A Live Probiotic Vaccine Prototype Based on Conserved Influenza a Virus Antigens Protect Mice against Lethal Influenza Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, Proteins and Peptides
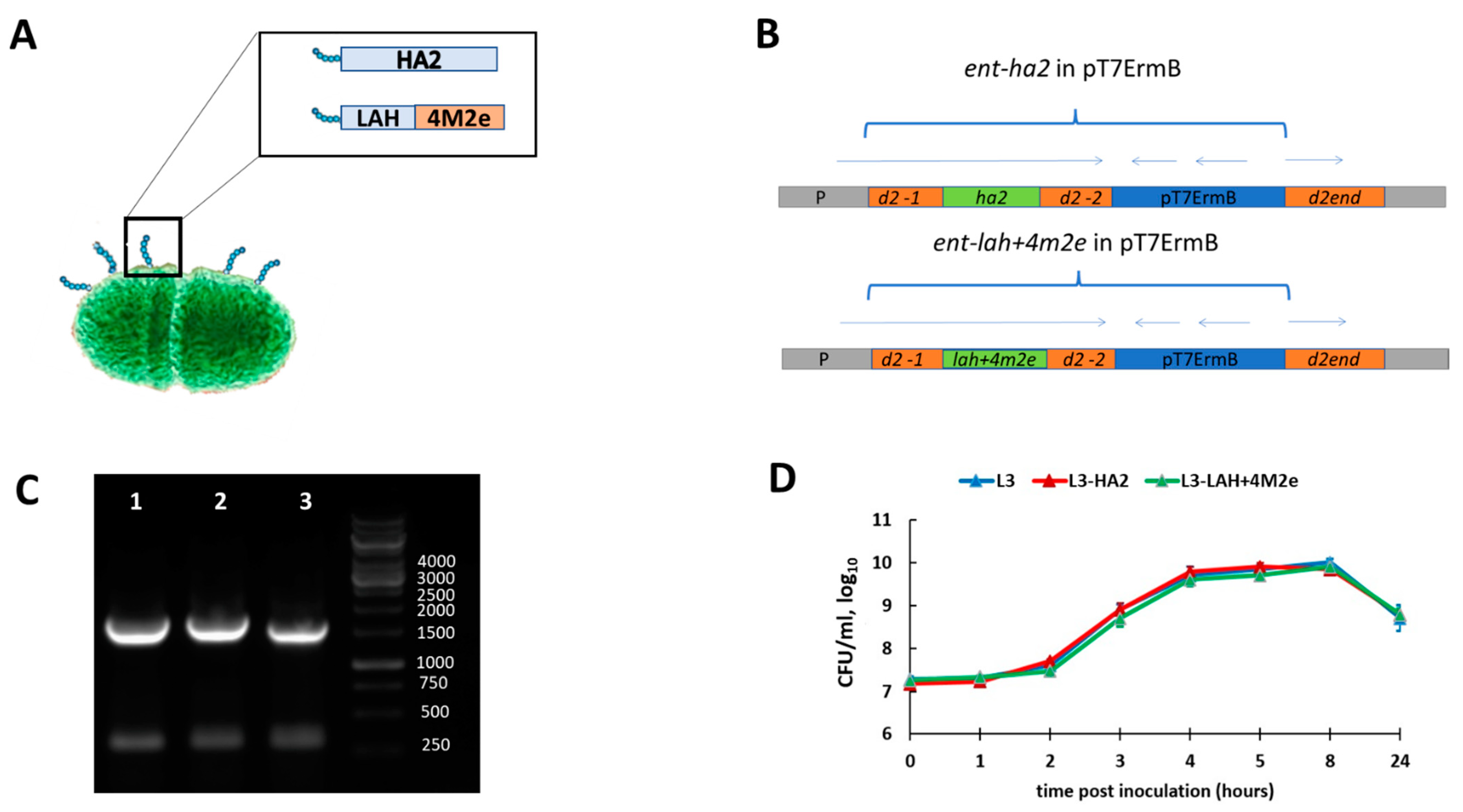
2.2. Generation of Enterococcus faecium Encoding Influenza Virus Fragments
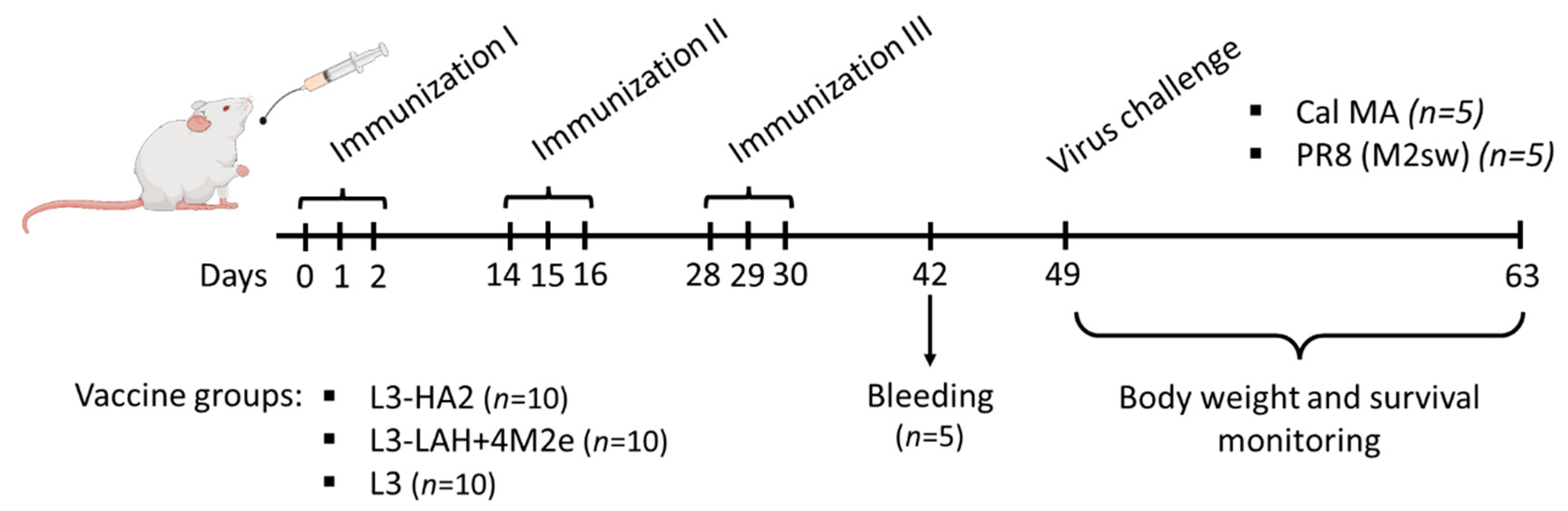
2.3. Immunization of Mice with Live Probiotic Vaccine and Assessment of Its Immunogenicity
2.4. Assessment of Protection against Influenza Virus Challenge
2.5. Statistical Analyses
3. Results
3.1. Generation and Characterization of Enterococcal Vaccines
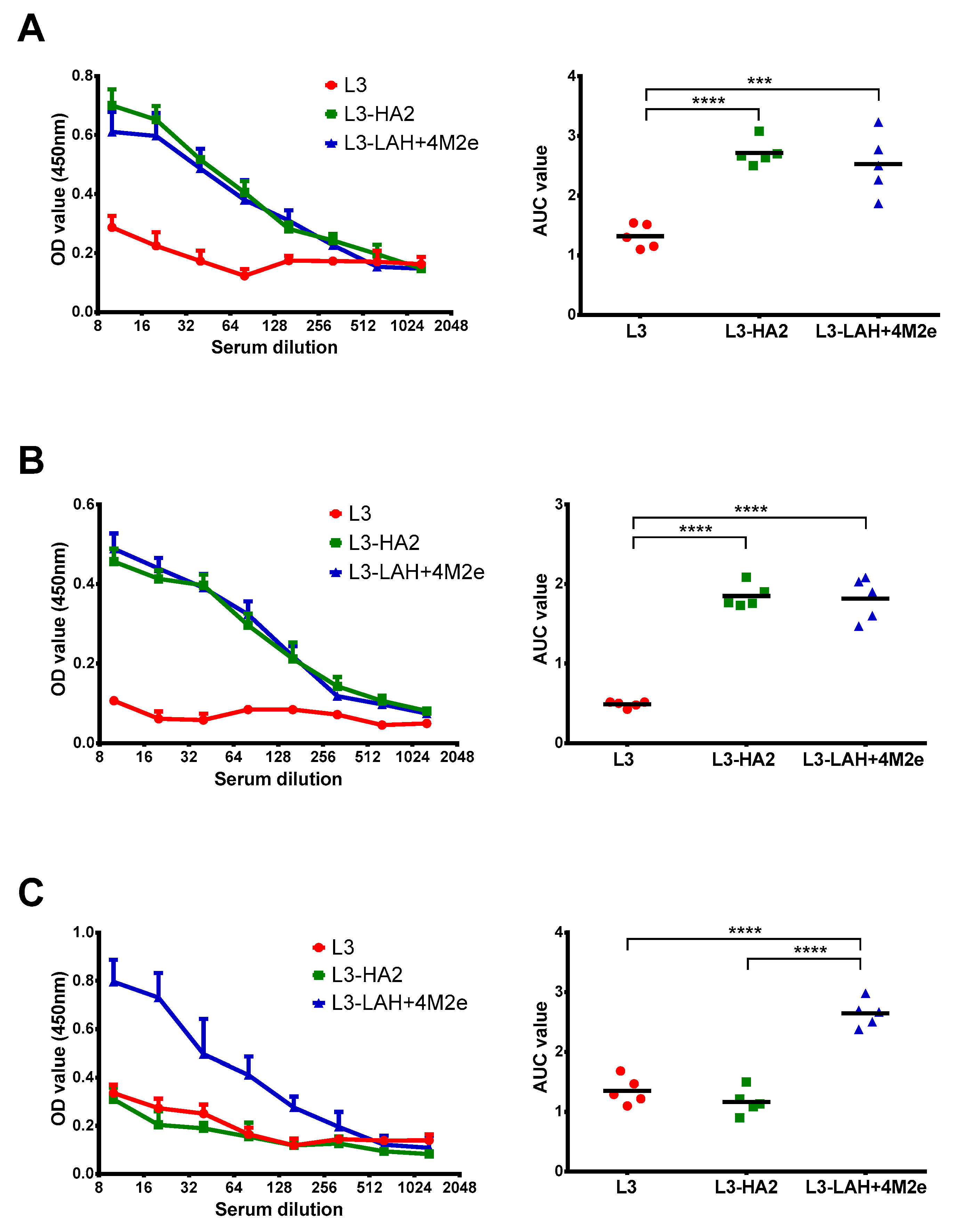
3.2. Immunogenicity of the Live Probiotic Influenza Vaccine Candidates in Mice
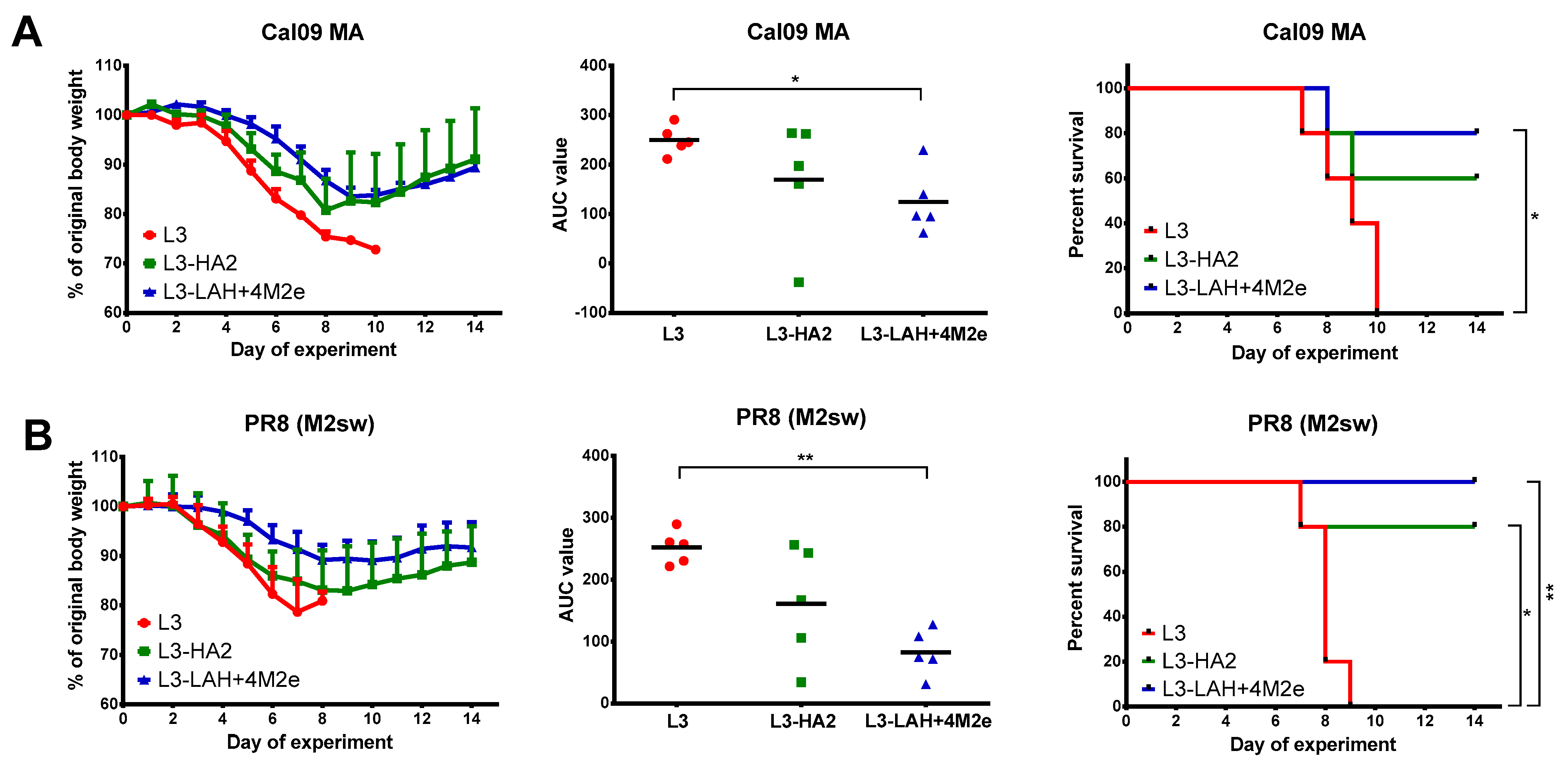
3.3. Protection against Lethal Influenza Virus Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belongia, E.A.; Kieke, B.A.; Donahue, J.G.; Greenlee, R.T.; Balish, A.; Foust, A.; Lindstrom, S.; Shay, D.K. Marshfield Influenza Study, G. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J. Infect. Dis. 2009, 199, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354. [Google Scholar] [CrossRef]
- Belongia, E.A.; McLean, H.Q. Influenza Vaccine Effectiveness: Defining the H3N2 Problem. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, 1817–1823. [Google Scholar] [CrossRef]
