Optimizing the Schedule of PARP Inhibitors in Combination with 177Lu-DOTATATE: A Dosimetry Rationale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gamma Camera Acquisition
2.2. Dosimetry
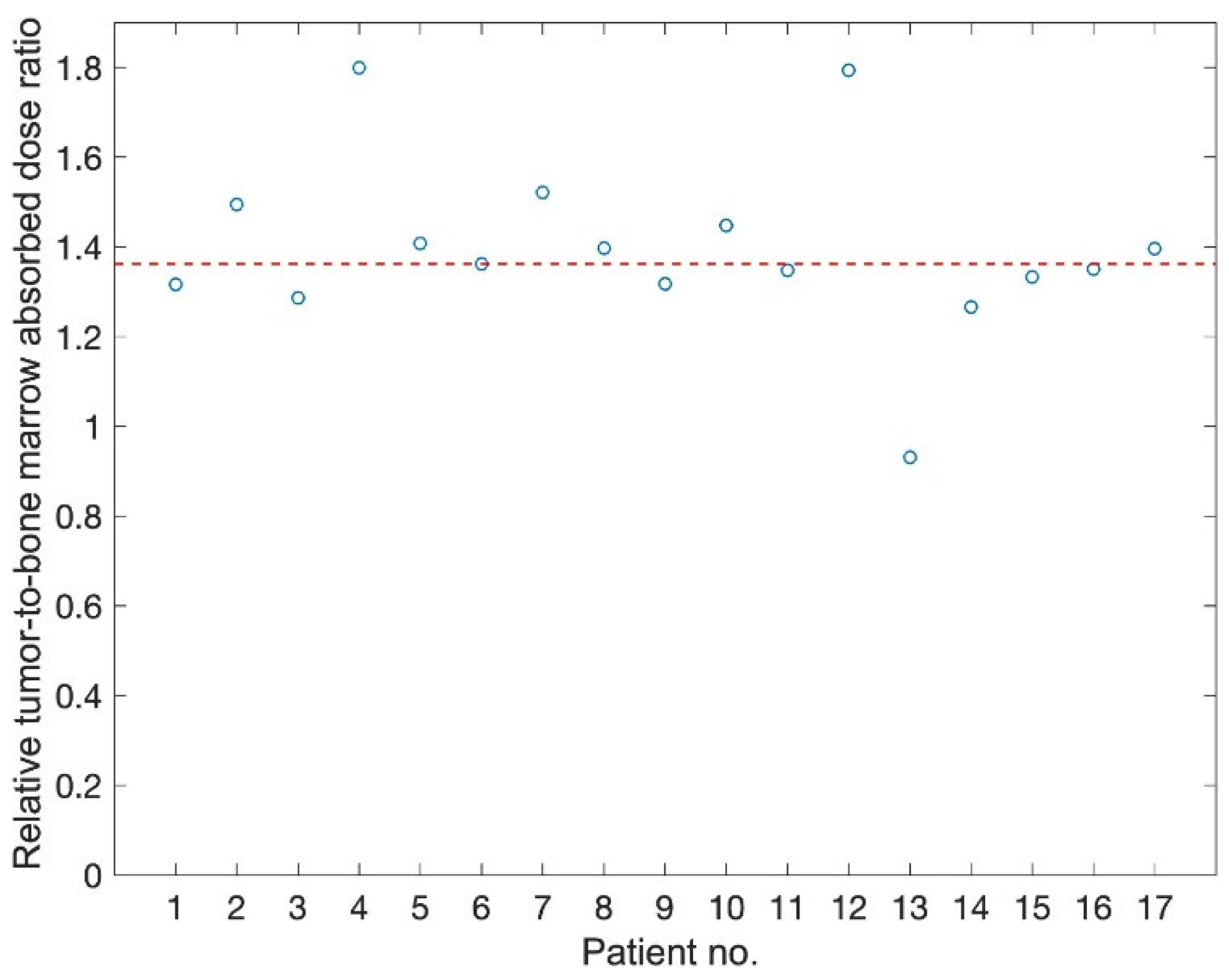
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cives, M.; Strosberg, J. Radionuclide Therapy for Neuroendocrine Tumors. Curr. Oncol. Rep. 2017, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Nonnekens, J.; van Kranenburg, M.; Beerens, C.E.; Suker, M.; Doukas, M.; van Eijck, C.H.; de Jong, M.; van Gent, D.C. Potentiation of Peptide Receptor Radionuclide Therapy by the PARP Inhibitor Olaparib. Theranostics 2016, 6, 1821–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Svensson, J.; Berg, G.; Wangberg, B.; Larsson, M.; Forssell-Aronsson, E.; Bernhardt, P. Renal function affects absorbed dose to the kidneys and haematological toxicity during 177Lu-DOTATATE treatment. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, J.; Ryden, T.; Hagmarker, L.; Hemmingsson, J.; Wangberg, B.; Bernhardt, P. A novel planar image-based method for bone marrow dosimetry in 177Lu-DOTATATE treatment correlates with haematological toxicity. EJNMMI Phys. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Ryden, T.; Heydorn Lagerlof, J.; Hemmingsson, J.; Marin, I.; Svensson, J.; Bath, M.; Gjertsson, P.; Bernhardt, P. Fast GPU-based Monte Carlo code for SPECT/CT reconstructions generates improved 177Lu images. EJNMMI Phys. 