Comparative Cancer Cell Signaling in Muscle-Invasive Urothelial Carcinoma of the Bladder in Dogs and Humans
Abstract
1. Introduction
1.1. Bladder Cancer and Its Treatment in Human Patients
1.2. Canine Patients as Naturally Occurring Models of Human MIUC
2. Muscle-Invasive Urothelial Carcinoma in the Dog
2.1. Standard Treatment for MIUC in Dogs
2.2. Similarities between Canine and Human MIUC
2.3. Differences between Canine and Human MIUC
3. Cell Cycle Regulation and Evasion of Apoptosis
3.1. p53 Family of Proteins—The Master Regulator of Cell Cycle
3.2. Evasion of Apoptosis—The Role of Survivin
3.3. Stratifin
3.4. Conclusions from the Comparative Analysis of Cell Cycle and Apoptosis Pathways
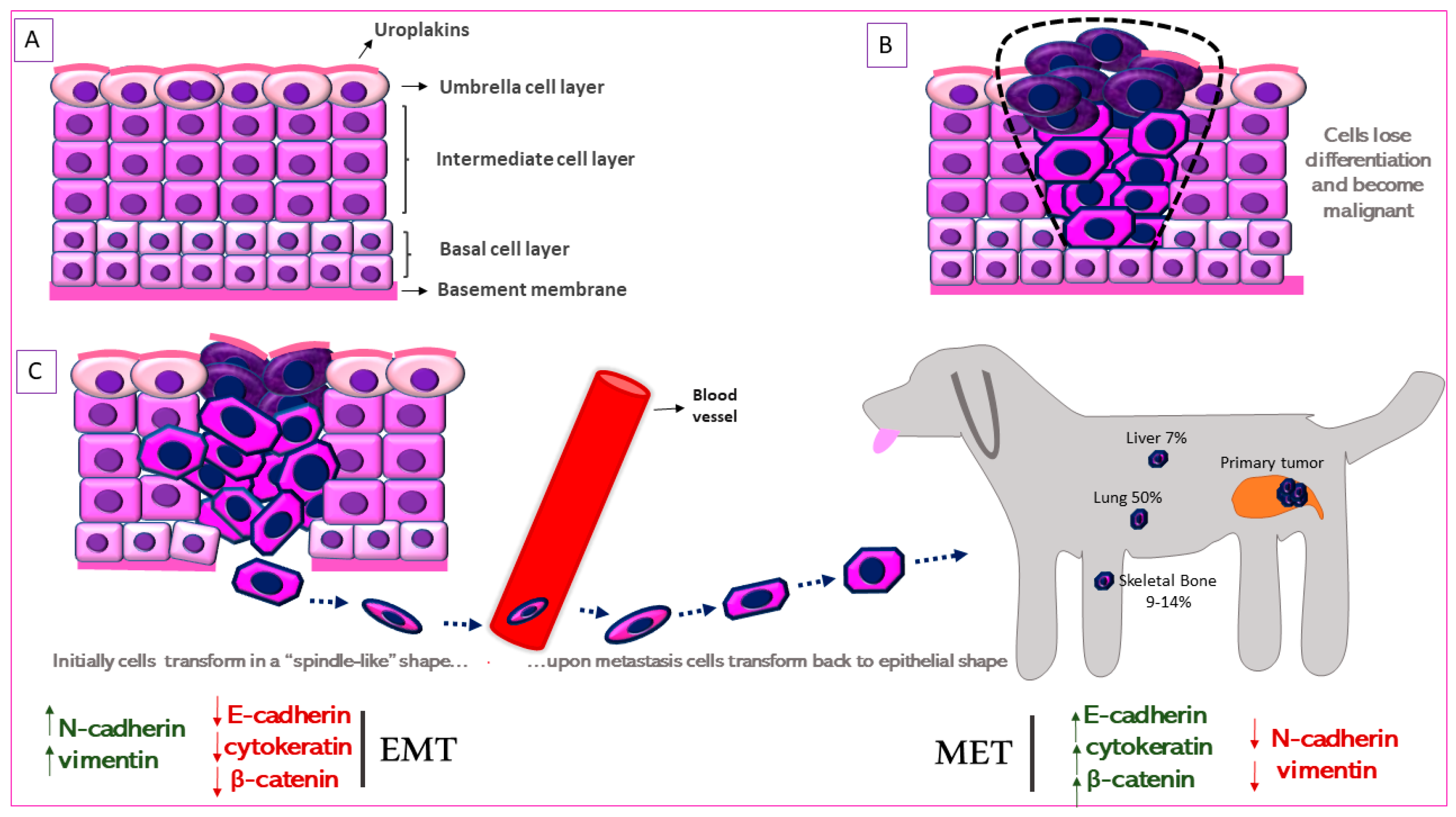
4. Identifying the Urothelial Origin of Metastatic UC Cells-Uroplakin Family of Proteins
5. Cell Signaling Pathways of Canine MIUC
5.1. Receptor Tyrosine Kinases
5.1.1. Fibroblast Growth Factor Receptor
5.1.2. ErbB Family of Receptors
5.1.3. ErbB Receptors in UC Diagnosis
5.1.4. Other Tyrosine Kinase Receptors
5.2. Arachidonic Acid Metabolism-Cyclooxygenases and Lipooxygenases
5.2.1. COX Inhibition—The “Gold” Standard Therapeutic Strategy in Canine UC
5.2.2. Cyclooxygenase Signaling and Multi-Drug Resistance
- Prostaglandin E2 (PGE2)
- P-glycoprotein (P-gp)
5.2.3. LOX Inhibition in Canine MIUC
5.3. Nectin 4 in Cell Adhesion, Migration and Invasion
5.4. Mitogen-Activated Protein Kinase (MAPK)/Extracellular Signal Regulated Kinase (ERK) Signaling Pathway
5.4.1. BRAFV595E Mutation as a Diagnostic Biomarker in Canine UC
5.4.2. BRAFV595E Mutation as a Therapeutic Target in Canine MIUC
6. Metastasis in Canine Urothelial Carcinoma: Epithelial-to-Mesenchymal Transition
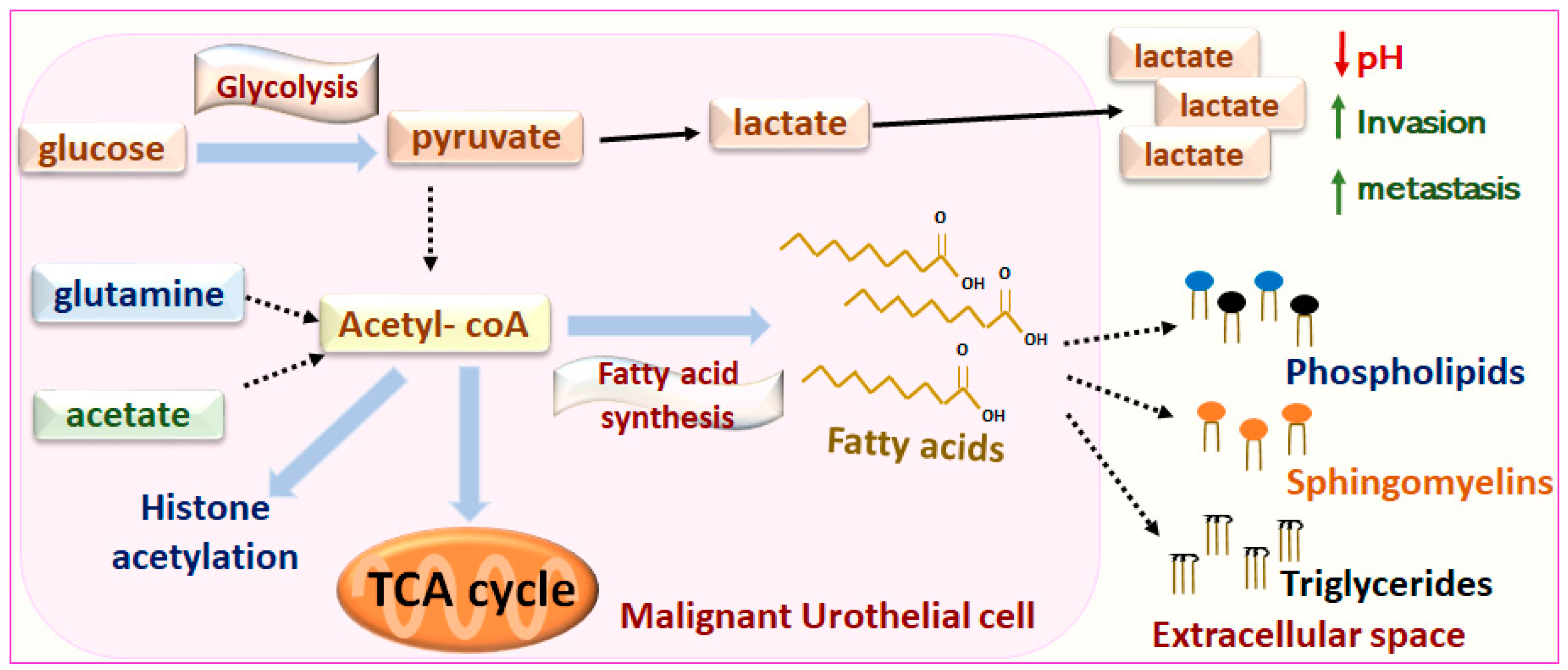
7. Metabolic Regulation of Canine MIUC
Metabolomic-Lipidomic Analyses for Biomarker Discovery
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Key Statistics for Bladder Cancer. Available online: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html (accessed on 29 March 2021).
