Footprints of microRNAs in Cancer Biology
Abstract
1. Introduction
2. Hallmark 1: Selective Proliferative Advantage
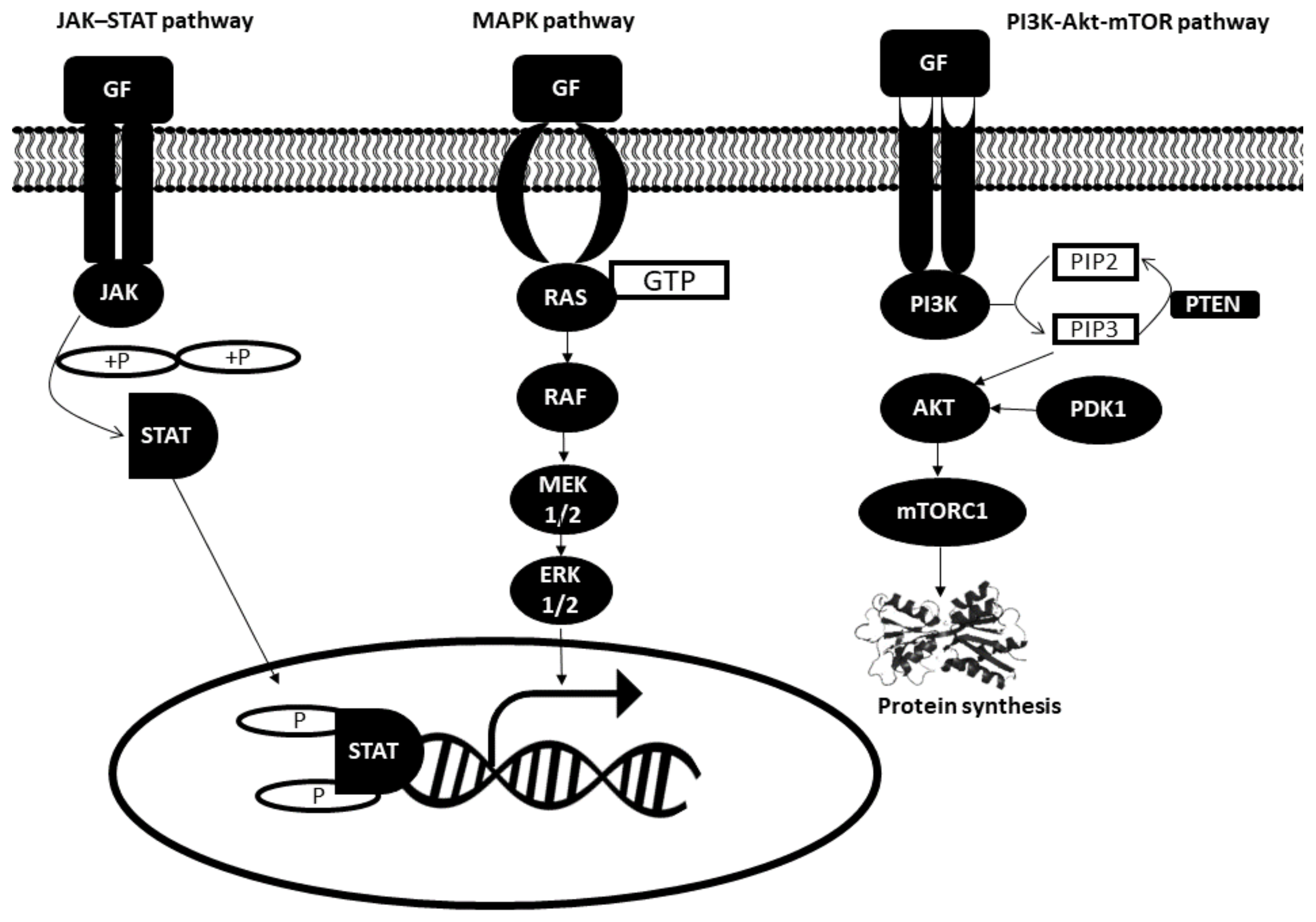
2.1. Intracellular Signal Pathways Dysregulation in Cancer Cells
2.1.1. JAK–STAT Pathway
2.1.2. MAPK Pathway
2.1.3. Phosphatidylinositol 3 Kinase (PI3K) Pathway
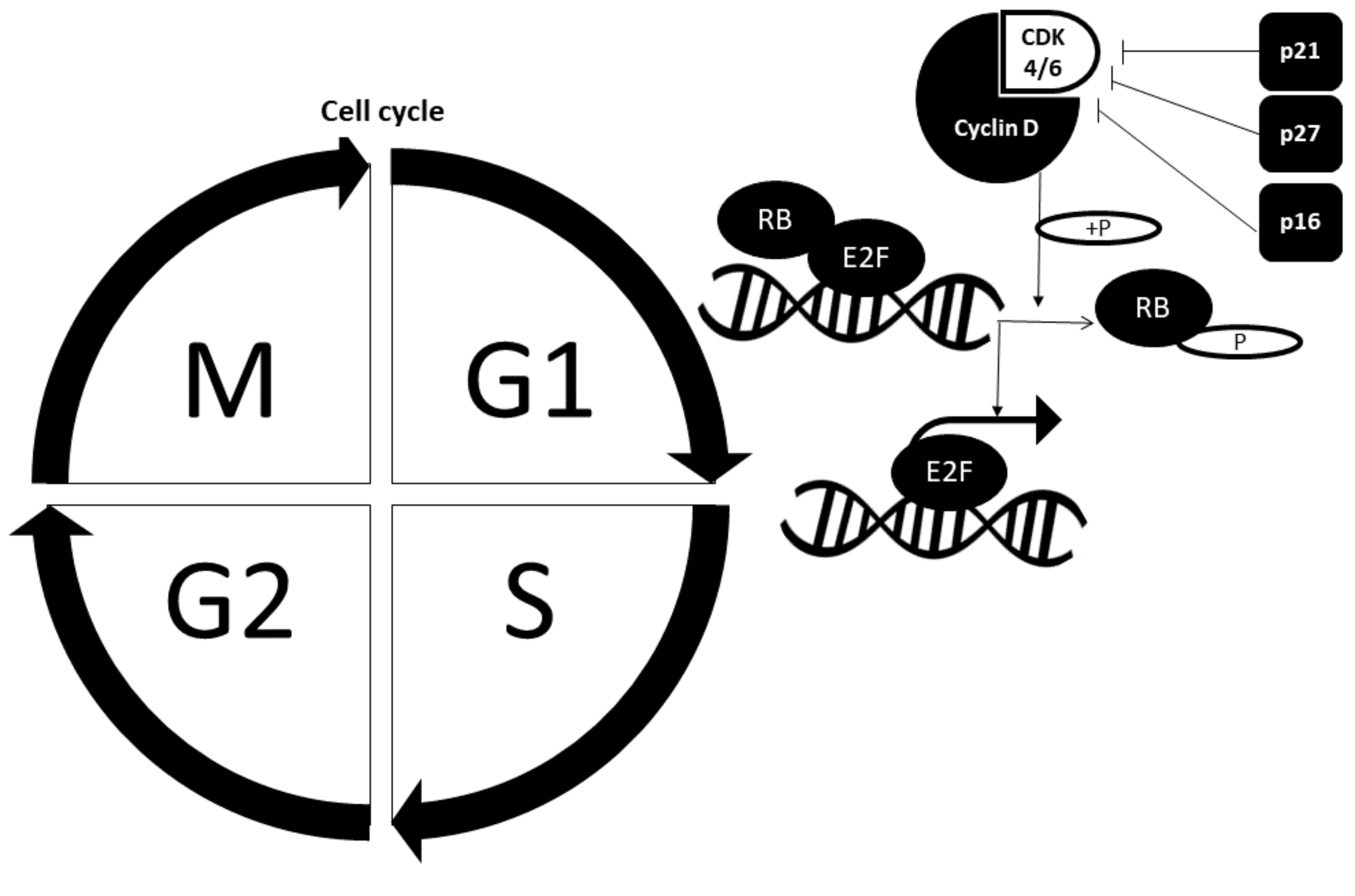
2.2. Cell Cycle Dysregulation in Cancer Cells
2.2.1. Retinoblastoma Pathway
2.2.2. Cell Cycle Checkpoint Proteins
2.3. The Role of miRNAs in Selective Proliferative Advantage
3. Hallmark 2: Altered Stress Response
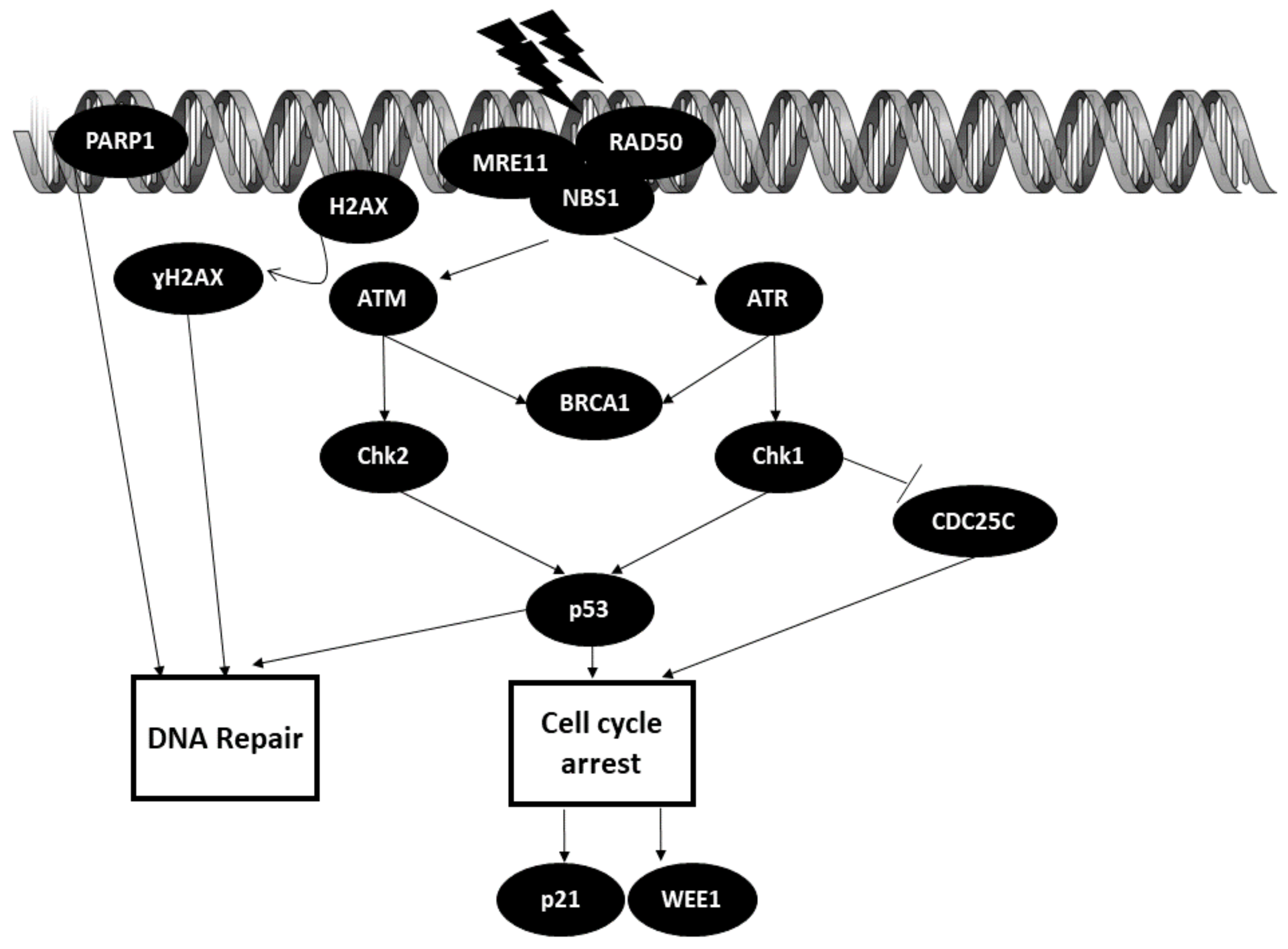
3.1. DNA Repair Pathways
The Role of miRNAs in DNA Repair Mechanisms
3.2. Autophagy
The Role of miRNAs in Altering Autophagy Mechanisms
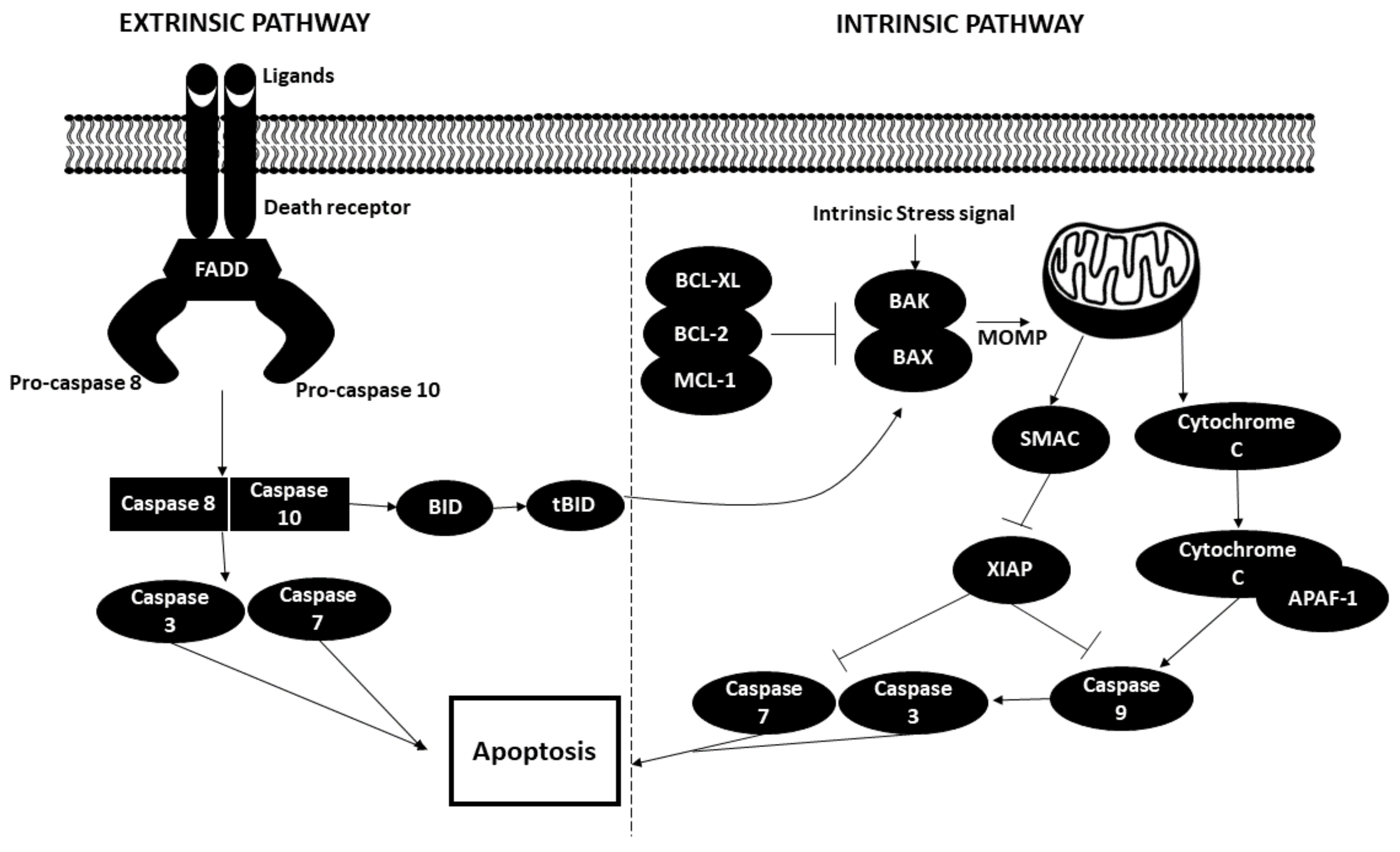
3.3. Apoptosis
The Role of miRNAs in Altering the Apoptotic State of Cancer Cells
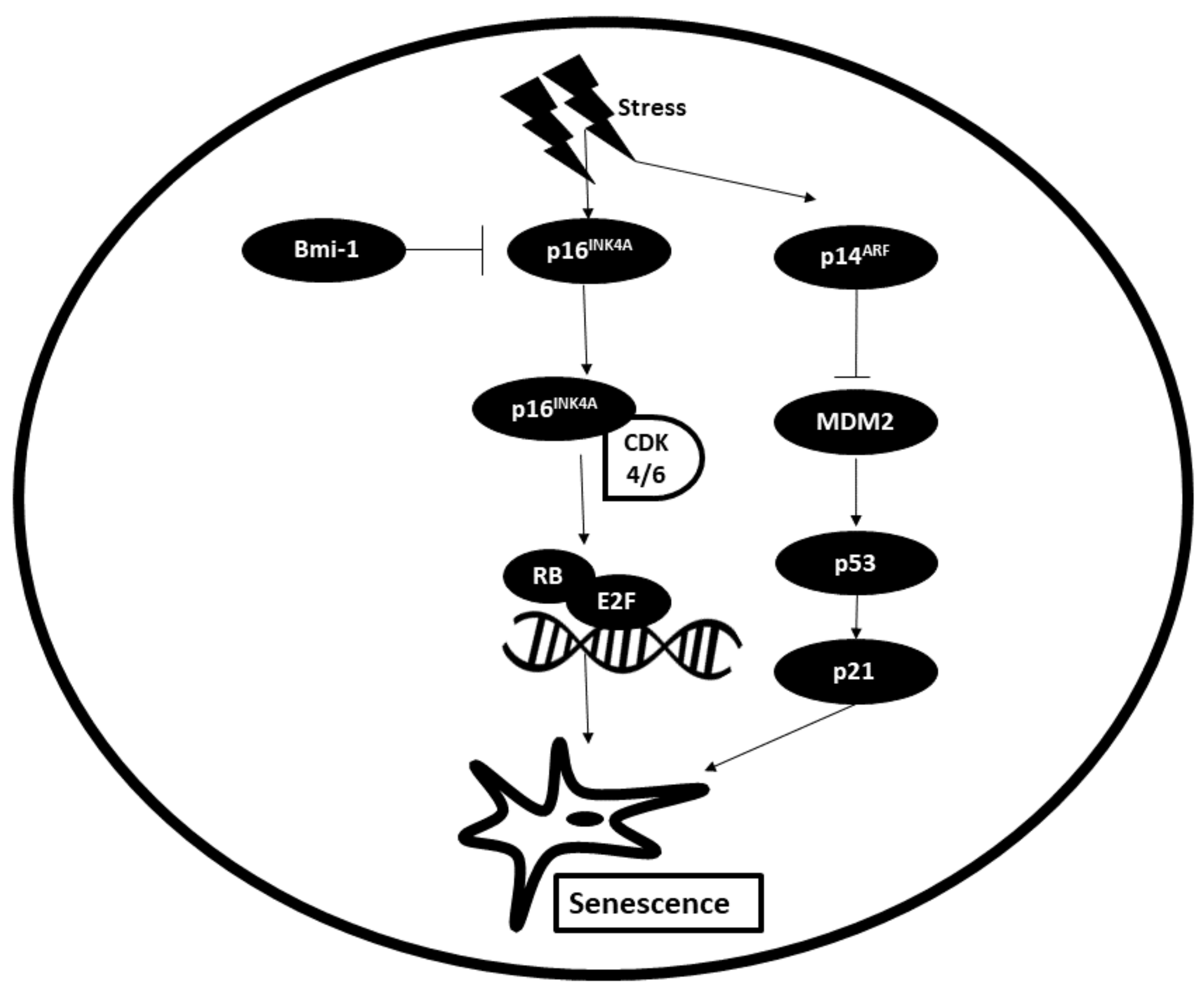
3.4. Senescence
The Role of miRNAs in Altering the Senescent State of Cancer Cells
4. Hallmark 3: Vascularization
4.1. Vascularization Mechanisms in Cancer Cells
4.2. The Role of miRNAs in the Vascularization of Cancer Cells
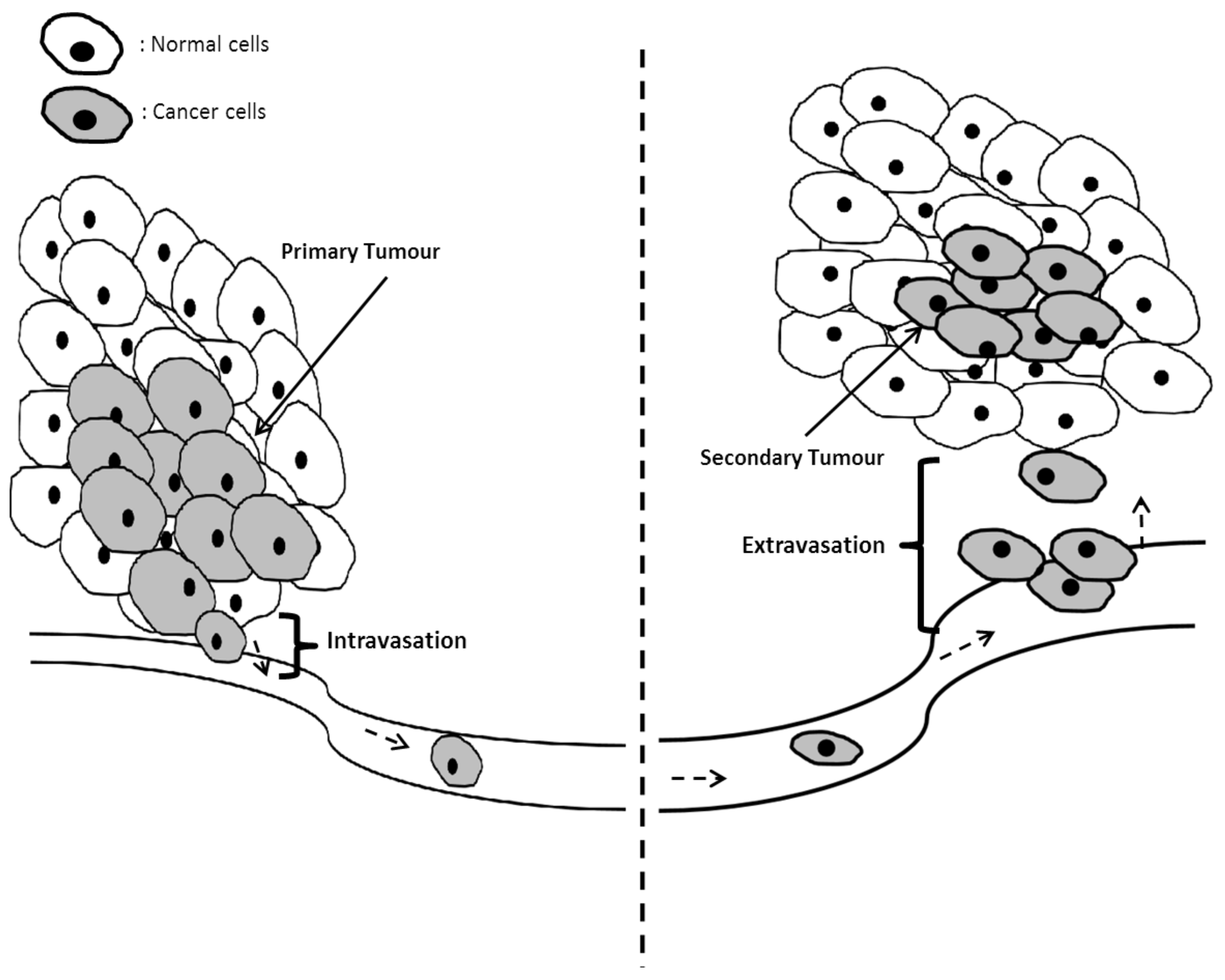
5. Hallmark 4: Invasion and Metastasis
5.1. Mechanisms of Invasion and Metastasis in Cancer Cells
5.2. The Role of miRNAs in Metastases and Invasion of Cancer Cells
6. Metabolic
6.1. Drivers of Metabolic Reprogramming
6.2. Alteration of Metabolic Pathways in Cancer Cells
6.3. The Role of miRNAs in Metabolic Rewiring
7. Hallmark: Tumor Microenvironment
7.1. miRNAs Involved in Cancer-Associated Fibroblasts (CAFs)
7.2. miRNAs in Hypoxia
8. Hallmark 7: Immune Modulation
8.1. miRNAs and Tumor-Associated Macrophages
8.2. miRNAs and Myeloid-Derived Suppressor Cells (MDSCs)
8.3. miRNAs and Natural Killer (NK) Cells
8.4. miRNAs and T Cells
9. The Role of microRNAs in Cancer Biology beyond the Hallmarks of Cancer
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Van Roosbroeck, K.; Calin, G.A. Cancer Hallmarks and MicroRNAs: The Therapeutic Connection. Adv. Cancer Res. 2017, 135, 119–149. [Google Scholar] [CrossRef] [PubMed]
- Stavast, C.; Erkeland, S. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef]
- Manasa, V.; Kannan, S. Impact of microRNA dynamics on cancer hallmarks: An oral cancer scenario. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Tan, W.; Liu, B.; Qu, S.; Liang, G.; Luo, W.; Gong, C. MicroRNAs and cancer: Key paradigms in molecular therapy (Review). Oncol. Lett. 2017, 15, 2735–2742. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Chantar, M.L.M.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Nogués, L.; Palacios-García, J.; Reglero, C.; Rivas, V.; Neves, M.; Ribas, C.; Penela, P.; Mayor, F. G protein-coupled receptor kinases (GRKs) in tumorigenesis and cancer progression: GPCR regulators and signaling hubs. Semin. Cancer Biol. 2018, 48, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Rodon, J.; Serra, V.; Tabernero, J. Picking the Point of Inhibition: A Comparative Review of PI3K/AKT/mTOR Pathway Inhibitors. Mol. Cancer Ther. 2014, 13, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol. Lett. 2018, 15, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.; Ichimura, K. Signal transduction pathways and resistance to targeted therapies in glioma. Semin. Cancer Biol. 2019, 58, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, Y.; Lin, L.; Ma, X.; Zheng, R. miR-101 regulates the cell proliferation and apoptosis in diffuse large B-cell lymphoma by targeting MEK1 via regulation of the ERK/MAPK signaling pathway. Oncol. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chang, H.; Chen, G. Effects of microRNA 20a on the proliferation, migration and apoptosis of multiple myeloma via the PTEN/PI3K/AKT signaling pathway. Oncol. Lett. 2018, 15, 10001–10007. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.-H.; Ji, X.-Q.; Zhang, H.; Xu, J.; Zhu, H.; Shao, X.-J. miR-590 promotes cell proliferation and invasion in T-cell acute lymphoblastic leukaemia by inhibiting RB1. Oncotarget 2016, 7, 39527–39534. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, L.; Tian, C.; Lu, F.; Wu, J.; Liu, L. microRNA-150 promotes cervical cancer cell growth and survival by targeting FOXO4. BMC Mol. Biol. 2015, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Hou, L.; Xiong, Y.-M.; Huang, J.-X.; Zhang, W.-H.; Pan, Y.-Y.; Song, X.-R. miR-132 targeting E2F5 suppresses cell proliferation, invasion, migration in ovarian cancer cells. Am. J. Transl. Res. 2016, 8, 1492. [Google Scholar] [PubMed]
- Li, Q.; Qiu, X.-M.; Li, Q.-H.; Wang, X.-Y.; Li, L.; Xu, M.; Dong, M.; Xiao, Y.-B. MicroRNA-424 may function as a tumor suppressor in endometrial carcinoma cells by targeting E2F7. Oncol. Rep. 2015, 33, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, T.; Huang, Z.; Qi, Y.; Du, S.; Di, T.; Lei, Z.; Zhang, X.; Yan, W. MicroRNA-133a Inhibits Osteosarcoma Cells Proliferation and Invasion via Targeting IGF-1R. Cell. Physiol. Biochem. 2016, 38, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, K.; Liu, S.; Ji, B.; Wang, Y.; Liu, Y. MicroRNA-133a functions as a tumor suppressor by targeting IGF-1R in hepatocellular carcinoma. Tumor Biol. 2015, 36, 9779–9788. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xiang, G.; Meng, Y.; Dong, R. MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting the PDCD4. Reprod. Biol. 2016, 16, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Deng, T.; Su, C.; Shang, Z. MicroRNA 217 inhibits cell proliferation and enhances chemosensitivity to doxorubicin in acute myeloid leukemia by targeting KRAS. Oncol. Lett. 2017, 13, 4986–4994. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, R.; Wang, H.; Luo, Y.; Wang, X.; Niu, W.; Zhou, Y.; Wen, Q.; Fan, S.; Li, X.; et al. miR-141 is involved in BRD7-mediated cell proliferation and tumor formation through suppression of the PTEN/AKT pathway in nasopharyngeal carcinoma. Cell Death Dis. 2016, 7, e2156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shao, S.; Pan, H.; Cheng, Z.; Rui, X. MicroRNA-136 inhibits prostate cancer cell proliferation and invasion by directly targeting mitogen-activated protein kinase kinase 4. Mol. Med. Rep. 2018, 17, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Chen, C.; Li, X.; Wei, Z.; Liu, Z.; Liu, Y. MicroRNA-124 suppresses cell proliferation and invasion of triple negative breast cancer cells by targeting STAT3. Mol. Med. Rep. 2019, 19, 3667–3675. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, W.; Bian, W.; Yang, H.; Wu, X.; Li, Y.; Feng, W.; Liu, X. MicroRNA-623 Targets Cyclin D1 to Inhibit Cell Proliferation and Enhance the Chemosensitivity of Cells to 5-Fluorouracil in Gastric Cancer. Oncol. Res. Featur. Preclin. Clin. 2018, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Moradimotlagh, A.; Arefian, E.; Valojerdi, R.R.; Ghaemi, S.; Adegani, F.J.; Soleimani, M. MicroRNA-129 Inhibits Glioma Cell Growth by Targeting CDK4, CDK6, and MDM2. Mol. Ther.-Nucleic Acids 2020, 19, 759–764. [Google Scholar] [CrossRef]
- He, Y.; Yu, B. MicroRNA-93 promotes cell proliferation by directly targeting P21 in osteosarcoma cells. Exp. Ther. Med. 2017, 13, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhang, Y.; Li, J. Upregulation of MiR-196a promotes cell proliferation by downregulating p27kip1 in laryngeal cancer. Biol. Res. 2016, 49, 1–8. [Google Scholar] [CrossRef][Green Version]
- Ye, C.-Y.; Zheng, C.-P.; Ying, W.-W.; Weng, S.-S. Up-regulation of microRNA-497 inhibits the proliferation, migration and invasion but increases the apoptosis of multiple myeloma cells through the MAPK/ERK signaling pathway by targeting Raf-1. Cell Cycle 2018, 17, 2666–2683. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Liu, X.; Hu, Y.; Ding, L.; Zhang, X.; Sun, Q.; Li, Y. Oncogenic microRNA-411 promotes lung carcinogenesis by directly targeting suppressor genes SPRY4 and TXNIP. Oncogene 2019, 38, 1892–1904. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Meng, Y.; Zhang, M.; Li, D. MRE11-RAD50-NBS1 complex alterations and DNA damage response: Implications for cancer treatment. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Tainer, J.A. The MRE11–RAD50–NBS1 Complex Conducts the Orchestration of Damage Signaling and Outcomes to Stress in DNA Replication and Repair. Annu. Rev. Biochem. 2018, 87, 263–294. [Google Scholar] [CrossRef] [PubMed]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104. [Google Scholar] [CrossRef]
- Tšuiko, O.; Jatsenko, T.; Grace LK, P.; Kurg, A.; Vermeesch, J.R.; Lanner, F.; Salumets, A. A speculative outlook on embryonic aneuploidy: Can molecular pathways be involved? Dev. Biol. 2019, 447, 3–13. [Google Scholar] [CrossRef]
- Pascal, J.M. The comings and goings of PARP-1 in response to DNA damage. DNA Repair 2018, 71, 177–182. [Google Scholar] [CrossRef]
- Ronco, C.; Martin, A.R.; Demange, L.; Benhida, R. ATM, ATR, CHK1, CHK2 and WEE1 inhibitors in cancer and cancer stem cells. MedChemComm 2017, 8, 295–319. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhou, W.; Li, C.; Guo, M. MicroRNAs, DNA damage response, and cancer treatment. Int. J. Mol. Sci. 2016, 17, 2087. [Google Scholar] [CrossRef]
- Majidinia, M.; Yousefi, B. DNA damage response regulation by microRNAs as a therapeutic target in cancer. DNA Repair 2016, 47, 1–11. [Google Scholar] [CrossRef]
- Lai, J.; Yang, H.; Zhu, Y.; Ruan, M.; Huang, Y.; Zhang, Q. MiR-7-5p-mediated downregulation of PARP1 impacts DNA homologous recombination repair and resistance to doxorubicin in small cell lung cancer. BMC Cancer 2019, 19, 602. [Google Scholar] [CrossRef]
- Yang, F.; Guo, L.; Cao, Y.; Li, S.; Li, J.; Liu, M. MicroRNA-7-5p Promotes Cisplatin Resistance of Cervical Cancer Cells and Modulation of Cellular Energy Homeostasis by Regulating the Expression of the PARP-1 and BCL2 Genes. Med. Sci. Monit. 2018, 24, 6506–6516. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Zhang, Y.-Y.; Zhu, B.-L.; Feng, F.-Z.; Zhang, H.-T.; Yan, H.; Zhou, B. MiR-203a-3p regulates the biological behaviors of ovarian cancer cells through mediating the Akt/GSK-3β/Snail signaling pathway by targeting ATM. J. Ovarian Res. 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Luo, J.; Liu, Z.; Zhou, R.; Luo, H. MicroRNA-138 Regulates DNA Damage Response in Small Cell Lung Cancer Cells by Directly Targeting H2AX. Cancer Investig. 2015, 33, 126–136. [Google Scholar] [CrossRef]
- Liao, X.-H.; Zheng, L.; He, H.-P.; Zheng, D.-L.; Wei, Z.-Q.; Wang, N.; Dong, J.; Ma, W.-J.; Zhang, T.-C. STAT3 regulated ATR via microRNA-383 to control DNA damage to affect apoptosis in A431 cells. Cell. Signal. 2015, 27, 2285–2295. [Google Scholar] [CrossRef]
