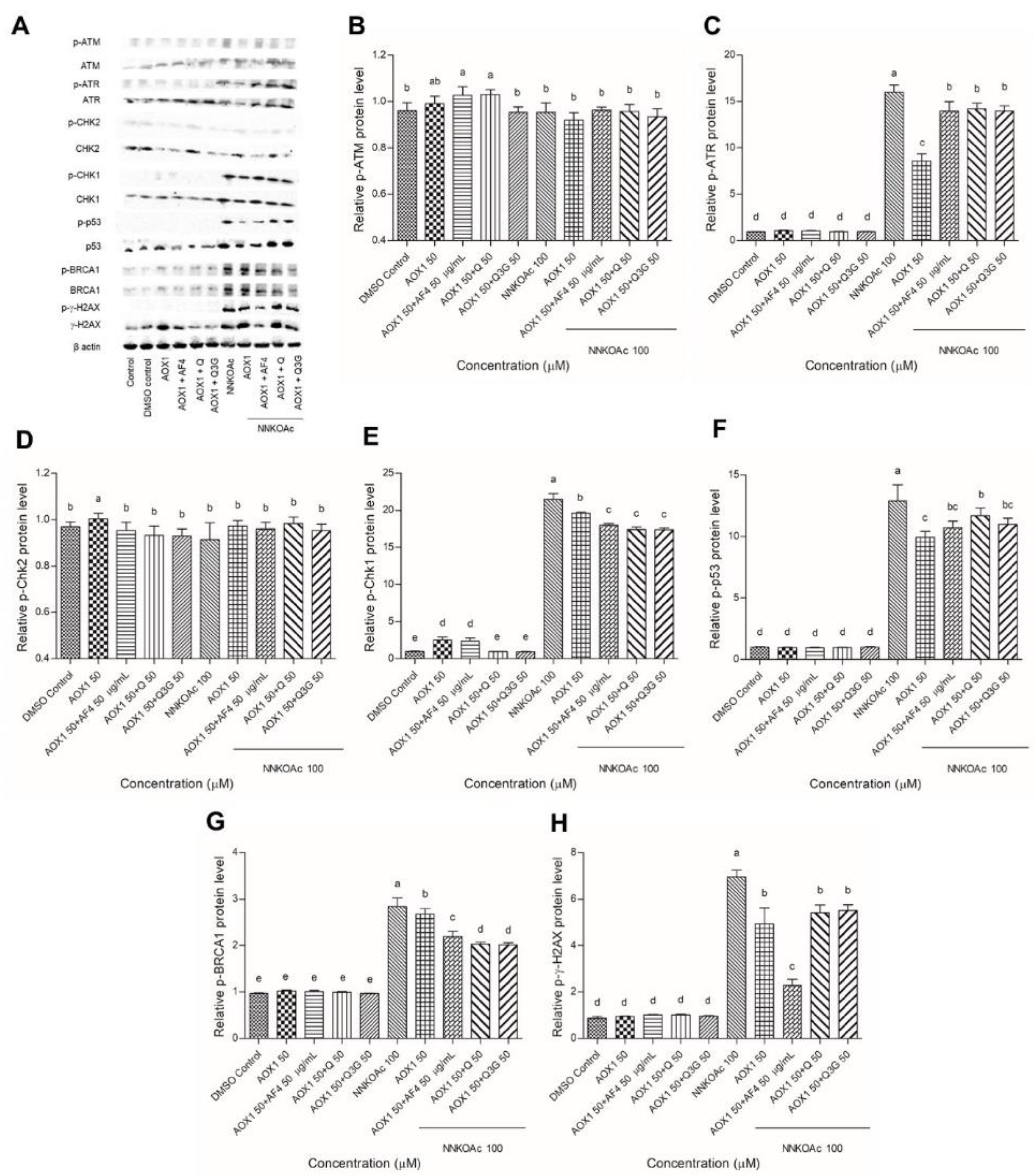
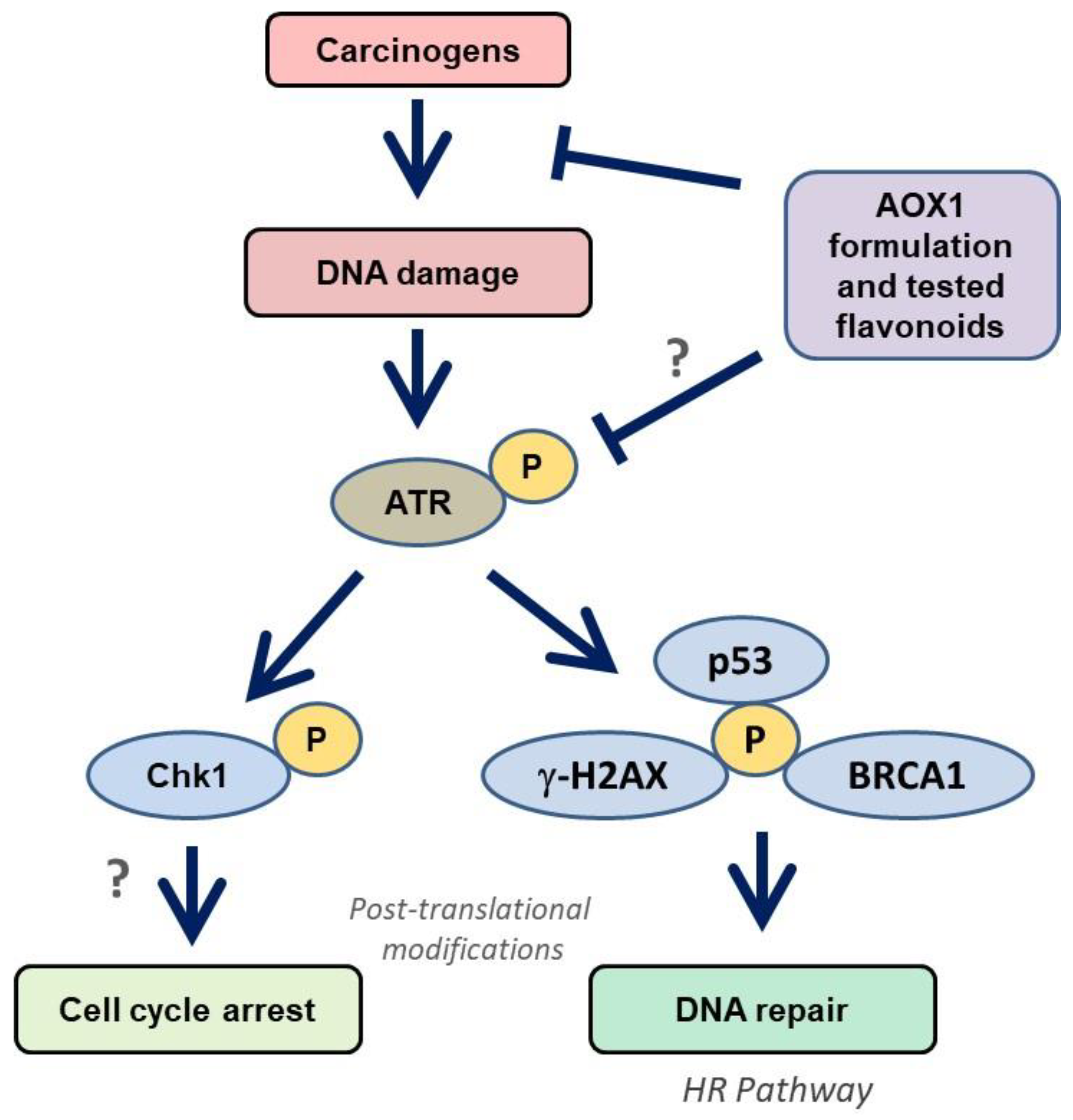
3.6. Dietary Antioxidants Contribute to DDR Cell Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| AF4 | Apple peel flavonoid fraction 4 |
| AOX1 | Antioxidant formulation |
| ATM | Ataxia telangiectasia mutated |
| ATR | ATM-Rad3-related |
| BC | beta-Carotene |
| BEAS-2B | Normal human bronchial epithelial cells |
| Chk | Checkpoint kinases |
| DDR | DNA damage response |
| DMSO | Dimethyl sulfoxide |
| DSBs | DNA double-strand breaks |
| FA | Folic acid |
| LA | alpha-Lipoic acid |
| MTX | Methotrexate |
| NAC | N-acetyl cysteine |
| NNK | 4-[(methyl)nitrosamino]-1-(3-pyridyl)-1-butanone |
| NNKOAc | 4-[(acetoxymethyl)nitrosamino]-1-(3-pyridyl)-1-butanone |
| Q | Quercetin |
| Q3G | Quercetin 3-O-d-glucoside |
| ROS | Reactive oxygen species |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Cecilia, Z.; Shaker, A.M. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar]
- Lou, Y.; Guo, Z.; Zhu, Y.; Kong, M.; Zhang, R.; Lu, L.; Wu, F.; Liu, Z.; Wu, J. Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 242. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef]
- Mok, T.S.; Zhou, Q.; Wu, Y.L. Research and standard care: Lung cancer in China. In American Society of Clinical Oncology Educational Book; ASCO: Alexandria, VA, USA, 2012; Volume 32, pp. 432–436. [Google Scholar]
- He, S.; Ou, R.; Wang, W.; Ji, L.; Gao, H.; Zhu, Y. Camptosorus sibiricus rupr aqueous extract prevents lung tumorigenesis via dual effects against ROS and DNA damage. J. Ethnopharmacol. 2018, 220, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Cramb, S.M.; Baade, P.D.; Moulden, D.R.; Nwogu, C.; Reid, M.E. The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J. Thorac. Oncol. 2016, 11, 1653–1671. [Google Scholar] [CrossRef]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Anthocyanin-rich haskap (Lonicera caerulea L.) berry extracts reduce nitrosamine-induced DNA damage in human normal lung epithelial cells in vitro. Food Chem. Toxicol. 2020, 141, 111404. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, Y.; Shikata, M.; Toyooka, T. γ-H2AX is a sensitive marker of DNA damage induced by metabolically activated 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Toxicol. In Vitro 2015, 29, 1831–1838. [Google Scholar] [CrossRef]
