Stable Gastric Pentadecapeptide BPC 157 as a Therapy for the Disable Myotendinous Junctions in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgery
2.3. Drugs
2.4. Experimental Protocol and Assessments
2.5. Functional Tests
2.5.1. Leg Contracture of Operated Experimental Leg (EL)
2.5.2. Walking Recovery Index WRI
2.5.3. Knee Joint Angle, Ankle Joint Angle, Hip Joint Angle
2.5.4. Extensor Postular Thrust EPT to Motor Function Index MFI, as Indicator of the Extent of the Function Failure
2.5.5. Tensiometry
2.6. Macroscopic Assessment
2.7. Microscopy
2.8. RNA Extraction and RT-PCR in the Tendon-Muscle Junction Samples
2.9. Oxidative Stress in the Tendon-Muscle Junction Samples
2.10. Nitric Oxide Determination in the Tendon-Muscle Junction Samples
2.11. Statistical Analysis
3. Results
3.1. Functional Recovery
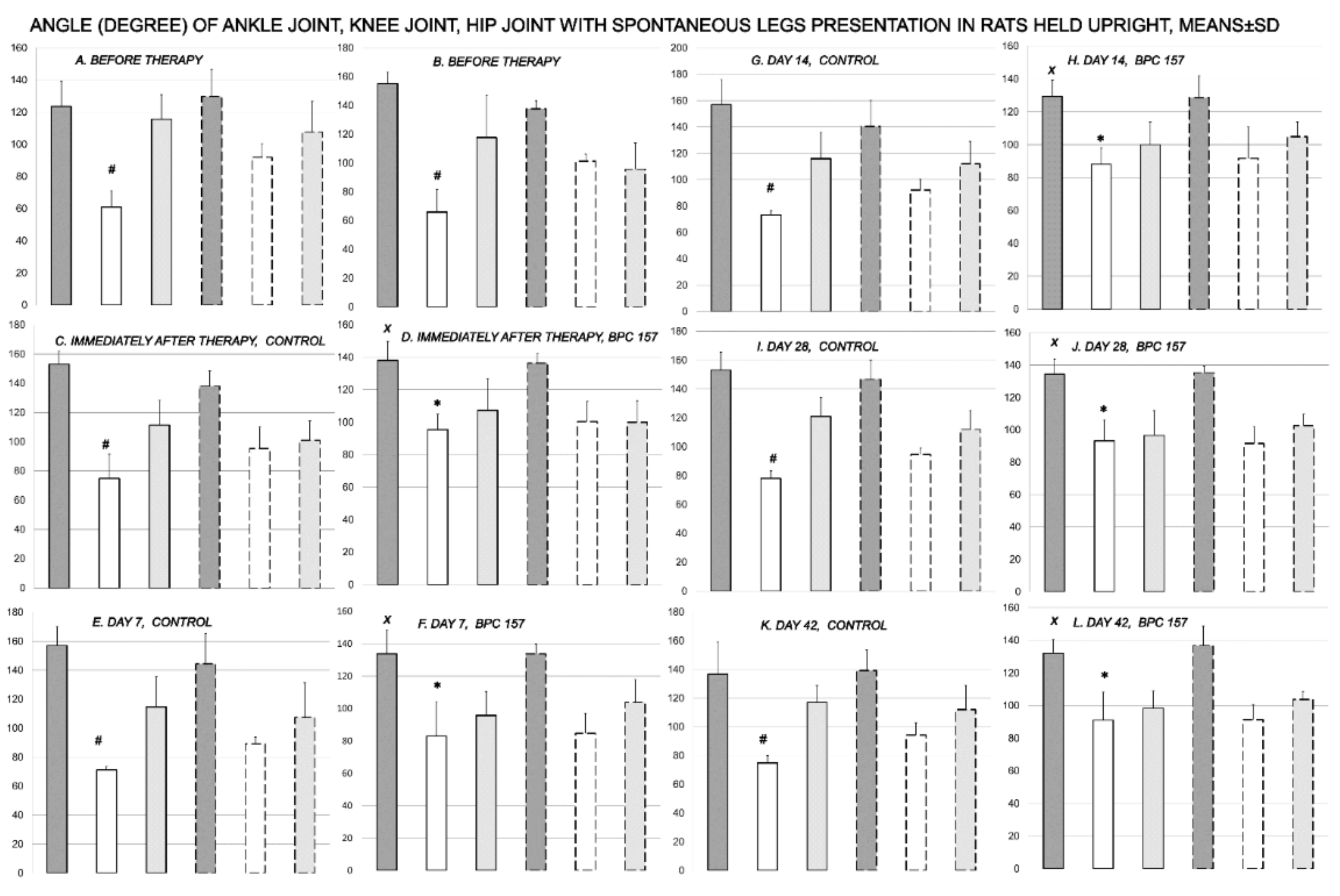
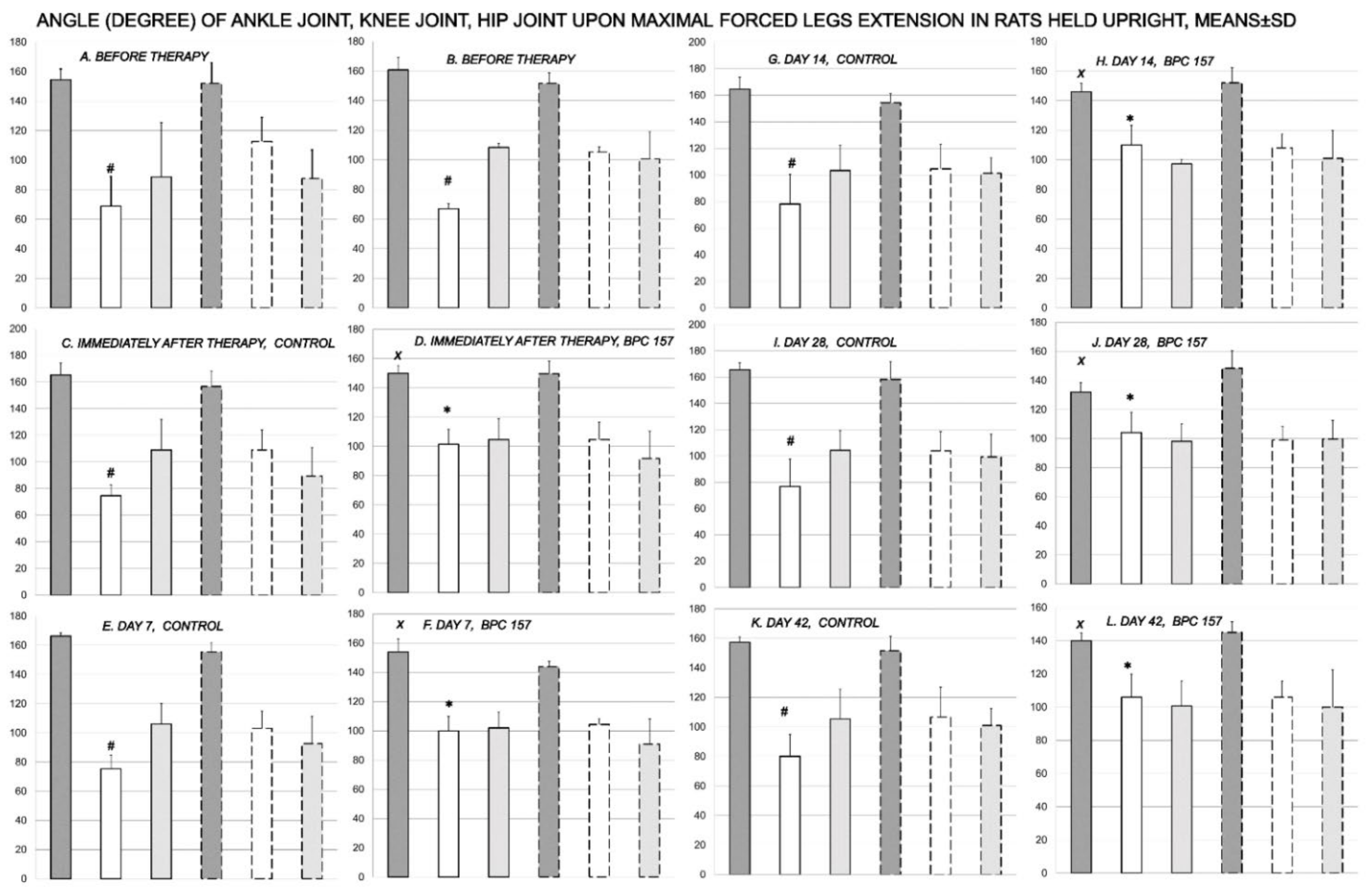

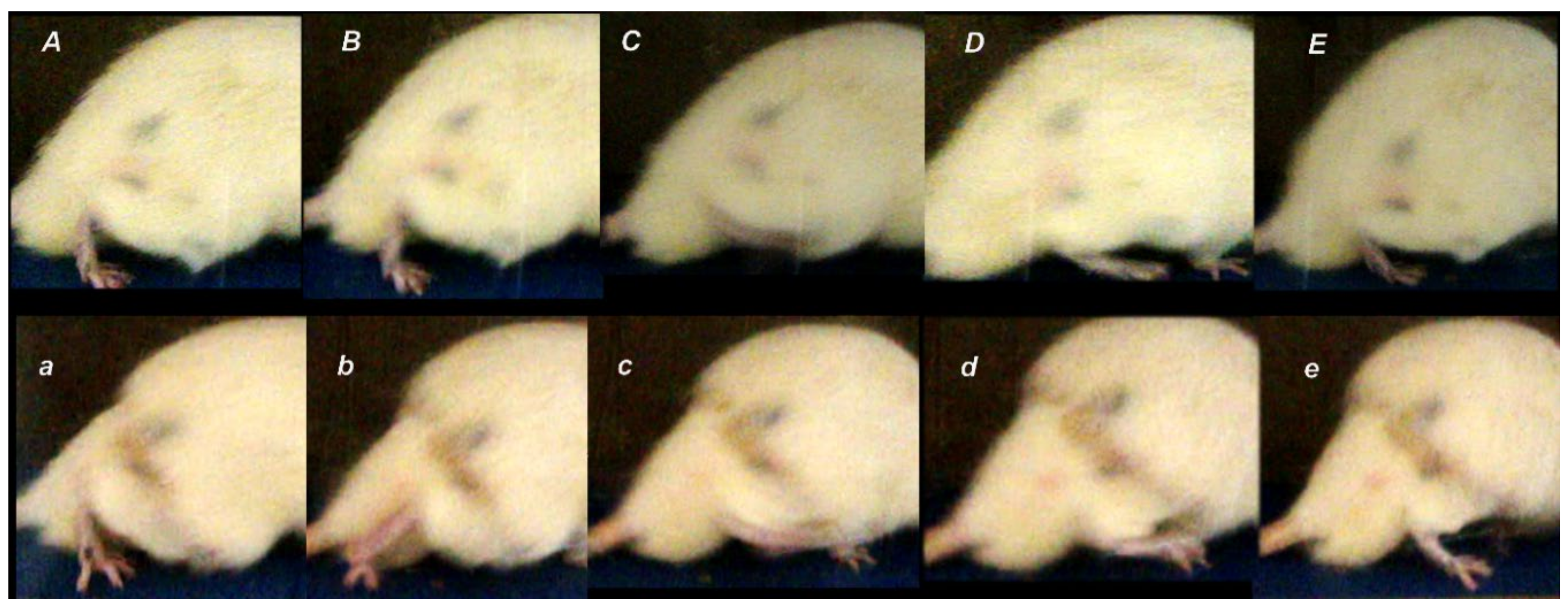


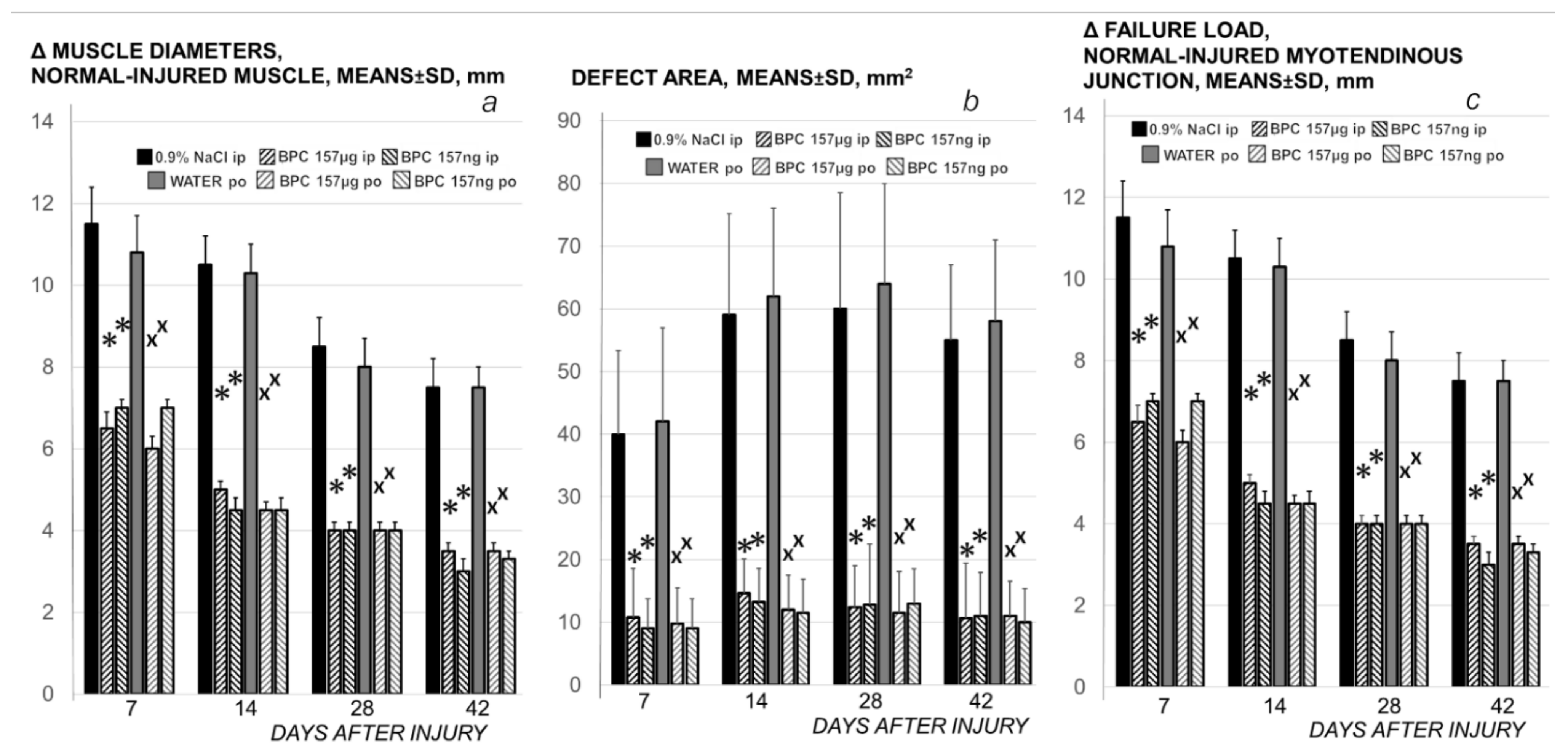
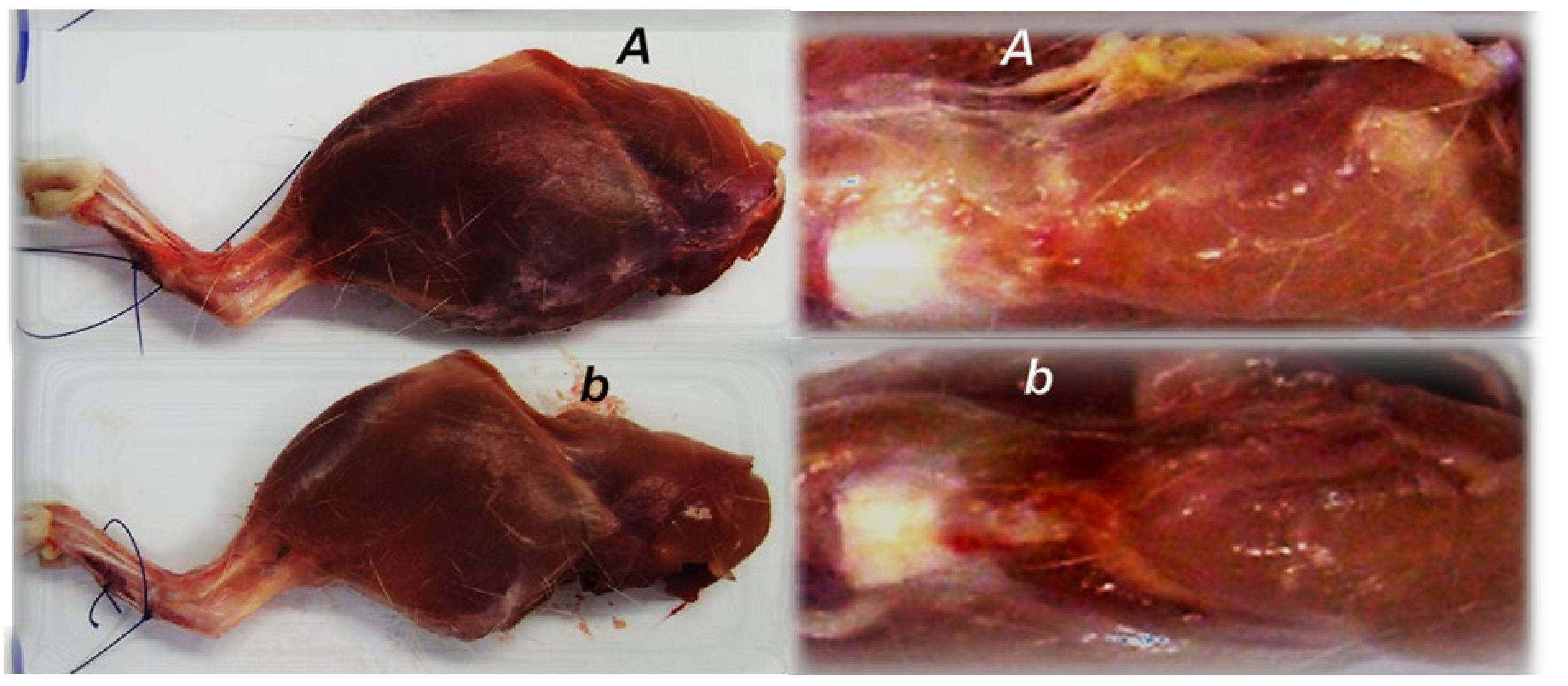
3.2. Muscle Size Recovery
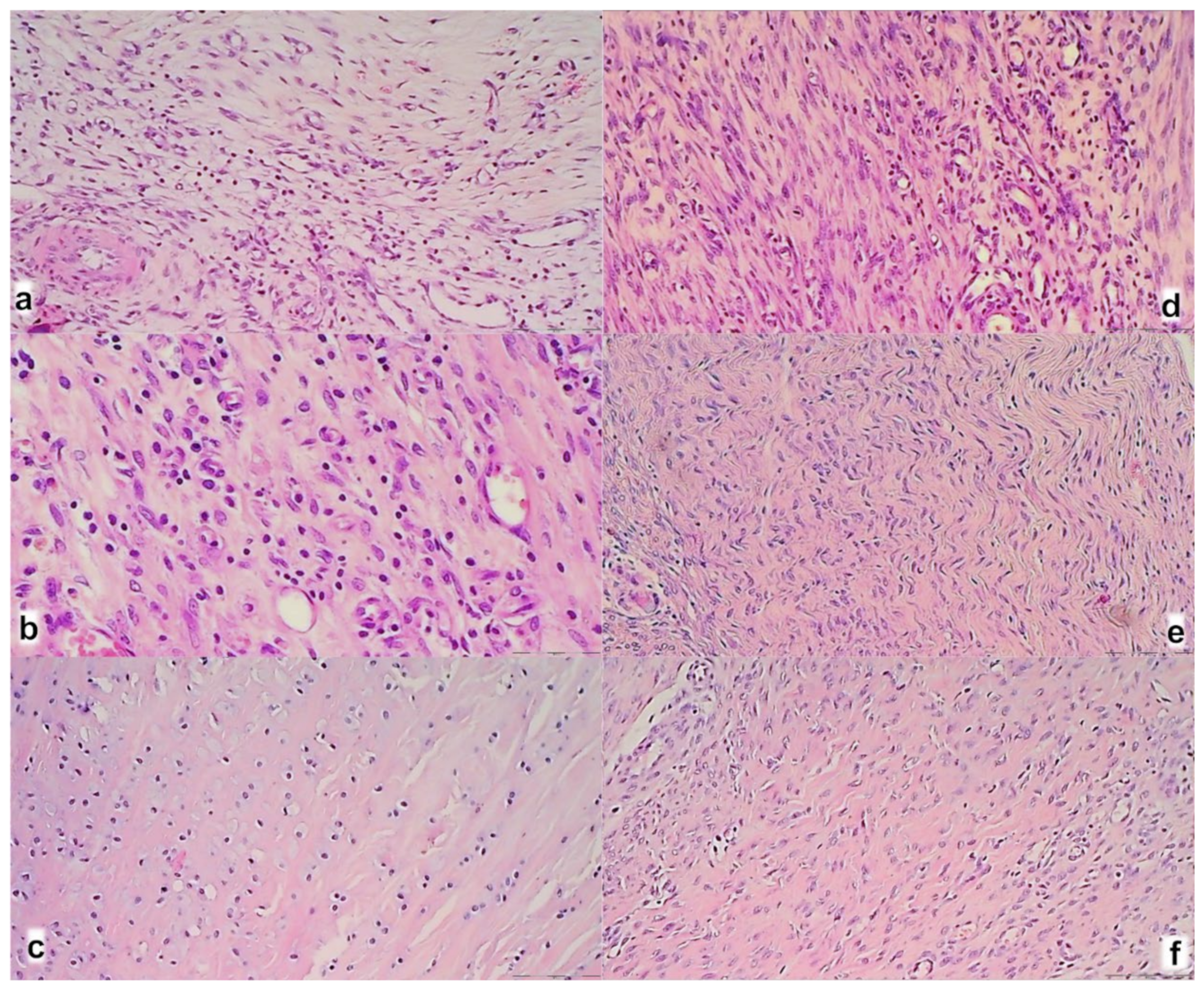
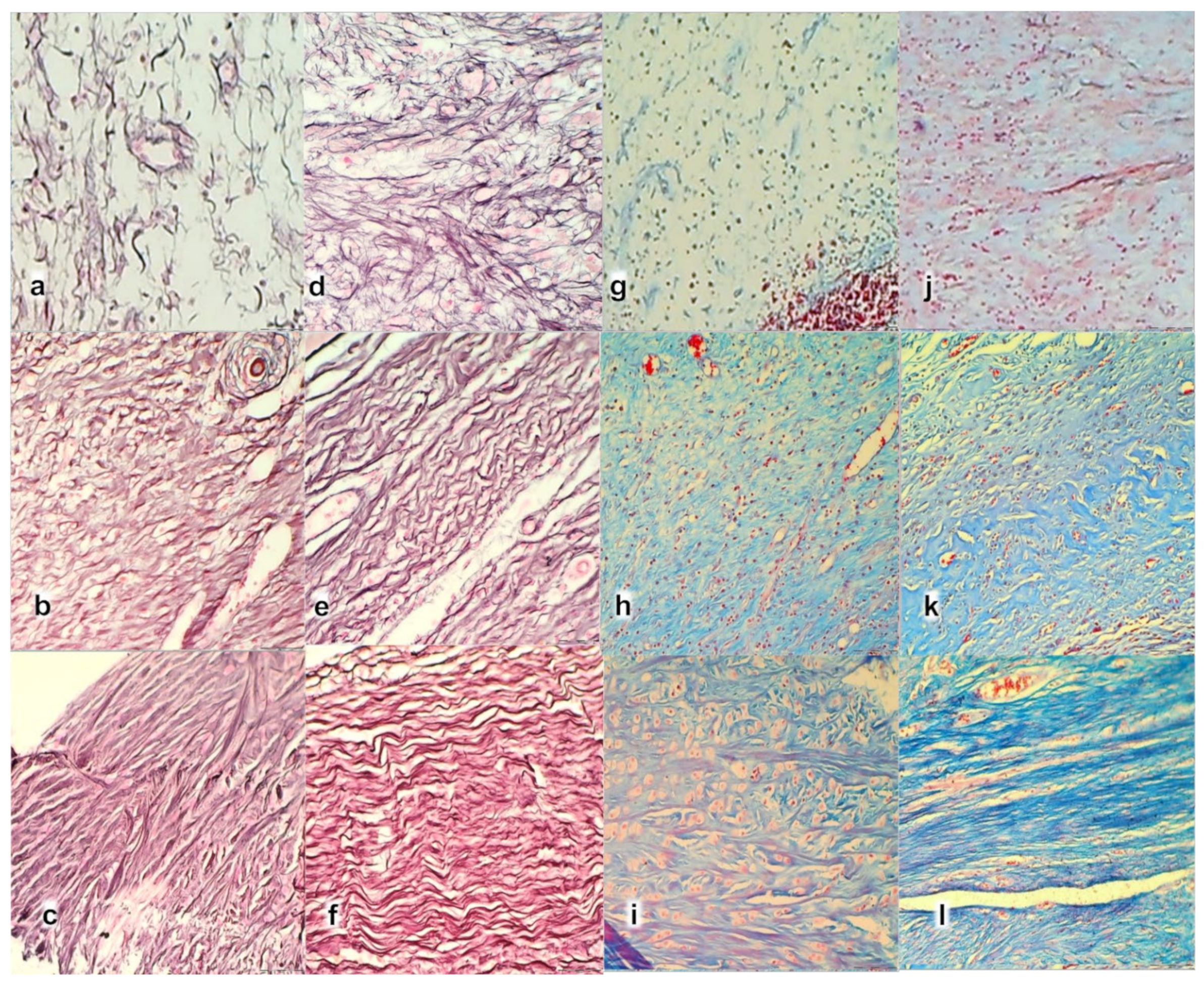
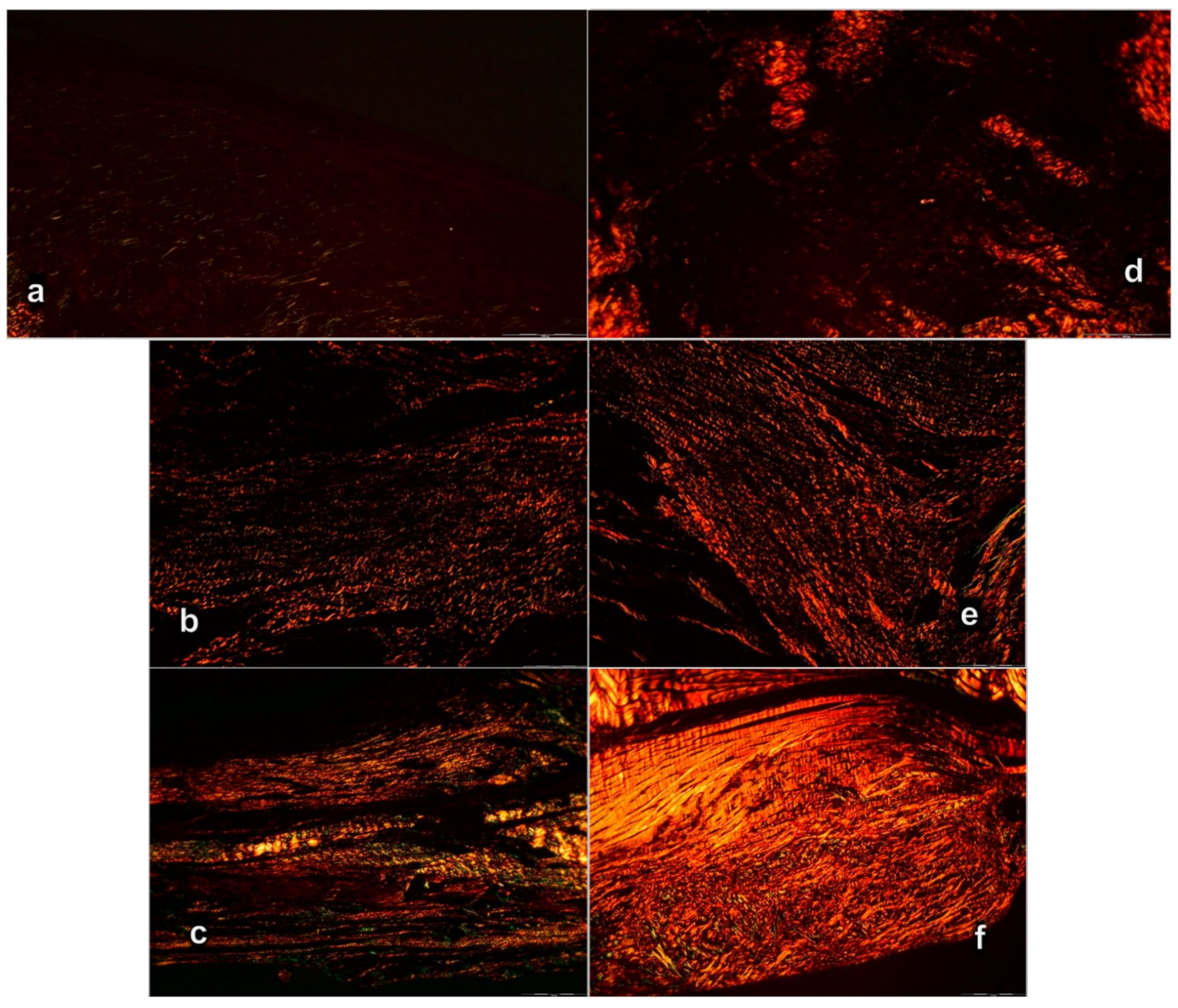

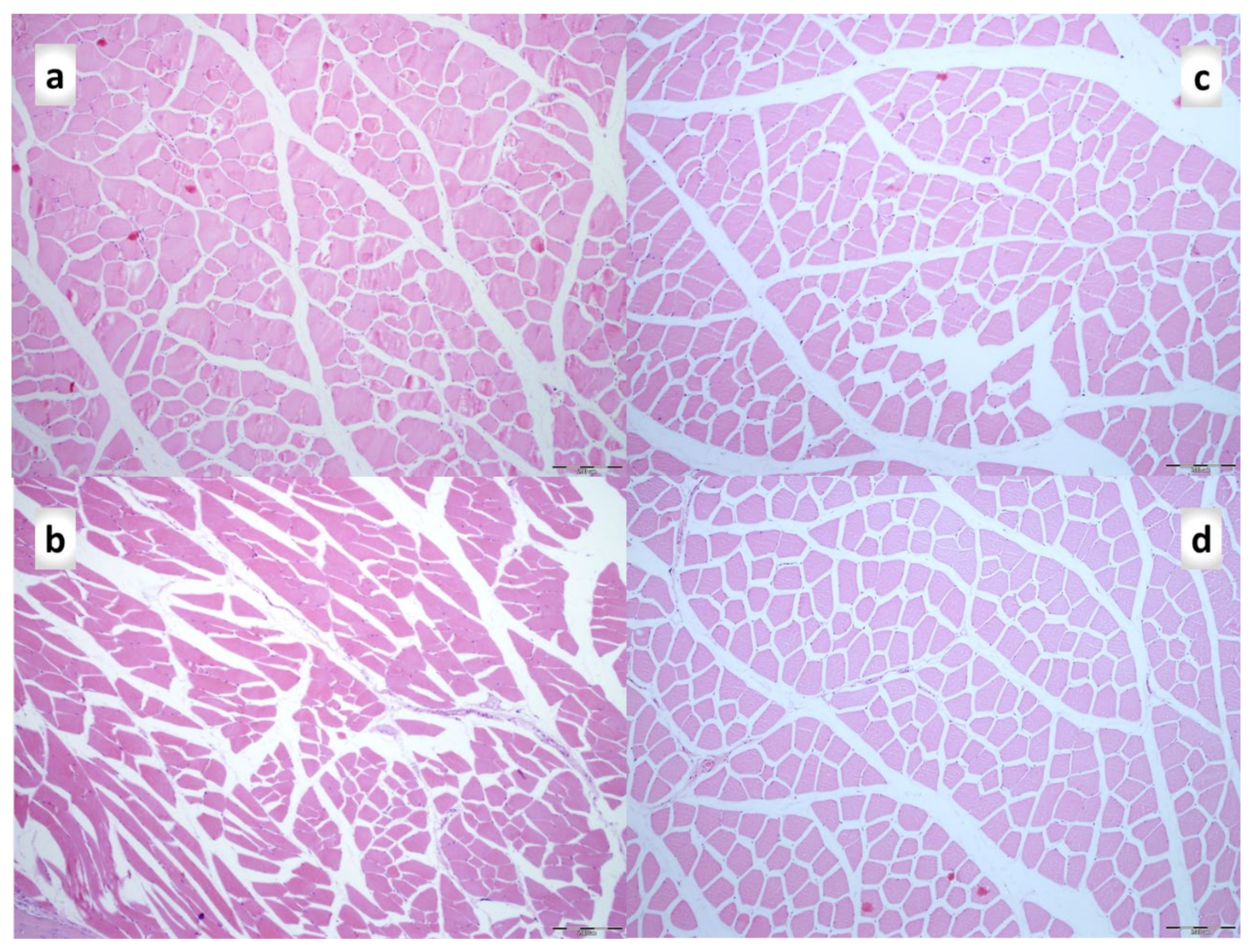
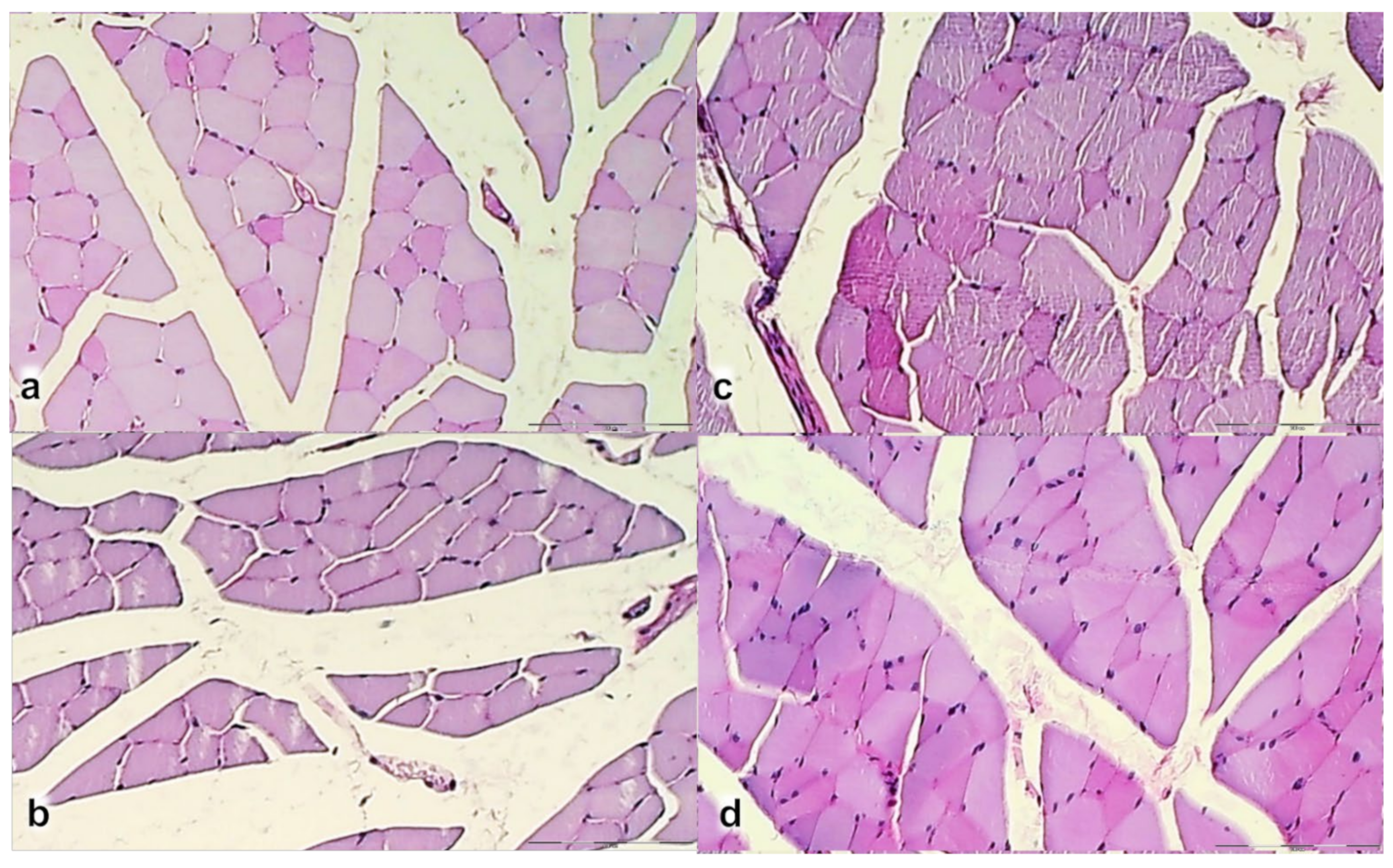
3.3. Oxidative Stress, and Particular Effect on COX 2, nNOS, iNOS, eNOS mRNA Levels
3.3.1. MDA, NO-Tissue Levels
3.3.2. COX 2, nNOS, iNOS, eNOS mRNA Levels

4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Finni, T. Structural and functional features of human muscle-tendon unit. Scand. J. Med. Sci. Sports 2006, 16, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Trotter, J.A.; Hsi, K.; Samora, A.; Wofsy, C. A morphometric analysis of the muscle-tendon junction. Anat. Rec. 1985, 213, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Gwyer, D.; Wragg, N.M.; Wilson, S.L. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019, 377, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Brcic, L.; Vuletic, L.B.; Kolenc, D.; Aralica, G.; Misic, M.; Zenko, A.; Drmic, D.; Rucman, R.; Sikiric, P. BPC 157 and blood vessels. Curr. Pharm. Des. 2014, 20, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Sever, M.; Klicek, R.; Blagaic, A.B.; Tvrdeic, A.; Kralj, T.; Kovac, K.K.; Vukojevic, J.; Siroglavic, M.; et al. Fistulas healing. Stable gastric pentadecapeptide BPC 157 therapy. Curr. Pharm. Des. 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Sikiric, P.; Hahm, K.B.; Blagaic, A.B.; Tvrdeic, A.; Pavlov, K.H.; Petrovic, A.; Kokot, A.; Gojkovic, S.; Krezic, I.