- Survival Rates for Bladder Cancer. Available online: https://www.cancer.org/cancer/bladder-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 5 July 2021).
- Treatment of Bladder Cancer, by Stage. Available online: https://www.cancer.org/cancer/bladder-cancer/treating/by-stage.html (accessed on 28 June 2021).
- Kunthur, A.; Siegel, E.R.; Govindarajan, R. Cisplatin and Gemcitabine versus Carboplatin and Gemcitabine in Metastatic Bladder Cancer: Survival Analysis of Veterans’ Health Care Data. J. Clin. Oncol. 2017, 35, e16023. [Google Scholar] [CrossRef]
- Immunotherapy for Bladder Cancer. Available online: https://www.cancer.org/cancer/bladder-cancer/treating/immunotherapy-for-bladder-cancer.html (accessed on 29 June 2021).
- Enfortumab Vedotin Approved for Recurrent Bladder Cancer - National Cancer Institute. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2020/enfortumab-vedotin-bladder-cancer-fda-approval (accessed on 9 June 2021).
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody–Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- FDA Approves Erdafitinib, First Targeted Therapy for Metastatic Bladder Cancer. Available online: https://www.ajmc.com/view/fda-approves-erdafitinib-first-targeted-therapy-for-metastatic-bladder-cancer (accessed on 24 June 2021).
- John, B.A.; Said, N. Insights from Animal Models of Bladder Cancer: Recent Advances, Challenges, and Opportunities. Oncotarget 2017, 8, 57766–57781. [Google Scholar] [CrossRef]
- Knapp, D.W.; Dhawan, D.; Ramos-Vara, J.A.; Ratliff, T.L.; Cresswell, G.M.; Utturkar, S.; Sommer, B.C.; Fulkerson, C.M.; Hahn, N.M. Naturally-Occurring Invasive Urothelial Carcinoma in Dogs, a Unique Model to Drive Advances in Managing Muscle Invasive Bladder Cancer in Humans. Front. Oncol. 2020, 9, 1493. [Google Scholar] [CrossRef]
- Sommer, B.C.; Dhawan, D.; Ratliff, T.L.; Knapp, D.W. Naturally-Occurring Canine Invasive Urothelial Carcinoma: A Model for Emerging Therapies. Bladder Cancer Amst. Neth. 2018, 4, 149–159. [Google Scholar] [CrossRef]
- Fulkerson, C.M.; Knapp, D.W. Management of Transitional Cell Carcinoma of the Urinary Bladder in Dogs: A Review. Vet. J. 2015, 205, 217–225. [Google Scholar] [CrossRef]
- Kent, M.S.; Zwingenberger, A.; Westropp, J.L.; Barrett, L.E.; Durbin-Johnson, B.P.; Ghosh, P.; Vinall, R.L. MicroRNA Profiling of Dogs with Transitional Cell Carcinoma of the Bladder Using Blood and Urine Samples. BMC Vet. Res. 2017, 13, 339. [Google Scholar] [CrossRef]
- Knapp, D.W.; Glickman, N.W.; DeNicola, D.B.; Bonney, P.L.; Lin, T.L.; Glickman, L.T. Naturally-Occurring Canine Transitional Cell Carcinoma of the Urinary Bladder A Relevant Model of Human Invasive Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2000, 5, 47–59. [Google Scholar] [CrossRef]
- Tsuboi, M.; Sakai, K.; Maeda, S.; Chambers, J.K.; Yonezawa, T.; Matsuki, N.; Uchida, K.; Nakayama, H. Assessment of HER2 Expression in Canine Urothelial Carcinoma of the Urinary Bladder. Vet. Pathol. 2019, 56, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W. Canine Bladder Cancer. Available online: https://www.vet.purdue.edu/pcop/files/docs/CanineUrinaryBladderCancer.pdf (accessed on 7 May 2021).
- Stone, E.A.; Withrow, S.J.; Page, R.L.; Schwarz, P.D.; Wheeler, S.L.; Seim, H.B. Ureterocolonic Anastomosis in Ten Dogs with Transitional Cell Carcinoma. Vet. Surg. VS 1988, 17, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Fries, C.L.; Binnington, A.G.; Valli, V.E.; Connolly, J.G.; Holmberg, D.L.; Pennock, P. Enterocystoplasty with Cystectomy and Subtotal Intracapsular Prostatectomy in the Male Dog. Vet. Surg. VS 1991, 20, 104–112. [Google Scholar] [CrossRef]
- Saulnier-Troff, F.-G.; Busoni, V.; Hamaide, A. A Technique for Resection of Invasive Tumors Involving the Trigone Area of the Bladder in Dogs: Preliminary Results in Two Dogs. Vet. Surg. VS 2008, 37, 427–437. [Google Scholar] [CrossRef]
- Boston, S.; Singh, A. Total Cystectomy for Treatment of Transitional Cell Carcinoma of the Urethra and Bladder Trigone in a Dog. Vet. Surg. VS 2014, 43, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.A.; Culp, W.T.N.; Rebhun, R.B. Lower Urinary Tract Neoplasia. Vet. Sci. 2018, 5, 96. [Google Scholar] [CrossRef]
- Anderson, C.R.; McNiel, E.A.; Gillette, E.L.; Powers, B.E.; LaRue, S.M. Late Complications of Pelvic Irradiation in 16 Dogs. Vet. Radiol. Ultrasound 2002, 43, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.; Fidel, J. Tolerability and Tumor Response of a Novel Low-Dose Palliative Radiation Therapy Protocol in Dogs with Transitional Cell Carcinoma of the Bladder and Urethra. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2016, 57, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.W.; Kogan, L.; Griffin, L.R.; Custis, J.T.; Harmon, J.F.; Biller, B.J.; LaRue, S.M. Intensity-Modulated and Image-Guided Radiation Therapy for Treatment of Genitourinary Carcinomas in Dogs. J. Vet. Intern. Med. 2012, 26, 987–995. [Google Scholar] [CrossRef]
- Poirier, V.J.; Forrest, L.J.; Adams, W.M.; Vail, D.M. Piroxicam, Mitoxantrone, and Coarse Fraction Radiotherapy for the Treatment of Transitional Cell Carcinoma of the Bladder in 10 Dogs: A Pilot Study. J. Am. Anim. Hosp. Assoc. 2004, 40, 131–136. [Google Scholar] [CrossRef]
- Marconato, L.; Nitzl, D.B.; Melzer-Ruess, K.J.; Keller, M.A.; Buchholz, J. Chemotherapy and Radiation Therapy in 4 Dogs with Muscle-Invasive Transitional Cell Carcinoma of the Urinary Tract. Can. Vet. J. 2012, 53, 875–879. [Google Scholar]
- Alhalabi, O.; Shah, A.Y.; Lemke, E.A.; Gao, J. Current and Future Landscape of Immune Checkpoint Inhibitors in Urothelial Cancer. Oncol. Williston Park N 2019, 33, 11–18. [Google Scholar]
- Choi, J.W.; Withers, S.S.; Chang, H.; Spanier, J.A.; Trinidad, V.L.D.L.; Panesar, H.; Fife, B.T.; Sciammas, R.; Sparger, E.E.; Moore, P.F.; et al. Development of Canine PD-1/PD-L1 Specific Monoclonal Antibodies and Amplification of Canine T Cell Function. PLoS ONE 2020, 15, e0235518. [Google Scholar] [CrossRef] [PubMed]