- Ishida, M.; Ishida, T.; Tashiro, S.; Uchida, H.; Sakai, C.; Hironobe, N.; Miura, K.; Hashimoto, Y.; Arihiro, K.; Chayama, K. Smoking cessation reverses DNA double-strand breaks in human mononuclear cells. PLoS ONE 2014, 9, e103993. [Google Scholar] [CrossRef]
- Clague, J.; Shao, L.; Lin, J.; Chang, S.; Zhu, Y.; Wang, W.; Wood, C.G.; Wu, X. Sensitivity to NNKOAc is associated with renal cancer risk. Carcinogenesis 2009, 30, 706–710. [Google Scholar] [CrossRef]
- Cloutier, J.F.; Drouin, R.; Weinfeld, M.; O’Connor, T.R.; Castonguay, A. Characterization and mapping of DNA damage induced by reactive metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK) at nucleotide resolution in human genomic DNA. J. Mol. Biol. 2001, 313, 539–557. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, M.; He, J.; Wu, W.; Jin, L.; Zheng, W.; Lou, J.; Wang, B. Investigating genetic damage in workers occupationally exposed to methotrexate using three genetic end-points. Mutagenesis 2005, 20, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhao, T.; Cai, J.; Su, Y.; Wang, Z.; Dong, W. Methotrexate induces DNA damage and inhibits homologous recombination repair in choriocarcinoma cells. Onco Targets Ther. 2016, 9, 7115–7122. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; McCarthy, A.; Barber, L.J.; Burgess, D.J.; Parry, S.; Lord, C.J.; Ashworth, A. Methotrexate induces oxidative DNA damage and is selectively lethal to tumour cells with defects in the DNA mismatch repair gene MSH2. EMBO Mol. Med. 2009, 1, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Yang, P.M.; Chang, Y.F.; Marquez, V.E.; Chen, C.C. Methotrexate induces apoptosis through p53/p21-dependent pathway and increases E-cadherin expression through downregulation of HDAC/EZH2. Biochem. Pharmacol. 2011, 81, 510–517. [Google Scholar] [CrossRef]
- George, V.C.; Rupasinghe, H.P.V. Apple flavonoids suppress carcinogen-induced DNA damage in normal human bronchial epithelial cells. Oxid. Med. Cell. Longev. 2017, 2017, 1767198. [Google Scholar] [CrossRef]
- Wohlbold, L.; Fisher, R.P. Behind the wheel and under the hood: Functions of cyclin-dependent kinases in response to DNA damage. DNA Repair 2009, 8, 1018–1024. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The trinity at the heart of the DNA damage response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Clouaire, T.; Marnef, A.; Legube, G. Taming tricky DSBs: ATM on duty. DNA Repair 2017, 56, 84–91. [Google Scholar] [CrossRef]
- Lyu, K.; Kumagai, A.; Dunphy, W.G. RPA-coated single-stranded DNA promotes the ETAA1-dependent activation of ATR. Cell Cycle. 2019, 18, 898–913. [Google Scholar] [CrossRef]