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagmarker, L.; Svensson, J.; Ryden, T.; Gjertsson, P.; Bernhardt, P. Segmentation of Whole-Body Images into Two Compartments in Model for Bone Marrow Dosimetry Increases the Correlation with Hematological Response in 177Lu-DOTATATE Treatments. Cancer Biother. Radiopharm. 2017, 32, 335–343. [Google Scholar] [CrossRef]
- Hagmarker, L.; Svensson, J.; Ryden, T.; van Essen, M.; Sundlov, A.; Sjogreen Gleisner, K.; Gjertsson, P.; Bernhardt, P. Bone marrow absorbed doses and correlations with hematological response during 177Lu-DOTATATE treatments are influenced by image-based dosimetry method and presence of skeletal metastases. J. Nucl. Med. 2019, 60, 1406–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joiner, M.C.; van der Kogel, A.J. Basic Clinical Radiobiology, 5th ed.; Taylor & Francis Ltd.: London, UK, 2019. [Google Scholar]
- Bernhardt, P.; Benjegard, S.A.; Kolby, L.; Johanson, V.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Dosimetric comparison of radionuclides for therapy of somatostatin receptor-expressing tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 514–524. [Google Scholar] [CrossRef]
- Garske-Roman, U.; Sandstrom, M.; Fross Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, D.L.; Bodeker, K.L. Overview and Current Status of Peptide Receptor Radionuclide Therapy. Surg. Oncol. Clin. N. Am. 2020, 29, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Otte, A.; Herrmann, R.; Heppeler, A.; Behe, M.; Jermann, E.; Powell, P.; Maecke, H.R.; Muller, J. Yttrium-90 DOTATOC: First clinical results. Eur. J. Nucl. Med. 1999, 26, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Adant, S.; Shah, G.M.; Beauregard, J.M. Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Brayshaw, P.A.; Price, R.A.; Turner, J.H. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Price, R.A.; Turner, J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012, 27, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Turner, J.H. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother. Radiopharm. 2015, 30, 261–269. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Turner, J.H. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology 2016, 103, 432–439. [Google Scholar] [CrossRef] [PubMed]
- van Essen, M.; Krenning, E.P.; Kam, B.L.; de Herder, W.W.; van Aken, M.O.; Kwekkeboom, D.J. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nile, D.L.; Rae, C.; Hyndman, I.J.; Gaze, M.N.; Mairs, R.J. An evaluation in vitro of PARP-1 inhibitors, rucaparib and olaparib, as radiosensitisers for the treatment of neuroblastoma. BMC Cancer 2016, 16, 621. [Google Scholar] [CrossRef] [Green Version]
