Unprecedented Monoterpenoid Polyprenylated Acylphloroglucinols with a Rare 6/6/5/4 Tetracyclic Core, Enhanced MCF-7 Cells’ Sensitivity to Camptothecin by Inhibiting the DNA Damage Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Plant Material
2.3. Extraction and Isolation
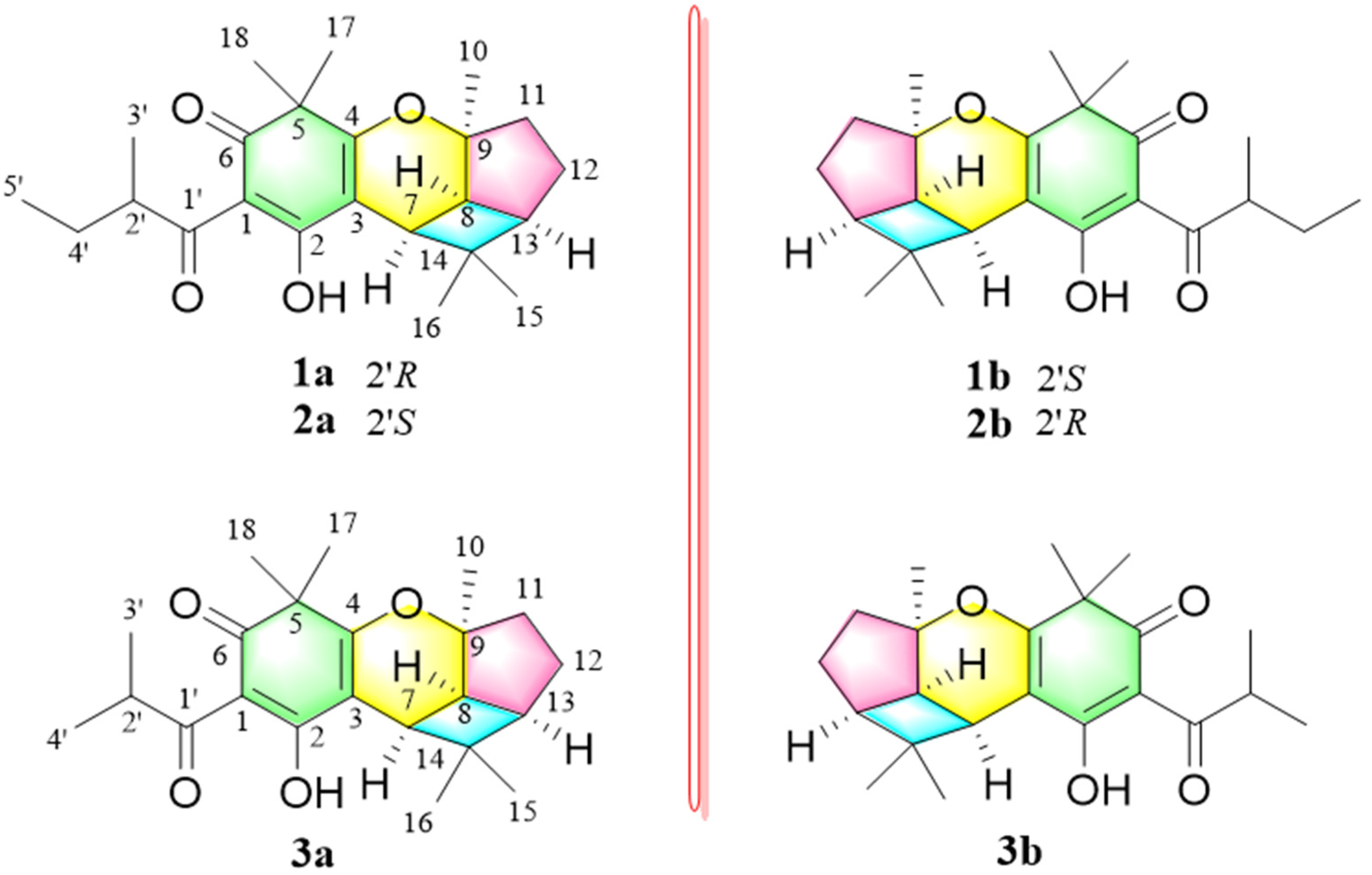
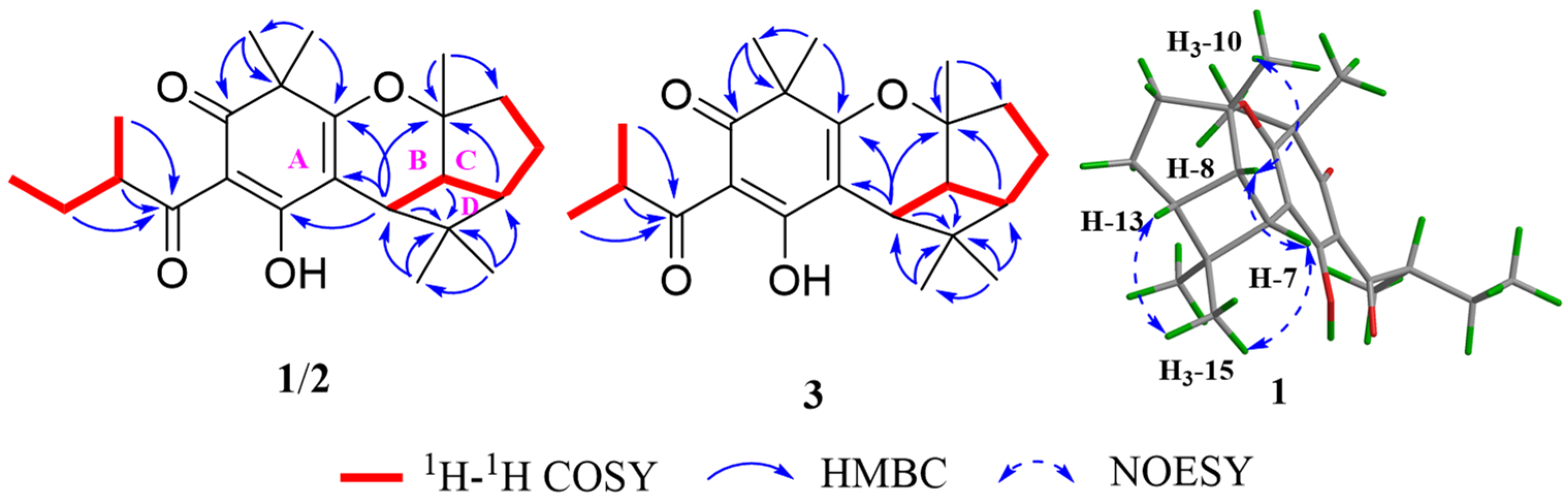
2.3.1. Hypersine A
2.3.2. Hypersine B
2.3.3. Hypersine C
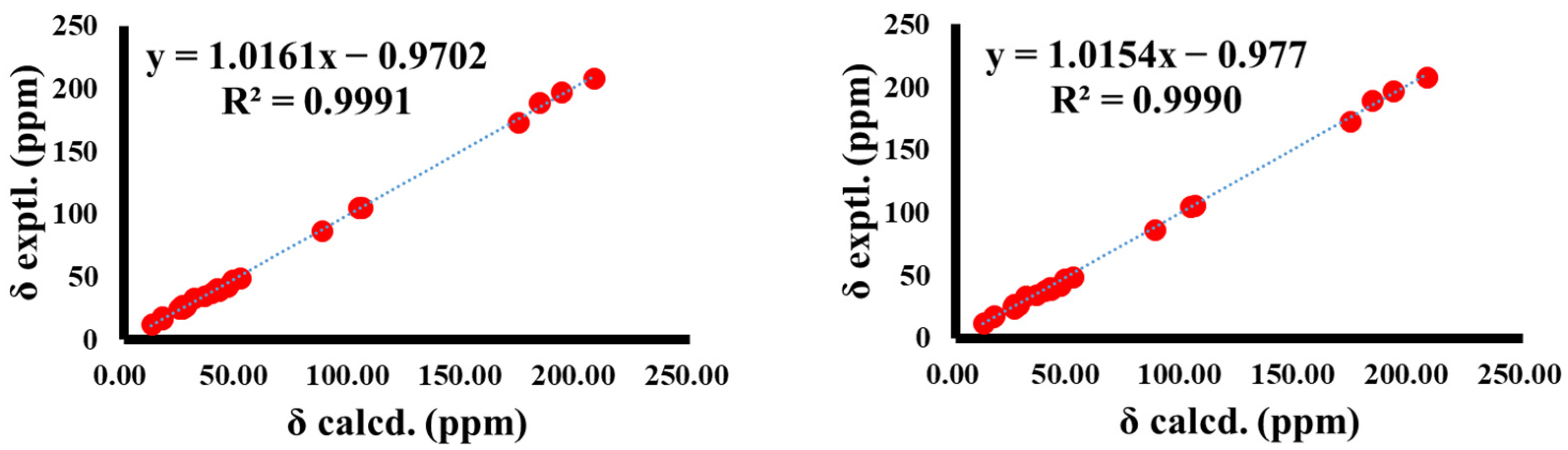
2.4. Quantum Chemical Calculation of 13C NMR
2.5. Quantum Chemical Calculation of ECD Spectra
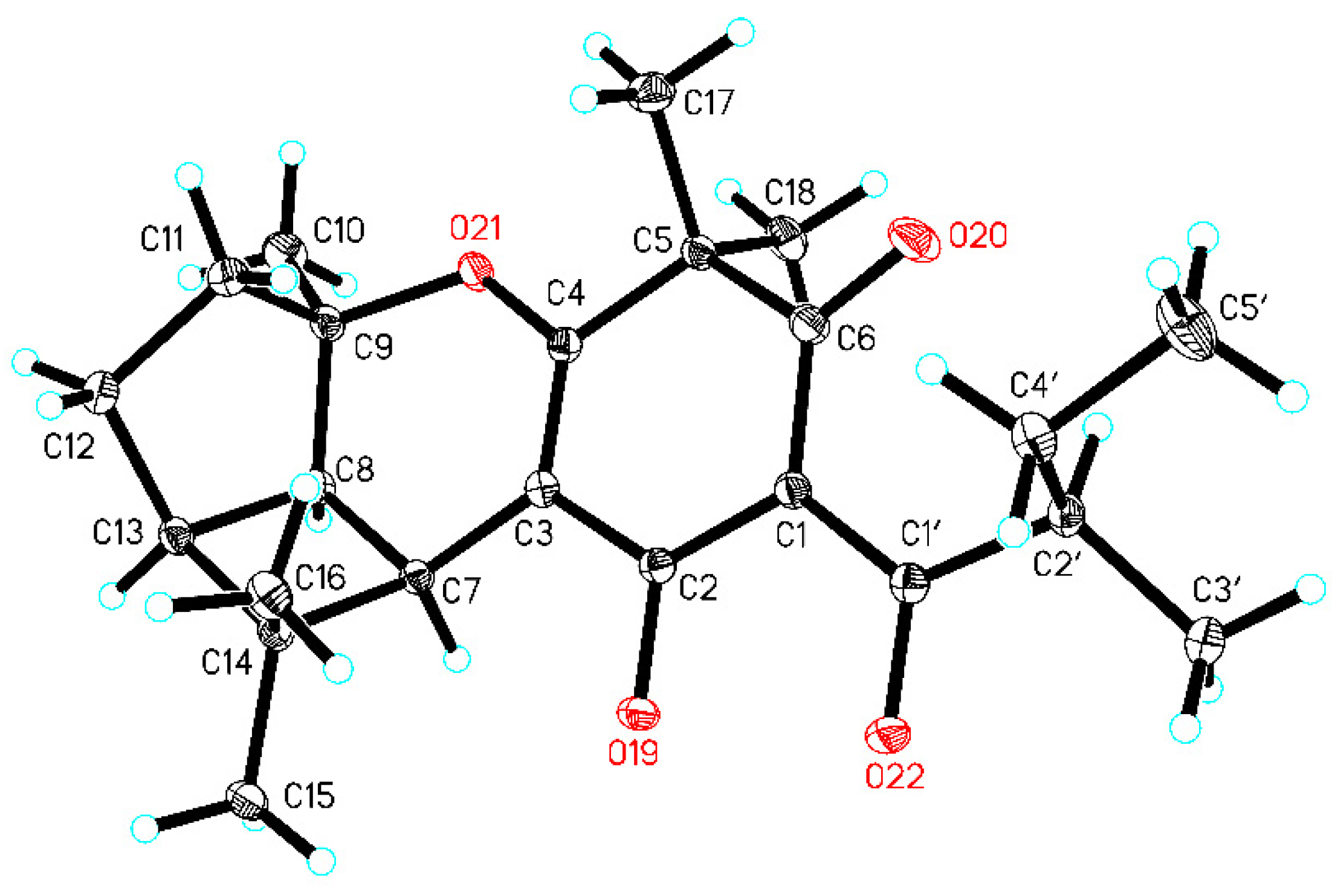
2.6. Single-Crystal X-ray Diffraction Analysis of 2b
2.7. Cell Culture
2.8. MTT Assay
2.9. Western Blotting
2.10. Cell Cycle Analysis
2.11. Statistical Analysis
3. Results
3.1. Isolation of Compounds 1–3
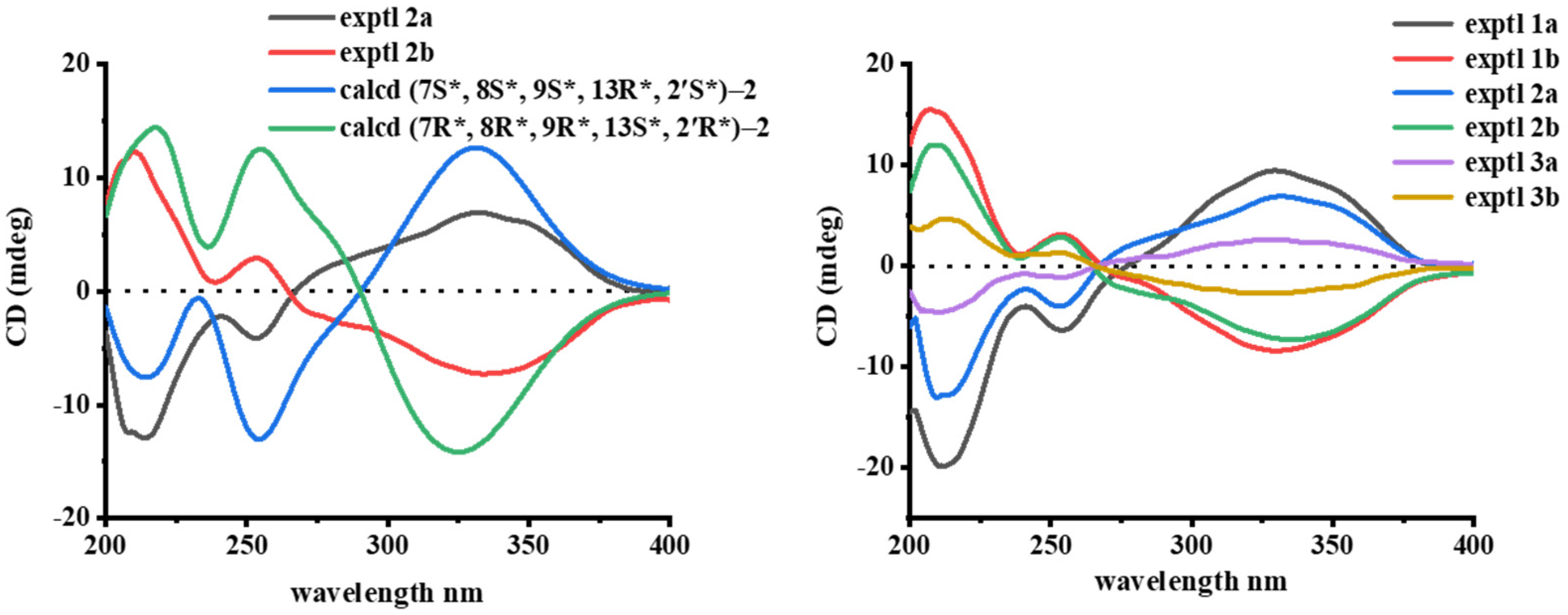
3.2. Structural Elucidation of Compounds 1−3
3.3. Plausible Biosynthetic Pathway to 1–3
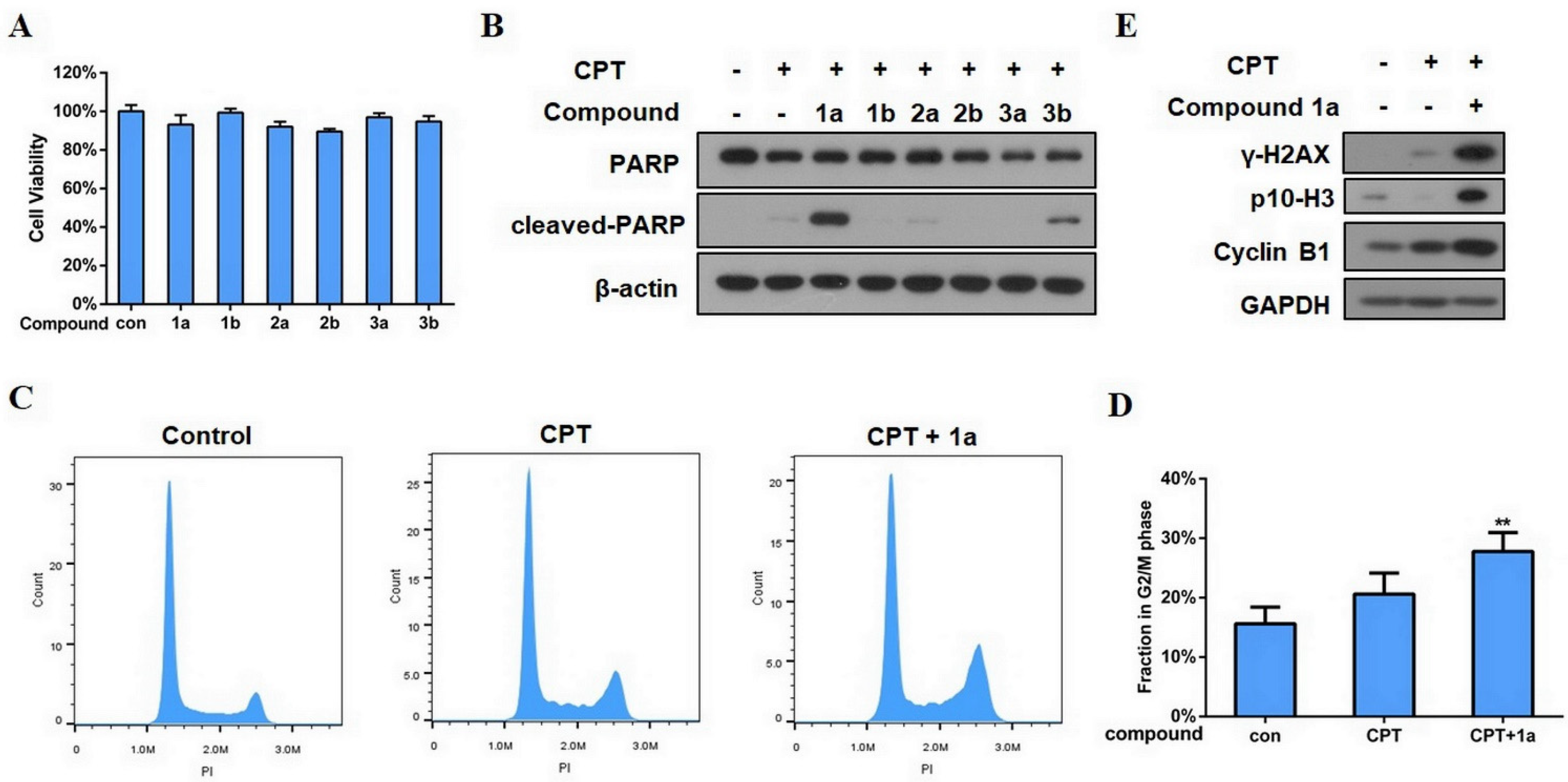
3.4. Biological Activity Evaluation of the Isolated Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tian, W.J.; Qiu, Y.Q.; Yao, X.J.; Chen, H.F.; Dai, Y.; Zhang, X.K.; Yao, X.S. Dioxasampsones A and B, two polycyclic polyprenylated acylphloroglucinols with unusual epoxy-ring-fused skeleton from Hypericum sampsonii. Org. Lett. 2014, 16, 6346–6349. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Grossman, R.B.; Xu, G. Research progress of polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2018, 118, 3508–3558. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.Y.; Li, Y.N.; Yi, P.; Jian, J.Y.; Hu, Z.X.; Gu, W.; Huang, L.J.; Li, Y.M.; Yuan, C.M.; Hao, X.J. Hyperfols A and B: Two highly modified polycyclic polyprenylated acylphloroglucinols from hypericum perforatum. Org. Lett. 2020, 22, 6903–6906. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xie, S.; Bu, P.; Guo, Y.; Shi, Z.; Guo, Y.; Cao, Y.; Sun, W.; Qi, C.; Zhang, Y. Hypaluton A, an immunosuppressive 3,4-nor-polycyclic polyprenylated acylphloroglucinol from hypericum patulum. J. Org. Chem. 2021, 86, 6478–6485. [Google Scholar] [CrossRef]
- Li, Y.R.; Xu, W.J.; Wei, S.S.; Lu, W.J.; Luo, J.; Kong, L.Y. Hyperbeanols F-Q, diverse monoterpenoid polyprenylated acylphloroglucinols from the flowers of hypericum beanie. Phytochemistry 2019, 159, 56–64. [Google Scholar] [CrossRef]
- Qiu, D.; Zhou, M.; Chen, J.; Wang, G.; Lin, T.; Huang, Y.; Yu, F.; Ding, R.; Sun, C.; Tian, W.; et al. Hyperelodiones A-C, monoterpenoid polyprenylated acylphoroglucinols from hypericum elodeoides, induce cancer cells apoptosis by targeting RXRα. Phytochemistry 2020, 170, 112216. [Google Scholar] [CrossRef]
- Zhang, Z.; Elsohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. Natural products inhibiting candida albicans secreted aspartic proteases from tovomita krukovii. Planta Medica 2002, 68, 49–54. [Google Scholar] [CrossRef]
- Crockett, S.L.; Wenzig, E.M.; Kunert, O.; Bauer, R. Anti-inflammatory phloroglucinol derivatives from hypericum empetrifolium. Phytochem Lett. 2008, 1, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Xue, Y.; Zhang, J.; Zhu, H.; Chen, C.; Li, X.N.; Liu, J.; Wang, Z.; Zhang, Y.; Zhang, Y. (+/-)-Japonicols A-D, acylphloroglucinol-based meroterpenoid enantiomers with anti-KSHV activities from hypericum japonicum. J. Nat. Prod. 2016, 79, 1322–1328. [Google Scholar] [CrossRef]
