Highlights of Immunomodulation in Salmonella-Based Cancer Therapy
Abstract
1. Introduction
2. Salmonella as a Viable Option for Bacteria-Mediated Cancer Therapy
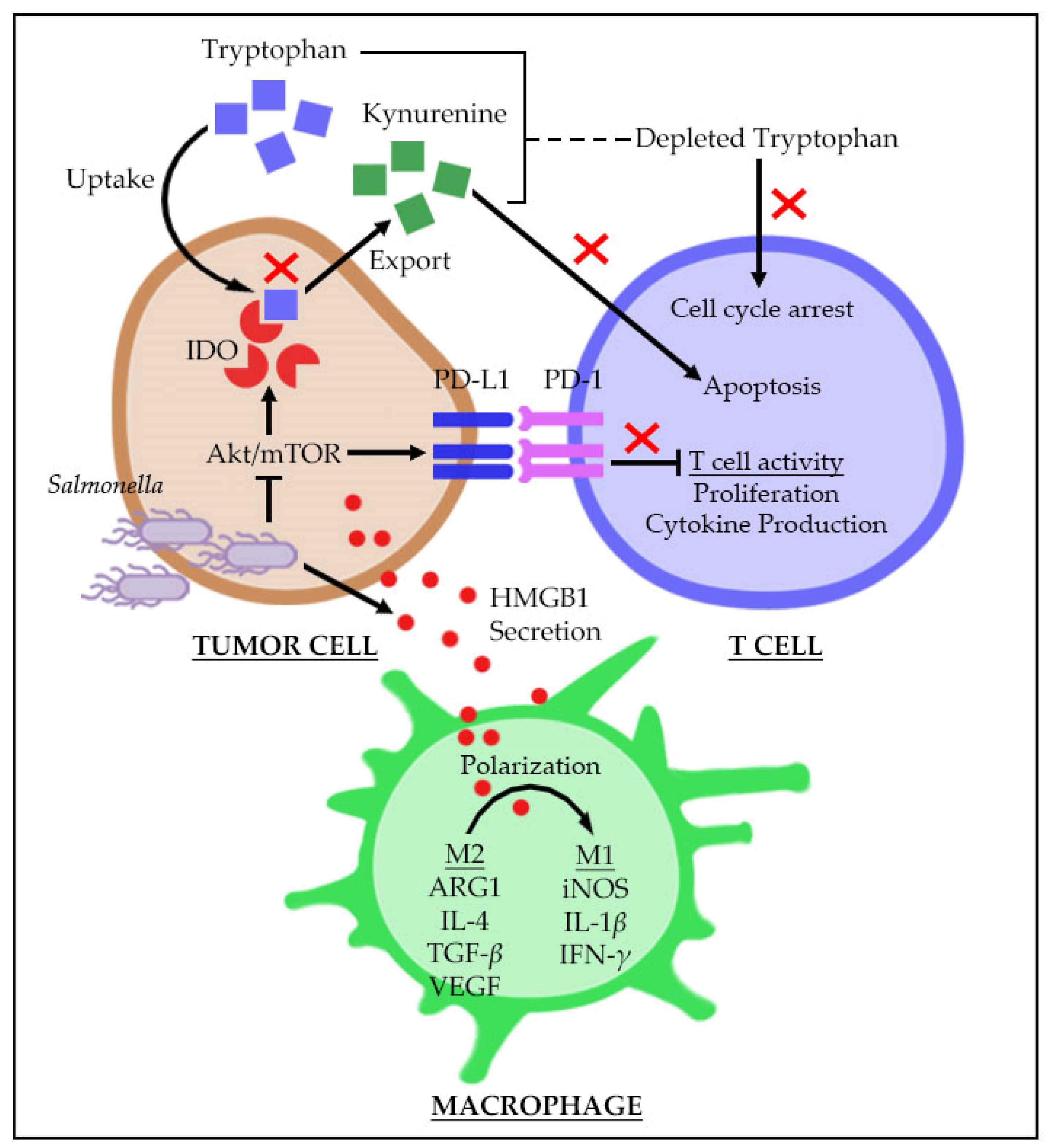
3. Breaking the Immunosuppressive Nature of Tumor Microenvironment
4. Directing Immune Infiltrates toward Antitumor Responses
4.1. Adaptive Immunity
4.2. Innate Immunity
5. Antitumor Immune Modulation via Salmonella Delivery System
6. Salmonella as Tool for Anticancer Vaccine
7. In Tandem Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-1BBL | TNF ligand superfamily member 9 |
| AIDA-1 | Adhesin involved in diffuse adherence |
| Akt | Protein kinase B |
| APC | Antigen-presenting cells |
| ARG1 | Arginase 1 |
| BMCT | Bacteria-mediated cancer therapy |
| CCL | Chemokine (C-C motif) ligand |
| CD | Cluster of differentiation |
| CTL | Cytotoxic T cells |
| Cx43 | Connexin 43 |
| CXCL | Chemokine (C-X-C motif) ligand |
| CXCR | Chemokine receptor |
| DCs | Dendritic cells |
| ECM | Extracellular matrix |
| FlaB | Flagellin B |
| FLK1 | Fetal Liver Kinase-1 |
| HMGB1 | High mobility group box 1 protein |
| HPV16 E7 | Human papillomavirus type 16 protein E7 |
| IDO | Indoleamine-2,3-dioxygenase |
| IFN-γ | Interferon-gamma |
| IL | Interleukins |
| iNOS | Inducible nitric oxide synthase |
| LIGHT | Lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells |
| KO | Knockout |
| LPS | Lipopolysaccharide |
| mTOR | Mammalian target of rapamycin |
| MYCN | MYCN protooncogene |
| MyD88 | Myeloid differentiation primary response 88 |
| NK | Natural killer |
| NY-ESO-1 | New York esophageal squamous cell carcinoma 1 |
| PD-1 | Programmed Death-1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PNP | Purine nucleoside phosphorylase |
| PMNs | Polymorphonuclear leukocytes |
| shRNA | Short hairpin ribonucleic acid |
| TAA | Tumor-associated antigens |
| TAMs | Tumor-associated macrophages |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| TME | Tumor microenvironment |
| Treg | Regulatory T cells |
| TTSS | Type three secretion system |
| VEGF | Vascular endothelial growth factor |
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Bickels, J.; Kollender, Y.; Meller, I. Coley’s Toxin: Historical Perspective. IMAJ 2002, 6, 471–472. [Google Scholar]
- McCarthy, E.F. The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Sedighi, M.; Zahedi Bialvaei, A.; Hamblin, M.R.; Ohadi, E.; Asadi, A.; Halajzadeh, M.; Lohrasbi, V.; Mohammadzadeh, N.; Amiriani, T.; Krutova, M.; et al. Therapeutic Bacteria to Combat Cancer; Current Advances, Challenges, and Opportunities. Cancer Med. 2019, 8, 3167–3181. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.Y.; le Minor, L.E. Salmonella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Pangilinan, C.R.; Lee, C.-H. Salmonella-Based Targeted Cancer Therapy: Updates on A Promising and Innovative Tumor Immunotherapeutic Strategy. Biomedicines 2019, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium Specifically Chemotax and Proliferate in Heterogeneous Tumor Tissue in Vitro. Biotechnol. Bioeng. 2006, 94, 710–721. [Google Scholar] [CrossRef]
- Silva-Valenzuela, C.A.; Desai, P.T.; Molina-Quiroz, R.C.; Pezoa, D.; Zhang, Y.; Porwollik, S.; Zhao, M.; Hoffman, R.M.; Contreras, I.; Santiviago, C.A.; et al. Solid Tumors Provide Niche-Specific Conditions That Lead to Preferential Growth of Salmonella. Oncotarget 2016, 7, 35169–35180. [Google Scholar] [CrossRef][Green Version]
- Wei, M.Q.; Ellem, K.A.O.; Dunn, P.; West, M.J.; Bai, C.X.; Vogelstein, B. Facultative or Obligate Anaerobic Bacteria Have the Potential for Multimodality Therapy of Solid Tumours. Eur. J. Cancer 2007, 43, 490–496. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-Targeted Salmonella as a Novel Anticancer Vector. Cancer Res. 1997, 57, 4537–4544. [Google Scholar]
- Avogadri, F.; Martinoli, C.; Petrovska, L.; Chiodoni, C.; Transidico, P.; Bronte, V.; Longhi, R.; Colombo, M.P.; Dougan, G.; Rescigno, M. Cancer Immunotherapy Based on Killing of Salmonella -Infected Tumor Cells. Cancer Res. 2005, 65, 3920–3927. [Google Scholar] [CrossRef]