- Purohit, N.K.; Shah, R.G.; Adant, S.; Hoepfner, M.; Shah, G.M.; Beauregard, J.M. Potentiation of 177Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget 2018, 9, 24693–24706. [Google Scholar] [CrossRef] [PubMed]
- Elf, A.K.; Bernhardt, P.; Hofving, T.; Arvidsson, Y.; Forssell-Aronsson, E.; Wangberg, B.; Nilsson, O.; Johanson, V. NAMPT Inhibitor GMX1778 Enhances the Efficacy of 177Lu-DOTATATE Treatment of Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
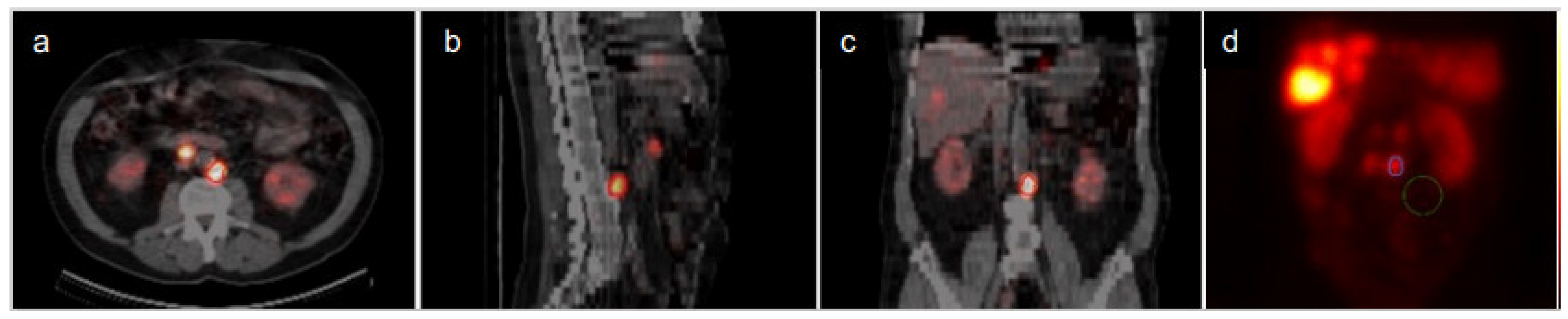
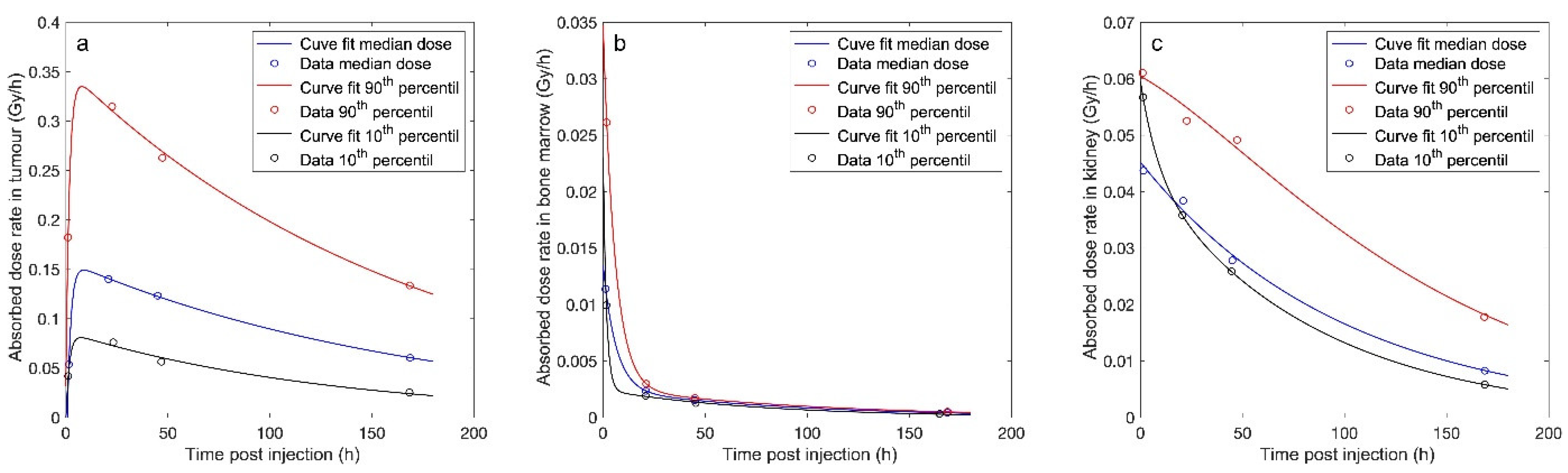
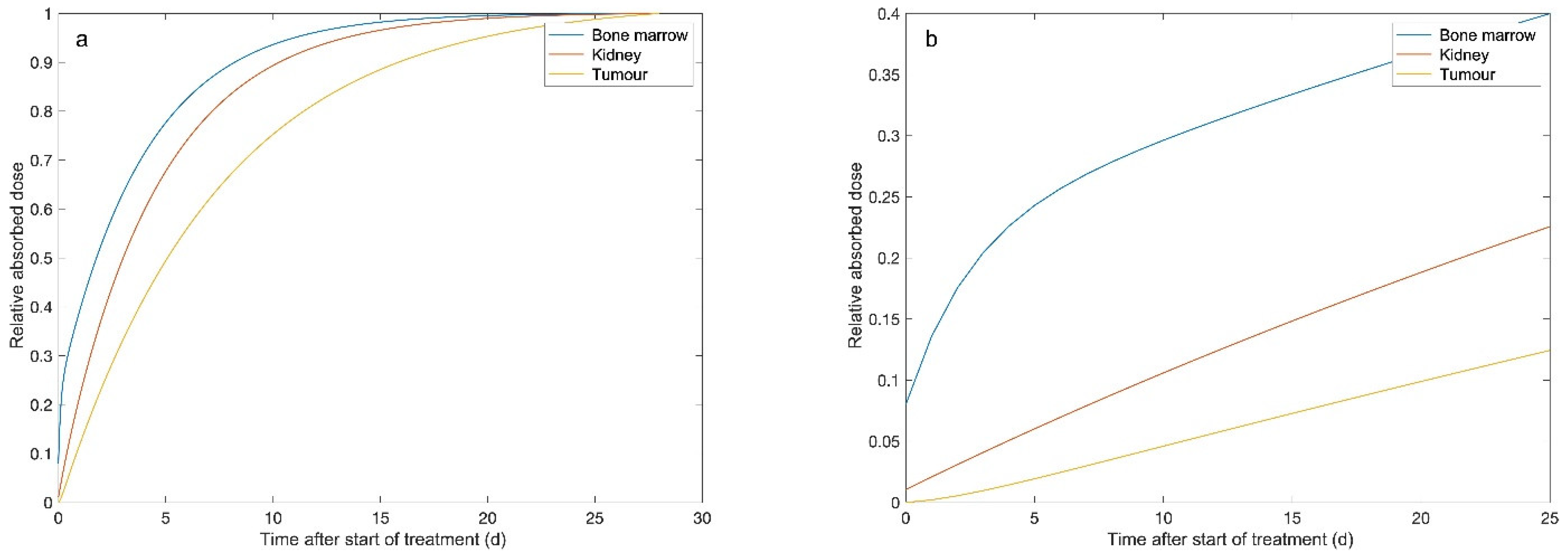
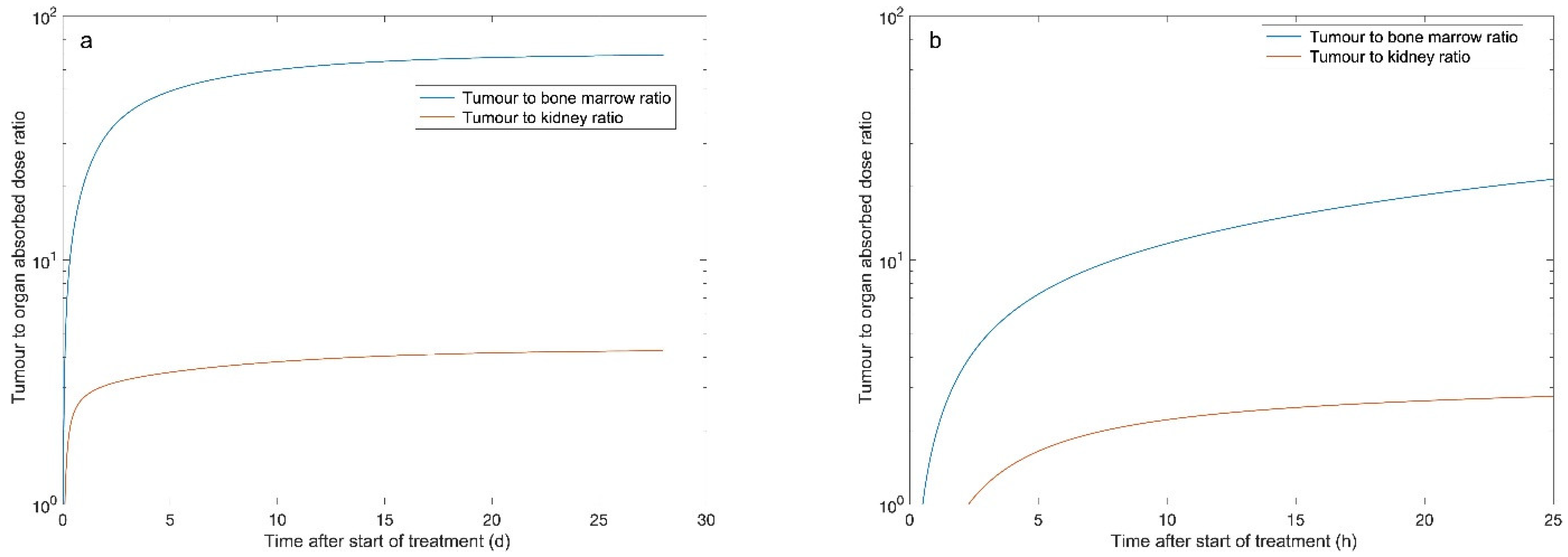
| Organ | Median Absorbed Dose within the Time Intervals (Range) (Gy/7.4 GBq) | ||||
|---|---|---|---|---|---|
| 0–inf | 6 h–inf | 6 h–28 d | 24 h–inf | 24 h–28 d | |
| Tumour | 27.3 (8.9–76.6) | 26.8 (8.3–73.4) | 26.2 (8.3–73.2) | 24.2 (6.6–63.1) | 23.6 (6.6–62.8) |
| 97% (93–99) | 89% (96–98) | 87% (75–92) | 85% (75–87) | ||
| Bone marrow | 0.30 (0.21–0.52) | 0.23 (0.18–0.42) | 0.23 (0.18–0.42) | 0.18 (0.14–0.33) | 0.18 (0.14–0.33) |
| 79% (66–95) | 79% (66–95) | 62% (48–80) | 62% (48–80) | ||
| Kidney | 4.5 (3.1–9.8) | 4.2 (2.9–9.2) | 4.2 (2.9–9.2) | 3.5 (2.4–7.5) | 4.33 (79%) |