- Ao, Z.; Liu, Y.Y.; Lin, Y.L.; Chen, X.l.; Chen, K.; Kong, L.Y.; Luo, J.G. Hyperpatulones A and B, two new peroxide polyprenylated acylphloroglucinols from the leaves of hypericum patulum. Tetrahedron Lett. 2020, 61, 151385. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Li, Y.; Zhang, M.; Tang, P.; Lu, W.; Li, Q.; Zhang, H.; Luo, J.; Kong, L. Anti-inflammatory, antioxidant, and anti-nonalcoholic steatohepatitis acylphloroglucinol meroterpenoids from hypericum bellum flowers. J. Agric. Food Chem. 2021, 69, 646–654. [Google Scholar] [CrossRef]
- Mamemura, T.; Tanaka, N.; Shibazaki, A.; Gonoi, T.; Kobayashi, J.I. Yojironins A–D, meroterpenoids and prenylated acylphloroglucinols from Hypericum yojiroanum. Tetrahedron Lett. 2011, 52, 3575–3578. [Google Scholar] [CrossRef]
- Schmidt, S.; Jurgenliemk, G.; Schmidt, T.J.; Skaltsa, H.; Heilmann, J. Bi-, tri-, and polycyclic acylphloroglucinols from hypericum empetrifolium. J. Nat. Prod. 2012, 75, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Fobofou, S.A.; Franke, K.; Sanna, G.; Porzel, A.; Bullita, E.; la Colla, P.; Wessjohann, L.A. Isolation and anticancer, anthelminthic, and antiviral (HIV) activity of acylphloroglucinols, and regioselective synthesis of empetrifranzinans from hypericum roeperianum. Bioorg. Med. Chem. 2015, 23, 6327–6334. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Liu, J.; Sun, B.; Wei, G.; Li, Y.; Zhang, J.; Yao, G.; Luo, Z.; Xue, Y.; et al. Hyperascyrones A-H, polyprenylated spirocyclic acylphloroglucinol derivatives from hypericum ascyron linn. Phytochemistry 2015, 115, 222–230. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ao, Z.; Xue, G.M.; Wang, X.B.; Luo, J.G.; Kong, L.Y. Hypatulone A, a Homoadamantane-type acylphloroglucinol with an intricately caged core from hypericum patulum. Org. Lett. 2018, 20, 7953–7956. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-N.; Niu, Q.-W.; Zhang, Y.-B.; Luo, D.; Li, Q.-G.; Li, Y.-Y.; Kuang, G.-K.; He, L.-J.; Wang, G.-C.; Li, Y.-L. Hyperpatulones A–F, polycyclic polyprenylated acylphloroglucinols from hypericum patulum and their cytotoxic activities. RSC Adv. 2019, 9, 7961–7966. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.; Zhou, M.; Lin, T.; Chen, J.; Wang, G.; Huang, Y.; Jiang, X.; Tian, W.; Chen, H. Cytotoxic components from hypericum elodeoides targeting RXRα and inducing HeLa cell apoptosis through caspase-8 activation and PARP cleavage. J. Nat. Prod. 2019, 82, 1072–1080. [Google Scholar] [CrossRef]
- Li, Q.J.; Tang, P.F.; Zhou, X.; Lu, W.J.; Xu, W.J.; Luo, J.; Kong, L.Y. Dimethylated acylphloroglucinol meroterpenoids with anti-oral-bacterial and anti-inflammatory activities from hypericum elodeoides. Bioorg. Chem. 2020, 104, 104275. [Google Scholar] [CrossRef]
- Li, Q.J.; Tang, P.F.; Zhou, X.; Lu, W.J.; Xu, W.J.; Luo, J.; Kong, L.Y. Elodeoidins A–H, acylphloroglucinol meroterpenoids possessing diverse rearranged skeletons from hypericum elodeoides. Org. Chem. Front. 2021, 8, 1409–1414. [Google Scholar] [CrossRef]