- Petrie, J.G.; Malosh, R.E.; Cheng, C.K.; Ohmit, S.E.; Martin, E.T.; Johnson, E.; Truscon, R.; Eichelberger, M.C.; Gubareva, L.V.; Fry, A.M.; et al. The Household Influenza Vaccine Effectiveness Study: Lack of Antibody Response and Protection following Receipt of 2014–2015 Influenza Vaccine. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 1644–1651. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Kawaoka, Y. Current and future influenza vaccines. Nat. Med. 2019, 25, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Isakova-Sivak, I.; Stepanova, E.; Mezhenskaya, D.; Matyushenko, V.; Prokopenko, P.; Sychev, I.; Wong, P.F.; Rudenko, L. Influenza vaccine: Progress in a vaccine that elicits a broad immune response. Expert Rev. Vaccines 2021, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol. J. 2015, 10, 690–701. [Google Scholar] [CrossRef]
- Krammer, F.; Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015, 14, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Hashem, A.M.; Chen, Z.; Li, C.; Doyle, T.; Zhang, Y.; Yi, Y.; Farnsworth, A.; Xu, K.; Li, Z.; et al. Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol. 2015, 8, 211–220. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, S.; Qu, D.; Chen, J.; Wang, F.; Zhang, R.; Chen, Z. Influenza H7N9 LAH-HBc virus-like particle vaccine with adjuvant protects mice against homologous and heterologous influenza viruses. Vaccine 2016, 34, 6464–6471. [Google Scholar] [CrossRef] [PubMed]
- Mezhenskaya, D.; Isakova-Sivak, I.; Rudenko, L. M2e-based universal influenza vaccines: A historical overview and new approaches to development. J. Biomed. Sci. 2019, 26, 76. [Google Scholar] [CrossRef]