- Lai, T.-H.; Ewald, B.; Zecevic, A.; Liu, C.; Sulda, M.; Papaioannou, D.; Garzon, R.; Blachly, J.S.; Plunkett, W.; Sampath, D. HDAC Inhibition Induces MicroRNA-182, which Targets RAD51 and Impairs HR Repair to Sensitize Cells to Sapacitabine in Acute Myelogenous Leukemia. Clin. Cancer Res. 2016, 22, 3537–3549. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-L.; Dong, Y.; Deng, Y.-Z.; Wang, W.-J.; Li, W.-D. Tumor suppressor miR-145 reverses drug resistance by directly targeting DNA damage-related gene RAD18 in colorectal cancer. Tumor Biol. 2015, 36, 5011–5019. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fan, S. hsa-miR-212 modulates the radiosensitivity of glioma cells by targeting BRCA1. Oncol. Rep. 2018, 39, 977–984. [Google Scholar] [CrossRef]
- Valenti, F.; Sacconi, A.; Ganci, F.; Grasso, G.; Strano, S.; Blandino, G.; Di Agostino, S. The miR-205-5p/BRCA1/RAD17 Axis Promotes Genomic Instability in Head and Neck Squamous Cell Carcinomas. Cancers 2019, 11, 1347. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Zhang, J.; Shao, H.-Y.; Chen, J.-P.; Zhao, H.-Y. MicroRNA-191 promotes osteosarcoma cells proliferation by targeting checkpoint kinase 2. Tumor Biol. 2015, 36, 6095–6101. [Google Scholar] [CrossRef]
- Peng, D.; Dong, J.; Zhao, Y.; Peng, X.; Tang, J.; Chen, X.; Wang, L.; Hu, D.-N.; Reinach, P.S.; Qu, J.; et al. miR-142-3p suppresses uveal melanoma by targeting CDC25C, TGFβR1, GNAQ, WASL, and RAC1. Cancer Manag. Res. 2019, 11, 4729–4742. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, H.; Chen, Y.-N.; Deng, Y.-T.; Zhang, B.; Xiong, X.-D.; Yuan, Y.; Zhu, Y.; Huang, H.; Xie, L.; et al. DNA damage responsive miR-33b-3p promoted lung cancer cells survival and cisplatin resistance by targeting p21WAF1/CIP1. Cell Cycle 2016, 15, 2920–2930. [Google Scholar] [CrossRef]
- Besse, A.; Sana, J.; Lakomy, R.; Kren, L.; Fadrus, P.; Smrcka, M.; Hermanova, M.; Jancalek, R.; Reguli, S.; Lipina, R.; et al. MiR-338-5p sensitizes glioblastoma cells to radiation through regulation of genes involved in DNA damage response. Tumor Biol. 2015, 37, 7719–7727. [Google Scholar] [CrossRef]
- Towers, C.G.; Wodetzki, D.; Thorburn, A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations Autophagy and cancer. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Stiuso, P.; Potenza, N.; Lombardi, A.; Ferrandino, I.; Monaco, A.; Zappavigna, S.; Vanacore, D.; Mosca, N.; Castiello, F.; Porto, S.; et al. MicroRNA-423-5p Promotes Autophagy in Cancer Cells and Is Increased in Serum from Hepatocarcinoma Patients Treated with Sorafenib. Mol. Ther.-Nucleic Acids 2015, 4, e233. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Katz, S.G. Non-apoptotic functions of BCL-2 family proteins. Cell Death Differ. 2017, 24, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Zhu, X.; Geng, Q.; Xia, L.; Sun, X.; Chen, Y.; Li, W.; Zhou, Z.; Zhan, Y.; Xu, D. The microRNA-423-3p-Bim Axis Promotes Cancer Progression and Activates Oncogenic Autophagy in Gastric Cancer. Mol. Ther. 2017, 25, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ban, R.; Liu, W.; Wang, H.; Li, S.; Yue, Z.; Zhu, G.; Zhuan, Y.; Wang, C. MiRNA-409-3p enhances cisplatin-sensitivity of ovarian cancer cells by blocking the autophagy mediated by Fip200. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2020. [Google Scholar] [CrossRef]
- Jin, F.; Wang, Y.; Li, M.; Zhu, Y.; Liang, H.; Wang, C.; Wang, F.; Zhang, C.-Y.; Zen, K.; Li, L. MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy. Cell Death Dis. 2018, 8, e2540. [Google Scholar] [CrossRef]
- Zheng, B.; Zhu, H.; Gu, D.; Pan, X.; Qian, L.; Xue, B.; Yang, D.; Zhou, J.; Shan, Y. MiRNA-30a-mediated autophagy inhibition sensitizes renal cell carcinoma cells to sorafenib. Biochem. Biophys. Res. Commun. 2015, 459, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Nie, Y.; Wang, H.; Lin, Y. miR-181a suppresses autophagy and sensitizes gastric cancer cells to cisplatin. Gene 2016, 576, 828–833. [Google Scholar] [CrossRef]
- Li, S.; Qiang, Q.; Shan, H.; Shi, M.; Gan, G.; Ma, F.; Chen, B. MiR-20a and miR-20b negatively regulate autophagy by targeting RB1CC1/FIP200 in breast cancer cells. Life Sci. 2016, 147, 143–152. [Google Scholar] [CrossRef]
- Huangfu, L.; Liang, H.; Wang, G.; Su, X.; Li, L.; Du, Z.; Hu, M.; Dong, Y.; Bai, X.; Liu, T.; et al. miR-183 regulates autophagy and apoptosis in colorectal cancer through targeting of UVRAG. Oncotarget 2015, 7, 4735–4745. [Google Scholar] [CrossRef]
- Guo, X.; Xue, H.; Guo, X.; Gao, X.; Xu, S.; Yan, S.; Han, X.; Li, T.; Shen, J.; Li, G. MiR224-3p inhibits hypoxia-induced autophagy by targeting autophagy-related genes in human glioblastoma cells. Oncotarget 2015, 6, 41620–41637. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, Z.; Yan, X.; Dai, X.; Hu, C.; Zheng, Y.; He, F.; Lian, J. Targeting the MIR34C-5p-ATG4B-autophagy axis enhances the sensitivity of cervical cancer cells to pirarubicin. Autophagy 2016, 12, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Feng, X.; Zhao, X.; Ma, F.; Liu, N.; Guo, H.; Li, C.; Du, H.; Zhang, B. Role of Beclin-1-Mediated Autophagy in the Survival of Pediatric Leukemia Cells. Cell. Physiol. Biochem. 2016, 39, 1827–1836. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Fang, F.; Liu, K. MALAT1 modulates the autophagy of retinoblastoma cell through miR-124-mediated stx17 regulation. J. Cell. Biochem. 2018, 119, 3853–3863. [Google Scholar] [CrossRef]
- Hua, L.; Zhu, G.; Wei, J. MicroRNA-1 overexpression increases chemosensitivity of non-small cell lung cancer cells by inhibiting autophagy related 3-mediated autophagy. Cell Biol. Int. 2018, 42, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Tait, S.W. Apoptosis and cancer: Force awakens, phantom menace, or both? Int. Rev. Cell Mol. Biol. 2018, 337, 135–152. [Google Scholar]
- Ichim, G.; Tait, S. A fate worse than death: Apoptosis as an oncogenic process. Nat. Rev. Cancer 2016, 16, 539–548. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.-E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Wang, X.; He, M.; Qiao, S. MicroRNA-224 Promotes Tumorigenesis through Downregulation of Caspase-9 in Triple-Negative Breast Cancer. Dis. Markers 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Yoo, J.K.; Lee, J.M.; Kang, S.H.; Jeon, S.H.; Kim, C.M.; Oh, S.-H.; Kim, J.K. The novel microRNA hsa-miR-CHA1 regulates cell proliferation and apoptosis in human lung cancer by targeting XIAP. Lung Cancer 2019, 132, 99–106. [Google Scholar] [CrossRef]
- Li, T.; Ding, Z.-L.; Zheng, Y.-L.; Wang, W. MiR-484 promotes non-small-cell lung cancer (NSCLC) progression through inhibiting Apaf-1 associated with the suppression of apoptosis. Biomed. Pharmacother. 2017, 96, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Mobarra, N.; Shafiee, A.; Rad, S.M.A.H.; Tasharrofi, N.; Soufi-Zomorod, M.; Hafizi, M.; Movahed, M.; Kouhkan, F.; Soleimani, M. Overexpression of microRNA-16 declines cellular growth, proliferation and induces apoptosis in human breast cancer cells. Vitr. Cell. Dev. Biol.-Anim. 2015, 51, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Zeng, W.; Wan, C.; Duan, S.; Jiang, S. microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP. Br. J. Cancer 2017, 116, 66–76. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Bhardwaj, A.; Arora, S.; Tyagi, N.; Singh, S.; Andrews, J.; McClellan, S.; Wang, B.; Singh, A. MicroRNA-345 induces apoptosis in pancreatic cancer cells through potentiation of caspase-dependent and -independent pathways. Br. J. Cancer 2015, 113, 660–668. [Google Scholar] [CrossRef]
- Zhou, C.; Tan, W.; Lv, H.; Gao, F.; Sun, J. Hypoxia-inducible microRNA-488 regulates apoptosis by targeting Bim in osteosarcoma. Cell. Oncol. 2016, 39, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-M.; Luo, Y.; Li, D.-F.; Wei, C.-K.; Hua, K.-Y.; Song, J.-L.; Xu, H.; Maskey, N.; Fang, L. MicroRNA-96 plays an oncogenic role by targeting FOXO1 and regulating AKT/FOXO1/Bim pathway in papillary thyroid carcinoma cells. Int. J. Clin. Exp. Pathol. 2015, 8, 9889–9900. [Google Scholar]
- He, H.; Tian, W.; Chen, H.; Deng, Y. MicroRNA-101 sensitizes hepatocellular carcinoma cells to doxorubicin-induced apoptosis via targeting Mcl-1. Mol. Med. Rep. 2016, 13, 1923–1929. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, R.; Wang, Y.; Zhou, M.; Ding, Z. Loss of BAX by miR-365 Promotes Cutaneous Squamous Cell Carcinoma Progression by Suppressing Apoptosis. Int. J. Mol. Sci. 2017, 18, 1157. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Yan, L. Inhibition of MicroRNA-149-5p Induces Apoptosis of Acute Myeloid Leukemia Cell Line THP-1 by Targeting Fas Ligand (FASLG). Med. Sci. Monit. 2016, 22, 5116–5123. [Google Scholar] [CrossRef]
- Xue, F.; Zhu, Y.; Xu, F.; Zhou, L.-J.; Han, F.; Wang, S.-C. MicroRNA-199 inhibits proliferation and promotes apoptosis in children with acute myeloid leukemia by mediating caspase-3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3584–3593. [Google Scholar]
- Yan, J.; Zhang, Y.; She, Q.; Li, X.; Peng, L.; Wang, X.; Liu, S.; Shen, X.; Zhang, W.; Dong, Y.; et al. Long Noncoding RNA H19/miR-675 Axis Promotes Gastric Cancer via FADD/Caspase 8/Caspase 3 Signaling Pathway. Cell. Physiol. Biochem. 2017, 42, 2364–2376. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Pratsinis, H.; Kletsas, D. The role of senescence in cancer development. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Munk, R.; Panda, A.C.; Grammatikakis, I.; Gorospe, M.; Abdelmohsen, K. Senescence-associated microRNAs. Int. Rev. Cell Mol. Biol. 2017, 334, 177–205. [Google Scholar]
- Lee, S.; Lee, J.-S. Cellular senescence: A promising strategy for cancer therapy. BMB Rep. 2019, 52, 35. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ye, L.; Wang, C.; Li, N.; Wang, D.; Li, X. MicroRNA-128 increases glioma cell radio-sensitivity by suppressing senescent evasion through oncogene Bmi-1. Int. J. Clin. Exp. Pathol. 2018, 11, 1423–1430. [Google Scholar]