- Qiu, D.R.; Zhou, M.; Liu, X.Z.; Chen, J.J.; Wang, G.H.; Lin, T.; Yu, F.R.; Ding, R.; Sun, C.L.; Tian, W.J.; et al. Cytotoxic polyprenylated phloroglucinol derivatives from hypericum elodeoides choisy modulating the transactivation of RXRα. Bioorganic Chem. 2021, 107, 104578. [Google Scholar] [CrossRef]
- Hashida, C.; Tanaka, N.; Kawazoe, K.; Murakami, K.; Sun, H.D.; Takaishi, Y.; Kashiwada, Y. Hypelodins A and B, polyprenylated benzophenones from hypericum elodeoides. J. Nat. Med. 2014, 68, 737–742. [Google Scholar] [CrossRef]
- Li, Y.P.; Yang, X.W.; Xia, F.; Yan, H.; Ma, W.G.; Xu, G. Hyperjapones F–I, terpenoid polymethylated acylphloroglucinols from hypericum japonicum. Tetrahedron Lett. 2016, 57, 5868–5871. [Google Scholar] [CrossRef]
- Yang, X.W.; Li, Y.P.; Su, J.; Ma, W.G.; Xu, G. Hyperjapones A-E, terpenoid polymethylated acylphloroglucinols from hypericum japonicum. Org. Lett. 2016, 18, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. C 01, Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Wolinski, K.; Hinton, J.F.; Pulay, P. 17O NMR chemical shifts of polyoxides in gas phase and in solution. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Li, F.; Kozono, D.; Deraska, P.; Branigan, T.; Dunn, C.; Zheng, X.F.; Parmar, K.; Nguyen, H.; DeCaprio, J.; Shapiro, G.I. CHK1 inhibitor blocks phosphorylation of FAM122A and promotes replication stress. Mol. Cell 2020, 80, 410–422.e6. [Google Scholar] [CrossRef] [PubMed]