- Gao, S.; Jung, J.-H.; Lin, S.-M.; Jang, A.-Y.; Zhi, Y.; Bum Ahn, K.; Ji, H.-J.; Hyang Lim, J.; Guo, H.; Choy, H.E.; et al. Development of Oxytolerant Salmonella typhimurium Using Radiation Mutation Technology (RMT) for Cancer Therapy. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Badie, F.; Ghandali, M.; Tabatabaei, S.A.; Safari, M.; Khorshidi, A.; Shayestehpour, M.; Mahjoubin-Tehran, M.; Morshedi, K.; Jalili, A.; Tajiknia, V.; et al. Use of Salmonella Bacteria in Cancer Therapy: Direct, Drug Delivery and Combination Approaches. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Chang, W.-W.; Lee, C.-H. Salmonella as an Innovative Therapeutic Antitumor Agent. Int. J. Mol. Sci. 2014, 15, 14546–14554. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Lei, Q.; Zhang, Y. Tumor-Intrinsic Signaling Pathways: Key Roles in the Regulation of the Immunosuppressive Tumor Microenvironment. J. Hematol. Oncol. 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Damsky, W.E.; Theodosakis, N.; Bosenberg, M. Melanoma Metastasis: New Concepts and Evolving Paradigms. Oncogene 2014, 33, 2413–2422. [Google Scholar] [CrossRef]
- Jorge, N.A.N.; Cruz, J.G.V.; Pretti, M.A.M.; Bonamino, M.H.; Possik, P.A.; Boroni, M. Poor Clinical Outcome in Metastatic Melanoma Is Associated with a MicroRNA-Modulated Immunosuppressive Tumor Microenvironment. J. Transl. Med. 2020, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The Clinical Role of the TME in Solid Cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-Intrinsic β-Catenin Signalling Prevents Anti-Tumour Immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]
- Wang, T.; Niu, G.; Kortylewski, M.; Burdelya, L.; Shain, K.; Zhang, S.; Bhattacharya, R.; Gabrilovich, D.; Heller, R.; Coppola, D.; et al. Regulation of the Innate and Adaptive Immune Responses by Stat-3 Signaling in Tumor Cells. Nat. Med. 2004, 10, 48–54. [Google Scholar] [CrossRef]
- Schmid, M.C.; Avraamides, C.J.; Dippold, H.C.; Franco, I.; Foubert, P.; Ellies, L.G.; Acevedo, L.M.; Manglicmot, J.R.E.; Song, X.; Wrasidlo, W.; et al. Receptor Tyrosine Kinases and TLR/IL1Rs Unexpectedly Activate Myeloid Cell PI3Kγ, A Single Convergent Point Promoting Tumor Inflammation and Progression. Cancer Cell 2011, 19, 715–727. [Google Scholar] [CrossRef]
- Kuan, Y.-D.; Lee, C.-H. Salmonella Overcomes Tumor Immune Tolerance by Inhibition of Tumor Indoleamine 2, 3-Dioxygenase 1 Expression. Oncotarget 2016, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Bishnupuri, K.S.; Alvarado, D.M.; Khouri, A.N.; Shabsovich, M.; Chen, B.; Dieckgraefe, B.K.; Ciorba, M.A. IDO1 and Kynurenine Pathway Metabolites Activate PI3K-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019, 79, 1138–1150. [Google Scholar] [CrossRef]
- Mbongue, J.; Nicholas, D.; Torrez, T.; Kim, N.-S.; Firek, A.; Langridge, W. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines 2015, 3, 703–729. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef]
- Munn, D.H.; Sharma, M.D.; Baban, B.; Harding, H.P.; Zhang, Y.; Ron, D.; Mellor, A.L. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity 2005, 22, 633–642. [Google Scholar] [CrossRef]
- Lin, H.-C.; Yang, C.-J.; Kuan, Y.-D.; Wang, W.-K.; Chang, W.-W.; Lee, C.-H. The Inhibition of Indoleamine 2, 3-Dioxygenase 1 by Connexin 43. Int. J. Med Sci. 2017, 14, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, Z.; Wang, Z.; Wang, X.; Zhang, H.; Qin, J.; Qin, X.; Xu, J.; Sun, Y. Increased Expression of IDO Associates with Poor Postoperative Clinical Outcome of Patients with Gastric Adenocarcinoma. Sci. Rep. 2016, 6, 21319. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Shen, H.; Wang, J. The Prognostic Value of IDO Expression in Solid Tumors: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Chen, M.-C.; Pangilinan, C.R.; Lee, C.-H. Salmonella Breaks Tumor Immune Tolerance by Downregulating Tumor Programmed Death-Ligand 1 Expression. Cancers 2019, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Gajewski, T.F.; Mackensen, A. Interaction of PD-L1 on Tumor Cells with PD-1 on Tumor-Specific T Cells as a Mechanism of Immune Evasion: Implications for Tumor Immunotherapy. Cancer Immunol. Immunother. 2005, 54, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kaimala, S.; Mohamed, Y.A.; Nader, N.; Issac, J.; Elkord, E.; Chouaib, S.; Fernandez-Cabezudo, M.J.; al-Ramadi, B.K. Salmonella-Mediated Tumor Regression Involves Targeting of Tumor Myeloid Suppressor Cells Causing a Shift to M1-like Phenotype and Reduction in Suppressive Capacity. Cancer Immunol. Immunother. 2014, 63, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Res. 2011, 71, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The Immune Contexture in Cancer Prognosis and Treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Wang, L.; Zhang, C. Interplay between Inflammatory Tumor Microenvironment and Cancer Stem Cells (Review). Oncol. Lett. 2018, 16, 679–686. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hsieh, J.-L.; Wu, C.-L.; Hsu, P.-Y.; Shiau, A.-L. T Cell Augments the Antitumor Activity of Tumor-Targeting Salmonella. Appl. Microbiol. Biotechnol. 2011, 90, 1381–1388. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, C.-L.; Shiau, A.-L. Toll-like Receptor 4 Mediates an Antitumor Host Response Induced by Salmonella Choleraesuis. Clin. Cancer Res. 2008, 14, 1905–1912. [Google Scholar] [CrossRef]