- Su, W.; Hong, L.; Xu, X.; Huang, S.; Herpai, D.; Li, L.; Xu, Y.; Truong, L.; Hu, W.-Y.; Wu, X.; et al. miR-30 disrupts senescence and promotes cancer by targeting both p16INK4A and DNA damage pathways. Oncogene 2018, 37, 5618–5632. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Carvalho, J.; Graça, I.; Gomez, A.; Oliveira, J.; Henrique, R.; Esteller, M.; Jerónimo, C. Downregulation of miR-130b~301b cluster is mediated by aberrant promoter methylation and impairs cellular senescence in prostate cancer. J. Hematol. Oncol. 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Nucera, S.; Giustacchini, A.; Boccalatte, F.; Calabria, A.; Fanciullo, C.; Plati, T.; Ranghetti, A.; Garcia-Manteiga, J.; Cittaro, D.; Benedicenti, F.; et al. miRNA-126 Orchestrates an Oncogenic Program in B Cell Precursor Acute Lymphoblastic Leukemia. Cancer Cell 2016, 29, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.-Y.; Liu, Q.-Y.; Yuan, L.; Xuan, S.-Y. Upregulation of microRNA-132 in gastric cancer promotes cell proliferation via retinoblastoma 1 targeting. Mol. Med. Rep. 2015, 12, 7005–7010. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Hu, X.; Chen, J.; Hu, D.; Chen, L.-F. BRD4 regulates cellular senescence in gastric cancer cells via E2F/miR-106b/p21 axis. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-H.; Yu, C.-C.; Lee, Y.-C.; Lin, C.-W.; Chang, W.-W.; Kuo, Y.-L. miR-494-3p Induces Cellular Senescence and Enhances Radiosensitivity in Human Oral Squamous Carcinoma Cells. Int. J. Mol. Sci. 2016, 17, 1092. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, M.; Zhang, J.; Xue, L.; Zhang, G.; Hu, C.; Wang, Z.; He, S.; Chen, L.; Ma, K.; et al. Overexpression of KLF4 promotes cell senescence through microRNA-203-survivin-p21 pathway. Oncotarget 2016, 7, 60290–60302. [Google Scholar] [CrossRef][Green Version]
- Neault, M.; Mallette, F.A.; Richard, S. miR-137 Modulates a Tumor Suppressor Network-Inducing Senescence in Pancreatic Cancer Cells. Cell Rep. 2016, 14, 1966–1978. [Google Scholar] [CrossRef]
- He, X.; Yang, A.; McDonald, D.G.; Riemer, E.C.; Vanek, K.N.; Schulte, B.A.; Wang, G.Y. MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells. Oncotarget 2017, 8, 69797–69807. [Google Scholar] [CrossRef]
- Loizzi, V.; Del Vecchio, V.; Gargano, G.; De Liso, M.; Kardashi, A.; Naglieri, E.; Resta, L.; Cicinelli, E.; Cormio, G. Biological Pathways Involved in Tumor Angiogenesis and Bevacizumab Based Anti-Angiogenic Therapy with Special References to Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1967. [Google Scholar] [CrossRef] [PubMed]
- Dome, B.; Hendrix, M.J.; Paku, S.; Tóvári, J.; Tímár, J. Alternative Vascularization Mechanisms in Cancer: Pathology and Therapeutic Implications. Am. J. Pathol. 2007, 170, 1–15. [Google Scholar] [CrossRef]
- Doktorova, H.; Hrabeta, J.; Khalil, M.A.; Eckschlager, T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed. Pap. 2015, 159, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qin, T.; Li, J.; Wang, L.; Zhang, Q.; Jiang, Z.; Mao, J. MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther. 2017, 24, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Batty, M.; Pugh, R.; Rathinam, I.; Simmonds, J.; Walker, E.; Forbes, A.; Anoopkumar-Dukie, S.; McDermott, C.M.; Spencer, B.; Christie, D.; et al. The Role of α1-Adrenoceptor Antagonists in the Treatment of Prostate and Other Cancers. Int. J. Mol. Sci. 2016, 17, 1339. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chan, Y.-C.; Chang, W.-M.; Lin, Y.-F.; Yang, C.-J.; Su, C.-Y.; Huang, M.-S.; Wu, A.T.; Hsiao, M. Feedback regulation of ALDOA activates the HIF-1α/MMP9 axis to promote lung cancer progression. Cancer Lett. 2017, 403, 28–36. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Zheng, Y.-L. Hypoxia promotes invasion of retinoblastoma cells in vitro by upregulating HIF-1α/MMP9 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5361–5369. [Google Scholar]
- Oren, R.; Addadi, S.; Haziza, L.N.; Dafni, H.; Rotkopf, R.; Meir, G.; Fishman, A.; Neeman, M. Fibroblast recruitment as a tool for ovarian cancer detection and targeted therapy. Int. J. Cancer 2016, 139, 1788–1798. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in In Vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Gómez-Escudero, J.; Clemente, C.; García-Weber, D.; Acín-Pérez, R.; Millán, J.; Enríquez, J.A.; Bentley, K.; Carmeliet, P.; Arroyo, A.G. PKM2 regulates endothelial cell junction dynamics and angiogenesis via ATP production. Sci. Rep. 2019, 9, 1–18. [Google Scholar]
- Roux, Q.; Gavard, J. Endothelial Cell-Cell Junctions in Tumor Angiogenesis. In Tumor Angiogenesis Key Target for Cancer Therapy; Springer: Cham, Switzerland, 2019; pp. 91–119. [Google Scholar] [CrossRef]
- Wong, H.-K.A.; El Fatimy, R.; Onodera, C.; Wei, Z.; Yi, M.; Mohan, A.; Gowrisankaran, S.; Karmali, P.; Marcusson, E.; Wakimoto, H.; et al. The Cancer Genome Atlas Analysis Predicts MicroRNA for Targeting Cancer Growth and Vascularization in Glioblastoma. Mol. Ther. 2015, 23, 1234–1247. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-J.; Fang, J.-H.; Yang, X.-J.; Zhang, C.; Yuan, Y.; Zheng, L.; Zhuang, S.-M. Hepatocellular Carcinoma Cell-Secreted Exosomal MicroRNA-210 Promotes Angiogenesis In Vitro and In Vivo. Mol. Ther.-Nucleic Acids 2018, 11, 243–252. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Khan, A.A.; Li, B.; Gu, B.; Lin, F.; Su, X.; Yan, J. miRNA-124-3p/neuropilin-1(NRP-1) axis plays an important role in mediating glioblastoma growth and angiogenesis. Int. J. Cancer 2018, 143, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Nault, B.; Ugwuagbo, K.; Maiti, S.; Majumder, M. Mir526b and Mir655 Promote Tumour Associated Angiogenesis and Lymphangiogenesis in Breast Cancer. Cancers 2019, 11, 938. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Q.-H.; Wang, F.; Tan, J.-J.; Deng, Y.-Q.; Peng, X.-H.; Liu, X.; Zhang, B.; Xu, X.; Li, X.-P. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wu, X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 2019, 9, 8206–8220. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Lei, Z.; Huang, M.; Li, Z.; Wang, L.; Cao, Q.; Han, D.; Chang, Y.; Chen, Y.; et al. Dysregulation of miR-6868-5p/FOXM1 circuit contributes to colorectal cancer angiogenesis. J. Exp. Clin. Cancer Res. 2018, 37, 292. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-P.; Hu, Y.-P.; Wu, X.-S.; Wu, Y.-S.; Ye, Y.-Y.; Li, H.-F.; Liu, Y.-C.; Jiang, L.; Liu, F.-T.; Zhang, Y.-J.; et al. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.Q.; He, Y.H.; Wang, S.B.; Yang, S.; Wang, Y.J.; Nan, C.J.; Bao, Y.F.; Xie, Q.P.; Chen, Y.H. MiR-130b/TNF-α/NF-κB/VEGFA loop inhibits prostate cancer angiogenesis. Clin. Transl. Oncol. 2020, 22, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; You, B.; Shi, S.; Shan, Y.; Zhang, Q.; Yue, H.; Zhang, J.; Zhang, W.; Shi, Y.; Liu, Y.; et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 2018, 37, 2873–2889. [Google Scholar] [CrossRef]
- Fan, B.; Jin, Y.; Zhang, H.; Zhao, R.; Sun, M.; Sun, M.; Yuan, X.; Wang, W.; Wang, X.; Chen, Z.; et al. MicroRNA-21 contributes to renal cell carcinoma cell invasiveness and angiogenesis via the PDCD4/c-Jun (AP-1) signalling pathway. Int. J. Oncol. 2020, 56, 178–192. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Xu, R.; Shang, J.; He, H.; Yang, Q. MicroRNA-574-5p in gastric cancer cells promotes angiogenesis by targeting protein tyrosine phosphatase non-receptor type 3 (PTPN3). Gene 2020, 733, 144383. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Xie, C.; Hu, J.; Tan, J.; Yuan, Y.; Liu, Z.; Yang, Z. Pancreatic cancer cell–derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. J. Cell. Mol. Med. 2020, 24, 588–604. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, H.; Yang, H.; Wang, H.; Bai, M.; Sun, W.; Wang, X.; Si, Y.; Ning, T.; Zhang, L.; et al. Exosome miR-155 Derived from Gastric Carcinoma Promotes Angiogenesis by Targeting the c-MYB/VEGF Axis of Endothelial Cells. Mol. Ther.-Nucleic Acids 2020, 19, 1449–1459. [Google Scholar] [CrossRef]
- Shang, A.; Wang, X.; Gu, C.; Liu, W.; Sun, J.; Zeng, B.; Chen, C.; Ji, P.; Wu, J.; Quan, W.; et al. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging 2020, 12, 8352–8371. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, S.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Sung, K.J.; Sung, Y.H.; Choi, C.-M.; Yun, M.; Yi, Y.-S.; et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020, 475, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ha, M.; Li, L.; Huang, X.; Liu, C. MicroRNA-3064-5p sponged by MALAT1 suppresses angiogenesis in human hepatocellular carcinoma by targeting the FOXA1/CD24/Src pathway. FASEB J. 2020, 34, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, F.; Wu, K.; Xu, X.; Zeng, K.; An, Y.; Xu, F.; Xun, J.; Lv, X.; Zhang, X.; et al. Lost miR-141 and upregulated TM4SF1 expressions associate with poor prognosis of pancreatic cancer: Regulation of EMT and angiogenesis by miR-141 and TM4SF1 via AKT. Cancer Biol. Ther. 2020, 21, 354–363. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Li, Y.; Li, C.; Qin, T.; Bai, M.; Zhang, Z.; Jia, R.; Su, Y.; Wang, C. miR-195 suppresses metastasis and angiogenesis of squamous cell lung cancer by inhibiting the expression of VEGF. Mol. Med. Rep. 2019, 20, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, Z.; Li, F. Overexpressed microRNA-136 works as a cancer suppressor in gallbladder cancer through suppression of JNK signaling pathway via inhibition of MAP2K4. Am. J. Physiol. Liver Physiol. 2019, 317, G670–G681. [Google Scholar] [CrossRef]
- Cao, J.; Li, L.; Han, X.; Cheng, H.; Chen, W.; Qi, K.; Chen, C.; Wu, Q.; Niu, M.; Zeng, L.; et al. miR-302 cluster inhibits angiogenesis and growth of K562 leukemia cells by targeting VEGFA. OncoTargets Ther. 2019, 12, 433–441. [Google Scholar] [CrossRef]
- Li, Y.; Cai, B.; Shen, L.; Dong, Y.; Lu, Q.; Sun, S.; Liu, S.; Ma, S.; Ma, P.X.; Chen, J. MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett. 2017, 397, 111–119. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, S.-S.; Zong, M.; Fan, S.-S.; Lu, T.-B.; Gong, R.-H.; Sun, L.-S.; Fan, L.-Y. Glucose-6-Phosphate Isomerase (G6PI) Mediates Hypoxia-Induced Angiogenesis in Rheumatoid Arthritis. Sci. Rep. 2017, 7, 40274. [Google Scholar] [CrossRef]
- Xie, M.; Dart, D.A.; Guo, T.; Xing, X.-F.; Cheng, X.-J.; Du, H.; Jiang, W.G.; Wen, X.-Z.; Ji, J.-F. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer 2017, 21, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-Y.; Chen, G.; Zhang, Y.-Q.; He, H.-C.; Liang, Y.-X.; Ye, J.-H.; Liang, Y.-K.; Mo, R.-J.; Lu, J.-M.; Zhuo, Y.-J.; et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol. Cancer 2017, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Campbell, K. Contribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasis. Curr. Opin. Cell Biol. 2018, 55, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Mayor, R. Tuning Collective Cell Migration by Cell–Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 2017, 9, a029199. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Yeung, K.T.; Yang, J. Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Cell junctions. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2020. [Google Scholar]