- Le, J.; Perez, E.; Nemzow, L.; Gong, F. Role of deubiquitinases in DNA damage response. DNA Repair 2019, 76, 89–98. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V. Flavonoids and their disease prevention and treatment potential: Recent advances and future perspectives. Molecules 2020, 25, 4746. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.S.; Rahman, S.; Rupasinghe, H.P.V.; Vazhappilly, C.G. Dietary flavonoids in p53—Mediated immune dysfunctions linking to cancer prevention. Biomedicines 2020, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE pathway by dietary flavonoids: A friend or foe for cancer management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.-O.; Divya, S.P.; Wang, L.; Turcios, L.; Roy, R.V.; Hitron, J.A.; Kim, D.; Dai, J.; Asha, P.; et al. Quercetin inhibits Cr(VI)-induced malignant cell transformation by targeting miR-21-PDCD4 signaling pathway. Oncotarget 2017, 8, 52118–52131. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.G.; Dunlop, K.; Warford, J.; Samson, M.L.; Jones, Q.R.; Rupasinghe, H.P.V.; Robertson, G.S. Neuroprotective and anti-inflammatory effects of the flavonoid-enriched fraction AF4 in a mouse model of hypoxic-ischemic brain injury. PLoS ONE 2012, 7, e51324. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the gamma-H2AX assay to monitor DNA damage and repair in cancer research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef]
- Vadnais, C.; Chen, R.; Fraszczak, J.; Yu, Z.; Boulais, J.; Pinder, J.; Frank, D.; Khandanpour, C.; Hebert, J.; Dellaire, G.; et al. GFI1 facilitates efficient DNA repair by regulating PRMT1 dependent methylation of MRE11 and 53BP1. Nat. Commun. 2018, 9, 1418. [Google Scholar] [CrossRef]
- Mozaffarieh, M.; Schoetzau, A.; Sauter, M.; Grieshaber, M.; Orgul, S.; Golubnitschaja, O.; Flammer, J. Comet assay analysis of single–stranded DNA breaks in circulating leukocytes of glaucoma patients. Mol. Vis. 2008, 14, 1584–1588. [Google Scholar]
- Boo, H.J.; Min, H.Y.; Jang, H.J.; Yun, H.J.; Smith, J.K.; Jin, Q.; Lee, H.J.; Liu, D.; Kweon, H.S.; Behrens, C. The tobacco-specific carcinogen-operated calcium channel promotes lung tumorigenesis via IGF2 exocytosis in lung epithelial cells. Nat. Commun. 2016, 7, 12961. [Google Scholar] [CrossRef]