- Grille, S.; Moreno, M.; Bascuas, T.; Marqués, J.M.; Muñoz, N.; Lens, D.; Chabalgoity, J.A. Salmonella enterica Serovar Typhimurium Immunotherapy for B-Cell Lymphoma Induces Broad Anti-Tumour Immunity with Therapeutic Effect. Immunology 2014, 143, 428–437. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Liu, C. Salmonella Promote T Cell-Mediated Anti-Tumor Immuno-Therapy. J. Immunol. 2016, 196. [Google Scholar]
- Drees, J.; Mertensotto, M.; Liu, G.; Panyam, J.; Leonard, A.; Agustin, L.; Schottel, J.; Saltzman, D. Attenuated Salmonella enterica Typhimurium Reduces Tumor Burden in an Autochthonous Breast Cancer Model. Anticancer Res. 2015, 35, 843–850. [Google Scholar] [PubMed]
- Kocijancic, D.; Leschner, S.; Felgner, S.; Komoll, R.-M.; Frahm, M.; Pawar, V.; Weiss, S. Therapeutic Benefit of Salmonella Attributed to LPS and TNF-α Is Exhaustible and Dictated by Tumor Susceptibility. Oncotarget 2017, 8, 36492–36508. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.-H.; Chang, S.-Y.; Lee, B.-R.; Pyun, A.-R.; Kim, J.-W.; Kweon, M.-N.; Ko, H.-J. Intratumoral Injection of Attenuated Salmonella Vaccine Can Induce Tumor Microenvironmental Shift from Immune Suppressive to Immunogenic. Vaccine 2013, 31, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiao, Y.; Chen, G.; Chang, C.; Dong, H.; Tang, B.; Cheng, X.; Liu, X.; Hua, Z. Salmonella Flagella Confer Anti-Tumor Immunological Effect via Activating Flagellin/TLR5 Signalling within Tumor Microenvironment. Acta Pharm. Sin. B 2021, 11, 3165–3177. [Google Scholar] [CrossRef]
- Ma, J.; Liu, L.; Che, G.; Yu, N.; Dai, F.; You, Z. The M1 Form of Tumor-Associated Macrophages in Non-Small Cell Lung Cancer is Positively Associated with Survival Time. BMC Cancer 2010, 10, 112. [Google Scholar] [CrossRef]
- Yuan, R.; Li, S.; Geng, H.; Wang, X.; Guan, Q.; Li, X.; Ren, C.; Yuan, X. Reversing the Polarization of Tumor-Associated Macrophages Inhibits Tumor Metastasis. Int. Immunopharmacol. 2017, 49, 30–37. [Google Scholar] [CrossRef]
- Pangilinan, C.R.; Wu, L.-H.; Lee, C.-H. Salmonella Impacts Tumor-Induced Macrophage Polarization, and Inhibits SNAI1-Mediated Metastasis in Melanoma. Cancers 2021, 13, 2894. [Google Scholar] [CrossRef]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Weagel, E.; Smith, C.; Liu, P.G.; Robison, R.; O’Neill, K. Macrophage Polarization and Its Role in Cancer. J. Clin. Cell. Immunol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Riboldi, E.; Ippolito, A.; Sica, A. Molecular and Epigenetic Basis of Macrophage Polarized Activation. Semin. Immunol. 2015, 27, 237–248. [Google Scholar] [CrossRef]
- Guerriero, J.L.; Ditsworth, D.; Catanzaro, J.M.; Sabino, G.; Furie, M.B.; Kew, R.R.; Crawford, H.C.; Zong, W.-X. DNA Alkylating Therapy Induces Tumor Regression through an HMGB1-Mediated Activation of Innate Immunity. J. Immunol. 2011, 186, 3517–3526. [Google Scholar] [CrossRef] [PubMed]
- Falleni, M.; Savi, F.; Tosi, D.; Agape, E.; Cerri, A.; Moneghini, L.; Bulfamante, G.P. M1 and M2 Macrophages’ Clinicopathological Significance in Cutaneous Melanoma. Melanoma Res. 2017, 27, 200–210. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, P.; Yu, Y.; Lu, H.; Liu, Y.; Ni, P.; Su, X.; Wang, D.; Liu, Y.; Wang, J.; et al. HMGB1 Facilitated Macrophage Reprogramming towards a Proinflammatory M1-like Phenotype in Experimental Autoimmune Myocarditis Development. Sci. Rep. 2016, 6, 21884. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Peng, Z.; Hu, B.; Rao, X.; Li, J. HMGB1 Participates in LPS-induced Acute Lung Injury By Activating the AIM2 Inflammasome in Macrophages and Inducing Polarization of M1 Macrophages via TLR2, TLR4, and RAGE/NF-κB Signaling Pathways. Int. J. Mol. Med. 2019, 45, 61–80. [Google Scholar] [CrossRef]
- Kim, J.-E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.-V.; Zheng, J.H.; Yun, M.; Park, S.-G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics 2015, 5, 1328–1342. [Google Scholar] [CrossRef]