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157, robert’s stomach cytoprotection/adaptive cytoprotection/organoprotection, and selye’s stress coping response: Progress, achievements, and the future. Gut Liver 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Ilic, S.; Kolenc, D. Revised robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr. Pharm. Des. 2010, 16, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Drmic, D.; Stupnisek, M.; Kokot, A.; Sever, M.; Zoricic, I.; Zoricic, Z.; Batelja, L.; et al. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a Solution? Curr. Pharm. Des. 2017, 23, 4012–4028. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Grgic, T.; Strbe, S.; Zukanovic, G.; Crvenkovic, D.; et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr. Neuropharmacol. 2016, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Ilic, S.; et al. Focus on ulcerative colitis: Stable gastric pentadecapeptide BPC 157. Curr. Med. Chem. 2012, 19, 126–132. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157: Novel therapy in gastrointestinal tract. Curr. Pharm. Des. 2011, 17, 1612–1632. [Google Scholar] [CrossRef]
- Brcic, L.; Brcic, I.; Staresinic, M.; Novinscak, T.; Sikiric, P.; Seiwerth, S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J. Physiol. Pharmacol. 2009, 60, 191–196. [Google Scholar]
- Krivic, A.; Anic, T.; Seiwerth, S.; Huljev, D.; Sikiric, P. Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: Promoted tendon-to-bone healing and opposed corticosteroid aggravation. J. Orthop. Res. 2006, 24, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Krivic, A.; Majerovic, M.; Jelic, I.; Seiwerth, S.; Sikiric, P. Modulation of early functional recovery of achilles tendon to bone unit after transection by BPC 157 and methylprednisolone. Inflamm. Res. 2008, 57, 205–210. [Google Scholar] [CrossRef]
- Staresinic, M.; Petrovic, I.; Novinscak, T.; Jukic, I.; Pevec, D.; Suknaic, S.; Kokic, N.; Batelja, L.; Brcic, L.; Boban-Blagaic, A.; et al. Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. J. Orthop. Res. 2006, 24, 1109–1117. [Google Scholar] [CrossRef]
- Staresinic, M.; Sebecic, B.; Patrlj, L.; Jadrijevic, S.; Suknaic, S.; Perovic, D.; Aralica, G.; Zarkovic, N.; Borovic, S.; Srdjak, M.; et al. Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth. J. Orthop. Res. 2003, 21, 976–983. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Yang, Q.-Q.; Yan, Y.Y.; Zhang, L.; Wang, Q.H.; Ju, F.; Tang, J.B. Gene-loaded nanoparticle-coated sutures provide effective gene delivery to enhance tendon healing. Mol. Ther. 2019, 27, 1534–1546. [Google Scholar] [CrossRef]
- Gross, G.; Hoffmann, A. Therapeutic strategies for tendon healing based on novel biomaterials, factors and cells. Pathobiology 2013, 80, 203–210. [Google Scholar] [CrossRef]
