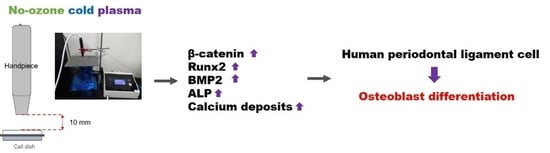
Enhancement of Osteoblast Differentiation Using No-Ozone Cold Plasma on Human Periodontal Ligament Cells
Abstract
:1. Introduction
2. Materials and Methods
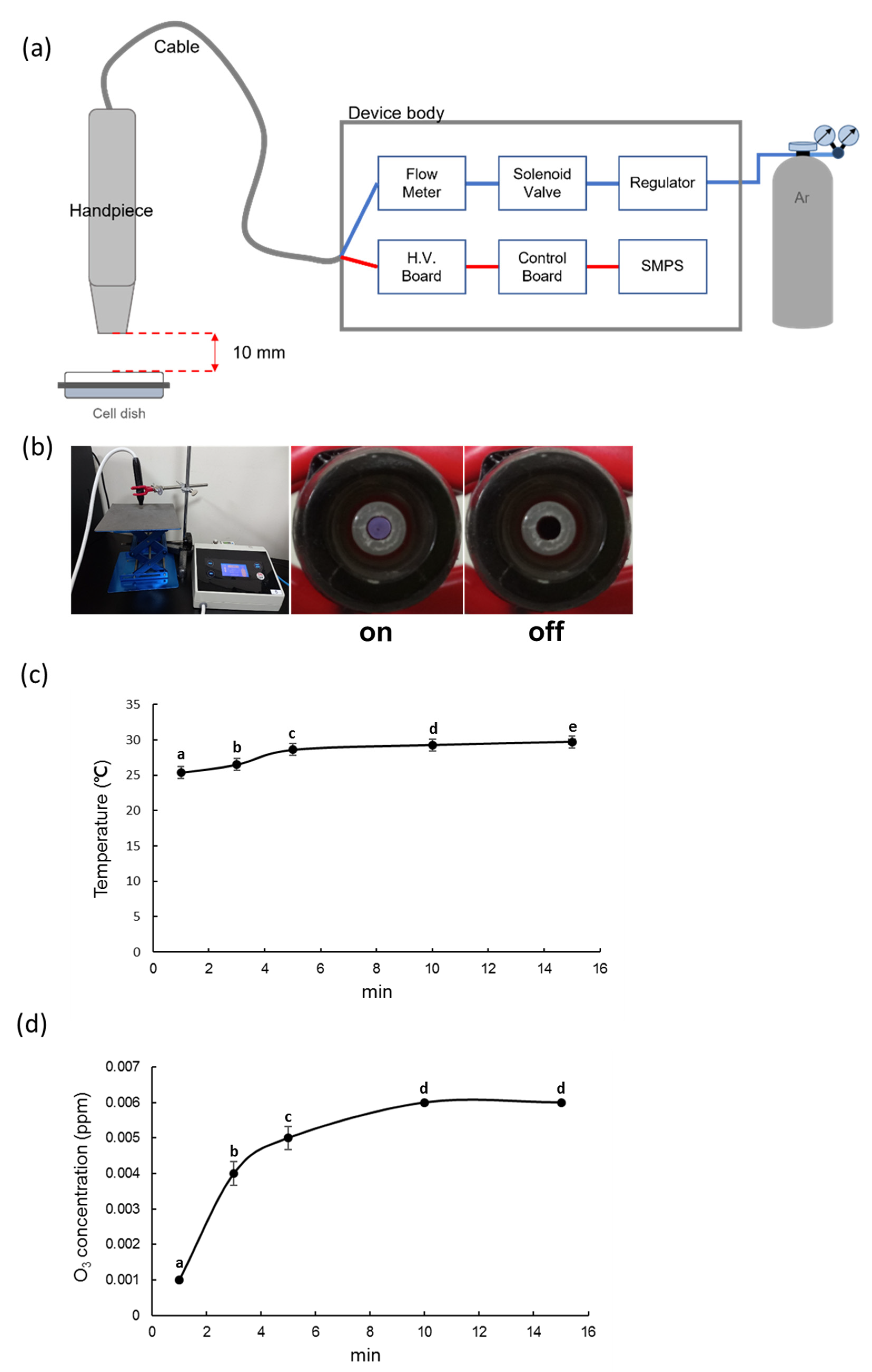
2.1. Plasma Device
2.2. NCP Temperature Measurement
2.3. NCP Ozone Concentration Measurement
2.4. Culture and Differentiation of the PDL Cells
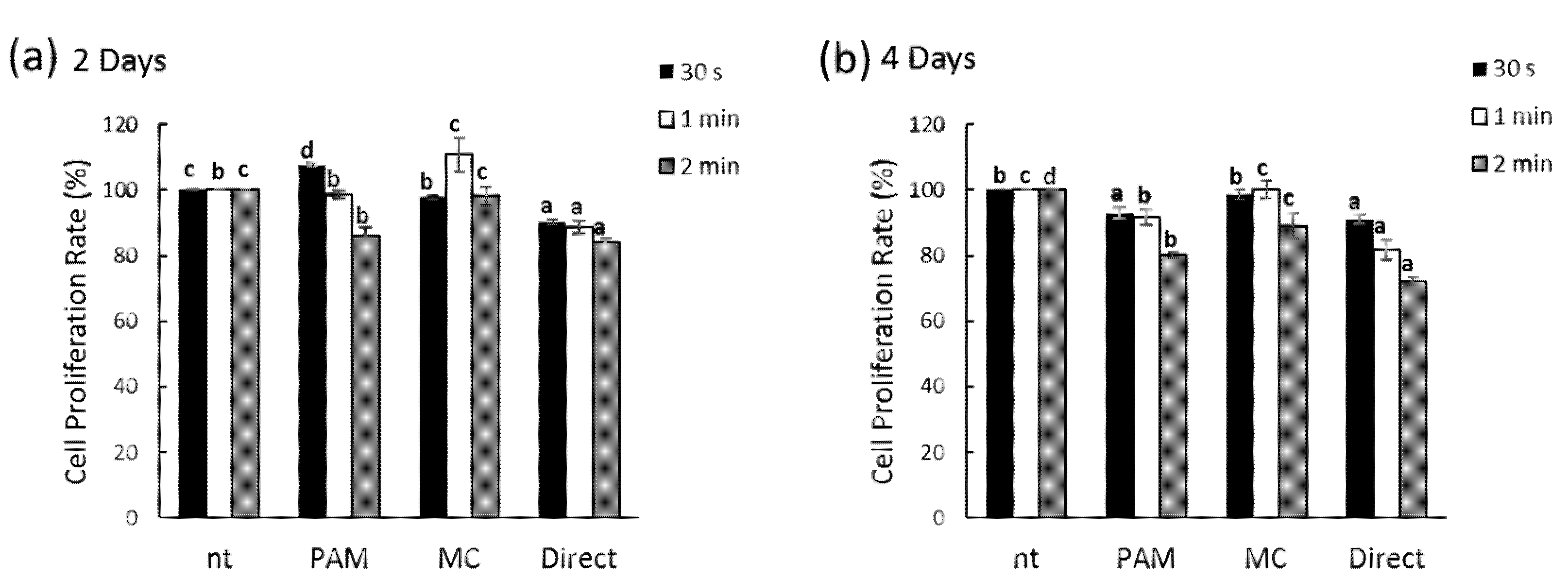
2.5. Cell Proliferation
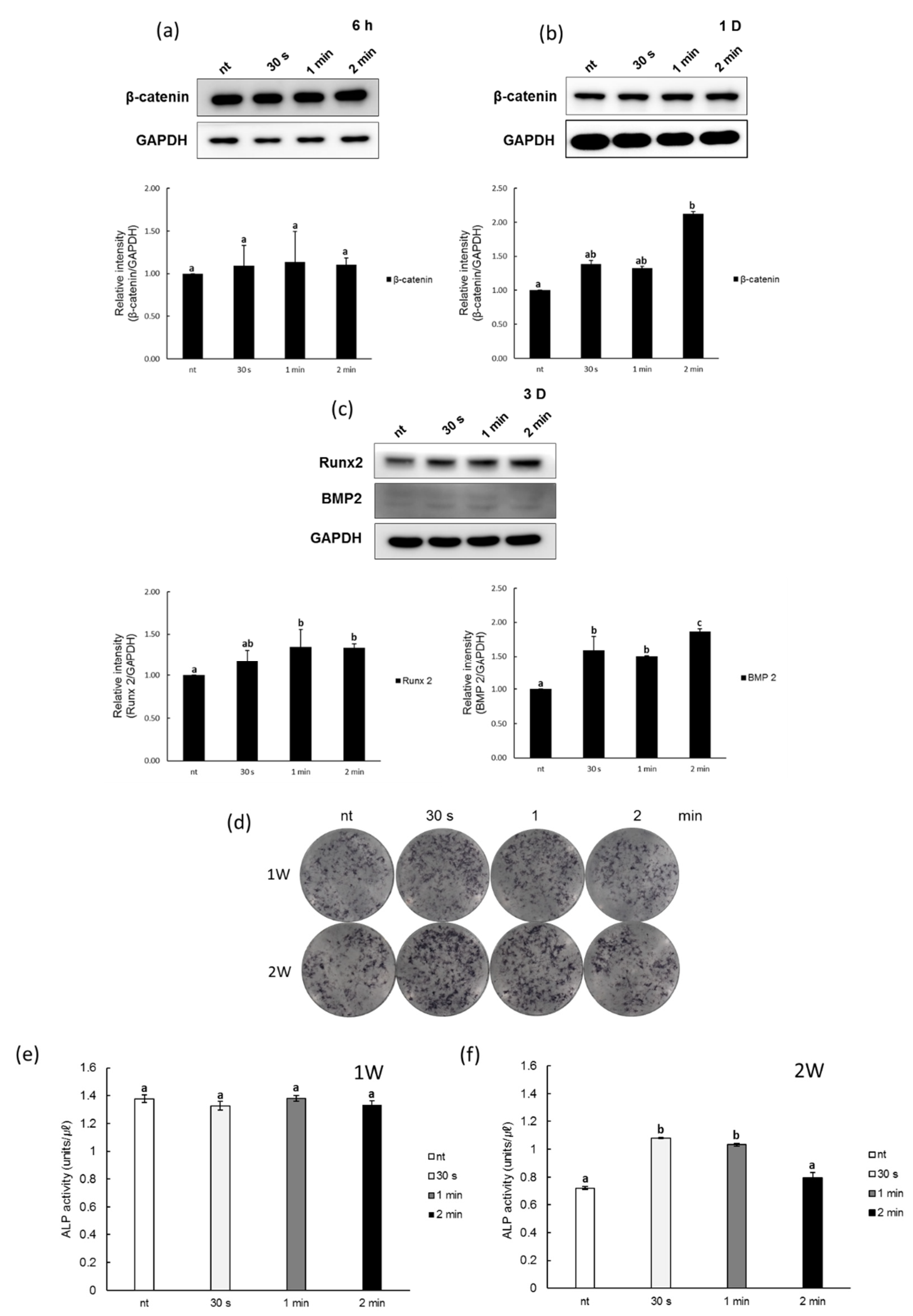
2.6. Western Blot Assay
2.7. Alkaline Phosphatase Staining
2.8. Alkaline Phosphatase Activity
2.9. Quantitative Real Time PCR
- Alkaline phosphatase-1 Forward, 5′-TTTGGTGGATACACCCCC-3′
- Alkaline phosphatase-1 Reverse, 5′-GCCTGGTAGTTGTTGTGAGC-3′
- Osteocalcin Forward, 5′-AGAGACCCAGGCGCTACCT-3′
- Osteocalcin Reverse 5′-CTGGGAGGTCAGGGCAAG-3′
- Osteopontin Forward, 5′-GGTCACTGATTTTCCCACGG-3′
