Comparison of the Confluence-Initiated Neurogenic Differentiation Tendency of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Expansion of BMSCs and ADSCs
2.2. Confluence-Initiated Differentiation
2.3. Factor-Induced Neurogenic Differentiation
2.4. Osteogenic Induction Medium (OIM)-Induced Differentiation
2.5. Adipogenic Induction Medium (AIM)-Induced Differentiation
2.6. RT-qPCR Analysis
2.7. Immunofluorescence (IF) Staining and Intensity Quantification
2.8. Statistical Analysis
3. Results
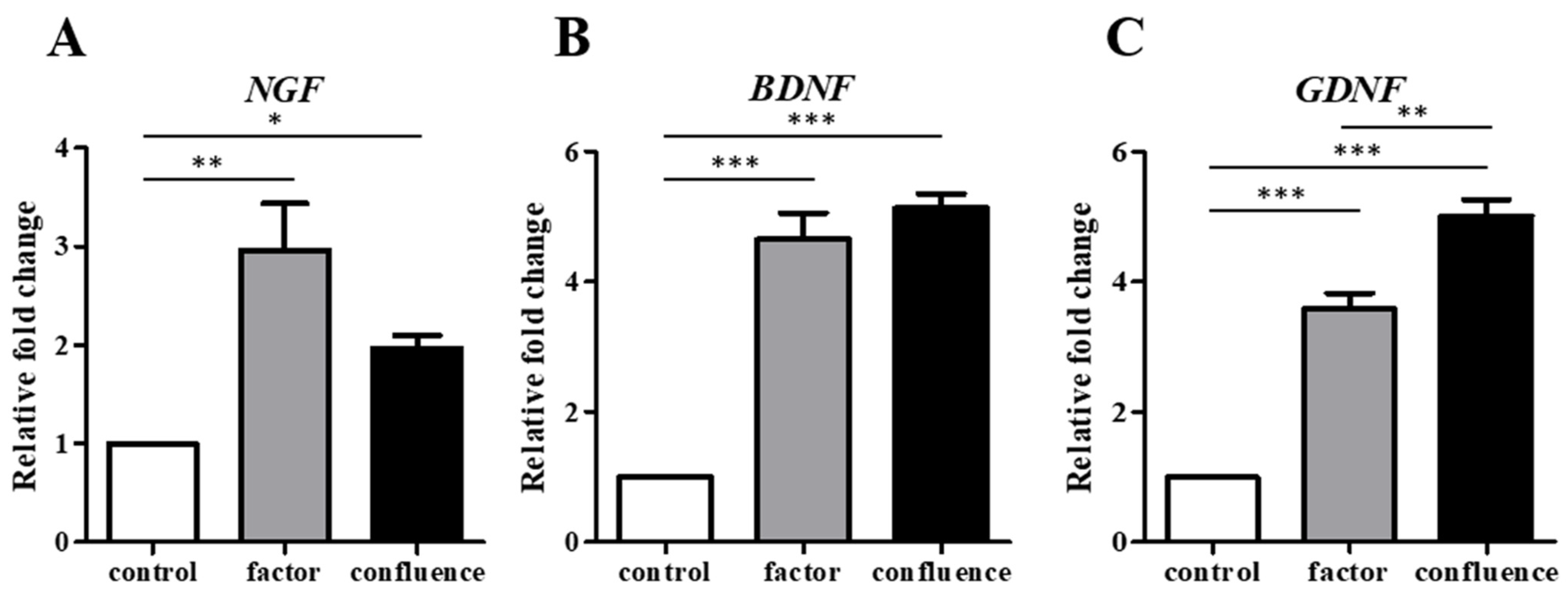
3.1. Confluence-Initiated and Factor-Induced Neurogenic Differentiation Effects on Human ADSCs In Vitro
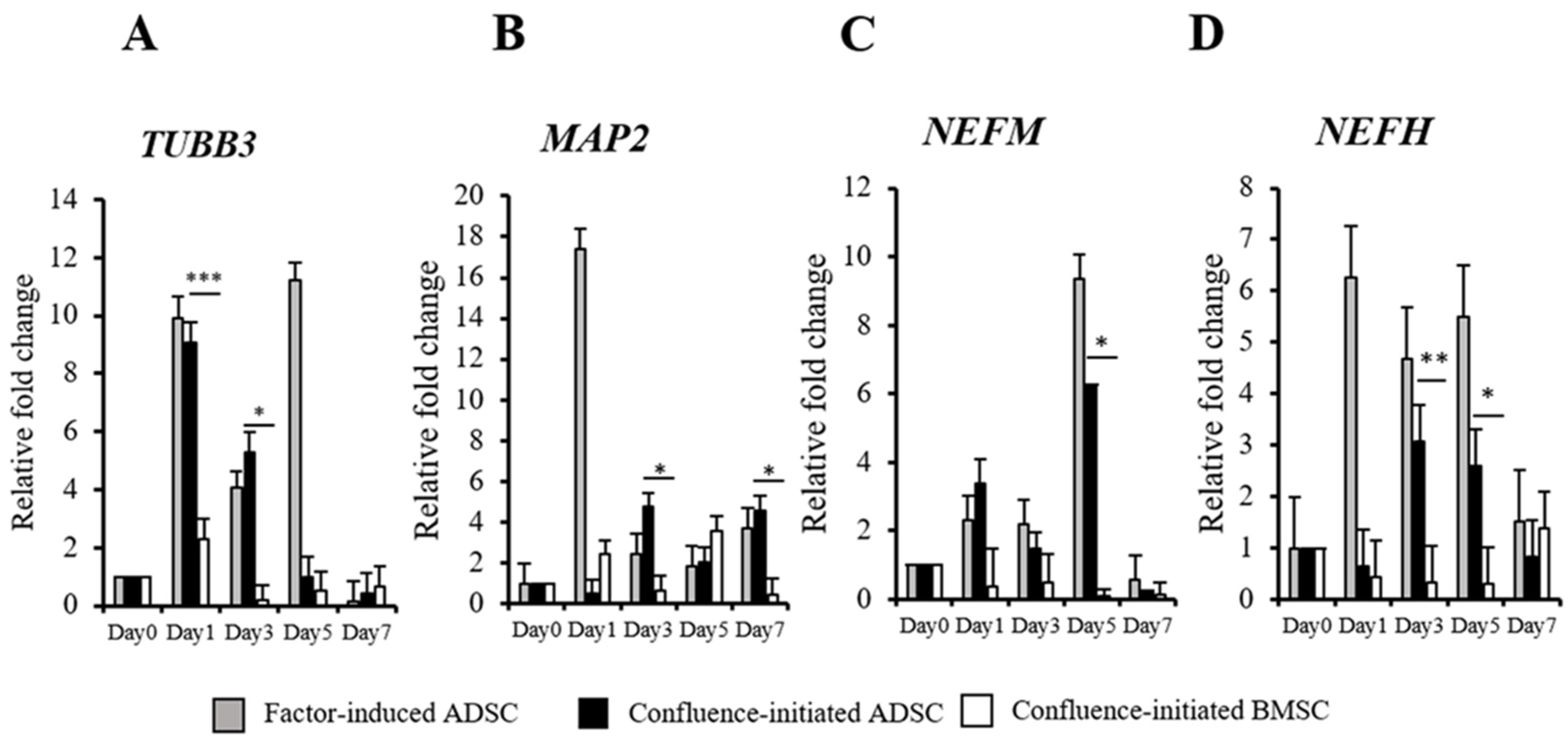
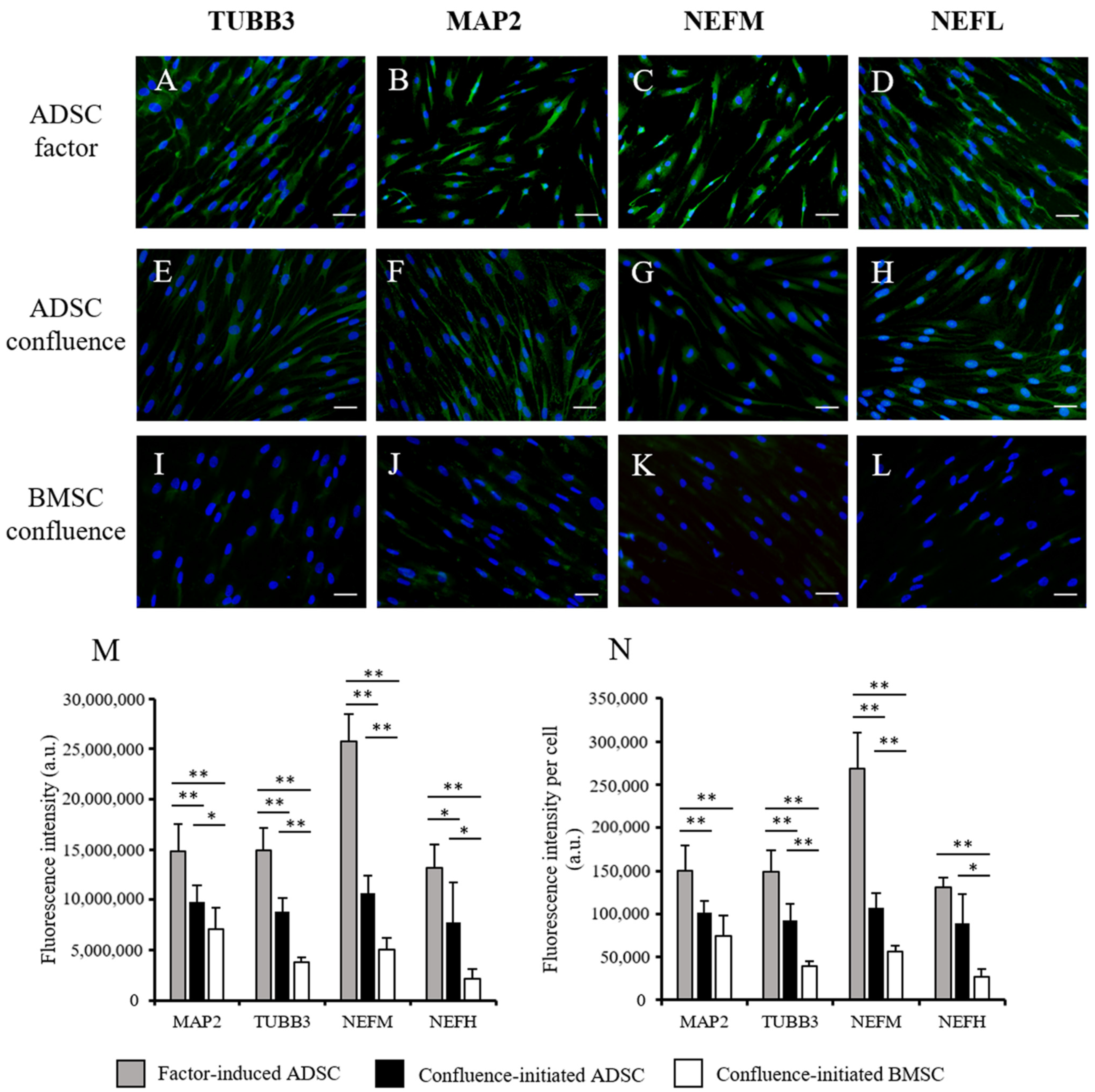
3.2. Comparison of Confluence-Initiated Neurogenic Differentiation on the ADSCs and BMSCs
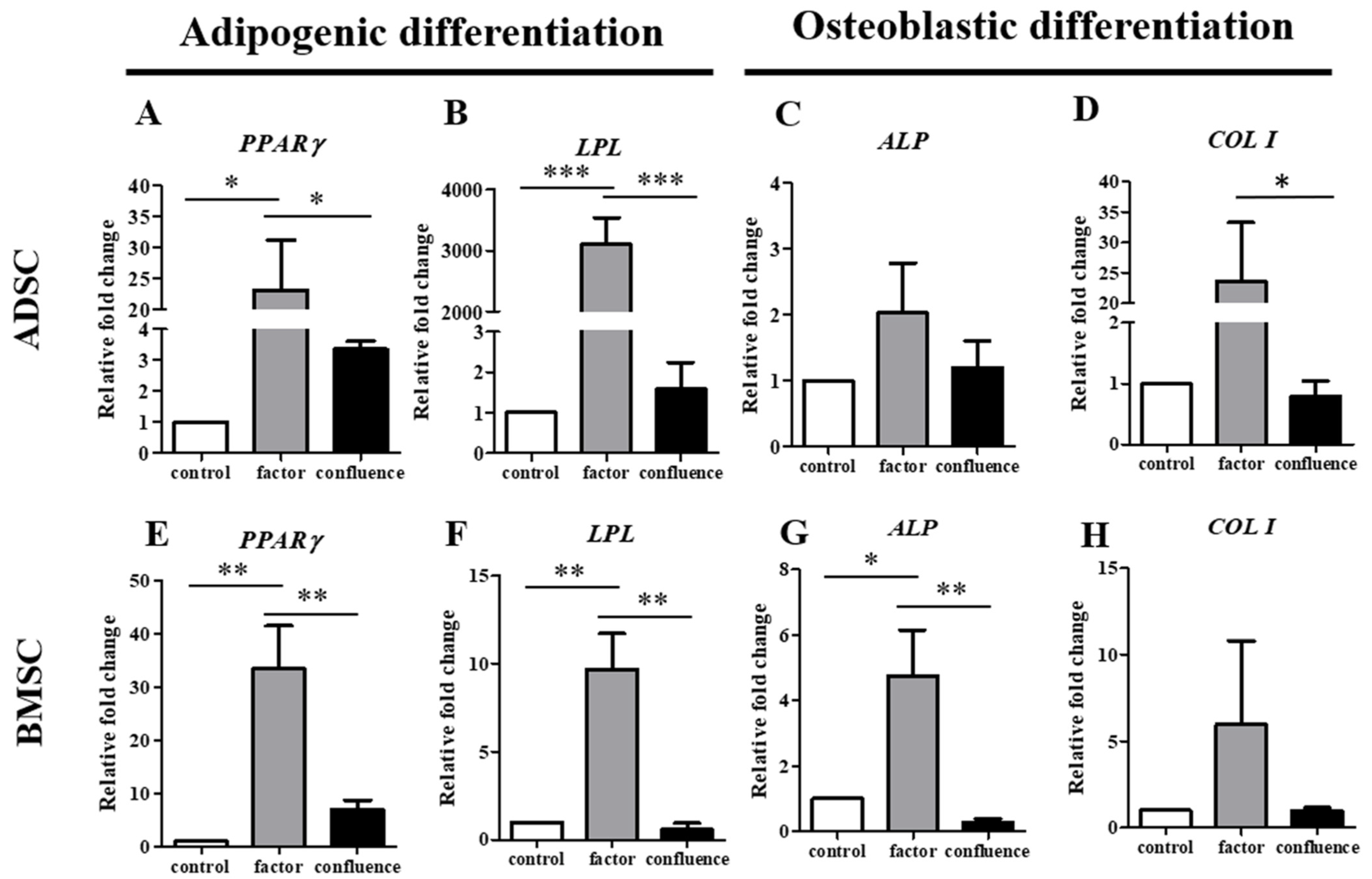
3.3. Comparison of the ADSCs and BMSCs in Confluence-Initiated Adipogenic and Osteogenic Differentiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Scioli, M.G.; Bielli, A.; Gentile, P.; Mazzaglia, D.; Cervelli, V.; Orlandi, A. The Biomolecular Basis of Adipogenic Differentiation of Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2014, 15, 6517–6526. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Prockop, D.J.; Spees, J.L. Non-hematopoietic bone marrow stem cells: Molecular control of expansion and differentiation. Exp. Cell Res. 2005, 306, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, H.; Liu, X.W.; Chen, J.T.; Xiang, L.B.; Zhou, D.P. Neurogenic differentiation from adipose-derived stem cells and application for autologous transplantation in spinal cord injury. Cell Tissue Bank. 2015, 16, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Kim, E.J.; Bharti, D.; Jang, S.J.; Park, J.S.; Shivakumar, S.B.; Lee, S.L.; Kang, D.; Byun, J.H.; et al. In vitro comparative analysis of human dental stem cells from