- Coy, J.; Caldwell, A.; Chow, L.; Guth, A.; Dow, S. PD-1 Expression by Canine T Cells and Functional Effects of PD-1 Blockade. Vet. Comp. Oncol. 2017, 15, 1487–1502. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H.; Kim, S.; Okagawa, T.; Izumi, Y.; et al. PD-L1 Immunohistochemistry for Canine Cancers and Clinical Benefit of Anti-PD-L1 Antibody in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Npj Precis. Oncol. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Maeda, S.; Murakami, K.; Inoue, A.; Yonezawa, T.; Matsuki, N. CCR4 Blockade Depletes Regulatory T Cells and Prolongs Survival in a Canine Model of Bladder Cancer. Cancer Immunol. Res. 2019, 7, 1175–1187. [Google Scholar] [CrossRef]
- Fulkerson, C.M.; Dhawan, D.; Ratliff, T.L.; Hahn, N.M.; Knapp, D.W. Naturally Occurring Canine Invasive Urinary Bladder Cancer: A Complementary Animal Model to Improve the Success Rate in Human Clinical Trials of New Cancer Drugs. Int. J. Genom. 2017, 2017, 6589529. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Breen, M. Comparative Oncology: What Dogs and Other Species Can Teach Us about Humans with Cancer. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef]
- Dow, S. A Role for Dogs in Advancing Cancer Immunotherapy Research. Front. Immunol. 2020, 10. [Google Scholar] [CrossRef]
- Sciarra, A.; De Matteis, A.; Mariotti, G.; Voria, G.; Lucera, R.; Di Silverio, F. Histopathological Aspects of Transitional Cell Carcinoma of the Bladder: Analysis of 20 Years Experience. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2004, 11, 467–475. [Google Scholar] [CrossRef]
- De Brot, S.; Robinson, B.D.; Scase, T.; Grau-Roma, L.; Wilkinson, E.; Boorjian, S.A.; Gardner, D.; Mongan, N.P. The Dog as an Animal Model for Bladder and Urethral Urothelial Carcinoma: Comparative Epidemiology and Histology. Oncol. Lett. 2018, 16, 1641–1649. [Google Scholar] [CrossRef]
- Sun, W.; Wilhelmina Aalders, T.; Oosterwijk, E. Identification of Potential Bladder Progenitor Cells in the Trigone. Dev. Biol. 2014, 393, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, G.; Li, P.; Zhou, X.; Zhang, Y. Urine-Derived Stem Cells: A Novel and Versatile Progenitor Source for Cell-Based Therapy and Regenerative Medicine. Genes Dis. 2014, 1, 8–17. [Google Scholar] [CrossRef]
- Katleba, K.; Lombard, A.P.; Tsamouri, M.-M.; Baek, H.B.; Nishida, K.S.; Libertini, S.J.; Platero, A.J.; Ma, A.-H.; Pan, C.-X.; Ghosh, P.M.; et al. Depletion of Androgen Receptor Low Molecular Weight Isoform Reduces Bladder Tumor Cell Viability and Induces Apoptosis. Cancer Lett. 2021, 504, 49–57. [Google Scholar] [CrossRef]
- Mitra, A.P.; Datar, R.H.; Cote, R.J. Molecular Pathways in Invasive Bladder Cancer: New Insights Into Mechanisms, Progression, and Target Identification. J. Clin. Oncol. 2006, 24, 5552–5564. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Goebell, P.J.; Knowles, M.A. Bladder Cancer or Bladder Cancers? Genetically Distinct Malignant Conditions of the Urothelium. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 409–428. [Google Scholar] [CrossRef]
- Mitra, A.P.; Hansel, D.E.; Cote, R.J. Prognostic Value of Cell-Cycle Regulation Biomarkers in Bladder Cancer. Semin. Oncol. 2012, 39, 524–533. [Google Scholar] [CrossRef][Green Version]
- Wu, G.; Wang, F.; Li, K.; Li, S.; Zhao, C.; Fan, C.; Wang, J. Significance of TP53 Mutation in Bladder Cancer Disease Progression and Drug Selection. PeerJ 2019, 7, e8261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X. P53 Tumor Suppressor and Iron Homeostasis. FEBS J. 2019, 286, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Kent, M.S.; Rodriguez, C.O.; Chen, X. Establishment of a Dog Model for the P53 Family Pathway and Identification of a Novel Isoform of P21 Cyclin-Dependent Kinase Inhibitor. Mol. Cancer Res. MCR 2009, 7, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Bonnet, A.; Herráez, P.; Aguirre, M.; Suárez-Bonnet, E.; Andrada, M.; Rodríguez, F.; Espinosa de Los Monteros, A. Expression of Cell Cycle Regulators, 14-3-3σ and P53 Proteins, and Vimentin in Canine Transitional Cell Carcinoma of the Urinary Bladder. Urol. Oncol. 2015, 33, 332.e1–332.e7. [Google Scholar] [CrossRef] [PubMed]
- Hanazono, K.; Nishimori, T.; Fukumoto, S.; Kawamura, Y.; Endo, Y.; Kadosawa, T.; Uchide, T. Immunohistochemical Expression of P63, Ki67 and β-Catenin in Canine Transitional Cell Carcinoma and Polypoid Cystitis of the Urinary Bladder. Vet. Comp. Oncol. 2016, 14, 263–269. [Google Scholar] [CrossRef]
- Maeda, S.; Tomiyasu, H.; Tsuboi, M.; Inoue, A.; Ishihara, G.; Uchikai, T.; Chambers, J.K.; Uchida, K.; Yonezawa, T.; Matsuki, N. Comprehensive Gene Expression Analysis of Canine Invasive Urothelial Bladder Carcinoma by RNA-Seq. BMC Cancer 2018, 18, 472. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Zhou, Y.; Li, Y.; Xie, J.; Lee, R.J.; Cai, Y.; Teng, L. Silencing of Survivin Expression Leads to Reduced Proliferation and Cell Cycle Arrest in Cancer Cells. J. Cancer 2015, 6, 1187–1194. [Google Scholar] [CrossRef]
- Suzuki, A.; Hayashida, M.; Ito, T.; Kawano, H.; Nakano, T.; Miura, M.; Akahane, K.; Shiraki, K. Survivin Initiates Cell Cycle Entry by the Competitive Interaction with Cdk4/P16 INK4a and Cdk2/Cyclin E Complex Activation. Oncogene 2000, 19, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Pennati, M.; Folini, M.; Zaffaroni, N. Targeting Survivin in Cancer Therapy: Fulfilled Promises and Open Questions. Carcinogenesis 2007, 28, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Makboul, R.; Refaiy, A.E.-R.M.; Badary, F.A.M.; Abdelkawi, I.F.; Merseburger, A.S.; Mohammed, R.A.A. Expression of Survivin in Squamous Cell Carcinoma and Transitional Cell Carcinoma of the Urinary Bladder: A Comparative Immunohistochemical Study. Korean J. Urol. 2015, 56, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Swana, H.S.; Grossman, D.; Anthony, J.N.; Weiss, R.M.; Altieri, D.C. Tumor Content of the Antiapoptosis Molecule Survivin and Recurrence of Bladder Cancer. N. Engl. J. Med. 1999, 341, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-A.; Su, C.-M.; Hsieh, H.-Y.; Tung, C.-L.; Hsu, C.-D.; Wang, Y.-H.; Shen, C.-H. Clinical Significance of Survivin Expression in Patients with Urothelial Carcinoma. Dis. Markers 2014, 2014, 574985. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Kim, M.; Kwak, C.; Kim, H.H.; Ku, J.H. Prognostic Role of Survivin in Bladder Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Skagias, L.; Politi, E.; Karameris, A.; Sambaziotis, D.; Archondakis, A.; Ntinis, A.; Moreas, I.; Vasou, O.; Koutselini, H.; Patsouris, E. Survivin Expression as a Strong Indicator of Recurrence in Urothelial Bladder Cancer. Predictive Value of Nuclear versus Cytoplasmic Staining. Anticancer Res. 2009, 29, 4163–4167. [Google Scholar]