- Mezhenskaya, D.; Isakova-Sivak, I.; Matyushenko, V.; Donina, S.; Rekstin, A.; Sivak, K.; Yakovlev, K.; Katelnikova, A.; Kryshen, K.; Makarov, V.; et al. Universal Live-Attenuated Influenza Vaccine Candidates Expressing Multiple M2e Epitopes Protect Ferrets against a High-Dose Heterologous Virus Challenge. Viruses 2021, 13, 1280. [Google Scholar] [CrossRef] [PubMed]
- Lu, I.N.; Kirsteina, A.; Farinelle, S.; Willieme, S.; Tars, K.; Muller, C.P.; Kazaks, A. Structure and applications of novel influenza HA tri-stalk protein for evaluation of HA stem-specific immunity. PLoS ONE 2018, 13, e0204776. [Google Scholar] [CrossRef]
- Mezhenskaya, D.; Isakova-Sivak, I.; Kotomina, T.; Matyushenko, V.; Kim, M.C.; Bhatnagar, N.; Kim, K.H.; Kang, S.M.; Rudenko, L. A Strategy to Elicit M2e-Specific Antibodies Using a Recombinant H7N9 Live Attenuated Influenza Vaccine Expressing Multiple M2e Tandem Repeats. Biomedicines 2021, 9, 133. [Google Scholar] [CrossRef]
- Gupalova, T.; Leontieva, G.; Kramskaya, T.; Grabovskaya, K.; Kuleshevich, E.; Suvorov, A. Development of experimental pneumococcal vaccine for mucosal immunization. PLoS ONE 2019, 14, e0218679. [Google Scholar] [CrossRef]
- Ermolenko, E.I.; Desheva, Y.A.; Kolobov, A.A.; Kotyleva, M.P.; Sychev, I.A.; Suvorov, A.N. Anti-Influenza Activity of Enterocin B In vitro and Protective Effect of Bacteriocinogenic Enterococcal Probiotic Strain on Influenza Infection in Mouse Model. Probiotics Antimicrob. Proteins 2019, 11, 705–712. [Google Scholar] [CrossRef]