a single donor and its neuronal differentiation potential evaluated by electrophysiology. Life Sci. 2016, 154, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Araki, H.; Kato, J.; Nakamura, K.; Kawano, Y.; Kobune, M.; Sato, T.; Miyanishi, K.; Takayama, T.; Takahashi, M.; et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 2005, 106, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. Rep. 2011, 7, 269–291. [Google Scholar] [CrossRef]
- Hildner, F.; Albrecht, C.; Gabriel, C.; Redl, H.; van Griensven, M. State of the art and future perspectives of articular cartilage regeneration: A focus on adipose-derived stem cells and platelet-derived products. J. Tissue Eng. Regen. Med. 2011, 5, e36–e51. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult Bone Marrow Stromal Cells Differentiate into Neural Cells in Vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef]
- Scintu, F.; Reali, C.; Pillai, R.; Badiali, M.; Sanna, M.A.; Argiolu, F.; Ristaldi, M.S.; Sogos, V. Differentiation of human bone marrow stem cells into cells with a neural phenotype: Diverse effects of two specific treatments. BMC Neurosci. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Moochhala, S.; Moore, X.-L.; Ng, K.C.; Tan, M.H.; Lee, L.K.H.; He, B.; Wong, M.C.; Ling, E.-A. Adult bone marrow cells differentiate into neural phenotypes and improve functional recovery in rats following traumatic brain injury. Neurosci. Lett. 2006, 398, 12–17. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Liqing, Y.; Jia, G.; Jiqing, C.; Ran, G.; Fei, C.; Jie, K.; Yanyun, W.; Cheng, Z. Directed differentiation of motor neuron cell-like cells from human adipose-derived stem cells in vitro. NeuroReport 2011, 22, 370–373. [Google Scholar] [CrossRef]