- Phan, T.X.; Nguyen, V.H.; Duong, M.T.-Q.; Hong, Y.; Choy, H.E.; Min, J.-J. Activation of Inflammasome by Attenuated Salmonella typhimurium in Bacteria-Mediated Cancer Therapy. Microbiol. Immunol. 2015, 59, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 Inflammasome in Dendritic Cells Induces IL-1β–Dependent Adaptive Immunity against Tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Lee, P.-H.; Yamamoto, T.N.; Gurusamy, D.; Sukumar, M.; Yu, Z.; Hu-Li, J.; Kawabe, T.; Gangaplara, A.; Kishton, R.J.; Henning, A.N.; et al. Host Conditioning with IL-1β Improves the Antitumor Function of Adoptively Transferred T Cells. J. Exp. Med. 2019, 216, 2619–2634. [Google Scholar] [CrossRef]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I Study of the Intravenous Administration of Attenuated Salmonella typhimurium to Patients With Metastatic Melanoma. J. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Liu, Q.; Li, P.; Luo, H.; Wang, H.; Kong, Q. Genetically Engineered Salmonella typhimurium: Recent Advances in Cancer Therapy. Cancer Lett. 2019, 448, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H. Engineering Bacteria toward Tumor Targeting for Cancer Treatment: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 517–523. [Google Scholar] [CrossRef]
- Lee, C.-H. Employment of Salmonella in Cancer Gene Therapy. In Bacterial Therapy of Cancer: Methods and Protocols; Humana Press: New York, NY, USA, 2016; Volume 1409, pp. 79–83. [Google Scholar]
- Zhang, L.; Gao, L.; Li, Y.; Lin, G.; Shao, Y.; Ji, K.; Yu, H.; Hu, J.; Kalvakolanu, D.V.; Kopecko, D.J.; et al. Effects of Plasmid-Based Stat3-Specific Short Hairpin RNA and GRIM-19 on PC-3M Tumor Cell Growth. Clin. Cancer Res. 2008, 14, 559–568. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, X.; Chen, L.; Li, S.; Ren, D. Oral Administration of Attenuated S. typhimurium Carrying ShRNA-Expressing Vectors as a Cancer Therapeutic. Cancer Biol. Ther. 2008, 7, 145–151. [Google Scholar] [CrossRef]
- Deng, J.; Guo, Y.; Jiang, Z.; Yang, M.; Li, H.; Wang, J. Enhancement of Ovarian Cancer Chemotherapy by Delivery of Multidrug-Resistance Gene Small Interfering RNA Using Tumor Targeting Salmonella. J. Obstet. Gynaecol. Res. 2015, 41, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, Y.; Zeng, J.; Wang, B.; Ji, K.; Tang, Y.; Sun, Q. Knockdown of HIF-1α by SiRNA-Expressing Plasmid Delivered by Attenuated Salmonella Enhances the Antitumor Effects of Cisplatin on Prostate Cancer. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Blache, C.A.; Manuel, E.R.; Kaltcheva, T.I.; Wong, A.N.; Ellenhorn, J.D.I.; Blazar, B.R.; Diamond, D.J. Systemic Delivery of Salmonella typhimurium Transformed with IDO ShRNA Enhances Intratumoral Vector Colonization and Suppresses Tumor Growth. Cancer Res. 2012, 72, 6447–6456. [Google Scholar] [CrossRef]
- Agorio, C.; Schreiber, F.; Sheppard, M.; Mastroeni, P.; Fernandez, M.; Martinez, M.A.; Chabalgoity, J.A. Live Attenuated Salmonella as a Vector for Oral Cytokine Gene Therapy in Melanoma. J. Gene Med. 2007, 9, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Yuhua, L.; Kunyuan, G.; Hui, C.; Yongmei, X.; Chaoyang, S.; Xun, T.; Daming, R. Oral Cytokine Gene Therapy against Murine Tumor Using Attenuated Salmonella typhimurium. Int. J. Cancer 2001, 94, 438–443. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Attenuated Salmonella Engineered to Produce Human Cytokine LIGHT Inhibit Tumor Growth. Proc. Natl. Acad. Sci. USA 2007, 104, 12879–12883. [Google Scholar] [CrossRef]
- Sorenson, B.S.; Banton, K.L.; Frykman, N.L.; Leonard, A.S.; Saltzman, D.A. Attenuated Salmonella typhimurium with IL-2 Gene Reduces Pulmonary Metastases in Murine Osteosarcoma. Clin. Orthop. Relat. Res. 2008, 466, 1285–1291. [Google Scholar] [CrossRef]
- Fritz, S.E.; Henson, M.S.; Greengard, E.; Winter, A.L.; Stuebner, K.M.; Yoon, U.; Wilk, V.L.; Borgatti, A.; Augustin, L.B.; Modiano, J.F.; et al. A Phase I Clinical Study to Evaluate Safety of Orally Administered, Genetically Engineered Salmonella enterica Serovar Typhimurium for Canine Osteosarcoma. Vet. Med. Sci. 2016, 2, 179–190. [Google Scholar] [CrossRef]