- Lshiguro, K.; Yamak, M.; Kashihara, M.; Takagi, S.; Isoi, K. Sarothralin G: A new antimicrobial compound from hypericum japonicum. Planta Med. 1990, 56, 274–276. [Google Scholar] [CrossRef]
- Ishiguro, K.; Nagat, S.; Fukumoto, H.; Yamaki, M.; Isoi, K. Phloroglucinol derivatives from hypericum japonicum. Phytochemistry 1994, 35, 469–471. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Zhu, H.; Liu, J.; Li, H.; Li, X.N.; Sun, W.; Zeng, J.; Xue, Y.; Zhang, Y. Filicinic acid based meroterpenoids with anti-epstein-barr virus activities from hypericum japonicum. Org. Lett. 2016, 18, 2272–2275. [Google Scholar] [CrossRef]
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar] [CrossRef]
- Chopra, S.S.; Jenney, A.; Palmer, A.; Niepel, M.; Chung, M.; Mills, C.; Sivakumaren, S.C.; Liu, Q.; Chen, J.Y.; Yapp, C.; et al. Torin2 exploits replication and checkpoint vulnerabilities to cause death of pi3k-activated triple-negative breast cancer cells. Cell Syst. 2020, 10, 66–81.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, G.; Zhou, Y.; Tu, X.; Ye, X.; Xu, L.; Xiao, Z.; Wang, Q.; Wang, X.; Du, M.; Chen, Z.; et al. Centrosomal localization of rxralpha promotes plk1 activation and mitotic progression and constitutes a tumor vulnerability. Dev. Cell 2020, 55, 707–722.e9. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xu, H.; George, E.; Hallberg, D.; Kumar, S.; Jagannathan, V.; Medvedev, S.; Kinose, Y.; Devins, K.; Verma, P.; et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 2020, 11, 3726. [Google Scholar] [CrossRef] [PubMed]