- Langie, S.A.; Koppen, G.; Desaulniers, D. Causes of genome instability: The effect of low dose chemical exposures in modern society. Carcinogenesis 2015, 36, S61–S88. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Villalta, P.W.; Zarth, A.T.; Kotandeniya, D.; Upadhyaya, P.; Stepanov, I.; Hecht, S.S. Comprehensive high-resolution mass spectrometric analysis of DNA phosphate adducts formed by the tobacco-specific lung carcinogen 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanone. Chem. Res. Toxicol. 2015, 28, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K. Free radicals, antioxidants and nutraceuticals in health, disease & radiation biology. Preface. Indian J. Biochem. Bio. 2012, 49, 291–292. [Google Scholar]
- Sudan, S.; Rupasinghe, H.P.V. Flavonoid-enriched apple fraction AF4 induces cell cycle arrest, DNA topoisomerase II inhibition, and apoptosis in human liver cancer HepG2 cells. Nutri. Cancer 2014, 66, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Reang, J.; Sharma, P.C.; Thakur, V.K.; Majeed, J. Understanding the therapeutic potential of ascorbic acid in the battle to overcome cancer. Biomolecules 2021, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Cartmel, B.; Silva, F.; Kim, C.S.; Fallon, B.G.; Briskin, K.; Zheng, T.; Baum, M.; Shor-Posner, G.; Goodwin, W.J., Jr. Effect of supplemental b-carotene on plasma concentrations of carotenoids, retinol, and a-tocopherol in humans1–3. Am. J. Clin. Nutr. 1998, 68, 642–647. [Google Scholar] [CrossRef][Green Version]
- de Lau, L.M.L.; Refsum, H.; Smith, A.D.; Johnston, C.; Breteler, M.M.B. Plasma folate concentration and cognitive performance: Rotterdam scan study1-3. Am. J. Clin. Nutr. 2007, 86, 728–734. [Google Scholar] [CrossRef]
- Chwatko, G.; Krawczyk, M.; Iciek, M.; Kaminska, A.; Bilska-Wilkosz, A.; Marcykiewicz, B.; Glowacki, R. Determination of lipoic acid in human plasma by high-performance liquid chromatography with ultraviolet detection. Arab. J. Chem. 2019, 12, 4878–4886. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Gil, H.W.; Yang, J.-O.; Lee, E.-Y.; Kim, H.-K.; Kim, S.-H.; Chung, Y.-H.; Lee, E.-M.; Hwang, S.-K. Effect of high-dose intravenous N-acetylcysteine on the concentration of plasma sulfur-containing amino acids. Korean J. Intern. Med. 2005, 20, 217–223. [Google Scholar] [CrossRef]