- Majewski, M.; Betz, O.; Ochsner, P.E.; Liu, F.; Porter, R.M.; Evans, C.H. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008, 15, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Majewski, M.; Heisterbach, P.; Jaquiéry, C.; Dürselen, L.; Todorov, A.; Martin, I.; Evans, C.H.; Müller, S.A. Improved tendon healing using bFGF, BMP-12 and TGFβ1 in a rat model. Eur. Cell Mater. 2018, 35, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H.S. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.; Seneviratne, A.M.; Izawa, K.; Atkinson, B.L.; Potter, H.G.; Rodeo, S.A. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am. J. Sports Med. 2001, 29, 689–698. [Google Scholar] [CrossRef]
- Martinek, V.; Latterman, C.; Usas, A.; Abramowitch, S.; Woo, S.L.Y.; Fu, F.H.; Huard, J. Enhancement of tendon–bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-II gene transfer: A histological and biomechanical study. J. Bone Jt. Surg. 2002, 84, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Cerovecki, T.; Bojanic, I.; Brcic, L.; Radic, B.; Vukoja, I.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 (PL 14736) improves ligament healing in the rat. J. Orthop. Res. 2010, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Gamulin, O.; Serec, V.; Bilic, V.; Balarin, M.; Kosovic, M.; Drmic, D.; Brcic, L.; Seiwerth, S.; Sikiric, P. Monitoring the healing process of rat bones using Raman spectroscopy. J. Mol. Struct. 2013, 1044, 308–313. [Google Scholar] [CrossRef]
- Keremi, B.; Lohinai, Z.; Komora, P.; Duhaj, S.; Borsi, K.; Jobbagy-Ovari, G.; Kallo, K.; Szekely, A.D.; Fazekas, A.; Dobo-Nagy, C.; et al. Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. J. Physiol. Pharmacol. 2009, 60, 115–122. [Google Scholar] [PubMed]
- Sebecic, B.; Nikolic, V.; Sikiric, P.; Seiwerth, S.; Sosa, T.; Patrlj, L.; Grabarevic, Z.; Rucman, R.; Petek, M.; Konjevoda, P.; et al. Osteogenic effect of a gastric pentadecapeptide, BPC-157, on the healing of segmental bone defect in rabbits: A comparison with bone marrow and autologous cortical bone implantation. Bone 1999, 24, 195–202. [Google Scholar] [CrossRef]
- Mihovil, I.; Radic, B.; Brcic, L.; Brcic, I.; Vukoja, I.; Ilic, S.; Boban Blagaic, A.; Seiwerth, S.; Sikiric, P. Beneficial effect of pentadecapeptide BPC 157 on denervated muscle in rats. J. Physiol. Pharmacol. 2009, 60, 69. [Google Scholar]
- Novinscak, T.; Brcic, L.; Staresinic, M.; Jukic, I.; Radic, B.; Pevec, D.; Mise, S.; Tomasovic, S.; Brcic, I.; Banic, T.; et al. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg. Today 2008, 38, 716–725. [Google Scholar] [CrossRef]
- Pevec, D.; Novinscak, T.; Brcic, L.; Sipos, K.; Jukic, I.; Staresinic, M.; Mise, S.; Brcic, I.; Kolenc, D.; Klicek, R.; et al. Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Med. Sci. Monit. 2010, 16, 81–88. [Google Scholar]
- Kvist, M.; Hurme, T.; Kannus, P.; Järvinen, T.; Maunu, V.M.; Jozsa, L.; Järvinen, M. Vascular density at the myotendinous junction of the rat gastrocnemius muscle after immobilization and remobilization. Am. J. Sports Med. 1995, 23, 359–364. [Google Scholar] [CrossRef]