- Wang, T.T.; Tan, G.S.; Hai, R.; Pica, N.; Ngai, L.; Ekiert, D.C.; Wilson, I.A.; Garcia-Sastre, A.; Moran, T.M.; Palese, P. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl. Acad. Sci. USA 2010, 107, 18979–18984. [Google Scholar] [CrossRef] [PubMed]
- Schotsaert, M.; Ysenbaert, T.; Smet, A.; Schepens, B.; Vanderschaeghe, D.; Stegalkina, S.; Vogel, T.U.; Callewaert, N.; Fiers, W.; Saelens, X. Long-Lasting Cross-Protection against Influenza A by Neuraminidase and M2e-based immunization strategies. Sci. Rep. 2016, 6, 24402. [Google Scholar] [CrossRef]
- Fan, J.; Liang, X.; Horton, M.S.; Perry, H.C.; Citron, M.P.; Heidecker, G.J.; Fu, T.M.; Joyce, J.; Przysiecki, C.T.; Keller, P.M.; et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine 2004, 22, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.M.; Freed, D.C.; Horton, M.S.; Fan, J.; Citron, M.P.; Joyce, J.G.; Garsky, V.M.; Casimiro, D.R.; Zhao, Q.; Shiver, J.W.; et al. Characterizations of four monoclonal antibodies against M2 protein ectodomain of influenza A virus. Virology 2009, 385, 218–226. [Google Scholar] [CrossRef]
- Byappanahalli, M.N.; Nevers, M.B.; Korajkic, A.; Staley, Z.R.; Harwood, V.J. Enterococci in the environment. Microbiol. Mol. Biol. Rev. MMBR 2012, 76, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Taghinezhad, S.S.; Mohseni, A.H.; Bermudez-Humaran, L.G.; Casolaro, V.; Cortes-Perez, N.G.; Keyvani, H.; Simal-Gandara, J. Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease. Vaccines 2021, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Casolaro, V.; Bermudez-Humaran, L.G.; Keyvani, H.; Taghinezhad, S.S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Aubry, C.; Cortes-Perez, N.G.; de Moreno de LeBlanc, A.; Vergnolle, N.; Langella, P.; Azevedo, V.; Chatel, J.M.; Miyoshi, A.; Bermudez-Humaran, L.G. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: An update. FEMS Microbiol. Lett. 2013, 344, 1–9. [Google Scholar] [CrossRef]
- Dieye, Y.; Usai, S.; Clier, F.; Gruss, A.; Piard, J.C. Design of a protein-targeting system for lactic acid bacteria. J. Bacteriol. 2001, 183, 4157–4166. [Google Scholar] [CrossRef]
- Michon, C.; Langella, P.; Eijsink, V.G.; Mathiesen, G.; Chatel, J.M. Display of recombinant proteins at the surface of lactic acid bacteria: Strategies and applications. Microb. Cell Factories 2016, 15, 70. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Taghinezhad, S.S.; Keyvani, H. The First Clinical Use of a Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E7 Oncogene Oral Vaccine: A Phase I Safety and Immunogenicity Trial in Healthy Women Volunteers. Mol. Cancer Ther. 2020, 19, 717–727. [Google Scholar] [CrossRef]
- Taghinezhad, S.S.; Mohseni, A.H.; Keyvani, H.; Razavi, M.R. Phase 1 Safety and Immunogenicity Trial of Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E6 Oncoprotein Vaccine. Mol. Ther. Methods Clin. Dev. 2019, 15, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Bahey-El-Din, M.; Gahan, C.G. Lactococcus lactis-based vaccines: Current status and future perspectives. Hum. Vaccines 