- Xu, X.; Li, P.; Fu, D.; Wei, Z.; Xu, S.; Xu, F.; Tian, F.; Ge, J.; Zhang, Z.; Cheng, W. Combined Use of Urinary Survivin Detection and Liquid-Based Cytology for the Early Diagnosis of Bladder Urothelial Carcinoma. Oncol. Lett. 2018, 15, 7739–7743. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Wheeler, M.A.; Plescia, J.; Colberg, J.W.; Weiss, R.M.; Altieri, D.C. Urine Detection of Survivin and Diagnosis of Bladder Cancer. JAMA 2001, 285, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Uchide, T.; Takatsu, N.; Fujimori, Y.; Fukushima, U.; Itoh, H. Expression of Survivin MRNA in Dog Tumors. DNA Seq. J. DNA Seq. Mapp. 2005, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Rankin, W.V.; Henry, C.J.; Turnquist, S.E.; Turk, J.R.; Beissenherz, M.E.; Tyler, J.W.; Rucker, E.B.; Knapp, D.W.; Rodriguez, C.O.; Green, J.A. Identification of Survivin, an Inhibitor of Apoptosis, in Canine Urinary Bladder Transitional Cell Carcinoma*. Vet. Comp. Oncol. 2008, 6, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Rankin, W.V.; Henry, C.J.; Turnquist, S.E.; Turk, J.R.; Beissenherz, M.E.; Tyler, J.W.; Green, J.A. Comparison of Distributions of Survivin among Tissues from Urinary Bladders of Dogs with Cystitis, Transitional Cell Carcinoma, or Histologically Normal Urinary Bladders. Am. J. Vet. Res. 2008, 69, 1073–1078. [Google Scholar] [CrossRef]
- Lehner, R.; Lucia, M.S.; Jarboe, E.A.; Orlicky, D.; Shroyer, A.L.; McGregor, J.A.; Shroyer, K.R. Immunohistochemical Localization of the IAP Protein Survivin in Bladder Mucosa and Transitional Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. AIMM 2002, 10, 134–138. [Google Scholar] [CrossRef]
- Kavya, N.; Rao, S.; Sathyanarayana, M.L.; Narayanaswamy, H.D.; Byregowda, S.M.; Ranganath, L.; Kamaran, A.; Purushotham, K.M.; Kishore, T.K. Survivin Expression in Canine Spontaneous Cutaneous and Subcutaneous Tumors and Its Prognostic Importance. Vet. World 2017, 10, 1286–1291. [Google Scholar] [CrossRef][Green Version]
- Thamm, D.H.; Joseph, J.K.; Rose, B.J.; Meuten, T.K.; Weishaar, K.M. Phase-I Trial of Survivin Inhibition with EZN-3042 in Dogs with Spontaneous Lymphoma. BMC Vet. Res. 2020, 16, 97. [Google Scholar] [CrossRef]
- Ghaffari, A.; Li, Y.; Kilani, R.T.; Ghahary, A. 14-3-3σ Associates with Cell Surface Aminopeptidase N in the Regulation of Matrix Metalloproteinase-1. J. Cell Sci. 2010, 123, 2996–3005. [Google Scholar] [CrossRef][Green Version]
- Moreira, J.M.A.; Gromov, P.; Celis, J.E. Expression of the Tumor Suppressor Protein 14-3-3σ Is down-Regulated in Invasive Transitional Cell Carcinomas of the Urinary Bladder Undergoing Epithelial-to-Mesenchymal Transition. Mol. Cell. Proteomics 2004, 3, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A. Everything You Wanted to Know about the Bladder Epithelium but Were Afraid to Ask. Am. J. Physiol. Renal Physiol. 2000, 278, F867–F874. [Google Scholar] [CrossRef]
- Bolla, S.R.; Odeluga, N.; Jetti, R. Histology, Bladder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wu, X.-R.; Kong, X.-P.; Pellicer, A.; Kreibich, G.; Sun, T.-T. Uroplakins in Urothelial Biology, Function, and Disease. Kidney Int. 2009, 75, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Olsburgh, J.; Harnden, P.; Weeks, R.; Smith, B.; Joyce, A.; Hall, G.; Poulsom, R.; Selby, P.; Southgate, J. Uroplakin Gene Expression in Normal Human Tissues and Locally Advanced Bladder Cancer. J. Pathol. 2003, 199, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.M.; Amin, M.B.; Luthringer, D.J.; Westfall, D.; Arora, K.; Farver, C.F.; Osunkoya, A.O.; McKenney, J.K.; Hansel, D.E. Selective Immunohistochemical Markers to Distinguish Between Metastatic High-Grade Urothelial Carcinoma and Primary Poorly Differentiated Invasive Squamous Cell Carcinoma of the Lung. Arch. Pathol. Lab. Med. 2012, 136, 1339–1346. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Miller, M.A.; Boucher, M.; Roudabush, A.; Johnson, G.C. Immunohistochemical Detection of Uroplakin III, Cytokeratin 7, and Cytokeratin 20 in Canine Urothelial Tumors. Vet. Pathol. 2003, 40, 55–62. [Google Scholar] [CrossRef]
- Reed, L.T.; Knapp, D.W.; Miller, M.A. Cutaneous Metastasis of Transitional Cell Carcinoma in 12 Dogs. Vet. Pathol. 2013, 50, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Burcham, G.N.; Childress, M.O.; Rohleder, J.J.; Bonney, P.L.; Ramos-Vara, J.A.; Knapp, D.W. Characterization and Treatment of Transitional Cell Carcinoma of the Abdominal Wall in Dogs: 24 Cases (1985–2010). J. Am. Vet. Med. Assoc. 2013, 242, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Satoh, T.; Irie, A.; Ishii, J.; Kuwao, S.; Iwamura, M.; Baba, S. Loss Expression of Uroplakin III Is Associated with Clinicopathologic Features of Aggressive Bladder Cancer. Urology 2008, 72, 444–449. [Google Scholar] [CrossRef]
- Lai, Y.; Ye, J.; Chen, J.; Zhang, L.; Wasi, L.; He, Z.; Zhou, L.; Li, H.; Yan, Q.; Gui, Y.; et al. UPK3A: A Promising Novel Urinary Marker for the Detection of Bladder Cancer. Urology 2010, 76, 514.e6–514.e11. [Google Scholar] [CrossRef] [PubMed]
- Sledge, D.G.; Patrick, D.J.; Fitzgerald, S.D.; Xie, Y.; Kiupel, M. Differences in Expression of Uroplakin III, Cytokeratin 7, and Cyclooxygenase-2 in Canine Proliferative Urothelial Lesions of the Urinary Bladder. Vet. Pathol. 2015, 52, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bourn, J.; Cekanova, M. Cyclooxygenase Inhibitors Potentiate Receptor Tyrosine Kinase Therapies in Bladder Cancer Cells in Vitro. Drug Des. Devel. Ther. 2018, 12, 1727–1742. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Tomlinson, D.C.; Baldo, O.; Harnden, P.; Knowles, M.A. FGFR3 Protein Expression and Its Relationship to Mutation Status and Prognostic Variables in Bladder Cancer. J. Pathol. 2007, 213, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Allen, D.K.; Waters, D.J.; Knapp, D.W.; Kuczek, T. High Urine Concentrations of Basic Fibroblast Growth Factor in Dogs With Bladder Cancer. J. Vet. Intern. Med. 1996, 10, 231–234. [Google Scholar] [CrossRef]