- Murrel, G.A.C. Using nitric oxide to treat tendinopathy. Br. J. Sports Med. 2007, 41, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Bilic, M.; Bumber, Z.; Blagaic, A.B.; Batelja, L.; Seiwerth, S.; Sikiric, P. The stable gastric pentadecapeptide BPC 157, given locally, improves CO2 laser healing in mice. Burns 2005, 31, 310–315. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Dev. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikus, D.; Sikiric, P.; Seiwerth, S.; Petricevic, A.; Aralica, G.; Druzijancic, N.; Rucman, R.; Petek, M.; Pigac, B.; Perovic, D.; et al. Pentadecapeptide BPC 157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice. Burns 2001, 27, 817–827. [Google Scholar] [CrossRef]
- Seiwerth, S.; Sikiric, P.; Grabarevic, Z.; Zoricic, I.; Hanzevacki, M.; Ljubanovic, D.; Coric, V.; Konjevoda, P.; Petek, M.; Rucman, R.; et al. BPC 157′s effect on healing. J. Physiol. Paris 1997, 91, 173–178. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Mise, S.; Staresinic, M.; Bedekovic, V.; Zarkovic, N.; Borovic, S.; Gjurasin, M.; Boban-Blagaic, A.; Batelja, L.; et al. Corticosteroid-impairment of healing and gastric pentadecapeptide BPC-157 creams in burned mice. Burns 2003, 29, 323–334. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Separovic, J.; Anic, T.; Buljat, G.; Mikus, D.; Seiwerth, S.; Grabarevic, Z.; Stancic-Rokotov, D.; Pigac, B.; Hanzevacki, M.; et al. The effect of pentadecapeptide BPC 157, H2-blockers, omeprazole and sucralfate on new vessels and new granulation tissue formation. J. Physiol. Paris 1999, 93, 479–485. [Google Scholar] [CrossRef]
- Hrelec, M.; Klicek, R.; Brcic, L.; Brcic, I.; Cvjetko, I.; Seiwerth, S.; Sikiric, P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J. Physiol. Pharmacol. 2009, 60, 161–165. [Google Scholar]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Petek, M.; Rucman, R.; Turkovic, B.; Rotkvic, I.; Jagic, V.; Duvnjak, M.; Mise, S. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994, 54, PL63–PL68. [Google Scholar] [CrossRef]
- Amic, F.; Drmic, D.; Bilic, Z.; Krezic, I.; Zizek, H.; Peklic, M.; Klicek, R.; Pajtak, A.; Amic, E.; Vidovic, T.; et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5366–5378. [Google Scholar] [CrossRef]
- Drmic, D.; Samara, M.; Vidovic, T.; Malekinusic, D.; Antunovic, M.; Vrdoljak, B.; Ruzman, J.; Milkovic Perisa, M.; Horvat Pavlov, K.; Jeyakumar, J.; et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5462–5476. [Google Scholar] [CrossRef]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Pavlov, K.H.; Petrovic, A.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and the permanent occlusion of the superior sagittal sinus in rat. Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef]