- Osteopontin Reverse, 5′-GTCCTTCCCACGGCTGTC-3′
- Osteonectin Forward, 5′-TGGAGGCAGGAGACCACC-3′
- Osteonectin Reverse, 5′-TCCTTCTGCTTGATGCCG-3′
- GAPDH Forward 5′-ACTGGCATGGCCTTCCGT-3′
- GAPDH Reverse 5′-CCACCCTGTTGCTGTAGCC-3′
2.10. Alizarin Red S Staining
2.11. Statistical Analysis
3. Results
3.1. Measurement of the Gas Temperature and Ozone Concentration of NCP
3.2. Effects of NCP on PDL Cell Proliferation
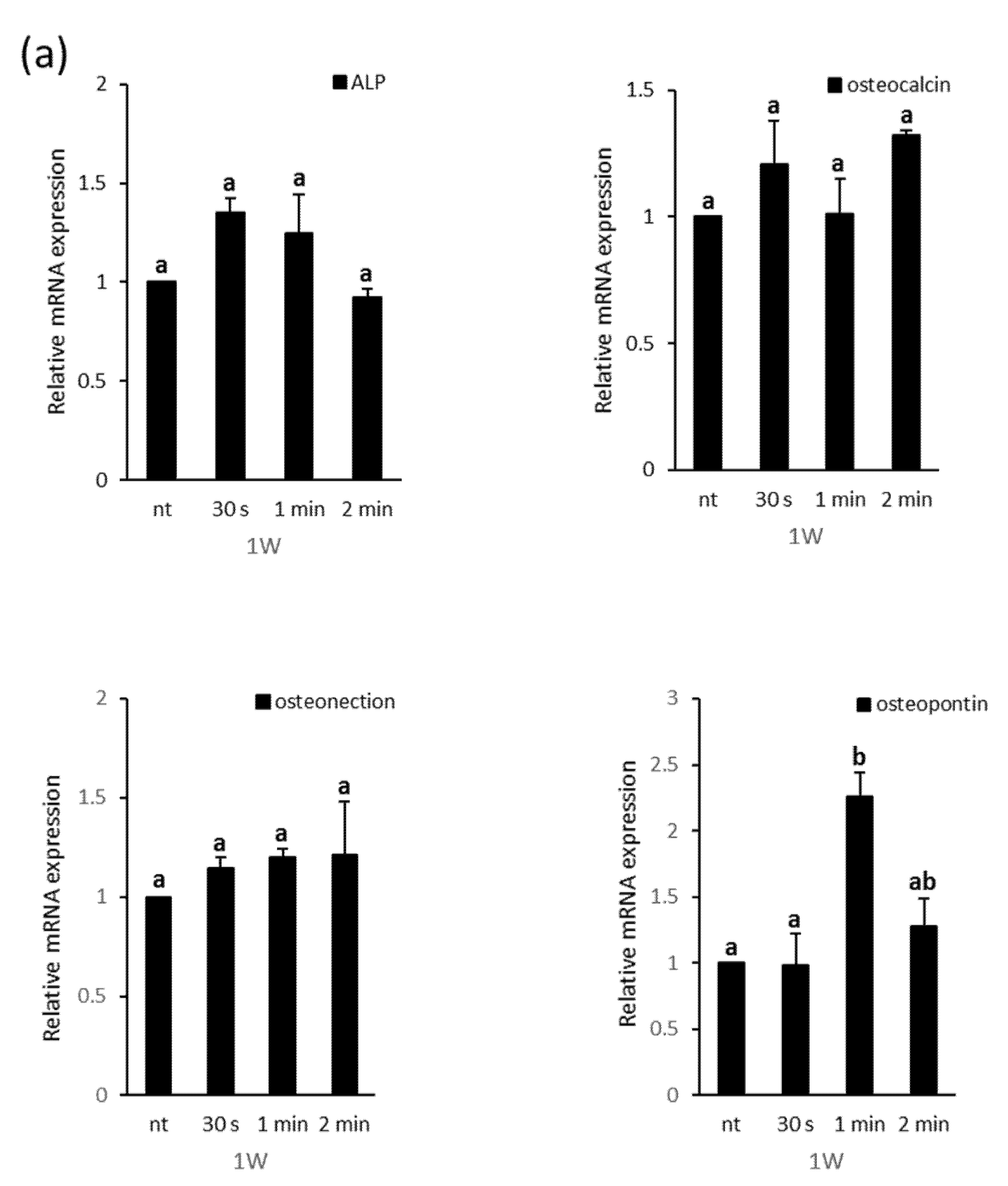
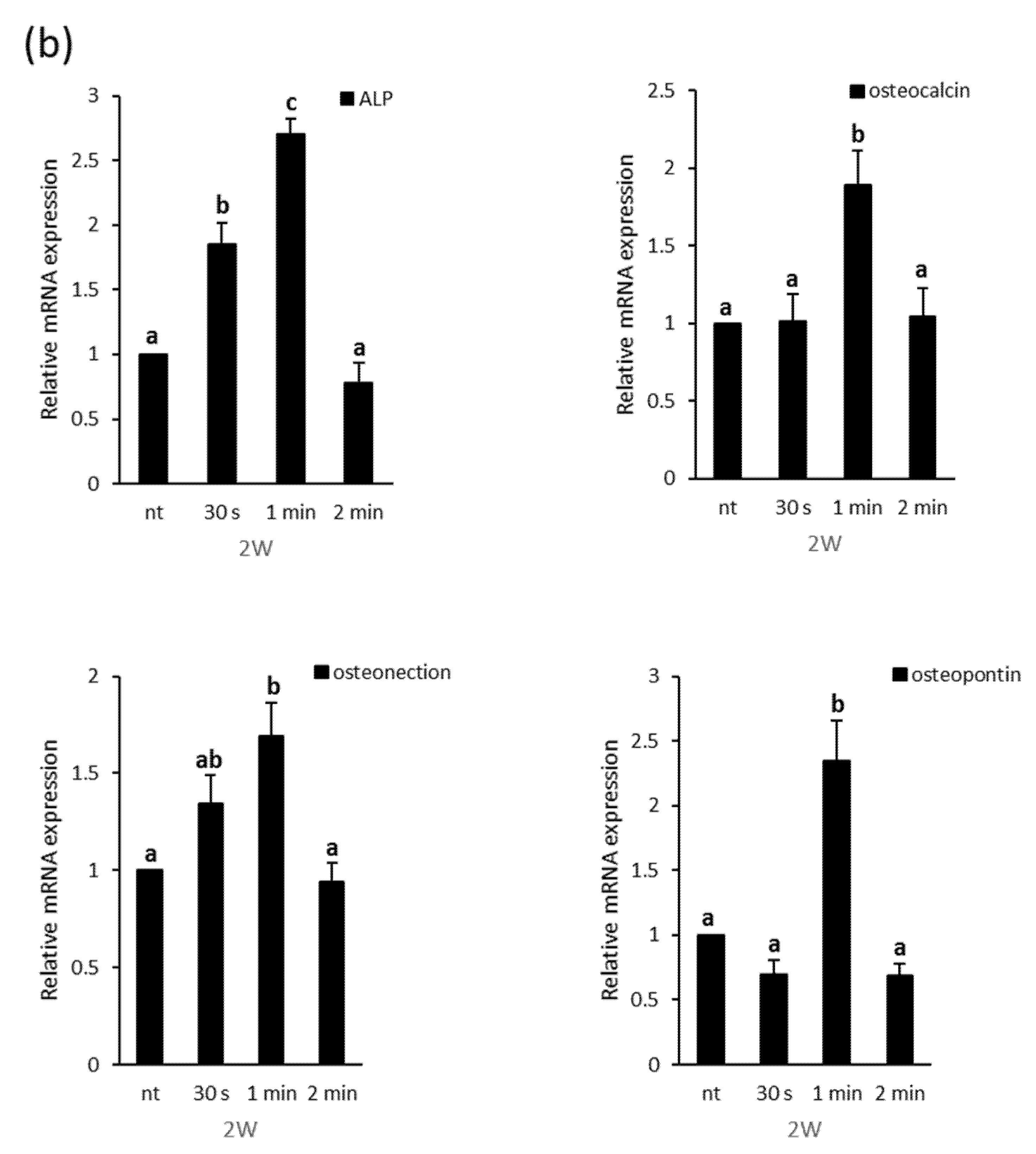
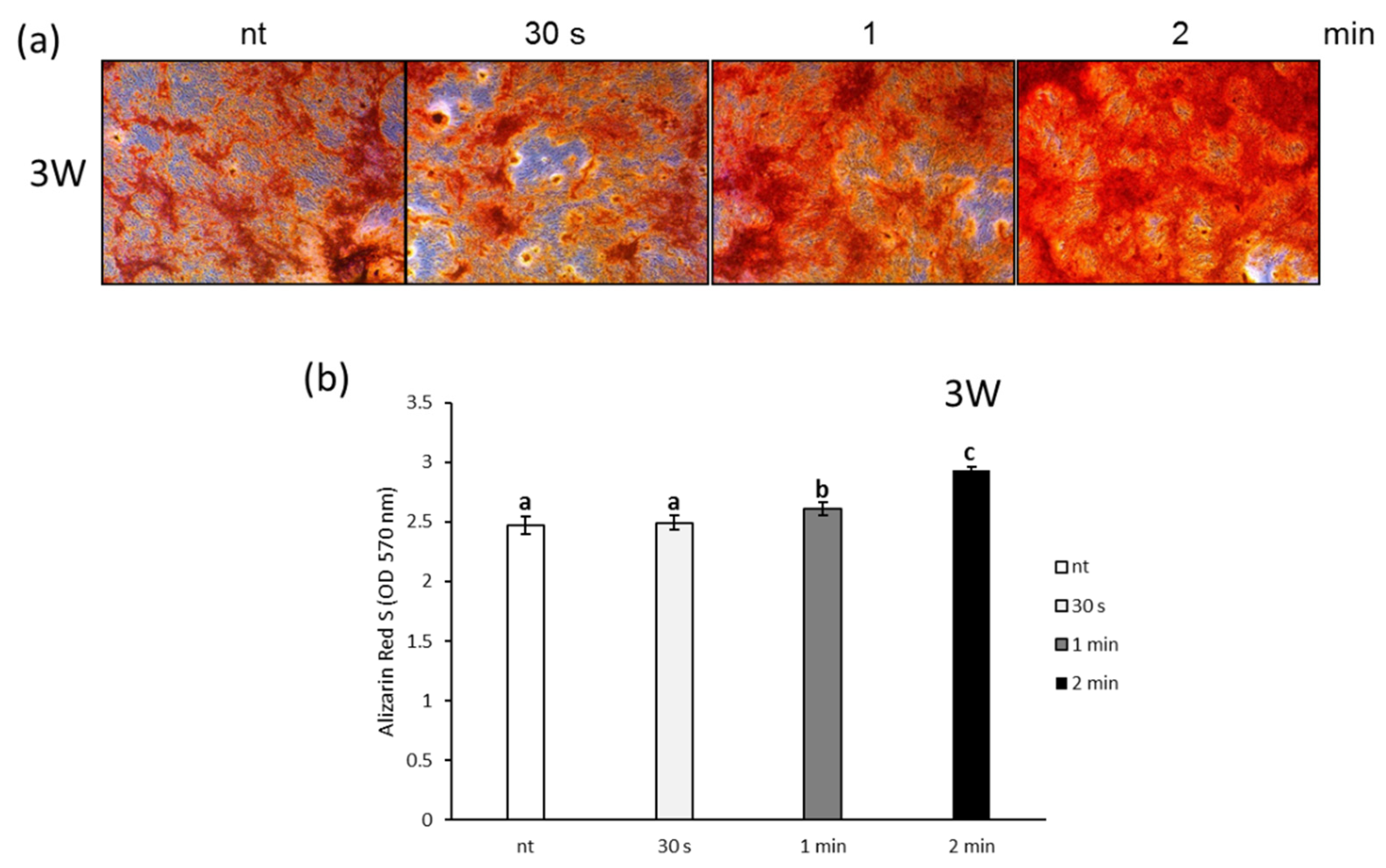
3.3. Effects of NCP on Osteogenic Differentiation of PDL Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graves, D.T.; Li, J.; Cochran, D.L. Inflammation and Uncoupling as Mechanisms of Periodontal Bone Loss. J. Dent. Res. 2011, 90, 143–153. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagalde, P.; Reininger, D. Oral tissues regeneration using intraoral mesenchymal stem cells. J. Clin. Exp. Dent. 2021, 13, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Paduano, F. The Regenerative Medicine in Oral and Maxillofacial Surgery: The Most Important Innovations in the Clinical Application of Mesenchymal Stem Cells. Int. J. Med. Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Nagatomo, K.; Komaki, M.; Sekiya, I.; Sakaguchi, Y.; Noguchi, K.; Oda, S.; Muneta, T.; Ishikawa, I. Stem cell properties of human periodontal ligament cells. J. Periodontal Res. 2006, 41, 303–310. [Google Scholar] [CrossRef]
- Roguljic, H.; Matthews, B.G.; Yang, W.; Cvija, H.; Mina, M.; Kalajzic, I. In vivo Identification of Periodontal Progenitor Cells. J. Dent. Res. 2013, 92, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Gronthos, S.; Mrozik, K.; Shi, S.; Bartold, P.M. Ovine periodontal ligament stem cells: Isolation, characterization, and differentiation potential. Calcif. Tissue Int. 2006, 79, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, A.A.; Al Mamun, A. Current advances in regulation of bone homeostasis. FASEB Bioadv. 2020, 2, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of gene expression in osteoblasts. Biofactors 2010, 36, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, T.; Ueda, T.; Tomita, K.; Matsumoto, S.; Takedachi, M.; Murakami, S.; Okada, T.; Ogura, T.; Wakisaka, S.; Noda, T.; et al. Osteoblastic lysosome plays a central role in mineralization. Sci. Adv. 2019, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Skillington, J.; Choy, L.; Derynck, R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J. Cell Biol. 2002, 159, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Kalghatgi, S.; Friedman, G.; Kelly, C.M.; Torabi, B.; Alekseev, O.; Azizkhan-Clifford, J.; Cerchar, E.; Fridman, A. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE. 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.H.; Lee, H.W.; Cho, S.H.; Lee, J.K.; Jeon, Y.C.; Kim, G.C. High-efficiency tooth bleaching using non-thermal atmospheric pressure plasma with low concentration of hydrogen peroxide. J. Appl. Oral Sci. 2013, 21, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Lee, H.Y.; Lee, H.J.; Kim, J.B.; Kim, S.; Joo, J.Y.; Kim, G.C. Retention Improvement in Fluoride Application with Cold Atmospheric Plasma. J. Dent. Res. 2018, 97, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Song, Y.S.; Song, K.; Lee, H.J.; Hong, J.W.; Kim, G.C. Skin renewal activity of non-thermal plasma through the activation of beta-catenin in keratinocytes. Sci. Rep. 2017, 7, 6146. [Google Scholar] [CrossRef] [Green Version]
- Kubinova, S.; Zaviskova, K.; Uherkova, L.; Zablotskii, V.; Churpita, O.; Lunov, O.; Dejneka, A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci. Rep. 2017, 7, 45183. [Google Scholar] [CrossRef] [Green Version]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jäger, A.; Polívka, L.; Syková, E.; Dejneka, A.; Kubinová, Š. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective Killing of Melanoma Cells With Non-Thermal Atmospheric Pressure Plasma and p-FAK Antibody Conjugated Gold Nanoparticles. Int. J. Med. Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.S.; Kim, Y.H.; Choi, E.H.; Kim, K.N. The effects of non-thermal atmospheric pressure plasma jet on attachment of osteoblast. Curr. Appl. Phys. 2013, 13, S42–S47. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G.; Pawlat, J.; Terebun, P.; Duday, D.; Thomann, J.-S.; Canal, C.; Labay, C.; Hermans, S.; Audemar, M. The effect of low temperature atmospheric nitrogen plasma on MC3T3-E1 preosteoblast proliferation and differentiation in vitro. J. Phys. D Appl. Phys. 2019, 52, 275401. [Google Scholar] [CrossRef]
- Lee, S.T.; Jang, Y.S.; Kim, U.K.; Kim, H.J.; Ryu, M.H.; Kim, G.C.; Hwang, D.S. Non-thermal plasma application enhances the recovery of transected sciatic nerves in rats. Exp. Biol. Med. 2021, 246, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Filippidou, E.C.; Koukouliata, A. Ozone effects on the respiratory system. Prog. Health Sci. 2011, 1, 144–155. [Google Scholar]
- Ozone Generators that are Sold as Air Cleaners: An Assessment of Effectiveness and Health Consequences. Available online: https://www.epa.gov/sites/default/files/2014-08/documents/ozone_generator.pdf (accessed on 3 August 2021).