- Heo, D.N.; Acquah, N.; Kim, J.; Lee, S.-J.; Castro, N.J.; Zhang, L.G. Directly Induced Neural Differentiation of Human Adipose-Derived Stem Cells Using Three-Dimensional Culture System of Conductive Microwell with Electrical Stimulation. Tissue. Eng. Part A 2017, 24, 537–545. [Google Scholar] [CrossRef]
- Frattini, F.; Lopes, F.R.; Almeida, F.M.; Rodrigues, R.F.; Boldrini, L.C.; Tomaz, M.A.; Baptista, A.F.; Melo, P.A.; Martinez, A.M. Mesenchymal stem cells in a polycaprolactone conduit promote sciatic nerve regeneration and sensory neuron survival after nerve injury. Tissue. Eng. Part A 2012, 18, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cui, H.F. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2012, 23, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Bittencourt-Navarrete, R.E.; de Almeida, F.M.; Tonda-Turo, C.; Martinez, A.M.; Franca, J.G. Enhancement of median nerve regeneration by mesenchymal stem cells engraftment in an absorbable conduit: Improvement of peripheral nerve morphology with enlargement of somatosensory cortical representation. Front. Neuroanat. 2014, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Dezawa, M.; Kanno, H.; Sawada, H.; Yamamoto, I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J. Neurosurg. 2004, 101, 806–812. [Google Scholar] [CrossRef]
- Liu, G.B.; Cheng, Y.X.; Feng, Y.K.; Pang, C.J.; Li, Q.; Wang, Y.; Jia, H.; Tong, X.J. Adipose-derived stem cells promote peripheral nerve repair. Arch. Med. Sci. 2011, 7, 592–596. [Google Scholar] [CrossRef]
- Di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef]
- Orbay, H.; Uysal, A.C.; Hyakusoku, H.; Mizuno, H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 657–664. [Google Scholar] [CrossRef]
- Sowa, Y.; Kishida, T.; Imura, T.; Numajiri, T.; Nishino, K.; Tabata, Y.; Mazda, O. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast. Reconstr. Surg. 2016, 137, 318e–330e. [Google Scholar] [CrossRef] [PubMed]