- Sever, A.Z.; Sever, M.; Vidovic, T.; Lojo, N.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Kokot, A.; Zoricic, I.; Coric, M.; et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur. J. Pharmacol. 2019, 847, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Vukojevic, J.; Siroglavic, M.; Kasnik, K.; Kralj, T.; Stancic, D.; Kokot, A.; Kolaric, D.; Drmic, D.; Sever, A.Z.; Barisic, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vasc. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavic, M.; Kolenc, D.; Boban Blagaic, A.; Batelja, L.; Drmic, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome; intracranial, portal and caval hypertension; and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Horvat Pavlov, K.; Drmic, D.; et al. Complex syndrome of the complete occlusion of the end of the superior mesenteric vein, opposed with the stable gastric pentadecapeptide BPC 157 in rats. Biomedicines 2021, 9, 1029. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded superior mesenteric artery and vein. Therapy with the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur. J. Pharmacol. 1997, 332, 23–33. [Google Scholar] [CrossRef]
- Perovic, D.; Kolenc, D.; Bilic, V.; Somun, N.; Drmic, D.; Elabjer, E.; Buljat, G.; Seiwerth, S.; Sikiric, P. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J. Orthop. Surg. Res. 2019, 14, 199. [Google Scholar] [CrossRef]
- Barisic, I.; Balenovic, D.; Klicek, R.; Radic, B.; Nikitovic, B.; Drmic, D.; Udovicic, M.; Strinic, D.; Bardak, D.; Berkopic, L.; et al. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul. Pept. 2013, 181, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Medvidovic-Grubisic, M.; Stambolija, V.; Kolenc, D.; Katancic, J.; Murselovic, T.; Plestina-Borjan, I.; Strbe, S.; Drmic, D.; Barisic, I.; Sindic, A.; et al. Hypermagnesemia disturbances in rats, NO-related: Pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology 2017, 25, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Stambolija, V.; Stambolija, T.P.; Holjevac, J.K.; Murselovic, T.; Radonic, J.; Duzel, V.; Duplancic, B.; Uzun, S.; Zivanovic-Posilovic, G.; Kolenc, D.; et al. BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur. J. Pharmacol. 2016, 781, 83–91. [Google Scholar] [CrossRef]
- Belosic Halle, Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Seiwerth, S.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Marovic, A.; Perovic, D.; Seiwerth, S.; Mise, S.; Turkovic, B.; Dodig, G.; et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: The effect on catalepsy and gastric ulcers in mice and rats. Eur. J. Pharmacol. 1999, 379, 19–31. [Google Scholar] [CrossRef]