- Wu, J.-I.; Wang, L.-H. Emerging roles of gap junction proteins connexins in cancer metastasis, chemoresistance and clinical application. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Balda, M.S.; Matter, K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell Sci. 2014, 127, 3401–3413. [Google Scholar] [CrossRef]
- Knights, A.; Funnell, A.P.W.; Crossley, M.; Pearson, R.C.M. Holding Tight: Cell Junctions and Cancer Spread. Trends Cancer Res. 2012, 8, 61–69. [Google Scholar]
- Yu, Y.; Elble, R.C. Homeostatic Signaling by Cell–Cell Junctions and Its Dysregulation during Cancer Progression. J. Clin. Med. 2016, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Jacob, L.S.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma—Astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 533, 493–498. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; Zhang, Y.; Zhou, Z.; Cui, X.; Zhang, L.; Fung, K.-M.; Zheng, W.; Allard, F.D.; Yee, E.U.; et al. ZIP4 Promotes Pancreatic Cancer Progression by Repressing ZO-1 and Claudin-1 through a ZEB1-Dependent Transcriptional Mechanism. Clin. Cancer Res. 2018, 24, 3186–3196. [Google Scholar] [CrossRef]
- Todd, M.C.; Petty, H.M.; King, J.; Marshall, B.N.P.; Sheller, R.A.; Cuevas, M.E. Overexpression and delocalization of claudin-3 protein in MCF-7 and MDA-MB-415 breast cancer cell lines. Oncol. Lett. 2015, 10, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Xin, S.; Qin, X.; Li, H.; Lin, L.; You, Y. Zoledronic acid suppresses metastasis of esophageal squamous cell carcinoma cells through upregulating the tight junction protein occludin. Cytotechnology 2016, 68, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Sinkevicius, K.W.; Bellaria, K.J.; Barrios, J.; Pessina, P.; Gupta, M.; Brainson, C.F.; Bronson, R.T.; Kim, C.F. E-Cadherin Loss Accelerates Tumor Progression and Metastasis in a Mouse Model of Lung Adenocarcinoma. Am. J. Respir. Cell Mol. Biol. 2018, 59, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xie, C.; Pei, T.; Wang, J.; Lan, Y.; Huang, K.; Cui, Y.; Wang, F.; Zhang, J.; Pan, S.; et al. Deregulated AJAP1/β-catenin/ZEB1 signaling promotes hepatocellular carcinoma carcinogenesis and metastasis. Cell Death Dis. 2017, 8, e2736. [Google Scholar] [CrossRef]
- Lee, S.-H.; Min, J.-K. Abstract 1082: Loss of desmoglein 2 promotes tumor growth and progression through the activation of Src and facilitates the internalization of EGFR in biliary tract carcinoma cells. Cancer Res. 2018, 78, 1082. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Mei, H.; Fang, E.; Wang, X.; Yang, F.; Li, H.; Chen, Y.; Huang, K.; Zheng, L.; et al. Armadillo repeat containing 12 promotes neuroblastoma progression through interaction with retinoblastoma binding protein 4. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Sang, Y.; Sun, L.; Wu, Y.; Yuan, W.; Liu, Y.; Li, S.-W. Histone deacetylase 7 inhibits plakoglobin expression to promote lung cancer cell growth and metastasis. Int. J. Oncol. 2019, 54, 1112–1122. [Google Scholar] [CrossRef]
- Cui, T.; Yang, L.; Ma, Y.; Petersen, I.; Chen, Y. Desmocollin 3 has a tumor suppressive activity through inhibition of AKT pathway in colorectal cancer. Exp. Cell Res. 2019, 378, 124–130. [Google Scholar] [CrossRef]
- Croset, M.; Pantano, F.; Kan, C.W.S.; Bonnelye, E.; Descotes, F.; Alix-Panabières, C.; Lecellier, C.-H.; Bachelier, R.; Allioli, N.; Hong, S.-S.; et al. miRNA-30 Family Members Inhibit Breast Cancer Invasion, Osteomimicry, and Bone Destruction by Directly Targeting Multiple Bone Metastasis–Associated Genes. Cancer Res. 2018, 78, 5259–5273. [Google Scholar] [CrossRef] [PubMed]
- McAnena, P.; Tanriverdi, K.; Curran, C.; Gilligan, K.; Freedman, J.E.; Brown, J.A.L.; Kerin, M.J. Circulating microRNAs miR-331 and miR-195 differentiate local luminal a from metastatic breast cancer. BMC Cancer 2019, 19, 436. [Google Scholar] [CrossRef]
- Luo, C.; Yin, D.; Zhan, H.; Borjigin, U.; Li, C.; Zhou, Z.; Hu, Z.; Wang, P.; Sun, Q.; Fan, J.; et al. microRNA-501-3p suppresses metastasis and progression of hepatocellular carcinoma through targeting LIN7A. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Hong, B.S.; Ryu, H.S.; Kim, N.; Kim, J.; Lee, E.; Moon, H.; Kim, K.H.; Jin, M.-S.; Kwon, N.H.; Kim, S.; et al. Tumor suppressor microRNA-204-5p regulates growth, metastasis, and immune microenvironment remodeling in breast cancer. Cancer Res. 2019, 79, 1520–1534. [Google Scholar] [CrossRef]
- Fu, X.-T.; Shi, Y.-H.; Zhou, J.; Peng, Y.-F.; Liu, W.-R.; Shi, G.-M.; Gao, Q.; Wang, X.-Y.; Song, K.; Fan, J.; et al. MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett. 2018, 412, 108–117. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Colden, M.; Dar, A.A.; Saini, S.; Dahiya, P.V.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; Stein, G.; Dahiya, R.; Majid, S. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2018, 8, e2572. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Das Mahapatra, K.; Pasquali, L.; Crudden, C.; Kular, L.; Ulum, Y.Z.A.; Zhang, L.; Landén, N.X.; Girnita, L.; Jagodic, M.; et al. Genome-Wide Screen for MicroRNAs Reveals a Role for miR-203 in Melanoma Metastasis. J. Investig. Dermatol. 2018, 138, 882–892. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Z.; Shang, L.; Luo, Y.; Lin, Y.; Yuan, Y.; Zhuang, S. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 2018, 68, 1459–1475. [Google Scholar] [CrossRef]
- Ke, J.; Shao, W.; Jiang, Y.; Xu, J.; Li, F.; Qin, J. MicroRNA-103 regulates tumorigenesis in colorectal cancer by targeting ZO-1. Mol. Med. Rep. 2018, 17, 783–788. [Google Scholar] [CrossRef]
- De Oliveira, J.G.; Marques, J.H.D.M.; Lacerda, J.Z.; Ferreira, L.C.; Coelho, M.M.C.; Zuccari, D.A.P.D.C. Melatonin down-regulates microRNA-10a and decreases invasion and migration of triple-negative breast cancer cells. Melatonin Res. 2019, 2, 86–99. [Google Scholar] [CrossRef]
- Wang, H.; Tan, Z.; Hu, H.; Liu, H.; Wu, T.; Zheng, C.; Wang, X.; Luo, Z.; Wang, J.; Liu, S.; et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, H.; Ren, F.; Feng, W.; Cao, Y.; Li, G.; Liu, Z.; Ji, P.; Zhang, M. MicroRNA-338-3p suppresses ovarian cancer cells growth and metastasis: Implication of Wnt/catenin beta and MEK/ERK signaling pathways. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grisard, E.; Coan, M.; Cesaratto, L.; Rigo, I.; Zandonà, L.; Paulitti, A.; Andreuzzi, E.; Vinciguerra, G.L.R.; Poletto, E.; Del Ben, F.; et al. Sleeping beauty genetic screen identifies miR-23b::BTBD7 gene interaction as crucial for colorectal cancer metastasis. EBioMedicine 2019, 46, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Xue, A.; Chi, Y.; Xue, J.; Wang, W.; Zhao, Z.; Fan, M.; Yang, C.H.; Shao, Z.-M.; Pfeffer, L.; et al. Induction of miRNA-181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene 2016, 35, 1302–1313. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, X.; Liu, Z.; Zhou, Z.; Wang, Y.; Tu, J.; Li, L.; Bao, H.; Yang, L.; Tu, K. MicroRNA-1296 inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting SRPK1-mediated PI3K/AKT pathway. Mol. Cancer 2017, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gimple, R.C.; Wang, X. RAS: Striking at the Core of the Oncogenic Circuitry. Front. Oncol. 2019, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Chen, M.; Assanah, M.; Canoll, P.; Manley, J. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 2009, 463, 364–368. [Google Scholar] [CrossRef]
- Shim, H.; Dolde, C.; Lewis, B.C.; Wu, C.-S.; Dang, G.; Jungmann, R.A.; Dalla-Favera, R.; Dang, C.V. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA 1997, 94, 6658–6663. [Google Scholar] [CrossRef]
- Wise, D.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Schwartzenberg-Bar-Yoseph, F.; Armoni, M.; Karnieli, E. The Tumor Suppressor p53 Down-Regulates Glucose Transporters GLUT1 and GLUT4 Gene Expression. Cancer Res. 2004, 64, 2627–2633. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, H.; Wu, F.; Zhang, Y.; Wang, J.; Zhao, L.; Guo, X.; Chang, L.-J.; Zhang, Y.; You, M.J.; et al. Hexokinase 2-Mediated Warburg Effect Is Required for PTEN- and p53-Deficiency-Driven Prostate Cancer Growth. Cell Rep. 2014, 8, 1461–1474. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zhao, Y.; Yue, X.; Wu, H.; Huang, S.; Chen, J.; Tomsky, K.; Xie, H.; Khella, C.A.; et al. Parkin targets HIF-1α for ubiquitination and degradation to inhibit breast tumor progression. Nat. Commun. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wieman, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine Stimulation Promotes Glucose Uptake via Phosphatidylinositol-3 Kinase/Akt Regulation of Glut1 Activity and Trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar] [CrossRef]
- Wu, N.; Zheng, B.; Shaywitz, A.; Dagon, Y.; Tower, C.; Bellinger, G.; Shen, C.-H.; Wen, J.; Asara, J.; McGraw, T.E.; et al. AMPK-Dependent Degradation of TXNIP upon Energy Stress Leads to Enhanced Glucose Uptake via GLUT1. Mol. Cell 2013, 49, 1167–1175. [Google Scholar] [CrossRef]
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of glut1 mRNA by hypoxia-inducible factor-1 Interaction between H-ras and hypoxia. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef]
- He, G.; Jiang, Y.; Zhang, B.; Wu, G. The effect of HIF-1alpha on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac. J. Clin. Nutr. 2014, 23, 174. [Google Scholar]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Aimee, Y.Y. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Maxwell, P.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.; Wykoff, C.C.; Pugh, C.; Maher, E.; Ratcliffe, P. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Soga, T. Cancer metabolism: Key players in metabolic reprogramming. Cancer Sci. 2013, 104, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, Q.; Halim, A.; Song, G. Targeting lipid metabolism of cancer cells: A promising therapeutic strategy for cancer. Cancer Lett. 2017, 401, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tsun, Z.-Y.; Possemato, R. Amino acid management in cancer. Semin. Cell Dev. Biol. 2015, 22–32. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, C.; Chu, W.; Zhang, B.; Zhen, Q.; Wang, R.; Zhang, Y.; Li, Z.; Lv, B.; Li, H. MicroRNA-124 suppresses proliferation and glycolysis in non–small cell lung cancer cells by targeting AKT–GLUT1/HKII. Tumor Biol. 2017, 39, 1010428317706215. [Google Scholar] [CrossRef]
- Zhu, B.; Cao, X.; Zhang, W.; Pan, G.; Yi, Q.; Zhong, W.; Yan, D. MicroRNA-31-5p enhances the Warburg effect via targeting FIH. FASEB J. 2019, 33, 545–556. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, Y.; Pan, Q.; Xiang, P.; Xie, N.; Yu, H. MicroRNA-98 Suppress Warburg Effect by Targeting HK2 in Colon Cancer Cells. Dig. Dis. Sci. 2017, 62, 660–668. [Google Scholar] [CrossRef]