- Gniadek, T.J.; Augustin, L.; Schottel, J.; Leonard, A.; Saltzman, D.; Greeno, E.; Batist, G. A Phase I, Dose Escalation, Single Dose Trial of Oral Attenuated Salmonella typhimurium Containing Human IL-2 in Patients with Metastatic Gastrointestinal Cancers. J. Immunother. 2020, 43, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Park, Y.C.; Kim, J.; Chae, Y.S.; Byeon, J.H.; Min, S.-H.; Park, S.; Yoo, Y.; Park, Y.K.; Kim, B.M. Application of Genetically Engineered Salmonella typhimurium for Interferon-Gamma–Induced Therapy against Melanoma. Eur. J. Cancer 2017, 70, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.-N.; Park, S.-H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.-J.; Hong, Y.; Bom, H.-S.; et al. Two-Step Enhanced Cancer Immunotherapy with Engineered Salmonella typhimurium Secreting Heterologous Flagellin. Sci. Transl. Med. 2017, 9, eaak9537. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Inhibition of Tumor Growth Using Salmonella Expressing Fas Ligand. JNCI J. Natl. Cancer Inst. 2008, 100, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. IL-18-Producing Salmonella Inhibit Tumor Growth. Cancer Gene Ther. 2008, 15, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Salmonella typhimurium Engineered to Produce CCL21 Inhibit Tumor Growth. Cancer Immunol. Immunother. 2009, 58, 769–775. [Google Scholar] [CrossRef]
- Chen, G.; Tang, B.; Yang, B.-Y.; Chen, J.-X.; Zhou, J.-H.; Li, J.-H.; Hua, Z.-C. Tumor-Targeting Salmonella typhimurium, a Natural Tool for Activation of Prodrug 6MePdR and Their Combination Therapy in Murine Melanoma Model. Appl. Microbiol. Biotechnol. 2013, 97, 4393–4401. [Google Scholar] [CrossRef]
- Yoon, W.; Choi, J.H.; Kim, S.; Park, Y.K. Engineered Salmonella typhimurium Expressing E7 Fusion Protein, Derived from Human Papillomavirus, Inhibits Tumor Growth in Cervical Tumor-Bearing Mice. Biotechnol. Lett. 2014, 36, 349–356. [Google Scholar] [CrossRef] [PubMed]
- al-Ramadi, B.K.; Fernandez-Cabezudo, M.J.; El-Hasasna, H.; Al-Salam, S.; Bashir, G.; Chouaib, S. Potent Anti-Tumor Activity of Systemically-Administered IL2-Expressing Salmonella Correlates with Decreased Angiogenesis and Enhanced Tumor Apoptosis. Clin. Immunol. 2009, 130, 89–97. [Google Scholar] [CrossRef] [PubMed]
- al-Ramadi, B.K.; Al-Dhaheri, M.H.; Mustafa, N.; AbouHaidar, M.; Xu, D.; Liew, F.Y.; Lukic, M.L.; Fernandez-Cabezudo, M.J. Influence of Vector-Encoded Cytokines on Anti- Salmonella Immunity: Divergent Effects of Interleukin-2 and Tumor Necrosis Factor Alpha. Infect. Immun. 2001, 69, 3980–3988. [Google Scholar] [CrossRef][Green Version]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Berendt, M.J.; North, R.J. T-Cell-Mediated Suppression of Anti-Tumor Immunity. An Explanation for Progressive Growth of an Immunogenic Tumor. J. Exp. Med. 1980, 151, 69–80. [Google Scholar] [CrossRef]
- Dye, E.S.; North, R.J. Specificity of the T Cells That Mediate and Suppress Adoptive Immunotherapy of Established Tumors. J. Leukoc. Biol. 1984, 36, 27–37. [Google Scholar] [CrossRef]
- Farashi-Bonab, S.; Khansari, N. Salmonella-Based Anticancer Vaccines and Their Efficacy. Vaccin. Res. 2019, 1, 65–71. [Google Scholar]
- Fan, Y.; Bai, T.; Tian, Y.; Zhou, B.; Wang, Y.; Yang, L. H2O2-Inactivated Salmonella typhimurium RE88 Strain as a New Cancer Vaccine Carrier: Evaluation in a Mouse Model of Cancer. Drug Des. Dev. Ther. 2021, 15, 209–222. [Google Scholar] [CrossRef]
- Stern, C.; Kasnitz, N.; Kocijancic, D.; Trittel, S.; Riese, P.; Guzman, C.A.; Leschner, S.; Weiss, S. Induction of CD4 + and CD8 + Anti-Tumor Effector T Cell Responses by Bacteria Mediated Tumor Therapy. Int. J. Cancer 2015, 137, 2019–2028. [Google Scholar] [CrossRef]
- Nishikawa, H. In Vivo Antigen Delivery by A Salmonella typhimurium Type III Secretion System for Therapeutic Cancer Vaccines. J. Clin. Investig. 2006, 116, 1946–1954. [Google Scholar] [CrossRef]
- Goyvaerts, C.; Breckpot, K. Pros and Cons of Antigen-Presenting Cell Targeted Tumor Vaccines. J. Immunol. Res. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, A.G.; Lubenau, H.; Mikus, G.; Knebel, P.; Hohmann, N.; Leowardi, C.; Beckhove, P.; Akhisaroglu, M.; Ge, Y.; Springer, M.; et al. Double-Blind, Placebo-Controlled First in Human Study to Investigate an Oral Vaccine Aimed to Elicit an Immune Reaction against the VEGF-Receptor 2 in Patients with Stage IV and Locally Advanced Pancreatic Cancer. BMC Cancer 2012, 12, 361. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Niethammer, A.G.; Friedrich, T.; Lubenau, H.; Springer, M.; Breiner, K.M.; Mikus, G.; Weitz, J.; Ulrich, A.; et al. Anti-Angiogenic Activity of VXM01, an Oral T-Cell Vaccine against VEGF Receptor 2, in Patients with Advanced Pancreatic Cancer: A Randomized, Placebo-Controlled, Phase 1 Trial. OncoImmunology 2015, 4, e1001217. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Schmidt, T.; Podola, L.; Friedrich, T.; Lubenau, H.; Springer, M.; Wieckowski, S.; Breiner, K.M.; Mikus, G.; et al. A Phase 1 Trial Extension to Assess Immunologic Efficacy and Safety of Prime-Boost Vaccination with VXM01, an Oral T Cell Vaccine against VEGFR2, in Patients with Advanced Pancreatic Cancer. OncoImmunology 2018, 7, e1303584. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health. Clinicaltrials.gov Multiple Myeloma Trial of Orally Administered Salmonella Based Survivin Vaccine (MAPSS). Available online: https://clinicaltrials.gov/ct2/show/NCT03762291 (accessed on 13 September 2021).
- U.S. National Institutes of Health. Clinicaltrials.gov DNA Vaccination against Neuroblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04049864 (accessed on 13 September 2021).
- Chou, C.-K.; Hung, J.-Y.; Liu, J.-C.; Chen, C.-T.; Hung, M.-C. An Attenuated Salmonella Oral DNA Vaccine Prevents the Growth of Hepatocellular Carcinoma and Colon Cancer That Express α-Fetoprotein. Cancer Gene Ther. 2006, 13, 746–752. [Google Scholar] [CrossRef]
- Lewēn, S.; Zhou, H.; Hu, H.; Cheng, T.; Markowitz, D.; Reisfeld, R.A.; Xiang, R.; Luo, Y. A Legumain-Based Minigene Vaccine Targets the Tumor Stroma and Suppresses Breast Cancer Growth and Angiogenesis. Cancer Immunol. Immunother. 2008, 57, 507–515. [Google Scholar] [CrossRef]
- Zuo, S.G.; Chen, Y.; Wu, Z.P.; Liu, X.; Liu, C.; Zhou, Y.C.; Wu, C.L.; Jin, C.G.; Gu, Y.L.; Li, J.; et al. Orally Administered DNA Vaccine Delivery by Attenuated Salmonella typhimurium Targeting Fetal Liver Kinase 1 Inhibits Murine Lewis Lung Carcinoma Growth and Metastasis. Biol. Pharm. Bull. 2010, 33, 174–182. [Google Scholar] [CrossRef]
- Xiong, G.; Husseiny, M.I.; Song, L.; Erdreich-Epstein, A.; Shackleford, G.M.; Seeger, R.C.; Jäckel, D.; Hensel, M.; Metelitsa, L.S. Novel Cancer Vaccine Based on Genes of Salmonella Pathogenicity Island 2. Int. J. Cancer 2009, 126, 2622–2634. [Google Scholar] [CrossRef]
- Xu, X.; Hegazy, W.A.H.; Guo, L.; Gao, X.; Courtney, A.N.; Kurbanov, S.; Liu, D.; Tian, G.; Manuel, E.R.; Diamond, D.J.; et al. Effective Cancer Vaccine Platform Based on Attenuated Salmonella and a Type III Secretion System. Cancer Res. 2014, 74, 6260–6270. [Google Scholar] [CrossRef]
- Xu, X.; Kurbanov, S.; Guo, L.; Gao, X.; Galen, J.E.; Metelitsa, L.S. 217. Development of an Effective Cancer Vaccine Platform Using Attenuated Salmonella to Deliver Recombinant Tumor-Associated Antigens. Mol. Ther. 2015, 23, S85. [Google Scholar] [CrossRef]
- Ahmad, S.; Casey, G.; Cronin, M.; Rajendran, S.; Sweeney, P.; Tangney, M.; O’Sullivan, G.C. Induction of Effective Antitumor Response After Mucosal Bacterial Vector Mediated DNA Vaccination with Endogenous Prostate Cancer Specific Antigen. J. Urol. 2011, 186, 687–693. [Google Scholar] [CrossRef]
- Berger, E.; Soldati, R.; Huebener, N.; Hohn, O.; Stermann, A.; Durmus, T.; Lobitz, S.; Zenclussen, A.C.; Christiansen, H.; Lode, H.N.; et al. Salmonella SL7207 Application Is the Most Effective DNA Vaccine Delivery Method for Successful Tumor Eradication in a Murine Model for Neuroblastoma. Cancer Lett. 2013, 331, 167–173. [Google Scholar] [CrossRef]
- Ye, J.; Li, L.; Zhang, Y.; Zhang, X.; Ren, D.; Chen, W. Recombinant Salmonella-Based 4-1BBL Vaccine Enhances T Cell Immunity and Inhibits the Development of Colorectal Cancer in Rats: In Vivo Effects of Vaccine Containing 4-1BBL. J. Biomed. Sci. 2013, 20, 8. [Google Scholar] [CrossRef]
- Binder, D.C.; Engels, B.; Arina, A.; Yu, P.; Slauch, J.M.; Fu, Y.-X.; Karrison, T.; Burnette, B.; Idel, C.; Zhao, M.; et al. Antigen-Specific Bacterial Vaccine Combined with Anti-PD-L1 Rescues Dysfunctional Endogenous T Cells to Reject Long-Established Cancer. Cancer Immunol. Res. 2013, 1, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhao, L.; Liu, Y.; Gong, H.; Song, Y.; Lei, L.; Zhu, Y.; Jin, Z.; Ma, S.; Hu, B.; et al. Combining DNA Vaccine and AIDA-1 in Attenuated Salmonella Activates Tumor-Specific CD4 + and CD8 + T-Cell Responses. Cancer Immunol. Res. 2017, 5, 503–514. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. Clinicaltrials.gov Treatment of Patients with Cancer with Genetically Modified Salmonella typhimurium Bacteria. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00004988?view=results (accessed on 22 October 2021).