- Xue, J.; Yang, S.; Seng, S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers 2014, 6, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Kumar, D.R.; Suresh, P.K.; Kumar, R.A. Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (soursop) extracts. J Food Sci. Technol. 2015, 52, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Ramdzan, Z.M.; Ginjala, V.; Pinder, J.B.; Chung, D.; Donovan, C.M.; Kaur, S.; Leduy, L.; Dellaire, G.; Ganesan, S.; Nepveu, A. The DNA repair function of CUX1 contributes to radioresistance. Oncotarget 2017, 8, 19021–19038. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the γ-H2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Cairns, B.R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009, 78, 273–304. [Google Scholar] [CrossRef]
- Olive, P.L.; Banath, J.P. Kinetics of H2AX phosphorylation after exposure to cisplatin. Cytometry B. Clin. Cytom. 2009, 76, 79–90. [Google Scholar] [CrossRef]
- Tanaka, T.; Kurose, A.; Huang, X.; Dai, W.; Darzynkiewicz, Z. ATM activation and histone H2AX phosphorylation as indicators of DNA damage by DNA topoisomerase I inhibitor topotecan and during apoptosis. Cell Prolif. 2006, 39, 49–60. [Google Scholar] [CrossRef]
- Hecht, S.S.; Stepanov, I.; Carmella, S.G. Expopsure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res. 2016, 49, 106–114. [Google Scholar] [CrossRef]
- Hecht, S.S.; Trushin, N.; Reid-Quinn, C.A.; Burak, E.S.; Jones, A.B.; Southers, J.L.; Gombar, C.T.; Carmella, S.G.; Anderson, L.M.; Rice, J.M. Metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone in the patas monkey: Pharmacokinetics and characterization of glucuronide metabolites. Carcinogenesis 1993, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hang, B.; Sarker, A.H.; Havel, C.; Saha, S.; Hazra, T.K.; Schick, S.; Jacob, P.; Rehan, V.K.; Chenna, A.; Sharan, D. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013, 28, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guntuku, S.; Cui, X.-S.; Matsuoka, S.; Cortez, D.; Tamai, K.; Luo, G.; Carattini-Rivera, S.; DeMayo, F.; Bradley, A.; et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000, 14, 1448–1459. [Google Scholar]
- Boudny, M.; Trbusek, M. ATR-CHK1 pathway as a therapeutic target for acute and chronic leukemias. Cancer Treat. Rev. 2020, 88, 102026. [Google Scholar] [CrossRef]
- Zhang, Y.; Hunter, T. Roles of Chk1 in cell biology and cancer therapy. Int. J. Cancer 2014, 134, 1013–1023. [Google Scholar] [CrossRef]
- Kasahara, K.; Goto, H.; Enomoto, M.; Tomono, Y.; Kiyono, T.; Inagaki, M. 14–3-3gamma mediates Cdc25A proteolysis to block premature mitotic entry after DNA damage. EMBO J. 2010, 29, 2802–2812. [Google Scholar] [CrossRef]