- Di Summa, P.G.; Kingham, P.J.; Campisi, C.C.; Raffoul, W.; Kalbermatten, D.F. Collagen (NeuraGen(R)) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci. Lett. 2014, 572, 26–31. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.; Alvarez-Gonzalez, C.; Vagaska, B.; Gomez, S.G.; Bulstrode, N.W.; Madrigal, A.; Ferretti, P. A matter of identity-Phenotype and differentiation potential of human somatic stem cells. Stem Cell Res. 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Faroni, A.; Smith, R.J.; Reid, A.J. Adipose derived stem cells and nerve regeneration. Neural Regen. Res. 2014, 9, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D.; Salibian, A.A.; Lalezari, S.; Evans, G.R. Neuromodulatory nerve regeneration: Adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J. Neurosci. Res. 2013, 91, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, C.; Liu, F.; Lin, H.; Yang, X.; Zhang, Z. Comparison of the neuronal differentiation abilities of bone marrowderived and adipose tissuederived mesenchymal stem cells. Mol. Med. Rep. 2017, 16, 3877–3886. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.T.; Liu, Z.L.; Yao, X.Q.; Yang, Z.J.; Xu, R.X. Neural differentiation ability of mesenchymal stromal cells from bone marrow and adipose tissue: A comparative study. Cytotherapy 2012, 14, 1203–1214. [Google Scholar] [CrossRef]
- Abo-Aziza, F.A.M.; Zaki, A.A. The Impact of Confluence on Bone Marrow Mesenchymal Stem (BMMSC) Proliferation and Osteogenic Differentiation. Int. J. Hematol. Oncol. Stem. Cell Res. 2017, 11, 121–132. [Google Scholar]
- Kim, D.S.; Lee, M.W.; Lee, T.H.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Cell culture density affects the stemness gene expression of adipose tissue-derived mesenchymal stem cells. Biomed. Rep. 2017, 6, 300–306. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, D.S.; Yoo, K.H.; Kim, H.R.; Jang, I.K.; Lee, J.H.; Kim, S.Y.; Son, M.H.; Lee, S.H.; Jung, H.L.; et al. Human bone marrow-derived mesenchymal stem cell gene expression patterns vary with culture conditions. Blood Res. 2013, 48, 107–114. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, M.W.; Yoo, K.H.; Lee, T.H.; Kim, H.J.; Jang, I.K.; Chun, Y.H.; Kim, H.J.; Park, S.J.; Lee, S.H.; et al. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS ONE 2014, 9, e83363. [Google Scholar] [CrossRef]
- Lin, Y.L.; Yet, S.F.; Hsu, Y.T.; Wang, G.J.; Hung, S.C. Mesenchymal Stem Cells Ameliorate Atherosclerotic Lesions via Restoring Endothelial Function. Stem Cells Transl. Med. 2015, 4, 44–55. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Wu, S.H.; Shirado, T.; Mashiko, T.; Feng, J.; Asahi, R.; Kanayama, K.; Mori, M.; Chi, D.; Sunaga, A.; Sarukawa, S.; et al. Therapeutic Effects of Human Adipose-Derived Products on Impaired Wound Healing in Irradiated Tissue. Plast. Reconstr. Surg. 2018, 142, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Cheng, H.; Pan, C.Y.; Tsai, M.J.; Kao, L.S.; Ma, H.L. In vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells 2002, 20, 522–529. [Google Scholar] [CrossRef]
- Guo, J.; Chan, K.M.; Zhang, J.F.; Li, G. Tendon-derived stem cells undergo spontaneous tenogenic differentiation. Exp. Cell Res. 2016, 341, 1–7. [Google Scholar] [CrossRef]
- Yang, D.; Li, N.; Zhang, G. Spontaneous adipogenic differentiation potential of adiposederived stem cells decreased with increasing cell passages. Mol. Med. Rep. 2018, 17, 6109–6115. [Google Scholar] [CrossRef]
- Roxburgh, J.; Metcalfe, A.D.; Martin, Y.H. The effect of medium selection on adipose-derived stem cell expansion and differentiation: Implications for application in regenerative medicine. Cytotechnology 2016, 68, 957–967. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Xie, Z.; Song, P.; Zhao, R.C.; Guo, L.; Liu, Z.; Wu, Y. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS ONE 2011, 6, e20526. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Zhang, C.; Deng, W.; Lv, Q.; Chen, X.; Huang, T.; Pan, L. Adipose-derived stem cells undergo spontaneous osteogenic differentiation in vitro when passaged serially or seeded at low density. Biotech. Histochem. 2016, 91, 369–376. [Google Scholar] [CrossRef]