| 93% (87–96) | 93% (87–96) | 75% (71–83) | 76% (71–83) | ||
| Organ | TND within the Time Intervals | ||||
|---|---|---|---|---|---|
| 0–inf | 6 h–inf | 6 h–28 d | 24 h–inf | 24 h–28 d | |
| Bone marrow | 66.8 (42.6–296) | 84.3 (41.5–410) | 81.7 (41.6–408) | 97.1 (39.3–461) | 93.9 (39.3–459) |
| 122% (98–149) | 121% (98–139) | 140% (93–190) | 134% (93–177) | ||
| Kidney | 6.1 (1.3–18.7) | 6.3 (1.4–19.4) | 6.2 (1.4–19.3) | 6.9 (1.3–21.4) | 6.7 (1.3–21.3) |
| 105% (102–107) | 103% (96–107) | 115% (100–123) | 112% (100–120) | ||
| Organ | Absorbed Dose (Gy) | Enhanced Dose (Gy) | ||||
|---|---|---|---|---|---|---|
| 0–24 h | 24 h–28 d | 28 d–inf | RBE = 1 | RBE = 1.5 | RBE = 2 | |
| Tumour | 3.1 (1.8–13.6) | 23.6 (6.6–62.8) | 0.3 (0.0–3.6) | 27.3 (8.9–76.6) | 39.1 (12.2–108) | 50.9 (15.6–139) |
| Bone marrow | 0.064 | 0.112 | 0.00 (0.00–0.00) | 0.30 (0.21–0.52) | 0.39 (0.29–0.69) | 0.48 (0.36–0.86) |
| Kidney | 1.17 (0.69–2.3) | 3.5 (2.4–7.5) | 0.00 (0.00–0.03) | 4.5 (3.1–9.8) | 6.2 (4.3–13.5) | 8.0 (5.4–17.3) |
| Organ | Tumour-to-Normal Tissue Enhanced Dose Ratio | |||
|---|---|---|---|---|
| TND | TNED2/2 * | TNED2/1.5 * | TNED2/1 * | |
| Bone marrow | 66.8 (42.6–296) | 77.0 (41.3–348) | 95.2 (53.1–423) | 125 (74.3–539) |
| 114% (97–126) | 142% (125–150) | 185% (175–187) | ||