- Mohammed, S.I.; Bennett, P.F.; Craig, B.A.; Glickman, N.W.; Mutsaers, A.J.; Snyder, P.W.; Widmer, W.R.; DeGortari, A.E.; Bonney, P.L.; Knapp, D.W. Effects of the Cyclooxygenase Inhibitor, Piroxicam, on Tumor Response, Apoptosis, and Angiogenesis in a Canine Model of Human Invasive Urinary Bladder Cancer. Cancer Res. 2002, 62, 356–358. [Google Scholar] [PubMed]
- Singer, J.; Weichselbaumer, M.; Stockner, T.; Mechtcheriakova, D.; Sobanov, Y.; Bajna, E.; Wrba, F.; Horvat, R.; Thalhammer, J.G.; Willmann, M.; et al. Comparative Oncology: ErbB-1 and ErbB-2 Homologues in Canine Cancer Are Susceptible to Cetuximab and Trastuzumab Targeting. Mol. Immunol. 2012, 50, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Tan, S.; Rao, Q.; Zhu, T.; Huang, G.; Li, Z.; Liu, G. Overexpression of Epidermal Growth Factor Receptor (EGFR) and HER-2 in Bladder Carcinoma and Its Association with Patients’ Clinical Features. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7178–7185. [Google Scholar] [CrossRef]
- Hanazono, K.; Fukumoto, S.; Kawamura, Y.; Endo, Y.; Kadosawa, T.; Iwano, H.; Uchide, T. Epidermal Growth Factor Receptor Expression in Canine Transitional Cell Carcinoma. J. Vet. Med. Sci. 2015, 77, 1–6. [Google Scholar] [CrossRef][Green Version]
- Dhawan, D.; Paoloni, M.; Shukradas, S.; Choudhury, D.R.; Craig, B.A.; Ramos-Vara, J.A.; Hahn, N.; Bonney, P.L.; Khanna, C.; Knapp, D.W. Comparative Gene Expression Analyses Identify Luminal and Basal Subtypes of Canine Invasive Urothelial Carcinoma That Mimic Patterns in Human Invasive Bladder Cancer. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Liedberg, F.; Anderson, H.; Chebil, G.; Fernö, M.; Gudjonsson, S.; Höglund, M.; Lindgren, D.; Lundberg, L.-M.; Lövgren, K.; Månsson, W. Tissue Microarray Based Analysis of Prognostic Markers in Invasive Bladder Cancer: Much Effort to No Avail? Urol. Oncol. 2008, 26, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Goutsouliak, K.; Veeraraghavan, J.; Sethunath, V.; De Angelis, C.; Osborne, C.K.; Rimawi, M.F.; Schiff, R. Towards Personalized Treatment for Early Stage HER2-Positive Breast Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Wuerstlein, R.; Harbeck, N. Neoadjuvant Therapy for HER2-Positive Breast Cancer. Rev. Recent Clin. Trials 2017, 12, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Burrai, G.P.; Tanca, A.; De Miglio, M.R.; Abbondio, M.; Pisanu, S.; Polinas, M.; Pirino, S.; Mohammed, S.I.; Uzzau, S.; Addis, M.F.; et al. Investigation of HER2 Expression in Canine Mammary Tumors by Antibody-Based, Transcriptomic and Mass Spectrometry Analysis: Is the Dog a Suitable Animal Model for Human Breast Cancer? Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 9083–9091. [Google Scholar] [CrossRef]
- Cherrington, J.M.; Strawn, L.M.; Shawver, L.K. New paradigms for the treatment of cancer: The role of anti-angiogenesis agents. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2000; Volume 79, pp. 1–38. [Google Scholar]
- Kopparapu, P.K.; Boorjian, S.A.; Robinson, B.D.; Downes, M.; Gudas, L.J.; Mongan, N.P.; Persson, J.L. Expression of VEGF and Its Receptors VEGFR1/VEGFR2 Is Associated with Invasiveness of Bladder Cancer. Anticancer Res. 2013, 33, 2381–2390. [Google Scholar]
- Yeh, C.-Y.; Shin, S.-M.; Yeh, H.-H.; Wu, T.-J.; Shin, J.-W.; Chang, T.-Y.; Raghavaraju, G.; Lee, C.-T.; Chiang, J.-H.; Tseng, V.S.; et al. Transcriptional Activation of the Axl and PDGFR-α by c-Met through a Ras- and Src-Independent Mechanism in Human Bladder Cancer. BMC Cancer 2011, 11, 139. [Google Scholar] [CrossRef]
- London, C.A.; Hannah, A.L.; Zadovoskaya, R.; Chien, M.B.; Kollias-Baker, C.; Rosenberg, M.; Downing, S.; Post, G.; Boucher, J.; Shenoy, N.; et al. Phase I Dose-Escalating Study of SU11654, a Small Molecule Receptor Tyrosine Kinase Inhibitor, in Dogs with Spontaneous Malignancies. Clin. Cancer Res. 2003, 9, 2755–2768. [Google Scholar]
- Walters, L.; Martin, O.; Price, J.; Sula, M.M. Expression of Receptor Tyrosine Kinase Targets PDGFR-β, VEGFR2 and KIT in Canine Transitional Cell Carcinoma. Vet. Comp. Oncol. 2018, 16, E117–E122. [Google Scholar] [CrossRef]
- Herschman, H.R. Prostaglandin Synthase 2. Biochim. Biophys. Acta BBA - Lipids Lipid Metab. 1996, 1299, 125–140. [Google Scholar] [CrossRef]
- Mann, J.R.; Backlund, M.G.; Buchanan, F.G.; Daikoku, T.; Holla, V.R.; Rosenberg, D.W.; Dey, S.K.; DuBois, R.N. Repression of Prostaglandin Dehydrogenase by Epidermal Growth Factor and Snail Increases Prostaglandin E2 and Promotes Cancer Progression. Cancer Res. 2006, 66, 6649–6656. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, Cellular, and Molecular Biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef]
- Adhim, Z.; Matsuoka, T.; Bito, T.; Shigemura, K.; Lee, K.-M.; Kawabata, M.; Fujisawa, M.; Nibu, K.; Shirakawa, T. In Vitro and in Vivo Inhibitory Effect of Three Cox-2 Inhibitors and Epithelial-to-Mesenchymal Transition in Human Bladder Cancer Cell Lines. Br. J. Cancer 2011, 105, 393–402. [Google Scholar] [CrossRef]
- Yoshimura, R.; Matsuyama, M.; Tsuchida, K.; Kawahito, Y.; Sano, H.; Nakatani, T. Expression of Lipoxygenase in Human Bladder Carcinoma and Growth Inhibition by Its Inhibitors. J. Urol. 2003, 170, 1994–1999. [Google Scholar] [CrossRef]
- Knapp, D.W.; Richardson, R.C.; Chan, T.C.K.; Bottoms, G.D.; Widmer, W.R.; DeNicola, D.B.; Teclaw, R.; Bonney, P.L.; Kuczek, T. Piroxicam Therapy in 34 Dogs With Transitional Cell Carcinoma of the Urinary Bladder. J. Vet. Intern. Med. 1994, 8, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.M.; Knapp, D.W.; Denicola, D.B.; Harris, R.K. Expression of Cyclooxygenase-2 in Transitional Cell Carcinoma of the Urinary Bladder in Dogs. Am. J. Vet. Res. 2000, 61, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.I.; Knapp, D.W.; Bostwick, D.G.; Foster, R.S.; Khan, K.N.M.; Masferrer, J.L.; Woerner, B.M.; Snyder, P.W.; Koki, A.T. Expression of Cyclooxygenase-2 (COX-2) in Human Invasive Transitional Cell Carcinoma (TCC) of the Urinary Bladder. Cancer Res. 1999, 59, 5647–5650. [Google Scholar] [PubMed]