- Minami, K.; Taniguchi, K.; Sugito, N.; Kuranaga, Y.; Inamoto, T.; Takahara, K.; Takai, T.; Yoshikawa, Y.; Kiyama, S.; Akao, Y.; et al. MiR-145 negatively regulates Warburg effect by silencing KLF4 and PTBP1 in bladder cancer cells. Oncotarget 2017, 8, 33064–33077. [Google Scholar] [CrossRef]
- Li, B.; He, L.; Zuo, D.; He, W.; Wang, Y.; Zhang, Y.; Liu, W.; Yuan, Y. Mutual Regulation of MiR-199a-5p and HIF-1α Modulates the Warburg Effect in Hepatocellular Carcinoma. J. Cancer 2017, 8, 940–949. [Google Scholar] [CrossRef]
- Chen, H.; Gao, S.; Cheng, C. MiR-323a-3p suppressed the glycolysis of osteosarcoma via targeting LDHA. Hum. Cell 2018, 31, 300–309. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Li, Y.; Fan, J.; Chen, L.; Xu, R. MicroRNA-153 regulates glutamine metabolism in glioblastoma through targeting glutaminase. Tumor Biol. 2017, 39, 1010428317691429. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Bai, R.; Yang, K.; Tian, Z. MiR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1α regulation. Oncogenesis 2016, 5, e224. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wu, X.; Zhou, W.; Fong, M.Y.; Cao, M.; Liu, J.; Liu, X.; Chen, C.-H.; Fadare, O.; Pizzo, D.P.; et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nature 2018, 20, 597–609. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Lim, S.; Yu, S.; Ku, G. microRNA 181a-5p Reprogramed Glucose and Lipid Metabolism in Non-Small Cell Lung Cancer. J. Cancer Sci. Clin. Oncol. 2018, 5, 205. [Google Scholar]
- Yang, Y.; Gabra, M.B.I.; Hanse, E.A.; Lowman, X.H.; Tran, T.Q.; Li, H.; Milman, N.; Liu, J.; Reid, M.; Locasale, J.W.; et al. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Xu, F.; Yan, J.-J.; Gan, Y.; Chang, Y.; Wang, H.-L.; He, X.-X.; Zhao, Q. miR-885-5p Negatively Regulates Warburg Effect by Silencing Hexokinase 2 in Liver Cancer. Mol. Ther.-Nucleic Acids 2019, 18, 308–319. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Calin, G.; Lopez-Berestein, G.; Sood, A.K. miRNA Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discov. 2016, 6, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Soon, P.; Kiaris, H. MicroRNAs in the tumour microenvironment: Big role for small players. Endocr.-Relat. Cancer 2013, 20, R257–R267. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Zou, L.; Zhu, Z. Role of Exosomes in Crosstalk between Cancer-Associated Fibroblasts and Cancer Cells. Front. Oncol. 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, K.; Cui, J.; Yuan, D.; Zou, B.; Li, J.; Liu, J.; Li, K.; Meng, Z.; Zhang, B. Cancer-associated fibroblast-derived exosomal miR-382-5p promotes the migration and invasion of oral squamous cell carcinoma. Oncol. Rep. 2019, 42, 1319–1328. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Tao, Y.-W.; Gao, S.; Li, P.; Zheng, J.-M.; Zhang, S.-E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, B.; Ye, J.; Cao, S.; Shi, J.; Zhao, Y.; Wang, Y.; Sang, J.; Yao, Y.; Guan, W.; et al. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int. J. Biol. Sci. 2019, 15, 2320–2329. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Y.; Yu, W.; Yan, Y.; Qiao, M.; Jiang, R.; Guan, W.; Wang, L. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J. Exp. Clin. Cancer Res. 2019, 38, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, H.; Chen, S.; Sun, D.; Zhang, D.; He, Y. Exosomal transfer of miR-124 inhibits normal fibroblasts to cancer-associated fibroblasts transition by targeting sphingosine kinase 1 in ovarian cancer. J. Cell. Biochem. 2019, 120, 13187–13201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhong, J.-H.; Gong, F.-S.; Xiao, J. MiR-141-3p suppresses gastric cancer induced transition of normal fibroblast and BMSC to cancer-associated fibroblasts via targeting STAT4. Exp. Mol. Pathol. 2019, 107, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Oh, E.-T.; Kim, J.M.; Park, J.-S.; Lee, D.H.; Lee, J.-S.; Kim, K.K.; Park, H.J. Hypoxia-induced microRNA-590-5p promotes colorectal cancer progression by modulating matrix metalloproteinase activity. Cancer Lett. 2018, 416, 31–41. [Google Scholar] [CrossRef]
- Zheng, H.; Bi, F.-R.; Yang, Y.; Hong, Y.-G.; Ni, J.-S.; Ma, L.; Liu, M.-H.; Hao, L.-Q.; Zhou, W.-P.; Song, L.-H.; et al. Downregulation of miR-196-5p Induced by Hypoxia Drives Tumorigenesis and Metastasis in Hepatocellular Carcinoma. Horm. Cancer 2019, 10, 177–189. [Google Scholar] [CrossRef]
- Ni, J.; Zhou, S.; Yuan, W.; Cen, F.; Yan, Q. Mechanism of miR-210 involved in epithelial–mesenchymal transition of pancreatic cancer cells under hypoxia. J. Recept. Signal Transduct. 2019, 39, 399–406. [Google Scholar] [CrossRef]
- Angel, C.Z.; Lynch, S.M.; Nesbitt, H.; McKenna, M.M.; Walsh, C.P.; McKenna, D.J. miR-210 is induced by hypoxia and regulates neural cell adhesion molecule in prostate cells. J. Cell. Physiol. 2020, 235, 6194–6203. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, H.; Dai, B.; Li, J.; Shang, L.; Huang, J.; Shi, X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J. Exp. Clin. Cancer Res. 2018, 37, 1–18. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Yan, M.; Shi, J.; Xu, Q.; Li, Z.; Yang, W.; Zhang, J.; Chen, W. Loss of exosomal miR-3188 in cancer-associated fibroblasts contributes to HNC progression. J. Exp. Clin. Cancer Res. 2019, 38, 1–23. [Google Scholar] [CrossRef]
- Medeiros, M.; Ribeiro, A.; Lupi, L.A.; Romualdo, G.R.; Pinhal, D.; Chuffa, L.G.D.A.; Delella, F.K. Mimicking the tumor microenvironment: Fibroblasts reduce miR-29b expression and increase the motility of ovarian cancer cells in a co-culture model. Biochem. Biophys. Res. Commun. 2019, 516, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.; Liu, W.; Li, X. SNHG3 Functions as miRNA Sponge to Promote Breast Cancer Cells Growth through the Metabolic Reprogramming. Appl. Biochem. Biotechnol. 2020, 191, 1084–1099. [Google Scholar] [CrossRef]
- Vu, L.T.; Peng, B.; Zhang, D.X.; Ma, V.; Mathey-Andrews, C.A.; Lam, C.K.; Kiomourtzis, T.; Jin, J.; McReynolds, L.; Huang, L.; et al. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J. Extracell. Vesicles 2019, 8, 1599680. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guan, X.; Zhang, Y.; Ge, S.; Zhang, L.; Li, H.; Wang, X.; Liu, R.; Ning, T.; Deng, T.; et al. Exosomal miR-27a Derived from Gastric Cancer Cells Regulates the Transformation of Fibroblasts into Cancer-Associated Fibroblasts. Cell. Physiol. Biochem. 2018, 49, 869–883. [Google Scholar] [CrossRef]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Qin, X.; Guo, H.; Wang, X.; Zhu, X.; Yan, M.; Wang, X.; Xu, Q.; Shi, J.; Lu, E.; Chen, W.; et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019, 20, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J.; Fu, Z.; Dong, L.; Tang, Y.; Xu, C.; Wang, H.; Zhang, T.; Wu, Y.; Dong, C.; et al. Hypoxia-induced microRNA-10b-3p promotes esophageal squamous cell carcinoma growth and metastasis by targeting TSGA10. Aging 2019, 11, 10374–10384. [Google Scholar] [CrossRef]
- McCoach, C.E.; Bivona, T.G. The evolving understanding of immunoediting and the clinical impact of immune escape. J. Thorac. Dis. 2018, 10, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Eichmüller, S.; Osen, W.; Mandelboim, O.; Seliger, B. Immune Modulatory microRNAs Involved in Tumor Attack and Tumor Immune Escape. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.N.; Frank, A.-C.; Raue, R.; Brüne, B. MicroRNA—A Tumor Trojan Horse for Tumor-Associated Macrophages. Cells 2019, 8, 1482. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.-M.; Luo, Y.-P. MicroRNAs in tumor immunity: Functional regulation in tumor-associated macrophages. J. Zhejiang Univ. Sci. B 2019, 21, 12–28. [Google Scholar] [CrossRef]
- Omar, H.A.; El-Serafi, A.T.; Hersi, F.; Arafa, E.A.; Zaher, D.M.; Madkour, M.; Arab, H.H.; Tolba, M.F. Immunomodulatory MicroRNAs in cancer: Targeting immune checkpoints and the tumor microenvironment. FEBS J. 2019, 286, 3540–3557. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In Vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Sahraei, M.; Chaube, B.; Liu, Y.; Sun, J.; Kaplan, A.; Price, N.; Ding, W.; Oyaghire, S.; García-Milian, R.; Mehta, S.; et al. Suppressing miR-21 activity in tumor-associated macrophages promotes an antitumor immune response. J. Clin. Investig. 2019, 129, 5518–5536. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Pateras, I.S.; Jenkins, L.M.; Patel, K.M.; Robles, A.I.; Morris, J.; Forshew, T.; Appella, E.; Gorgoulis, V.; Harris, C.C. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Wu, X.; Zhang, T.; Zhu, Q.; Wang, X.; Wang, H.; Wang, K.; Lin, Y.; Wang, X. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol. Res. 2018, 6, 1578–1592. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, Z.; Cong, L.; Zhao, S.; Li, Y.; Wang, X.; Lv, Y.; Zhu, Y.; Dong, J. MicroRNA-148b-colony-stimulating factor-1 signaling-induced tumor-associated macrophage infiltration promotes hepatocellular carcinoma metastasis. Biomed. Pharmacother. 2019, 120, 109523. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, X.; Li, W.; Feng, Q.; Feng, H.; Tong, Y.; Rong, H.; Wang, W.; Zhang, D.; Zhang, Z.; et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag. Res. 2019, 11, 4023–4040. [Google Scholar] [CrossRef]
- Ding, L.; Li, Q.; Chakrabarti, J.; Muñoz, A.; Faure-Kumar, E.; Ocadiz-Ruiz, J.R.; Razumilava, N.; Zhang, G.; Hayes, M.H.; A Sontz, R.; et al. MiR130b from Schlafen4+ MDSCs stimulates epithelial proliferation and correlates with preneoplastic changes prior to gastric cancer. Gut 2020, 69, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Vallacchi, V.; Fleming, V.; Hu, X.; Cova, A.; Dugo, M.; Shahaj, E.; Sulsenti, R.; Vergani, E.; Filipazzi, P.; et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J. Clin. Investig. 2018, 128, 5505–5516. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, W.; Liu, Q.; Qian, M.; Wang, S.; Zhang, Z.; Gao, X.; Chen, Z.; Xue, H.; Li, G. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene 2018, 37, 4239–4259. [Google Scholar] [CrossRef]
- Tang, S.; Fu, H.; Xu, Q.; Zhou, Y. miR-20a regulates sensitivity of colorectal cancer cells to NK cells by targeting MICA. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Liu, J.; Zhang, Z.; Mao, X.; Zhou, H. MicroRNA-130a enhances the killing ability of natural killer cells against non-small cell lung cancer cells by targeting signal transducers and activators of transcription 3. Biochem. Biophys. Res. Commun. 2020, 523, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, J.; Hu, Y.; Tang, X.; Yu, L.; Dong, L.; Chen, D. MiR-218-5p Suppresses the Killing Effect of Natural Killer Cell to Lung Adenocarcinoma by Targeting SHMT1. Yonsei Med. J. 2019, 60, 500–508. [Google Scholar] [CrossRef]