- Strinic, D.; Belosic Halle, Z.; Luetic, K.; Nedic, A.; Petrovic, I.; Sucic, M.; Zivanovic Posilovic, G.; Balenovic, D.; Strbe, S.; Udovicic, M.; et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017, 186, 66–79. [Google Scholar] [CrossRef]
- Klicek, R.; Kolenc, D.; Suran, J.; Drmic, D.; Brcic, L.; Aralica, G.; Sever, M.; Holjevac, J.; Radic, B.; Turudic, T.; et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J. Physiol. Pharmacol. 2013, 64, 597–612. [Google Scholar]
- Jandric, I.; Vrcic, H.; Jandric Balen, M.; Kolenc, D.; Brcic, L.; Radic, B.; Drmic, D.; Seiwerth, S.; Sikiric, P. Salutary effect of gastric pentadecapeptide BPC 157 in two different stress urinary incontinence models in female rats. Med. Sci. Monit. Basic Res. 2013, 19, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokot, A.; Zlatar, M.; Stupnisek, M.; Drmic, D.; Radic, R.; Vcev, A.; Seiwerth, S.; Sikiric, P. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: Reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur. J. Pharmacol. 2016, 771, 211–219. [Google Scholar] [CrossRef]
- Sucic, M.; Luetic, K.; Jandric, I.; Drmic, D.; Sever, A.Z.; Vuletic, L.B.; Halle, Z.B.; Strinic, D.; Kokot, A.; Seiwerth, R.S.; et al. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur. J. Pharmacol. 2019, 861, 172593. [Google Scholar] [CrossRef] [PubMed]
- Cesarec, V.; Becejac, T.; Misic, M.; Djakovic, Z.; Olujic, D.; Drmic, D.; Brcic, L.; Rokotov, D.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur. J. Pharmacol. 2013, 701, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Radeljak, S.; Seiwerth, S.; Sikiric, P. BPC 157 inhibits cell growth and VEGF signalling via the MAPK kinase pathway in the human melanoma cell line. Melanoma Res. 2004, 14, 14–15. [Google Scholar] [CrossRef]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, I.; Abassi, Z.; Coleman, R.; Milman, F.; Winaver, J.; Better, O.S. Involvement of nitric oxide system in experimental muscle crush injury. J. Clin. Investig. 1998, 101, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Koka, R.; Hadlock, T.A. Quantification of functional recovery following rat sciatic nerve transection. Exp. Neurol. 2001, 168, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Funato, T.; Sato, Y.; Fujiki, S.; Sato, Y.; Aoi, S.; Tsuchiya, K.; Yanagihara, D. Postural control during quiet bipedal standing in rats. PLoS ONE 2017, 12, e0189248. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.; Szczecinski, N.S.; Arnold, D.; Anrada, E.; Fischer, M.S.; Qinn, R.D.; Hunt, A.J. Neuromechanical model of rat hindlimb walking with two-layer CPGs. Biomimetics 2019, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jazvinscak Jembrek, M.; Cipak Gasparovic, A.; Vukovic, L.; Vlainic, J.; Zarkovic, N.; Orsolic, N. Quercetin supplementation: Insight into the potentially harmful outcomes of neurodegenerative prevention. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 1185–1197. [Google Scholar] [CrossRef]