- Huang, Y.C.; Zhu, H.M.; Cai, J.Q.; Huang, Y.Z.; Xu, J.; Zhou, Y.; Chen, X.H.; Li, X.Q.; Yang, Z.M.; Deng, L. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1407–1415. [Google Scholar] [CrossRef]
- Heng, B.C.; Saxena, P.; Fussenegger, M. Heterogeneity of baseline neural marker expression by undifferentiated mesenchymal stem cells may be correlated to donor age. J. Biotechnol. 2014, 174, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Mabuchi, Y.; Niibe, K.; Suzuki, S.; Nagoshi, N.; Sunabori, T.; Shimmura, S.; Nagai, Y.; Nakagawa, T.; Okano, H.; et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem. Biophys. Res. Commun. 2009, 379, 1114–1119. [Google Scholar] [CrossRef]
- Croft, A.P.; Przyborski, S.A. Formation of neurons by non-neural adult stem cells: Potential mechanism implicates an artifact of growth in culture. Stem Cells 2006, 24, 1841–1851. [Google Scholar] [CrossRef]
- Tseng, P.Y.; Chen, C.J.; Sheu, C.C.; Yu, C.W.; Huang, Y.S. Spontaneous differentiation of adult rat marrow stromal cells in a long-term culture. J. Vet. Med. Sci. 2007, 69, 95–102. [Google Scholar] [CrossRef][Green Version]
- Li, N.; Yang, H.; Lu, L.; Duan, C.; Zhao, C.; Zhao, H. Spontaneous expression of neural phenotype and NGF, TrkA, TrkB genes in marrow stromal cells. Biochem. Biophys. Res. Commun. 2007, 356, 561–568. [Google Scholar] [CrossRef]

| Gene | Primer Sequence | Accession Number |
|---|---|---|
| MAP2 | F: CAAACGTCATTACTTTACAACTTGA R: CAGCTGCCTCTGTGAGTGGAG | X54100.1 |
| TUBB3 | F: GCCAAGTTCTGGGAGGTCATC R: GTAGTAGACAACTGATGCGTTCCA | NM_139254 |
| NEFM | F: AGTGGTTCAAATGCCGCTAC R: TTTTCCAGCTGCTGGATGGT | NM_017029 |