- Patel, V.A.; Dunn, M.J.; Sorokin, A. Regulation of MDR-1 (P-Glycoprotein) by Cyclooxygenase-2. J. Biol. Chem. 2002, 277, 38915–38920. [Google Scholar] [CrossRef]
- Boria, P.A.; Glickman, N.W.; Schmidt, B.R.; Widmer, W.R.; Mutsaers, A.J.; Adams, L.G.; Snyder, P.W.; DiBernardi, L.; de Gortari, A.E.; Bonney, P.L.; et al. Carboplatin and Piroxicam Therapy in 31 Dogs with Transitional Cell Carcinoma of the Urinary Bladder. Vet. Comp. Oncol. 2005, 3, 73–80. [Google Scholar] [CrossRef]
- Knapp, D.W.; Glickman, N.W.; Widmer, W.R.; DeNicola, D.B.; Adams, L.G.; Kuczek, T.; Bonney, P.L.; DeGortari, A.E.; Han, C.; Glickman, L.T. Cisplatin versus Cisplatin Combined with Piroxicam in a Canine Model of Human Invasive Urinary Bladder Cancer. Cancer Chemother. Pharmacol. 2000, 46, 221–226. [Google Scholar] [CrossRef]
- McMillan, S.K.; Boria, P.; Moore, G.E.; Widmer, W.R.; Bonney, P.L.; Knapp, D.W. Antitumor Effects of Deracoxib Treatment in 26 Dogs with Transitional Cell Carcinoma of the Urinary Bladder. J. Am. Vet. Med. Assoc. 2011, 239, 1084–1089. [Google Scholar] [CrossRef]
- Knapp, D.W.; Henry, C.J.; Widmer, W.R.; Tan, K.M.; Moore, G.E.; Ramos-Vara, J.A.; Lucroy, M.D.; Greenberg, C.B.; Greene, S.N.; Abbo, A.H.; et al. Randomized Trial of Cisplatin versus Firocoxib versus Cisplatin/Firocoxib in Dogs with Transitional Cell Carcinoma of the Urinary Bladder. J. Vet. Intern. Med. 2013, 27, 126–133. [Google Scholar] [CrossRef]
- Hurst, E.A.; Pang, L.Y.; Argyle, D.J. The Selective Cyclooxygenase-2 Inhibitor Mavacoxib (Trocoxil) Exerts Anti-Tumour Effects in Vitro Independent of Cyclooxygenase-2 Expression Levels. Vet. Comp. Oncol. 2019, 17, 194–207. [Google Scholar] [CrossRef]
- Mutsaers, A.J.; Mohammed, S.I.; DeNicola, D.B.; Snyder, P.W.; Glickman, N.W.; Bennett, P.F.; de Gortari, A.E.; Bonney, P.L.; Knapp, D.W. Pretreatment Tumor Prostaglandin E2 Concentration and Cyclooxygenase-2 Expression Are Not Associated with the Response of Canine Naturally Occurring Invasive Urinary Bladder Cancer to Cyclooxygenase Inhibitor Therapy. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cekanova, M.; Uddin, M.J.; Bartges, J.W.; Callens, A.; Legendre, A.M.; Rathore, K.; Wright, L.; Carter, A.; Marnett, L.J. Molecular Imaging of Cyclooxygenase-2 in Canine Transitional Cell Carcinomas In Vitro and In Vivo. Cancer Prev. Res. Phila. Pa 2013, 6, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Gurpinar, E.; Grizzle, W.E.; Piazza, G.A. COX-Independent Mechanisms of Cancer Chemoprevention by Anti-Inflammatory Drugs. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-Induced Tumour Repopulation Abrogates Bladder Cancer Chemoresistance. Nature 2015, 517, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.I.; Coffman, K.; Glickman, N.W.; Hayek, M.G.; Waters, D.J.; Schlittler, D.; DeNicola, D.B.; Knapp, D.W. Prostaglandin E2concentrations in Naturally Occurring Canine Cancer. Prostaglandins Leukot. Essent. Fat. Acids PLEFA 2001, 64, 1–4. [Google Scholar] [CrossRef]
- Cheng, X.-Z.; Zhou, H.-L.; Tang, S.-X.; Jiang, T.; Chen, Q.; Gao, R.; Ding, Y.-L. Intercellular Transfer of P-Glycoprotein Mediates the Formation of Stable Multi-Drug Resistance in Human Bladder Cancer BIU-87 Cells. Biol. Open 2019, 8. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, Y.; Cheng, X.; Lv, Y.; Gao, R.; Mao, H.; Chen, Q. Intercellular Transfer of P-Glycoprotein from the Drug Resistant Human Bladder Cancer Cell Line BIU-87 Does Not Require Cell-to-Cell Contact. J. Urol. 2013, 190, 1069–1075. [Google Scholar] [CrossRef]
- Hoffmann, A.-C.; Wild, P.; Leicht, C.; Bertz, S.; Danenberg, K.D.; Danenberg, P.V.; Stöhr, R.; Stöckle, M.; Lehmann, J.; Schuler, M.; et al. MDR1 and ERCC1 Expression Predict Outcome of Patients with Locally Advanced Bladder Cancer Receiving Adjuvant Chemotherapy. Neoplasia 2010, 12, 628–636. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, W.; Chang, J.; Zhao, Z.; Sun, G.; Han, R. MDR1/P-Glycoprotein Overexpression in Bladder Transitional Cell Carcinoma and Its Correlation with Expression of Survivin and Fas. Chin. J. Clin. Oncol. 2006, 3, 191–195. [Google Scholar] [CrossRef]
- Pagliarulo, V.; Ancona, P.; Niso, M.; Colabufo, N.A.; Contino, M.; Cormio, L.; Azzariti, A.; Pagliarulo, A. The Interaction of Celecoxib with MDR Transporters Enhances the Activity of Mitomycin C in a Bladder Cancer Cell Line. Mol. Cancer 2013, 12. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Tanabe, S.; Shimohira, H.; Kobayashi, Y.; Oomachi, T.; Azuma, S.; Ogihara, K.; Inokuma, H. Expression of Cyclooxygenase-2, P-Glycoprotein and Multi-Drug Resistance-Associated Protein in Canine Transitional Cell Carcinoma. Res. Vet. Sci. 2007, 83, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J.; Colorado, S.U.; Ogilvie, G.K.; Powers, B.E. Monoclonal Antibody C219 Immunohistochemistry against P-Glycoprotein: Sequential Analysis and Predictive Ability in Dogs with Lymphoma. J. Vet. Intern. Med. USA 1996, 10, 354–359. [Google Scholar] [CrossRef]
- Neff, M.W.; Robertson, K.R.; Wong, A.K.; Safra, N.; Broman, K.W.; Slatkin, M.; Mealey, K.L.; Pedersen, N.C. Breed Distribution and History of Canine Mdr1-1Δ, a Pharmacogenetic Mutation That Marks the Emergence of Breeds from the Collie Lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 11725–11730. [Google Scholar] [CrossRef] [PubMed]
- Roulet, A.; Puel, O.; Gesta, S.; Lepage, J.-F.; Drag, M.; Soll, M.; Alvinerie, M.; Pineau, T. MDR1-Deficient Genotype in Collie Dogs Hypersensitive to the P-Glycoprotein Substrate Ivermectin. Eur. J. Pharmacol. 2003, 460, 85–91. [Google Scholar] [CrossRef]