2011, 7, 106–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezhenskaya, D.; Isakova-Sivak, I.; Gupalova, T.; Bormotova, E.; Kuleshevich, E.; Kramskaya, T.; Leontieva, G.; Rudenko, L.; Suvorov, A. A Live Probiotic Vaccine Prototype Based on Conserved Influenza a Virus Antigens Protect Mice against Lethal Influenza Virus Infection. Biomedicines 2021, 9, 1515. https://doi.org/10.3390/biomedicines9111515
Mezhenskaya D, Isakova-Sivak I, Gupalova T, Bormotova E, Kuleshevich E, Kramskaya T, Leontieva G, Rudenko L, Suvorov A. A Live Probiotic Vaccine Prototype Based on Conserved Influenza a Virus Antigens Protect Mice against Lethal Influenza Virus Infection. Biomedicines. 2021; 9(11):1515. https://doi.org/10.3390/biomedicines9111515
Chicago/Turabian StyleMezhenskaya, Daria, Irina Isakova-Sivak, Tatiana Gupalova, Elena Bormotova, Eugenia Kuleshevich, Tatiana Kramskaya, Galina Leontieva, Larisa Rudenko, and Alexander Suvorov. 2021. "A Live Probiotic Vaccine Prototype Based on Conserved Influenza a Virus Antigens Protect Mice against Lethal Influenza Virus Infection" Biomedicines 9, no. 11: 1515. https://doi.org/10.3390/biomedicines9111515
APA StyleMezhenskaya, D., Isakova-Sivak, I., Gupalova, T., Bormotova, E., Kuleshevich, E., Kramskaya, T., Leontieva, G., Rudenko, L., & Suvorov, A. (2021). A Live Probiotic Vaccine Prototype Based on Conserved Influenza a Virus Antigens Protect Mice against Lethal Influenza Virus Infection. Biomedicines, 9(11), 1515. https://doi.org/10.3390/biomedicines9111515