- Liu, S.H.; Panossian, V.; al-Shaikh, R.; Tomin, E.; Shepherd, E.; Finerman, G.A.; Lane, J.M. Morphology and matrix composition during early tendon to bone healing. Clin. Orthop. Relat. Res. 1997, 339, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Forslund, C.; Aspenberg, P. Osteogenic protein-1 has more effect than mechanical signals in the control of tissue differentiation in healing rat tendons. Acta Orthop. Scand. 1998, 69, 622–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, J.; Tu, Y.; Burns, M.; Silva, M.J.; Manske, P. BMP-12 gene transfer augmentation of lacerated tendon repair. J. Orthop. Res. 2001, 19, 1199–1202. [Google Scholar] [CrossRef]
- Rodeo, S.A.; Izawa, K. Tendon-to-bone healing: Basic science aspects and enhancement techniques. Tech. Orthop. 1999, 14, 22–33. [Google Scholar] [CrossRef]
- Anderson, J.E.; Do, M.Q.; Daneshvar, N.; Suzuki, T.; Dort, J.; Mizunoya, W.; Tatsumi, R. The role of semaphorin3A in myogenic regeneration and the formation of functional neuromuscular junctions on new fibres. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1389–1405. [Google Scholar] [CrossRef]
- Daneshvar, N.; Tatsumi, R.; Peeler, J.; Anderson, J.E. Premature satellite cell activation before injury accelerates myogenesis and disrupts neuromuscular junction maturation in regenerating muscle. Am. J. Physiol. Cell Physiol. 2020, 319, 116–128. [Google Scholar] [CrossRef]
- Balenovic, D.; Bencic, M.L.; Udovicic, M.; Simonji, K.; Hanzevacki, J.S.; Barisic, I.; Kranjcevic, S.; Prkacin, I.; Coric, V.; Brcic, L.; et al. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: A relation with NO-system. Regul. Pept. 2009, 156, 83–89. [Google Scholar] [CrossRef]
- Lozic, M.; Stambolija, V.; Krezic, I.; Dugandzic, A.; Zivanovic-Posilovic, G.; Gojkovic, S.; Kovacevic, J.; Vrdoljak, L.; Mirkovic, I.; Kokot, A.; et al. In relation to NO-system, stable pentadecapeptide BPC 157 counteracts lidocaine-induced adverse effects in rats and depolarisation in vitro. Emerg. Med. Int. 2020, 2020, 6805354. [Google Scholar] [CrossRef]
- Zivanovic-Posilovic, G.; Balenovic, D.; Barisic, I.; Strinic, D.; Stambolija, V.; Udovicic, M.; Uzun, S.; Drmic, D.; Vlainic, J.; Bencic, M.L.; et al. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur. J. Pharmacol. 2016, 793, 56–65. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. Pentadecapeptide BPC 157 positively affects both non-steroidal anti-inflammatory agent-induced gastrointestinal lesions and adjuvant arthritis in rats. J. Physiol. Paris 1997, 91, 113–122. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. Beneficial effect of a novel pentadecapeptide BPC 157 on gastric lesions induced by restraint stress, ethanol, indomethacin, and capsaicin neurotoxicity. Dig. Dis. Sci. 1996, 41, 1604–1614. [Google Scholar] [CrossRef]