- Quan, H.; Dai, X.; Liu, M.; Wu, C.; Wang, D. Luteolin supports osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 2019, 19, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.H.; Wang, X.X.; Ma, D.; Zhang, L.N.; Qiao, Q.F.; Zhang, J. Rythropoietin enhances osteogenic differentiation of human periodontal ligament stem cells via Wnt/β-catenin signalling pathway. Drug Des. Devel. Ther. 2019, 13, 2543–2552. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.S.; Lee, S.Y.; Lee, J.C. Wnt/β-catenin signalling enhances osteogenic differentiation from human periodontal ligament fibroblasts. Mol. Cells 2010, 30, 449–454. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Sheikh, S.; Stains, J.P.; Salazar, V.S.; Cheng, S.-L.; Chen, D.; Civitelli, R. β-Catenin and BMP-2 Synergize to Promote Osteoblast Differentiation and New Bone Formation. J. Cell Biochem. 2005, 94, 403–418. [Google Scholar] [CrossRef] [Green Version]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.N.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signalling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wang, D.; Qin, Y.; Xu, W.; Liu, X.; Ye, L.; Zheng, Q.; Li, D.; Yue, S.; Xu, M.; et al. Astragalin Promotes Osteoblastic Differentiation in MC3T3-E1 Cells and Bone Formation in vivo. Front. Endocrinol. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Uemura, T.; Nemoto, A.; Yabe, T.; Fujii, N.; Ushida, T.; Tateishi, T. Osteopontin involvement in integrin-mediated cell signalling and regulation of expression of alkaline phosphatase during early differentiation of UMR cells. FEBS Lett. 1997, 420, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Papagerakis, P.; Berdal, A.; Mesbah, M.; Peuchmaur, M.; Malaval, L.; Nydegger, J.; Simmer, J.; Macdougall, M. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 2002, 30, 377–385. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, B.; Wang, Y.; Xian, C.; Yang, L.; Deng, M.; Peng, Q.; Li, Y. Mechano-growth factor E peptide inhibits the differentiation and mineralization of osteoblasts. Arch. Oral Biol. 2012, 57, 720–727. [Google Scholar]
- Steinbeck, M.J.; Chernets, N.; Zhang, J.; Kurpad, D.S.; Fridman, G.; Fridman, A.; Freeman, T.A. Skeletal Cell Differentiation Is Enhanced by Atmospheric Dielectric Barrier Discharge Plasma Treatment. PLoS ONE 2013, 8, e82143. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.-B.; Choi, J.-H.; Kang, T.-H.; Lee, S.-J.; Kim, G.-C. Enhancement of Osteoblast Differentiation Using No-Ozone Cold Plasma on Human Periodontal Ligament Cells. Biomedicines 2021, 9, 1542. https://doi.org/10.3390/biomedicines9111542
Choi B-B, Choi J-H, Kang T-H, Lee S-J, Kim G-C. Enhancement of Osteoblast Differentiation Using No-Ozone Cold Plasma on Human Periodontal Ligament Cells. Biomedicines. 2021; 9(11):1542. https://doi.org/10.3390/biomedicines9111542
Chicago/Turabian StyleChoi, Byul-Bora, Jeong-Hae Choi, Tae-Hyung Kang, Seok-Jun Lee, and Gyoo-Cheon Kim. 2021. "Enhancement of Osteoblast Differentiation Using No-Ozone Cold Plasma on Human Periodontal Ligament Cells" Biomedicines 9, no. 11: 1542. https://doi.org/10.3390/biomedicines9111542
APA StyleChoi, B.-B., Choi, J.-H., Kang, T.-H., Lee, S.-J., & Kim, G.-C. (2021). Enhancement of Osteoblast Differentiation Using No-Ozone Cold Plasma on Human Periodontal Ligament Cells. Biomedicines, 9(11), 1542. https://doi.org/10.3390/biomedicines9111542