| Kidney | 6.1 (1.3–18.7) | 6.4 (1.3–19.8) | 8.1 (1.7–25.0) | 11.3 (2.3–34.0) |
| 105% (100–109) | 134% (127–137) | 185% (175–187) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallqvist, A.; Svensson, J.; Hagmarker, L.; Marin, I.; Rydén, T.; Beauregard, J.-M.; Bernhardt, P. Optimizing the Schedule of PARP Inhibitors in Combination with 177Lu-DOTATATE: A Dosimetry Rationale. Biomedicines 2021, 9, 1570. https://doi.org/10.3390/biomedicines9111570
Hallqvist A, Svensson J, Hagmarker L, Marin I, Rydén T, Beauregard J-M, Bernhardt P. Optimizing the Schedule of PARP Inhibitors in Combination with 177Lu-DOTATATE: A Dosimetry Rationale. Biomedicines. 2021; 9(11):1570. https://doi.org/10.3390/biomedicines9111570
Chicago/Turabian StyleHallqvist, Andreas, Johanna Svensson, Linn Hagmarker, Ida Marin, Tobias Rydén, Jean-Mathieu Beauregard, and Peter Bernhardt. 2021. "Optimizing the Schedule of PARP Inhibitors in Combination with 177Lu-DOTATATE: A Dosimetry Rationale" Biomedicines 9, no. 11: 1570. https://doi.org/10.3390/biomedicines9111570
APA StyleHallqvist, A., Svensson, J., Hagmarker, L., Marin, I., Rydén, T., Beauregard, J.-M., & Bernhardt, P. (2021). Optimizing the Schedule of PARP Inhibitors in Combination with 177Lu-DOTATATE: A Dosimetry Rationale. Biomedicines, 9(11), 1570. https://doi.org/10.3390/biomedicines9111570