- Carrassa, L.; Damia, G. DNA damage response inhibitors: Mechanisms and potential applications in cancer therapy. Cancer Treat Rev. 2017, 60, 139–151. [Google Scholar] [CrossRef]

| No. | 1a/1b | 2a/2b | 3a/3b | |||
|---|---|---|---|---|---|---|
| δH (mult, J, Hz) | δC | δH (mult, J, Hz) | δC | δH (mult, J, Hz) | δC | |
| 1 | 105.6 | 105.5 | 104.9 | |||
| 2 | 189.4 | 189.3 | 189.3 | |||
| 3 | 105.0 | 105.0 | 104.8 | |||
| 4 | 172.7 | 172.7 | 172.8 | |||
| 5 | 48.8 | 48.8 | 48.7 | |||
| 6 | 197.3 | 197.3 | 197.2 | |||
| 7 | 2.90 d (9.6) | 34.8 | 2.90 d (9.6) | 34.8 | 2.90 d (9.6) | 34.8 |
| 8 | 2.55 dd (9.6, 7.2) | 37.6 | 2.55 t (7.8) | 37.6 | 2.55 t (7.2) | 37.5 |
| 9 | 86.7 | 86.7 | 86.7 | |||
| 10 | 1.40 s | 27.4 | 1.40 s | 27.4 | 1.40 s | 27.4 |
| 11 | α 1.77 m | 40.4 | α 1.76 m | 40.4 | α 1.76 m | 40.3 |
| β 1.88 m | β 1.88 m | β 1.88 m | ||||
| 12 | α 1.61 m | 26.2 | α 1.61 m | 26.2 | α 1.61 m | 26.1 |
| β 1.70 m | β 1.70 m | β 1.68 m | ||||
| 13 | 2.42 t (7.8) | 47.1 | 2.42 t (7.2) | 47.1 | 2.43 t (7.8) | 47.0 |
| 14 | 38.8 | 38.8 | 38.8 | |||
| 15 | 1.34 s | 33.5 | 1.35 s | 33.5 | 1.34 s | 33.5 |
| 16 | 0.80 s | 18.1 | 0.80 s | 18.0 | 0.80 s | 18.0 |
| 17 | 1.32 s | 24.5 | 1.32 s | 24.1 | 1.32 s | 24.3 |
| 18 | 1.36 s | 25.2 | 1.36 s | 25.6 | 1.36 s | 25.5 |
| 1’ | 207.9 | 208.0 | 208.6 | |||
| 2’ | 3.84 m | 42.1 | 3.83 m | 42.2 | 3.97 m | 35.9 |
| 3’ | 1.14 d (7.2) | 16.7 | 1.12 d (7.2) | 16.7 | 1.13 d (7.2) | 19.1 |
| 4’ | α 1.40 overlap | 26.9 | α 1.41 overlap | 26.8 | 1.15 d (7.2) | 19.2 |
| β 1.73 m | β 1.74 m | |||||