- U.S. National Institutes of Health. Clinicaltrials.gov VNP20009 in Treating Patients with Advanced Solid Tumors. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00006254 (accessed on 22 October 2021).
- U.S. National Institutes of Health. Clinicaltrials.gov VNP20009 in Treating Patients with Advanced or Metastatic Solid Tumors That Have Not Responded to Previous Therapy. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00004216 (accessed on 22 October 2021).
- U.S. National Institutes of Health. Clinicaltrials.gov IL-2 Expressing, Attenuated Salmonella typhimurium in Unresectable Hepatic Spread. Available online: https://clinicaltrials.gov/ct2/show/NCT01099631 (accessed on 22 October 2021).
- U.S. National Institutes of Health. Clinicaltrials.gov VXM01 Phase I Dose Escalation Study in Patients with Locally Advanced, Inoperable and Stage IV Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01486329 (accessed on 22 October 2021).
- Platt, J.; Sodi, S.; Kelley, M.; Rockwell, S.; Bermudes, D.; Low, K.B.; Pawelek, J. Antitumour Effects of Genetically Engineered Salmonella in Combination with Radiation. Eur. J. Cancer 2000, 36, 2397–2402. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Piao, L.; Yuan, F. Radiotherapy Combined with an Engineered Salmonella typhimurium Inhibits Tumor Growth in a Mouse Model of Colon Cancer. Exp. Anim. 2016, 65, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.S.; Kim, S.; Seo, S.; Park, Y. Salmonella typhimurium with γ-Radiation Induced H2AX Phosphorylation and Apoptosis in Melanoma. Biosci. Biotechnol. Biochem. 2014, 78, 1082–1085. [Google Scholar] [CrossRef]
- Beauford, S.S.; Kumari, A.; Garnett-Benson, C. Ionizing Radiation Modulates the Phenotype and Function of Human CD4+ Induced Regulatory T Cells. BMC Immunol. 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Al-Saafeen, B.H.; Fernandez-Cabezudo, M.J.; al-Ramadi, B.K. Integration of Salmonella into Combination Cancer Therapy. Cancers 2021, 13, 3228. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Lai, C.-H.; Chen, M.-C.; Liu, C.-F.; Kuan, Y.-D.; Lin, S.-T.; Lee, C.-H. Salmonella Enhance Chemosensitivity in Tumor through Connexin 43 Upregulation. Int. J. Cancer 2013, 133, 1926–1935. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, C.-L.; Tai, Y.-S.; Shiau, A.-L. Systemic Administration of Attenuated Salmonella choleraesuis in Combination with Cisplatin for Cancer Therapy. Mol. Ther. 2005, 11, 707–716. [Google Scholar] [CrossRef]
- Saltzman, D.; Augustin, L.; Leonard, A.; Mertensotto, M.; Schottel, J. Low Dose Chemotherapy Combined with Attenuated Salmonella Decreases Tumor Burden and Is Less Toxic than High Dose Chemotherapy in an Autochthonous Murine Model of Breast Cancer. Surgery 2018, 163, 509–514. [Google Scholar] [CrossRef]
- Bascuas, T.; Moreno, M.; Grille, S.; Chabalgoity, J.A. Salmonella Immunotherapy Improves the Outcome of CHOP Chemotherapy in Non-Hodgkin Lymphoma-Bearing Mice. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Chen, J.; Qiao, Y.; Tang, B.; Chen, G.; Liu, X.; Yang, B.; Wei, J.; Zhang, X.; Cheng, X.; Du, P.; et al. Modulation of Salmonella Tumor-Colonization and Intratumoral Anti-Angiogenesis by Triptolide and Its Mechanism. Theranostics 2017, 7, 2250–2260. [Google Scholar] [CrossRef]
- Binder, D.C.; Arina, A.; Wen, F.; Tu, T.; Zhao, M.; Hoffman, R.M.; Wainwright, D.A.; Schreiber, H. Tumor Relapse Prevented by Combining Adoptive T Cell Therapy with Salmonella typhimurium. OncoImmunology 2016, 5, e1130207. [Google Scholar] [CrossRef][Green Version]
| Strains | Cargo | Immune Modulation Effects | Cell Lines | Mouse Models | Ref. |
|---|---|---|---|---|---|
| S. typhimurium | LIGHT | Increased splenic CD11c + CD205+ DCs; CXCR3-mediated increase of tumor infiltration with T cells | CT-26 colon carcinoma cells; Lewis lung carcinoma cells | BALB/c; C57BL/6 mice | [74] |
| S. typhimurium | Fas ligand (FasL) | Neutrophil-dependent antitumor activity | CT-26 colon carcinoma cells; D2F2 breast cancer; B16F10 melanoma | BALB/c; C57BL/6 mice | [80] |
| S. typhimurium | IL-18 | Increased cytokine secretion by tumors; enhanced infiltration and accumulation of T cells, NK cells, and granulocytes | CT-26 colon carcinoma cells; D2F2 breast cancer; Lewis lung carcinoma cells | BALB/c; C57BL/6 mice | [81] |