- Nanbakhsh, A.; Srinivasamani, A.; Holzhauer, S.; Riese, M.J.; Zheng, Y.; Wang, D.; Burns, R.; Reimer, M.H.; Rao, S.; Lemke, A.; et al. Mirc11 Disrupts Inflammatory but Not Cytotoxic Responses of NK Cells. Cancer Immunol. Res. 2019, 7, 1647–1662. [Google Scholar] [CrossRef]
- Chen, E.-B.; Zhou, Z.-J.; Xiao, K.; Zhu, G.-Q.; Yang, Y.; Wang, B.; Zhou, S.-L.; Chen, Q.; Yin, D.; Wang, Z.; et al. The miR-561-5p/CX3CL1 Signaling Axis Regulates Pulmonary Metastasis in Hepatocellular Carcinoma Involving CX3CR1+ Natural Killer Cells Infiltration. Theranostics 2019, 9, 4779–4794. [Google Scholar] [CrossRef]
- Ye, S.-B.; Zhang, H.; Cai, T.-T.; Liu, Y.-N.; Ni, J.-J.; He, J.; Peng, J.-Y.; Chen, Q.-Y.; Mo, H.-Y.; Cui, J.; et al. Exosomal miR-24-3p impedes T-cell function by targetingFGF11and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016, 240, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, Q.; Liu, W.; Zhou, H.; Zang, X.; Lu, J. MicroRNA-140 suppresses Helicobacter pylori-positive gastric cancer growth by enhancing the antitumor immune response. Mol. Med. Rep. 2019, 20, 2484–2492. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, D.; Shi, Y.; Wang, Y.; Joshi, R.; Yu, Q.; Liu, D.; Alotaibi, F.; Zhang, Y.; Wang, H.; et al. miR-149-3p reverses CD8 + T-cell exhaustion by reducing inhibitory receptors and promoting cytokine secretion in breast cancer cells. Open Biol. 2019, 9, 190061. [Google Scholar] [CrossRef]
- Lou, Q.; Liu, R.-X.; Yang, X.; Li, W.; Huang, L.; Wei, L.; Tan, H.; Xiang, N.; Chan, K.; Chen, J.; et al. miR-448 targets IDO1 and regulates CD8+ T cell response in human colon cancer. J. Immunother. Cancer 2019, 7, 210. [Google Scholar] [CrossRef]
- Yuan, C.; Xiang, L.; Bai, R.; Cao, K.; Gao, Y.; Jiang, X.; Zhang, N.; Gong, Y.; Xie, C. MiR-195 restrains lung adenocarcinoma by regulating CD4+ T cell activation via the CCDC88C/Wnt signaling pathway: A study based on the Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO) and bioinformatic analysis. Ann. Transl. Med. 2019, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. The dualistic origin of human tumors. Semin. Cancer Biol. 2018, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Murray, D. Roles of Polyploid/Multinucleated Giant Cancer Cells in Metastasis and Disease Relapse Following Anticancer Treatment. Cancers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Intratumor Heterogeneity and Therapy Resistance: Contributions of Dormancy, Apoptosis Reversal (Anastasis) and Cell Fusion to Disease Recurrence. Int. J. Mol. Sci. 2020, 21, 1308. [Google Scholar] [CrossRef]
- War, A.R.; Dang, K.; Jiang, S.; Xiao, Z.; Miao, Z.; Yang, T.; Li, Y.; Qian, A. Role of cancer stem cells in the development of giant cell tumor of bone. Cancer Cell Int. 2020, 20, 135–137. [Google Scholar] [CrossRef]
- Xu, Y.; So, C.; Lam, H.-M.; Fung, M.-C.; Tsang, S.-Y. Apoptosis Reversal Promotes Cancer Stem Cell-Like Cell Formation. Neoplasia 2018, 20, 295–303. [Google Scholar] [CrossRef]
- Zaitceva, V.; Kopeina, G.; Zhivotovsky, B. Anastasis: Return Journey from Cell Death. Cancers 2021, 13, 3671. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chen, Y.; Zhou, X.; Yao, Q.; Chen, Y.; Zhou, X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]


| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-150 | Cervical cancer | FOXO4/PI3K-Akt | Downregulates FOXO4 level and promotes cell cycle progression from G1 to S phase | [21] |
| 2 | miR-132 | Ovarian cancer | E2F5/Rb | Inhibits cell proliferation by targeting E2F5 | [22] |
| 3 | miR-424 | Endometrial cancer | E2F7/Rb | Abrogates cell cycle progression mediated by E2F7 downregulation | [23] |
| 4 | miR-133a | Hepatocellular carcinoma, osteosarcoma | IGF-1R/PI3K/Akt, MAPK/ERK | Negatively regulates IGF-1R and contributes to impaired ERK and Akt signaling pathways, which leads to reduced cell proliferation | [24,25] |
| 5 | miR-183-5p | Breast cancer | PDCD4 | Downregulates the levels of p21 and p27 by targeting PDCD4 | [26] |
| 6 | miR-217 | Acute myeloid leukemia | KRAS/MAPK | Inhibits KRAS, which contributes to cell proliferation | [27] |
| 7 | miR-141 | Nasopharyngeal carcinoma | PTEN/PI3K/AKT | Attenuation of cell proliferation occurs by suppressing PTEN, which impairs Akt activation | [28] |
| 8 | miR-136 | Prostate cancer | MAP2K4/MAPK | Suppresses cell growth by inhibiting MAP2K4 | [29] |
| 9 | miR-124 | Breast cancer | STAT3/JAK | Impairs cell proliferation by negatively regulating STAT3 | [30] |
| 10 | miR-623 | Gastric cancer | Cyclin D1 (CCND1) | Renders cell cycle progression impaired by inhibiting CCND1 | [31] |
| 11 | miR-129 | Glioblastoma | CDK4, CDK6 | Overexpression of miR-129 disrupts cell proliferation by downregulating CDK4 and CDK6 | [32] |
| 12 | miR-93 | Osteosarcoma | CDKN1A (P21) | miR-93 supports cell cycle progression by inhibiting p21 | [33] |
| 13 | miR-196a | Laryngeal cancer | CDKN1B (P27) | Promotes cell growth by suppressing p27Kip1 | [34] |
| 14 | miR-497 | Multiple myeloma | Raf-1/MAPK | Overexpression of miR-497 suppresses cell proliferation by downregulating Raf-1 | [35] |
| 15 | miR-411 | Non-small cell lung cancer | SPRY4/Akt | Promotes cell proliferation by inducing Akt activation, which is suppressed by SPRY4 | [36] |
| 16 | miR-101 | Diffuse large B cell lymphoma (DLBL) | MAPK kinase 1 (MEK) | Abrogates cell proliferation by inhibiting MEK1 | [18] |
| 17 | miR-20a | Multiple myeloma | PTEN | Negatively regulates PTEN, leading to AKT activation and cell proliferation | [19] |
| 18 | miR-590 | Acute lymphoblastic leukemia (ALL) | pRB (Retinoblastoma) | Targets and downregulates Rb, leading to an increase in cell proliferation | [20] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-138 | Small cell lung cancer | H2AX | miR-138 overexpression inhibits DNA damage repair by suppressing H2AX. | [49] |
| 2 | miR-383 | Epidermoid carcinoma | ATR | Downregulates the expression of ATR, leading to defective DNA repair. Its overexpression also inhibits other DNA repair markers such as MDC-1 and GADD45 | [50] |
| 3 | miR-182 | Acute myelogenous leukemia | Rad51 | Impairs homologous recombination repair by negatively regulating Rad51 | [51] |
| 4 | miR-145 | Colorectal cancer | Rad18 | Negatively regulates Rad18, thereby enhancing DNA damage | [52] |
| 5 | miR-212 | Glioma | BRCA1 | Suppresses BRCA1, which positively regulates DNA damage repair | [53] |
| 6 | miR-205-5p | Head and neck squamous cell carcinoma | BRCA1, Rad17 | Abrogates DNA repair activity by downregulating DNA repair genes BRCA1 and Rad17 | [54] |
| 7 | miR-191 | Osteosarcoma | Checkpoint kinase 2 (Chk2) | Inhibits Chk2, which is crucial in DDR | [55] |
| 8 | miR-142-3p | Uveal melanoma | Cdc25c | Impairs cell cycle arrest induced by Cdc25c | [56] |
| 9 | miR-33b-3p | Non-small cell lung cancer | P21 | Promotes DNA damage repair by downregulating p21 | [57] |
| 10 | miR-338-5p | Glioblastoma | PP2R5a | PP2R5a, a negative regulator of ATM, is inhibited, thereby promoting DNA repair | [58] |
| 11 | miR-7-5p | Small cell lung cancer Cervical cancer | PARP1 | Abrogates DNA repair by downregulating PARP1 | [46,47] |
| 12 | miR-203a-3p | Ovarian cancer | ATM | Promotes cell cycle arrest by inhibiting ATM | [48] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-26 | Hepatocellular carcinoma | ULK1 | Abrogates autophagy initiation step by inhibiting ULK1 | [65] |
| 2 | miR-30a | Renal cell carcinoma | Beclin1 | Negatively regulates Beclin1 and inhibits autophagy | [66] |
| 3 | miR-181 | Gastric cancer | ATG5 | Impairs autophagosome formation by downregulating ATG5 | [67] |
| 4 | miR-20 | Breast cancer | FIP200 | ULK1 complex formation is impaired by suppressing FIP200 | [68] |
| 5 | miR-183 | Colorectal cancer | UVRAG | Overexpression of miR-183 inhibits UVRAG, which is needed for autophagy initiation | [69] |
| 6 | miR-224-3p | Glioblastoma | ATG5, FIP200 | Downregulates the expression of ATG5 and FIP200 | [70] |
| 7 | miR-34c-5p | Cervical cancer | ATG4B | Negatively regulates ATG4B, which is necessary for autophagosome formation | [71] |
| 8 | miR-93 | Pediatric leukemia | Beclin1 | Impairs autophagy by downregulating Beclin1 expression | [72] |
| 9 | miR-124 | Retinoblastoma | STX17 | Directly targets and suppresses STX17, which aids in the fusion of lysosomes with autophagosomes | [73] |
| 10 | miR-1 | Non-small cell lung cancer | ATG3 | Abolish autophagy by downregulating ATG3 involved in conjugation machinery | [74] |
| 11 | miR-423-5p | Hepatocellular carcinoma | Not identified in the study | Promotes autophagy by increasing ATG7 and LC3-II levels | [61] |
| Gastric cancer | BIM | Downregulates BIM, a negative regulator of Beclin1, and promotes autophagy | [63] | ||
| 12 | miR-409-5p | Ovarian cancer | FIP200 | Attenuates autophagy by inhibiting FIP200 | [64] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-16 | Breast cancer | BCL-2 | Abrogates the antiapoptotic effect of BCL-2 by downregulating it | [81] |
| 2 | miR-137 | Ovarian cancer | XIAP | Negatively regulates XIAP, a caspase inhibitor | [82] |
| 3 | miR-345 | Pancreatic cancer | BCL-2 | Promotes apoptosis by suppressing the antiapoptotic protein BCL-2 | [83] |
| 4 | miR-488 | Osteosarcoma | BIM | Impairs apoptosis by targeting the apoptosis mediator BIM | [84] |
| 5 | miR-96 | Papillary thyroid carcinoma | FOXO1/BIM axis | Indirect apoptosis suppression by negatively regulating FOXO1, as BIM is involved in downstream signalling of AKT/FOXO1 pathway | [85] |
| 6 | miR-101 | Hepatocellular carcinoma | MCL-1 | Promotes apoptosis by inhibiting antiapoptotic protein MCL-1 | [86] |
| 7 | miR-365 | Cutaneous squamous cell carcinoma | BAX | Impairs apoptosis by downregulating pro-apoptotic protein BAX | [87] |
| 8 | miR-149-5p | Acute myeloid leukemia | FASLG (Fas ligand) | Abrogates extrinsic apoptosis by negatively regulating FASLG | [88] |
| 9 | miR-199 | Acute myeloid leukemia | CASP3 (caspase 3) | Impairs apoptosis by downregulating caspase 3 | [89] |
| 10 | miR-675 | Gastric cancer | FADD | Inhibits apoptosis by negatively regulating FADD | [90] |
| 11 | miR-224 | Breast cancer | CASP9 | Directly targets and impairs CASP9, attenuating apoptosis | [78] |
| 12 | miR-CHA1 | Non-small cell lung cancer | XIAP | Promotes apoptosis by downregulating XIAP | [79] |
| 13 | miR-484 | Non-small cell lung cancer | APAF1 | Impairs apoptosis by targeting APAF1 | [80] |
| No | miRNA | Cancer | Target Gene | Action | Source |
|---|---|---|---|---|---|
| 1 | miR-130b~301b cluster | Prostate cancer | CDKN1A, CDKN1B, CDKN2A | Promotes cellular senescence by upregulating the expression of CDK inhibitors such as CDKN1A, CDKN1B and CDKN2A | [96] |