| 5’ | 0.90 t (7.2) | 11.9 | 0.93 t (7.2) | 12.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-Z.; Zhou, M.; Du, C.-C.; Zhu, H.-H.; Lu, X.; He, S.-L.; Wang, G.-H.; Lin, T.; Tian, W.-J.; Chen, H.-F. Unprecedented Monoterpenoid Polyprenylated Acylphloroglucinols with a Rare 6/6/5/4 Tetracyclic Core, Enhanced MCF-7 Cells’ Sensitivity to Camptothecin by Inhibiting the DNA Damage Response. Biomedicines 2021, 9, 1473. https://doi.org/10.3390/biomedicines9101473
Liu X-Z, Zhou M, Du C-C, Zhu H-H, Lu X, He S-L, Wang G-H, Lin T, Tian W-J, Chen H-F. Unprecedented Monoterpenoid Polyprenylated Acylphloroglucinols with a Rare 6/6/5/4 Tetracyclic Core, Enhanced MCF-7 Cells’ Sensitivity to Camptothecin by Inhibiting the DNA Damage Response. Biomedicines. 2021; 9(10):1473. https://doi.org/10.3390/biomedicines9101473
Chicago/Turabian StyleLiu, Xiang-Zhong, Mi Zhou, Chun-Chun Du, Hong-Hong Zhu, Xi Lu, Shou-Lun He, Guang-Hui Wang, Ting Lin, Wen-Jing Tian, and Hai-Feng Chen. 2021. "Unprecedented Monoterpenoid Polyprenylated Acylphloroglucinols with a Rare 6/6/5/4 Tetracyclic Core, Enhanced MCF-7 Cells’ Sensitivity to Camptothecin by Inhibiting the DNA Damage Response" Biomedicines 9, no. 10: 1473. https://doi.org/10.3390/biomedicines9101473
APA StyleLiu, X.-Z., Zhou, M., Du, C.-C., Zhu, H.-H., Lu, X., He, S.-L., Wang, G.-H., Lin, T., Tian, W.-J., & Chen, H.-F. (2021). Unprecedented Monoterpenoid Polyprenylated Acylphloroglucinols with a Rare 6/6/5/4 Tetracyclic Core, Enhanced MCF-7 Cells’ Sensitivity to Camptothecin by Inhibiting the DNA Damage Response. Biomedicines, 9(10), 1473. https://doi.org/10.3390/biomedicines9101473