- Turkovic, B.; Sikiric, P.; Seiwerth, S.; Mise, S.; Anic, T.; Petek, M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 2004, 126, 287. [Google Scholar]
- Masnec, S.; Kokot, A.; Zlatar, M.; Kalauz, M.; Kunjko, K.; Radic, B.; Klicek, R.; Drmic, D.; Lazic, R.; Brcic, L.; et al. Perforating corneal injury in rat and pentadecapeptide BPC 157. Exp. Eye Res. 2015, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Maffulli, N.; Reverchon, E. Regeneration techniques for bone-to-tendon and muscle-to-tendon interfaces reconstruction. Br. Med. Bull. 2016, 117, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Sequence | Size (bp) | |
|---|---|---|---|
| GAPDH | TGGCAAGTTCAACGGCACAGT | 193 | XM_221353 |
| TTTGGCCTCACCCTTCAGGT | |||
| eNOS | CTGGCAAGACCGATTACACGA | 206 | NM_021838 |
| TCAGGAGGTCTTGCACATAGG | |||
| iNOS | TTGGAGCGAGTTGTGGATTGTTGTTC | 126 | NM_012611 |
| GGTGAGGGCTTGCCTGAGTGAGC | |||
| nNOS | AACTGGGAGGGGAGAGGATTC | 517 | NM_052799.1 |
| GGGTGGGAGGCGAGATTCAT | |||
| COX-2 | TCAGGAGGTCTTGCACATAGG | 157 | AF233596 |
| CTGTATCCCGCCCTGCTGGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Japjec, M.; Horvat Pavlov, K.; Petrovic, A.; Staresinic, M.; Sebecic, B.; Buljan, M.; Vranes, H.; Giljanovic, A.; Drmic, D.; Japjec, M.; et al. Stable Gastric Pentadecapeptide BPC 157 as a Therapy for the Disable Myotendinous Junctions in Rats. Biomedicines 2021, 9, 1547. https://doi.org/10.3390/biomedicines9111547
Japjec M, Horvat Pavlov K, Petrovic A, Staresinic M, Sebecic B, Buljan M, Vranes H, Giljanovic A, Drmic D, Japjec M, et al. Stable Gastric Pentadecapeptide BPC 157 as a Therapy for the Disable Myotendinous Junctions in Rats. Biomedicines. 2021; 9(11):1547. https://doi.org/10.3390/biomedicines9111547
Chicago/Turabian StyleJapjec, Mladen, Katarina Horvat Pavlov, Andreja Petrovic, Mario Staresinic, Bozidar Sebecic, Matko Buljan, Hrvoje Vranes, Ana Giljanovic, Domagoj Drmic, Miroslav Japjec, and et al. 2021. "Stable Gastric Pentadecapeptide BPC 157 as a Therapy for the Disable Myotendinous Junctions in Rats" Biomedicines 9, no. 11: 1547. https://doi.org/10.3390/biomedicines9111547
APA StyleJapjec, M., Horvat Pavlov, K., Petrovic, A., Staresinic, M., Sebecic, B., Buljan, M., Vranes, H., Giljanovic, A., Drmic, D., Japjec, M., Prtoric, A., Lovric, E., Batelja Vuletic, L., Dobric, I., Boban Blagaic, A., Skrtic, A., Seiwerth, S., & Predrag, S. (2021). Stable Gastric Pentadecapeptide BPC 157 as a Therapy for the Disable Myotendinous Junctions in Rats. Biomedicines, 9(11), 1547. https://doi.org/10.3390/biomedicines9111547