- Mealey, K.L.; Fidel, J. P-Glycoprotein Mediated Drug Interactions in Animals and Humans with Cancer. J. Vet. Intern. Med. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Goodman, L.A.; Jarrett, C.L.; Krunkosky, T.M.; Budsberg, S.C.; Northrup, N.C.; Saba, C.F.; LeRoy, B.E. 5-Lipoxygenase Expression in Benign and Malignant Canine Prostate Tissues*. Vet. Comp. Oncol. 2011, 9, 149–157. [Google Scholar] [CrossRef]
- Goupil, R.C.; Bushey, J.J.; Peters-Kennedy, J.; Wakshlag, J.J. Prevalence of 5-Lipoxygenase Expression in Canine Osteosarcoma and the Effects of a Dual 5-Lipoxygenase/Cyclooxygenase Inhibitor on Osteosarcoma Cells In Vitro and In Vivo. Vet. Pathol. 2012. [Google Scholar] [CrossRef]
- Loftus, J.P.; Cavatorta, D.; Bushey, J.J.; Levine, C.B.; Sevier, C.S.; Wakshlag, J.J. The 5-Lipoxygenase Inhibitor Tepoxalin Induces Oxidative Damage and Altered PTEN Status Prior to Apoptosis in Canine Osteosarcoma Cell Lines. Vet. Comp. Oncol. 2016, 14, e17–e30. [Google Scholar] [CrossRef]
- Finotello, R.; Schiavo, L.; Ressel, L.; Frohmader, A.; Silvestrini, P.; Verin, R. Lipoxygenase-5 Expression in Canine Urinary Bladder: Normal Urothelium, Cystitis and Transitional Cell Carcinoma. J. Comp. Pathol. 2019, 170, 1–9. [Google Scholar] [CrossRef]
- Rikitake, Y.; Mandai, K.; Takai, Y. The Role of Nectins in Different Types of Cell–Cell Adhesion. J. Cell Sci. 2012, 125, 3713–3722. [Google Scholar] [CrossRef] [PubMed]
- OGITA, H.; RIKITAKE, Y.; MIYOSHI, J.; TAKAI, Y. Cell Adhesion Molecules Nectins and Associating Proteins: Implications for Physiology and Pathology. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Sethy, C.; Goutam, K.; Nayak, D.; Pradhan, R.; Molla, S.; Chatterjee, S.; Rout, N.; Wyatt, M.D.; Narayan, S.; Kundu, C.N. Clinical Significance of a Pvrl 4 Encoded Gene Nectin-4 in Metastasis and Angiogenesis for Tumor Relapse. J. Cancer Res. Clin. Oncol. 2020, 146, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, P.C.; Boylan, K.L.M.; Walcheck, B.; Heinze, R.; Geller, M.A.; Argenta, P.A.; Skubitz, A.P.N. Ectodomain Shedding of the Cell Adhesion Molecule Nectin-4 in Ovarian Cancer Is Mediated by ADAM10 and ADAM17. J. Biol. Chem. 2017, 292, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of Nectin-4 Oncoprotein as a Diagnostic and Therapeutic Target for Lung Cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, P.; Sauvan, R.; Adélaïde, J.; Geneix, J.; et al. Nectin-4 Is a New Histological and Serological Tumor Associated Marker for Breast Cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 Overexpression in Ovarian Cancer Tissues and Serum: Potential Role as a Serum Biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef]
- Deng, H.; Shi, H.; Chen, L.; Zhou, Y.; Jiang, J. Over-Expression of Nectin-4 Promotes Progression of Esophageal Cancer and Correlates with Poor Prognosis of the Patients. Cancer Cell Int. 2019, 19, 106. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 Expression Contributes to Tumor Proliferation, Angiogenesis and Patient Prognosis in Human Pancreatic Cancer. J. Exp. Clin. Cancer Res. 2015, 34, 30. [Google Scholar] [CrossRef] [PubMed]
- Siddharth, S.; Goutam, K.; Das, S.; Nayak, A.; Nayak, D.; Sethy, C.; Wyatt, M.D.; Kundu, C.N. Nectin-4 Is a Breast Cancer Stem Cell Marker That Induces WNT/β-Catenin Signaling via Pi3k/Akt Axis. Int. J. Biochem. Cell Biol. 2017, 89, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Pallasch, C.; Elia, A.E.; Braun, C.J.; Westbrook, T.F.; Hemann, M.; Elledge, S.J. A Role for PVRL4-Driven Cell–Cell Interactions in Tumorigenesis. eLife 2013, 2. [Google Scholar] [CrossRef]
- Pratakpiriya, W.; Seki, F.; Otsuki, N.; Sakai, K.; Fukuhara, H.; Katamoto, H.; Hirai, T.; Maenaka, K.; Techangamsuwan, S.; Lan, N.T.; et al. Nectin4 Is an Epithelial Cell Receptor for Canine Distemper Virus and Involved in Neurovirulence. J. Virol. 2012, 86, 10207–10210. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Shoji, K.; Fujiyuki, T.; Moritoh, K.; Tamura, K.; Yoshida, A.; Sato, H.; Yoneda, M.; Asano, K.; Kai, C. Antitumor Activity of an Oncolytic Measles Virus against Canine Urinary Bladder Transitional Cell Carcinoma Cells. Res. Vet. Sci. 2020, 133, 313–317. [Google Scholar] [CrossRef]
- Sebolt-Leopold, J.S.; Herrera, R. Targeting the Mitogen-Activated Protein Kinase Cascade to Treat Cancer. Nat. Rev. Cancer 2004, 4, 937–947. [Google Scholar] [CrossRef]
- Mochizuki, H.; Breen, M. Comparative Aspects of BRAF Mutations in Canine Cancers. Vet. Sci. 2015, 2, 231–245. [Google Scholar] [CrossRef]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF Mutations in Canine Cancers. PloS ONE 2015, 10, e0129534. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous Mutation to Human BRAF V600E Is Common in Naturally Occurring Canine Bladder Cancer--Evidence for a Relevant Model System and Urine-Based Diagnostic Test. Mol. Cancer Res. MCR 2015, 13, 993–1002. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Masliah-Planchon, J.; Garinet, S.; Pasmant, E. RAS-MAPK Pathway Epigenetic Activation in Cancer: MiRNAs in Action. Oncotarget 2015, 7, 38892–38907. [Google Scholar] [CrossRef]
- Aupperle-Lellbach, H.; Grassinger, J.; Hohloch, C.; Kehl, A.; Pantke, P. Diagnostic value of the BRAF variant V595E in urine samples, smears and biopsies from canine transitional cell carcinoma. Tierarztl. Prax. Ausg. K Klientiere Heimtiere 2018, 46, 289–295. [Google Scholar] [CrossRef]
- Mochizuki, H.; Shapiro, S.G.; Breen, M. Detection of BRAF Mutation in Urine DNA as a Molecular Diagnostic for Canine Urothelial and Prostatic Carcinoma. PLoS ONE 2015, 10, e0144170. [Google Scholar] [CrossRef]
- Grassinger, J.M.; Merz, S.; Aupperle-Lellbach, H.; Erhard, H.; Klopfleisch, R. Correlation of BRAF Variant V595E, Breed, Histological Grade and Cyclooxygenase-2 Expression in Canine Transitional Cell Carcinomas. Vet. Sci. 2019, 6, 31. [Google Scholar] [CrossRef]
- Parker, H.G.; Dhawan, D.; Harris, A.C.; Ramos-Vara, J.A.; Davis, B.W.; Knapp, D.W.; Ostrander, E.A. RNAseq Expression Patterns of Canine Invasive Urothelial Carcinoma Reveal Two Distinct Tumor Clusters and Shared Regions of Dysregulation with Human Bladder Tumors. BMC Cancer 2020, 20, 251. [Google Scholar] [CrossRef]