| S. typhimurium SalpIL2 | IL-2 | Increased NK cell population in both spleen and metastatic tumor mass | Osteosarcoma cells | BALB/c mice | [75] |
| S. typhimurium (pur-/msb-) | CCL21 | Elevated CXCL9, CXCL10, and IFN-γ in the TME; abundant mononuclear and polynuclear cell tumor infiltrates | CT-26 colon carcinoma and B16F10 melanoma cells | BALB/c; C57BL/6 mice | [82] |
| S. typhimurium | IDO shRNA | Recruits ROS-producing PMNs | B16F10 melanoma cells | C57BL/6, IDO-KO; Rag1-KO mice | [71] |
| S. typhimurium VNP20009 | PNP | Enhances CD8(+) T-cell infiltration | B16F10 melanoma cells | C57BL/6J mice | [83] |
| S. typhimurium BRD509 | HPV16 E7 | Increased serum IFN-γ and TNF-α; enhanced CTL activity | TC-1 cervical cancer cells | C57BL/6J mice | [84] |
| S. typhimurium SHJ2037 | FlaB | TLR5-dependent infiltration, and activation of immune cells thereafter | MC38; B16F10 cells; TLR5–negative colon cancer cells | TLR4−/−, TLR5−/−, and MyD88−/− KO mice (C57BL/6 genetic background) | [79] |
| S. typhimurium BRD509 | IFN-γ | Enhanced IFN-γ-mediated NK cell activity | B16F10 and A375SM human melanoma cells | C57BL/6 mice; RAG0.γc0 lacking NK cells (C57BL/6 background) | [78] |
| S. typhimurium BRD509 | IL-2 | NK and cytotoxic T cell-mediated tumor apoptosis | B16F10 melanoma cells | C57BL/6J mice | [85,86] |
| Species | Route | Cargo | Murine Cancer Type | Ref. |
|---|---|---|---|---|
| S. typhimurium-lux | Oral | Mouse α-fetoprotein gene | Colon carcinoma; Hepatoma | [100] |
| S. typhimurium (ΔphoP ΔphoQ) | Oral; Intratumoral | NY-ESO-1 tumor antigen | Sarcoma | [93] |
| S. typhimurium (Dam−; AroA−) | Oral | Legumain | Breast cancer | [101] |
| S. typhimurium SL3261 | Oral | VEGFR-2/FLK1 | Lung carcinoma | [102] |
| S. typhimurium MvP728 (purD/htrA) | Oral | Survivin; MYCN oncoproteins | Colon carcinoma; Glioblastoma; B cell lymphoma | [103,104,105] |
| S. typhimurium SL7207 | Oral | Mouse prostate stem cell antigen | TRAMPC1 prostate carcinoma | [106] |
| S. typhimurium SL7207 | Oral | Survivin | Neuroblastoma | [107] |
| Salmonella SL3261 | Oral | 4-1BBL | Colorectal cancer | [108] |
| S. typhimurium A1-R | Intravenous | Tumor-specific antigen ovalbumin (OVA) | Melanoma | [109] |
| S. typhimurium SL7207 | Intranasal | AIDA-1 autotransporter and DNA vaccine elements | Melanoma | [110] |
| Strains | Cargo | Route | Cancer Type | Phase and Status | No. of Enrolled Patients | NCT Number | Ref. |
|---|---|---|---|---|---|---|---|
| VNP20009 | n/a | Intratumoral | Neoplasm, metastatic | Phase I, completed | 45 | NCT00004988 | [111] |
| VNP20009 | n/a | Intratumoral | Metastatic melanoma and renal cancer | Phase I, completed | 45 | NCT00006254 | [112] |
| VNP20009 | n/a | Intratumoral | Unspecified adult solid tumors (advanced/metastatic) | Phase I, completed | 40 | NCT00004216 | [113] |
| S. typhimurium SalpIL2 | human IL-2 | Oral | Solid tumors (unresectable hepatic spread) | Phase I, completed | 22 | NCT01099631 | [114] |
| S. typhimurium Ty21a (VXM01 vaccine) | VEGFR2 | Oral | Advanced pancreatic cancer | Phase I, completed | 72 | NCT01486329 | [115] |
| Salmonella CVD908ssb (TXSVN vaccine) | Survivin | Oral | Multiple myeloma | Phase I, recruiting | 24 (est.) | NCT03762291 | [98] |
| S. typhimurium SS2017 | Tumor-associated antigens | Oral | Neuroblastoma | Early phase I, recruiting | 12 (est.) | NCT04049864 | [99] |
| S. typhimurium Saltikva | human IL-2 | Oral | Metastatic pancreatic cancer | Phase II, recruiting | 60 (est.) | NCT045892 | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pangilinan, C.R.; Lee, C.-H. Highlights of Immunomodulation in Salmonella-Based Cancer Therapy. Biomedicines 2021, 9, 1566. https://doi.org/10.3390/biomedicines9111566
Pangilinan CR, Lee C-H. Highlights of Immunomodulation in Salmonella-Based Cancer Therapy. Biomedicines. 2021; 9(11):1566. https://doi.org/10.3390/biomedicines9111566
Chicago/Turabian StylePangilinan, Christian R., and Che-Hsin Lee. 2021. "Highlights of Immunomodulation in Salmonella-Based Cancer Therapy" Biomedicines 9, no. 11: 1566. https://doi.org/10.3390/biomedicines9111566
APA StylePangilinan, C. R., & Lee, C.-H. (2021). Highlights of Immunomodulation in Salmonella-Based Cancer Therapy. Biomedicines, 9(11), 1566. https://doi.org/10.3390/biomedicines9111566