- Merlin, J.P.J.; Rupasinghe, H.P.V.; Dellaire, G.; Murphy, K. Role of dietary antioxidants in p53-mediated cancer chemoprevention and tumor suppression. Oxid. Med. Cell. Longev. 2021, 2021, 9924328. [Google Scholar] [CrossRef]
- Silva, J.P.; Gomes, A.C.; Coutinho, O.P. Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. Eur. J. Pharmacol. 2008, 601, 50–60. [Google Scholar] [CrossRef]
- Dong, P.; Fu, X.; Wang, X.; Wang, W.M.; Cao, W.M.; Zhang, W.Y. Protective effects of sesaminol on BEAS-2B cells impaired by cigarette smoke extract. Cell Biochem. Biophys. 2015, 71, 1207–1213. [Google Scholar] [CrossRef]
- Arora, A.; Willhite, C.A.; Liebler, D.C. Interactions of β-carotene and cigarette smoke in human bronchial epithelial cells. Carcinogenesis 2001, 22, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Podder, B.; Song, H.-Y. Cytoprotective effect of alpha-lipoic acid on paraquat-exposed human bronchial epithelial cells via activation of nuclear factor erythroid related factor-2 pathway. Biol. Pharm. Bull. 2013, 36, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Franzini, M.; Casini, A.F.; Paolicchi, A.; Pompella, A. Vitamin C supply to bronchial epithelial cells linked to glutathione availability in elf—A role for secreted γ-glutamyltransferase? J. Cyst. Fibros. 2008, 7, 174–178. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlin, J.P.J.; Dellaire, G.; Murphy, K.; Rupasinghe, H.P.V. Vitamin-Containing Antioxidant Formulation Reduces Carcinogen-Induced DNA Damage through ATR/Chk1 Signaling in Bronchial Epithelial Cells In Vitro. Biomedicines 2021, 9, 1665. https://doi.org/10.3390/biomedicines9111665
Merlin JPJ, Dellaire G, Murphy K, Rupasinghe HPV. Vitamin-Containing Antioxidant Formulation Reduces Carcinogen-Induced DNA Damage through ATR/Chk1 Signaling in Bronchial Epithelial Cells In Vitro. Biomedicines. 2021; 9(11):1665. https://doi.org/10.3390/biomedicines9111665
Chicago/Turabian StyleMerlin, J.P. Jose, Graham Dellaire, Kieran Murphy, and H.P. Vasantha Rupasinghe. 2021. "Vitamin-Containing Antioxidant Formulation Reduces Carcinogen-Induced DNA Damage through ATR/Chk1 Signaling in Bronchial Epithelial Cells In Vitro" Biomedicines 9, no. 11: 1665. https://doi.org/10.3390/biomedicines9111665
APA StyleMerlin, J. P. J., Dellaire, G., Murphy, K., & Rupasinghe, H. P. V. (2021). Vitamin-Containing Antioxidant Formulation Reduces Carcinogen-Induced DNA Damage through ATR/Chk1 Signaling in Bronchial Epithelial Cells In Vitro. Biomedicines, 9(11), 1665. https://doi.org/10.3390/biomedicines9111665