| 2 | miR-126 | B Cell Precursor Acute Lymphoblastic Leukemia (B-ALL) | P53-dependent pathway | Evades senescence by reducing the activity of p53 via targeting various p53 upstream or downstream regulators | [97] |
| 3 | miR-132 | Gastric cancer | pRB | Abrogates senescence by negatively regulating pRB | [98] |
| 4 | miR-106b | Gastric cancer | CDKN1A (p21) | Impairs cellular senescence by negatively regulating CDKN1A | [99] |
| 5 | miR-494-5p | Oral squamous carcinoma | Bmi-1 | Inhibits cellular senescence by suppressing Bmi-1 | [100] |
| 6 | miR-203 | Cervical cancer | KLF4/Survivin/p21 | KLF4 induces miR-203 expression which inhibits survivin and upregulates p21, thereby inducing senescence | [101] |
| 7 | miR-137 | Pancreatic cancer | KDM4A(lysine demethylase 4A)/p53/pRB | miR-137 induces pRB expression and inhibits KDM4A, a negative regulator of p53, thereby inducing senescence | [102] |
| 8 | miR-34a | Non-small cell lung cancer (NSCLC) | c-MYC | Promotes senescence by negatively regulating c-MYC, an oncogene | [103] |
| 9 | miR-128 | Glioma | Bmi-1 | Promotes senescence by downregulating Bmi-1 | [94] |
| 10 | miR-30 | Osteosarcoma | CHD7 TNRC6A | Evades senescence by downregulating CHD7 (cotranscriptional activator of p16) and TNRC6A (player in p53 activation) | [95] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-124-3p | Glioblastoma | NRP-1, transcriptional | Overexpression leads to the attenuation of angiogenesis | [119] |
| 2 | miR-526b/miR-655 | Breast cancer | PTEN tumor suppressor, transcriptional | Overexpression improved angiogenesis suggesting roles as oncomiR via PTEN-regulated HIF1-α pathway | [120] |
| 3 | miR-9 | Nasopharyngeal Carcinoma | MDK, exosomal secretion | Suppression of miR-9 in patient suggest its role as oncomiR. Overexpression attenuated tubal formation HUVECs | [121] |
| 4 | miR-205 | Ovarian Cancer | PTEN tumor suppressor, exosomal secretion | Treatment of HUVECs with miR-205 exosome leads to an increase in tubal formation | [122] |
| 5 | miR-6868-5p | Colorectal Cancer | FOXM1, transcriptional | Overexpression leads to the reduction in endothelial tubal formation | [123] |
| 6 | miR-143-3p | Gallbladder Carcinoma | ITGA6, transcriptional | Suppression was observed in bad overall survival patients. Overexpression leads to increased tubal formation | [124] |
| 7 | miR-130b | Prostate cancer | TNF-α, transcriptional | Inhibition leads to attenuation of VEGFA, a downstream target of TNF-α suppressing angiogenesis | [125] |
| 8 | mR-23a | Nasopharyngeal Carcinoma | TSGA10, exosomal secretion | Exosomal overexpression enhanced angiogenesis | [126] |
| 9 | miR-21 | Renal cell carcinoma | PCD4, proteomal | Inhibition of miR-21 attenuated MMP levels, besides inhibiting angiogenesis | [127] |
| 10 | miR-574-5p | Gastric Cancer Cells | PTPN3 proteomal | Binds to PTPN3, enhancing ERK/JNK activity and driving angiogenesis | [128] |
| 11 | miR-27a | Pancreatic Cancer | BTG2, Exosomal | miR-27a was highly expressed in cancer tissue. Exosomal mir-27a stimulates HMVEC tubal formation. | [129] |
| 12 | miR-155 | Gastric Carcinoma | C-MYB/, Exosomal | Stimulates VEGF expression, leading to enhanced angiogenesis observed on HUVEC | [130] |
| 13 | miR-183-5p | Colorectal Cancer | FOXO1, Exosomal | CRC-derived- exosome enhanced tubal formation of HMEC-1 cells | [131] |
| 14 | miR-619-5p | Non-Small Cell Lung Cancer | RCAN1.4, Exosomal | Mimic transfection and leads to the increase in HUVEC tube length and tube abundance | [132] |
| 15 | miR-3064-5p | Hepatocellular carcinoma | FOXA1, transcriptional | Overexpression improves overall survival of mice and reduces tumor size; angiogenic factor suppression observed | [133] |
| 16 | miR-141 | Pancreatic cancer | TM5SF1 transcriptional | Angiogenic factors were induced following inhibition of miR-141 | [134] |
| 17 | miR-195 | Squamous cell lung cancer | VEGF transcriptional | miRNA-195 attenuates tubal formation | [135] |
| 18 | miR-136 | Gall Bladder cancer | MAP2K4 transcriptional | Mimic treatment resulted in activation of angiopoiesis | [136] |
| 19 | miR-302 | Chronic Myeloid leukemia | VEGFA, secretome | Low expression was associated with bad OS. Treatment of K562 media on HUVECS attenuate capillary formation | [137] |
| 20 | miR-148a miR-30 | Glioblastoma | FIH1 | Regulates HIF1-α via binding directly to its inhibitor FIH1 and attenuating vascularization | [116] |
| 21 | miR-29b | Breast cancer | AKT3 | Overexpression resulted in the attenuation of vascularization by downregulating AKT3, which is crucial for VEGF activation | [138] |
| 22 | miR-140-5p | Breast cancer | VEGFA | Abrogates vascularization by binding and attenuating VEGFA | [139] |
| 23 | miR-1 | Gastric cancer | VEGFA | Inhibition of miR-1 leads to accumulation of VEGFA | [140] |
| 24 | miR-30d | Prostate cancer | MYPT1 | Downregulation resulted in the attenuation of angiogenesis, leading to reduction in endothelial capillary tube formation | [141] |
| 25 | miR-210 | Hepatocellular carcinoma | SMAD4, STAT6 | Promote angiogenesis by inhibiting SMAD4 and STAT6 | [118] |
| Cell Junctions | Adhering Factors | Implication in Cancer Metastases | Reference |
|---|---|---|---|
| Gap Junction | Connexin 43 | Brain cancer cells secrete cGAMP to astrocytes via connexion 43 channels, leading to STAT1-NF-κB-mediated metastasis | [152] |
| Zonula Occluden (ZO-1) | ZO-1 was downregulated following overexpression of upstream regulator ZIP4 expression. This induced tumor migration | [153] | |
| Tight Junction | Claudin | Crucial for cell anchorage. Breast cancer cells with high Claudin-2 have higher liver metastatic potential | [154] |
| Occludin | Occludin upregulation suppresses metastatic potential of squamous cell carcinoma | [155] | |
| Adherens Junction | Cadherins | Deletion of e-cadherin results in the development of both local and distant metastasis | [156] |
| Catenins | Β-catenin nuclear localization is crucial for ZEB1 transcriptional activation, which negatively regulates metastasis | [157] | |
| Desmosomes | Desmoglein 2 | Loss of desmoglein enhances tumor invasiveness and migration | [158] |
| Armadillo repeat units containing proteins (ARM)/plakoglobin | It was found that inhibition of proteins with the ARM structure and plakoglobin enhances the metastatic ability of bladder cancer and lung cancer, respectively | [159,160] | |
| Desmocollin | Desmocollin 3 downregulation leads to Akt pathway activation and decreases e-cadherin abundance in colorectal cancer, thereby enhancing metastatic potential. | [161] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-501-3p | Hepatocellular Carcinoma | LIN7A | Metastatic cell line downregulates miR-501-3p. Overexpression of the miR inhibits metastases and EMT | [164] |
| 2 | miR-204-5p | Breast cancer | PIK3CB | miR was found to be downregulated in tumor samples. Overexpression of miR leads to metastatic attenuation in mice | [165] |
| 3 | miR-30a | Hepatocellular Carcinoma | Beclin1 and ATG5 | miR-30a mediates anoikis (detachment mediated cell death) by inhibiting Beclin1 and Atg5; however, the loss of miR-30a expression in HCC leads to EMT and metastases | [166] |
| 4 | miR-193a-3p/miR-210-3p/miR5100 | Bone Marrow Mesenchymal Stem cells | Exosomal miRNA targeting breast cancer metastases | Exosomal miRNA secretion of BMSC influences breast cancer metastatic potential | [167] |
| 5 | miR-466 | Prostate cancer | RUNX2 | Inhibition of miR-466 leads to tumorigenic properties and enhances bone metastases via RUNX2 accumulation | [168] |
| 6 | miR-203 | Melanoma | SLUG | Poor OS of patients with low miR-203 expression. miR-203 overexpression leads to attenuation of early and late metastases | [169] |
| 7 | miR-103 | Hepatocellular Carcinoma | VE-cadherin/ZO-1 | Exosomal secretion of miR103 by HC attenuated the tight junction, resulting in an increase in metastatic potential | [170] |
| 8 | miR-103 | Colorectal cancer | Zonula Occludin-1 (ZO-1) | miR-103 binds directly to the 3′-UTR of ZO-1, suggesting its role in metastases via targeting the gap junction factor | [171] |
| 9 | miR-10a | Breast cancer | Affect EMT via e- cadherin/vimentin | miR-10a suppression inhibited vimentin, disrupting the EMT pathway | [172] |
| 10 | miR-21 | Breast Cancer | LZTFL1 | Tumor removal via surgery reduces miR-21 expression. Suppression of miR-21 leads to attenuation of metastases; overexpression mediates metastasis in vivo | [173] |
| 11 | miR-338-3p | Ovarian cancer cells | MACC1 | miR-338-3p induces metastasis via inhibiting MACC1 expression | [174] |
| 12 | miR-27b | Colorectal cancer | BTBD7 | miR-27b controls the post-metastatic process via binding to BTBD7 | [175] |
| 13 | miR-30 family (miR-30a, miR-30b, miR-30c, miR-30d, miR-30e) | Breast cancer | IL8, IL11, DKK-1, RUNX2, CDH11, CTGF, ITGA5, ITGB3 | Overexpression of miR abrogates bone invasion and osteomimicry | [162] |
| 14 | miR-181a | Breast cancer | BAX | Downregulation of miR leads to metastasis inhibition | [176] |
| 15 | miR-1296 | Hepatocellular carcinoma | SRPK1 | Results in metastasis attenuation by downregulating Akt, a downstream effector of SRPK1 | [177] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-98 | Colon cancer | Hexokinase 2 (HK2) | Impairs aerobic glycolysis by inhibiting glycolytic enzyme hexokinase 2 | [201] |
| 2 | miR-145 | Bladder cancer | KLF4 | Negatively regulates KLF4, a transcriptional activator of PTBP1 that regulates PKM2, which contributes to the Warburg effect | [202] |
| 3 | miR-199a | Hepatocellular carcinoma | HIF-1a | Suppresses Warburg effect by inhibiting HIF-1a | [203] |
| 4 | miR-323a-5p | Osteosarcoma | Lactate dehydrogenase A (LDHA) | Disrupts glycolytic pathway through the inhibition of LDHA | [204] |
| 5 | miR-153 | Glioblastoma | Glutaminase (GLS) | Abrogates glutamine utilization by downregulating GLS | [205] |
| 6 | miR-186 | Gastric cancer | HIF-1a | Inhibits aerobic glycolysis by negatively regulating HIF-1a | [206] |
| 7 | miR-105 | Breast cancer | Max-interacting protein 1 (MAXI1)/MYC | MAXI1, a transcriptional repressor of MYC, is inhibited by miR-105, thereby enhancing glucose and glutamine metabolism | [207] |
| 8 | miR-181a-5p | Non-small cell lung cancer | Acyl-CoA synthetase long-chain family member 4 (ACSL4),Sirtuin 1 (SIRT1) | Impairs lipid metabolism by inhibiting ACSL4 and abrogates glucose metabolism by suppressing SIRT1, a negative regulator of p53 | [208] |
| 9 | miR-135 | Pancreatic ductal adenocarcinoma (PDAC) | Phosphofructokinase-1 (PFK1) | Targets and downregulates glycolytic enzyme PFK1 and impairs aerobic glycolysis | [209] |