| NEFH | F: AGTGGTTCCGAGTGAGATTG R: CTGCTGAATTGCATCCTGGT | NM_012607 |
| NGF | F: AGCGCAGCGAGTTTTGGC R: CCGCCTGTATGCCGATCAGA | NM_002506 |
| BDNF | F: AGAGGCTTGACATCATTGGCTG R: CAAAGGCACTTGACTACTGAGCATC | EF689042 |
| GDNF | F: CACCAGATAAACAAATGGCAGTGC R: CGACAGGTCATCATCAAAGGCG | NM_000514 |
| PPARγ | F: TCAGGTTTGGGCGGATGC R: TCAGCGGGAAGGACTTTATGTATG | NM_138712 |
| LPL | F: TGTAGATTCGCCCAGTTTCAGC R: AAGTCAGAGCCAAAAGAAGCAGC | NM_000237 |
| ALP | F: CGCTTGTGCCTGGACGGAC R: GGGGTCTTTCTCTTTCTCTGGCAC | MN308913 |
| COL I | F: GAGGGCCAAGACGAAGACGAAGACATC R: CAGATCACGTCATCGCACAAC | NM_000088 |
| GAPDH | F: CAACTCCCTCAAGATTGTCAGCAA R: GGCATGACTGTGGTCATGA | NM_017008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-H.; Liao, Y.-T.; Huang, C.-H.; Chen, Y.-C.; Chiang, E.-R.; Wang, J.-P. Comparison of the Confluence-Initiated Neurogenic Differentiation Tendency of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines 2021, 9, 1503. https://doi.org/10.3390/biomedicines9111503
Wu S-H, Liao Y-T, Huang C-H, Chen Y-C, Chiang E-R, Wang J-P. Comparison of the Confluence-Initiated Neurogenic Differentiation Tendency of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines. 2021; 9(11):1503. https://doi.org/10.3390/biomedicines9111503
Chicago/Turabian StyleWu, Szu-Hsien, Yu-Ting Liao, Chi-Han Huang, Yi-Chou Chen, En-Rung Chiang, and Jung-Pan Wang. 2021. "Comparison of the Confluence-Initiated Neurogenic Differentiation Tendency of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells" Biomedicines 9, no. 11: 1503. https://doi.org/10.3390/biomedicines9111503
APA StyleWu, S.-H., Liao, Y.-T., Huang, C.-H., Chen, Y.-C., Chiang, E.-R., & Wang, J.-P. (2021). Comparison of the Confluence-Initiated Neurogenic Differentiation Tendency of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines, 9(11), 1503. https://doi.org/10.3390/biomedicines9111503