| 10 | miR-885-5p | Hepatocellular carcinoma | Hexokinase 2 (HK2) | Attenuates the Warburg effect by downregulating HK2 | [210] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| Cancer-associated fibroblasts (CAFs) | |||||
| 1 | miR-382-5p | Oral squamous cell carcinoma (OSCC) | Target not identified in study | Promotes the migration and invasion capabilities of OSCC | [214] |
| 2 | miR-34a-5p | Oral squamous cell carcinoma (OSCC) | AXL | Represses OCSS proliferation and metastasis | [215] |
| 3 | miR-139 | Gastric cancer | Matrix metalloproteinase 11 (MMP11) | Represses the progression and development of metastasis of gastric cancer by modulating the level of MMP11 | [216] |
| 4 | miR-214 | Gastric cancer | FGF9 | miR-214 is downregulated in CAFs of GC. Mimetics leads to expression of E-cadherin and suppression of Vimentin, N-cadherin and Snail, denoting repression of EMT of GC cells | [217] |
| 5 | miR-124 | Ovarian cancer | Sphingosine kinase 1 (SPHK1) | Downregulates α-SMA and FAP expression to arrest cellular motility | [218] |
| 6 | miR-141-3p | Gastric cancer | STAT4 | Inhibits the migration and invasion of gastric cancer and suppresses the conversion of normal fibroblasts and BMSC into CAFs by targeting regulation of the STAT4/wnt/β-catenin pathway | [219] |
| 7 | miR-21 | Hepatocellular carcinoma (HCC) | PTEN | Activates the PDK1/AKT pathway in hepatic stellate cells | [224] |
| 8 | miR-3188 | Head and neck cancer (HNC) | B cell lymphoma 2 (BCL2) | Regulates the proliferation and apoptosis of HNC by targeting BCL2 in vitro and in vivo. | [225] |
| 9 | miR-29b | Ovarian cancer (SKOV-3 cells) | MMP-2 | Remodels the extracellular matrix and induces changes to cellular motility | [226] |
| 10 | miR-330-5p | Breast cancer | Pyruvate Kinase M1/M2 (PKM) | Represses glycolysis metabolism and cell proliferation | [227] |
| 11 | miR-125b | Breast cancer (4Ti and 4TO7) | TP53 and TP53INP1 | Enhances the levels of multiple CAFs markers in resident fibroblasts leading to activation of CAF phenotypes | [228] |
| 12 | miR-27a | Gastric cancer | CSRP2 | Transform fibroblasts into CAFs and enhances proliferation, motility and metastasis of tumor cells in vitro and in vivo | [229] |
| 13 | miR-1247-3p | Hepatocellular carcinoma (HCC) | B4GALT3 | Activates the β1-integrin-NF-κB signaling in fibroblasts for conversion into CAFs, which secrete pro-inflammatory cytokines to enhance tumor progression | [230] |
| 14 | miR-196a | Head and neck cancer (HNC) | CDKN1B and ING5 | Confers cisplatin resistance to HNC | [231] |
| Hypoxia | |||||
| 15 | miR-590-5p | Colorectal cancer (CRC) | RECK | Enhances invasive and migratory ability of cancer cells by activating matrix metalloproteinases (MMPs) and filopodia protrusions | [220] |
| 16 | miR-196-5p | Hepatocellular carcinoma (HCC) | High-mobility group AT-hook 2 (HMGA2) | Regulates the expression of HMGA2 for the proliferation and metastasis of HCC | [221] |
| 17 | miR-10b-3p | Esophageal squamous cell carcinoma (ESCC) | Testis specific 10 (TSGA10) | Increases the proliferation, migration and invasion of ESCC in both in vitro and in vivo models | [232] |
| 18 | miR-210 | Pancreatic cancer (PANC-1) | HOXA9 | Suppresses levels of HOXA9 to activate NFκB pathway, which drives EMT in pancreatic cancer | [222] |
| 19 | miR-210 | Prostate cancer | Neural cell adhesion molecule (NCAM) | Is induced by hypoxia and functions to regulate NCAM for the progression of prostate cancer | [223] |
| 20 | miR-885-5p | Hepatocellular carcinoma (HCC) | Hexokinase 2 (HK2) | Regulates the glycometabolic activity of cancer cells via suppression of glycolytic enzymes | [210] |
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| Tumor-associated macrophages (TAMs) | |||||
| 1 | miR-1246 | Colon cancer | Upregulated CCL2, ADAM12, MMP2, CCL7Downregulated IL17A, IL7R, LEF1, S1PR1, BCL2, CD96 | Reprograms macrophages to an anti-inflammatory, immunosuppressive state | [240] |
| 2 | miR-21 | Lung carcinoma | IL-12 | Macrophage-mediated enhancement of T cell cytotoxicity via expression of cytokines and chemokines | [239] |
| 3 | miR-29a-3p and miR-21-5p | Epithelial ovarian cancer (EOC) and TAMs | STAT3 | Inhibits expression of STAT3 in CD4+ T cells and induces imbalance of Tregs/Th17 cell regulation | [241] |
| 4 | miR-148b | Hepatocellular carcinoma (HCC) | Colony-stimulating factor 1 (CSF1) | Progressive growth and metastasis of HCC via CSF1/CSF1 receptor-mediated TAM infiltration | [242] |
| 5 | miR-107 | Gastric cancer | DICER1 and PTEN | Regulates MDSC proliferation and activation of the PI3K pathway | [243] |
| 6 | miR-130b | Spasmolytic polypeptide-expressing metaplasia (SPEM) | Cylindromatosis (Cyld) gene | Induces NFκb activity and suppresses T cell proliferation | [244] |
| 7 | miR-99b, miR-100, miR-125a, miR-125b, miR-146a, miR-146b, miR-155, let-7e | Melanomas | TLR4, SHIP-1, PTEN, SOCS1, Lin28A | Myeloid cell differentiation and polarization through pathways linked to tumor-associated immunosuppression | [245] |
| 8 | miR-10a, miR-21 | Glioma | RAR-related orphan receptor alpha (RORA), phosphatase and tensin homolog (PTEN) | Initiation of MDSC-induced immunosuppressive microenvironment | [246] |
| Natural killer (NK) cells | |||||
| 9 | miR-20a | Colorectal cancer (CRC) | NKG2D ligand Major Histocompatibility Complex (MHC) class-I-related chain gene A (MICA) | Downregulates NKG2D ligand MICA levels in CRC, which promotes CRC proliferation | [247] |
| 10 | miR-130a | Non-small cell lung cancer (NSCLC) | STAT3 | Increases the cytotoxic capability of NK cells by targeting STAT3 of NSCLC cells | [248] |
| 11 | miR-218-5p | Lung adenocarcinoma (LA) | Serine hydroxymethyl transferase 1 (SHMT1) | Downregulates IL-2-induced cytokine levels and cytotoxicity of NK towards LA | [249] |
| 12 | miRNA-23a, miRNA-24a, miRNA-27a | Melanoma | Ubiquitin modifiers A20, Cbl-b, and Itch | Activation of NF-κB and AP-1 via TRAF6 | [250] |
| 13 | miR-561-5p | Hepatocellular carcinoma (HCC) | CX3CL1 | Attenuate anticancer activity of CX3CG1+ NK cells via downregulation of CX3CL1 | [251] |
| T cells | |||||
| 14 | miR-24-3p | Nasopharyngeal carcinoma (NPC) | FGF11 | Upregulates P-ERK, P-STAT1 and P-STAT3 levels and downregulates P-STAT5 levels during T cell propagation and differentiation | [252] |
| 15 | miR-140 | Gastric cancers | PD-L1 | Overexpression leads to increase in cytotoxic CD8+ T cells and reductions in MDSC and Tregs in the immediate tumor microenvironment | [253] |
| 16 | miR-149-3p | Breast tumor | T cell inhibitor receptors PD-1, TIM-3, BTLA and Foxp1 | Increase T cell inhibitor receptor expression, reduction of apoptosis and secretion of effector cytokines, including IL-2, TNF-α and IFN-γ | [254] |
| 17 | miR-448 | Colon cancer | Indoleamine 2,3-dioxygenase 1 (IDO1) | Regulates posttranscriptional levels of IDO1 protein and mRNA, inhibits apoptosis of CD8+ T cells by reducing IDO1 enzyme activity | [255] |
| 18 | miR-195 | Lung adenocarcinoma | CCDC88C/Wnt | Regulates CCDC88C expression for CD4+ T cell activation | [256] |
| No | miRNA | Overlap Hallmark | Cancer Types | References |
|---|---|---|---|---|
| 1 | miR-132 |
| Ovarian cancer Gastric cancer | [22] [98] |
| 2 | miR-183 |
| Breast cancer Colorectal cancer Colorectal cancer | [26] [69] [131] |
| 3 | miR-141 |
| Nasopharyngeal carcinoma Pancreatic cancer Gastric cancer | [28] [134] [219] |
| 4 | miR-136 |
| Prostate cancer Gall bladder cancer | [29] [136] |
| 5 | miR-124 |
| Breast cancer Retinoblastoma Glioblastoma Ovarian cancer | [30] [73] [119] [218] |
| 6 | miR-93 |
| Osteosarcoma Leukemia | [60] [72] |
| 7 | miR-196a |
| Laryngeal cancer Head and neck cancer | [34] [231] |
| 8 | miR-145 |
| Colorectal cancer Bladder cancer | [52] [202] |
| 9 | miR-205 |
| Head and neck squamous cell carcinoma Ovarian cancer | [54] [122] |
| 10 | miR-338 |
| Glioblastoma Ovarian cancer | [58] [174] |
| 11 | miR-30a |
| Renal cell carcinoma Hepatocellular carcinoma | [66] [166] |
| 12 | miR-181 |
| Gastric cancer Non-small cell lung cancer Breast cancer | [67] [208] [176] |
| 13 | miR-20 |
| Multiple myeloma Breast cancer Colorectal cancer | [19] [96] [247] |
| 14 | miR-224 |
| Glioblastoma Breast cancer | [70] [78] |
| 15 | miR-1 |
| Non-small cell lung cancer Gastric cancer | [74] [140] |
| 16 | miR-137 |
| Ovarian cancer Pancreatic cancer | [82] [102] |
| 17 | miR-101 |
| Diffuse large B cell lymphoma Hepatocellular carcinoma | [18] [86] |
| 18 | miR-149 |
| Acute myeloid leukemia Breast cancer | [88] [254] |
| 19 | miR-199 |
| Acute myeloid leukemia Hepatocellular carcinoma | [89] [203] |
| 20 | miR-130b |
| Prostate cancer Prostate cancer Spasmolytic polypeptide-expressing metaplasia (SPEM) | [96] [125] [244] |
| 21 | miR-203 |
| Ovarian cancer Cervical cancer Melanoma | [48] [101] [169] |
| 22 | miR-34a |
| Non-small cell lung cancer Oral squamous cell carcinoma | [103] [215] |
| 23 | miR-21 |
| Renal cell carcinoma Breast cancer Hepatocellular carcinoma Lung carcinoma | [127] [173] [224] [239] |
| 24 | miR-27a |
| Pancreatic cancer Gastric cancer Melanoma | [129] [229] [250] |
| 25 | miR-155 |
| Gastric carcinoma Melanoma | [155] [245] |
| 26 | miR-195 |
| Squamous cell lung cancer Breast cancer Lung adenocarcinoma | [135] [163] [256] |
| 27 | miR-210 |
| Hepatocellular carcinoma Bone Marrow Mesenchymal Stem cells Pancreatic cancer Prostate cancer | [118] [167] [222] [223] |
| 28 | miR-10a |
| Breast cancer Glioma | [172] [246] |
| 29 | miR-29b |
| Breast cancer Ovarian cancer | [138] [226] |
| 30 | miR-125b |
| Breast cancer Melanoma | [228] [245] |
| 31 | miR-590 |
| T cell acute lymphoblastic leukemia Colorectal cancer | [20] [220] |
| 32 | miR-140 |
| Breast cancer Gastric cancer | [107] [253] |
| 33 | miR-885-5p |
| Hepatocellular carcinoma | [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajasegaran, Y.; Azlan, A.; Rosli, A.A.; Yik, M.Y.; Kang Zi, K.; Yusoff, N.M.; Moses, E.J. Footprints of microRNAs in Cancer Biology. Biomedicines 2021, 9, 1494. https://doi.org/10.3390/biomedicines9101494
Rajasegaran Y, Azlan A, Rosli AA, Yik MY, Kang Zi K, Yusoff NM, Moses EJ. Footprints of microRNAs in Cancer Biology. Biomedicines. 2021; 9(10):1494. https://doi.org/10.3390/biomedicines9101494
Chicago/Turabian StyleRajasegaran, Yaashini, Adam Azlan, Aliaa Arina Rosli, Mot Yee Yik, Khor Kang Zi, Narazah Mohd Yusoff, and Emmanuel Jairaj Moses. 2021. "Footprints of microRNAs in Cancer Biology" Biomedicines 9, no. 10: 1494. https://doi.org/10.3390/biomedicines9101494
APA StyleRajasegaran, Y., Azlan, A., Rosli, A. A., Yik, M. Y., Kang Zi, K., Yusoff, N. M., & Moses, E. J. (2021). Footprints of microRNAs in Cancer Biology. Biomedicines, 9(10), 1494. https://doi.org/10.3390/biomedicines9101494