- Tagawa, M.; Tambo, N.; Maezawa, M.; Tomihari, M.; Watanabe, K.; Inokuma, H.; Miyahara, K. Quantitative Analysis of the BRAF V595E Mutation in Plasma Cell-Free DNA from Dogs with Urothelial Carcinoma. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Cronise, K.E.; Hernandez, B.G.; Gustafson, D.L.; Duval, D.L. Identifying the ErbB/MAPK Signaling Cascade as a Therapeutic Target in Canine Bladder Cancer. Mol. Pharmacol. 2019, 96, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hatzivassiliou, G.; Song, K.; Yen, I.; Brandhuber, B.J.; Anderson, D.J.; Alvarado, R.; Ludlam, M.J.C.; Stokoe, D.; Gloor, S.L.; Vigers, G.; et al. RAF Inhibitors Prime Wild-Type RAF to Activate the MAPK Pathway and Enhance Growth. Nature 2010, 464, 431–435. [Google Scholar] [CrossRef]
- Yoshitake, R.; Saeki, K.; Eto, S.; Shinada, M.; Nakano, R.; Sugiya, H.; Endo, Y.; Fujita, N.; Nishimura, R.; Nakagawa, T. Aberrant Expression of the COX2/PGE 2 Axis Is Induced by Activation of the RAF/MEK/ERK Pathway in BRAF V595E Canine Urothelial Carcinoma. Sci. Rep. 2020, 10, 7826. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary Bladder Cancer in Dogs, a Naturally Occurring Model for Cancer Biology and Drug Development. ILAR J. 2014, 55, 100–118. [Google Scholar] [CrossRef]
- Charney, V.A.; Miller, M.A.; Heng, H.G.; Weng, H.Y.; Knapp, D.W. Skeletal Metastasis of Canine Urothelial Carcinoma: Pathologic and Computed Tomographic Features. Vet. Pathol. 2017, 54, 380–386. [Google Scholar] [CrossRef]
- Garg, M.; Singh, R. Epithelial-to-Mesenchymal Transition: Event and Core Associates in Bladder Cancer. Front. Biosci. Elite Ed. 2019, 11, 150–165. [Google Scholar] [CrossRef]
- Weis, W.I. Cadherin Structure: A Revealing Zipper. Structure 1995, 3, 425–427. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.H.; Zheng, G. E-Cadherin/β-Catenin Complex and the Epithelial Barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef]
- Baumgart, E.; Cohen, M.S.; Neto, B.S.; Jacobs, M.A.; Wotkowicz, C.; Rieger-Christ, K.M.; Biolo, A.; Zeheb, R.; Loda, M.; Libertino, J.A.; et al. Identification and Prognostic Significance of an Epithelial-Mesenchymal Transition Expression Profile in Human Bladder Tumors. Clin. Cancer Res. 2007, 13, 1685–1694. [Google Scholar] [CrossRef]
- Dhawan, D.; Ramos-Vara, J.A.; Stewart, J.C.; Zheng, R.; Knapp, D.W. Canine Invasive Transitional Cell Carcinoma Cell Lines: In Vitro Tools to Complement a Relevant Animal Model of Invasive Urinary Bladder Cancer. Urol. Oncol. 2009, 27, 284–292. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the Hallmarks of Cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Fatty Acid, Triglyceride, Phospholipid Synthesis and Metabolism. Available online: https://themedicalbiochemistrypage.org/lipid-synthesis.php (accessed on 3 February 2020).
- Röhrig, F.; Schulze, A. The Multifaceted Roles of Fatty Acid Synthesis in Cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The Link between Genotypes and Phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- H, B. Lipid Metabolism Profiling and Bladder Cancer. J. Postgenomics Drug Biomark. Dev. 2015, 05. [Google Scholar] [CrossRef]
- Lee, M.Y.; Yeon, A.; Shahid, M.; Cho, E.; Sairam, V.; Figlin, R.; Kim, K.-H.; Kim, J. Reprogrammed Lipid Metabolism in Bladder Cancer with Cisplatin Resistance. Oncotarget 2018, 9, 13231–13243. [Google Scholar] [CrossRef]
- Miryaghoubzadeh, J.; Darabi, M.; Madaen, K.; Shaaker, M.; Mehdizadeh, A.; Hajihosseini, R. Tissue Fatty Acid Composition in Human Urothelial Carcinoma. Br. J. Biomed. Sci. 2013, 70, 1–5. [Google Scholar] [CrossRef]
- Dill, A.L.; Ifa, D.R.; Manicke, N.E.; Costa, A.B.; Ramos-Vara, J.A.; Knapp, D.W.; Cooks, R.G. Lipid Profiles of Canine Invasive Transitional Cell Carcinoma of the Urinary Bladder and Adjacent Normal Tissue by Desorption Electrospray Ionization Imaging Mass Spectrometry. Anal. Chem. 2009, 81, 8758–8764. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, S.; Liu, L.; Nagana Gowda, G.A.; Bonney, P.; Stewart, J.; Knapp, D.W.; Raftery, D. NMR-Based Metabolomics Study of Canine Bladder Cancer. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 2012, 1822, 1807–1814. [Google Scholar] [CrossRef]
- D’Hue, C.A.; Dhawan, D.; Peat, T.; Ramos-Vara, J.; Jarmusch, A.; Knapp, D.W.; Cooks, R.G. Fatty Acid Patterns Detected By Ambient Ionization Mass Spectrometry in Canine Invasive Urothelial Carcinoma From Dogs of Different Breeds. Bladder Cancer Amst. Neth. 2018, 4, 283–291. [Google Scholar] [CrossRef]


| Target | Drug |
|---|---|
| COX-1/ COX-2 | Piroxicam, Meloxicam, Carprofen |
| COX-2 | Firocoxib, Mavacoxib |
| COX- 5-LOX | Tepoxalin |
| DNA damage repair mechanisms | Cisplatin, Mitoxantrone, Doxorubicin |
| Microtubular proteins | Vinblastin |
| DNA synthesis | Gemcitabine |
| CCR4 | Mogamulizumab |
| Survivin | EZN-3042 |
| Pan- ErbB | Sapatinib |
| PDGFR, VEGFR, KIT, Flt3 | SU11654 |
| BRAF | Vemurafenib, Dabrafenib |
| Pan- RAF | LY3009120 |
| MEK | Selumetinib, Trametinib |
| ERK | SCH772984 |
| P-38 | SB239063 |
| JNK | SP600125 |
| Nectin-4 | rMV-SLAMblind |
| Biomarker | Method of Detection | Tissue/Biofluid | Function |
|---|---|---|---|
| p63 | IHC | Tumor | Prognosis |
| Survivin (nuclear) | IHC | Tumor (↑) * | Diagnosis |
| Stratifin | IHC | Diagnosis | Diagnosis |
| uroplakin | IHC | Tumor (↓) | Diagnosis |
| ELISA | Urine (↑) | ||
| FGF | ELISA | Urine (↑) | Diagnosis |
| EGFR, HER-2 | RT-qPCR | Tumor (↑) | Diagnosis |
| IHC | |||
| PDGFR-β, KIT | IHC | Tumor (↑) | Diagnosis |
| BRAFV595E | Droplet PCR | Urine (+) # | Diagnosis |
| PCR | Plasma (+) | ||
| Choline | NMR | Urine (↑) | Diagnosis |
| Urea | NMR | Urine (↑) | Diagnosis |
| Methylguanidine | NMR | Urine (↑) | Diagnosis |
| Citrate | NMR | Urine (↑) | Diagnosis |
| Acetone | NMR | Urine (↑) | Diagnosis |
| β-hydroxybutyrate | NMR | Urine (↑) | Diagnosis |
| Oleic acid | DESI-MS/ TS-MS | Tumor (↑) | Diagnosis |
| Stearic acid | DESI-MS/ TS-MS | Tumor (↓) | Diagnosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamouri, M.M.; Steele, T.M.; Mudryj, M.; Kent, M.S.; Ghosh, P.M. Comparative Cancer Cell Signaling in Muscle-Invasive Urothelial Carcinoma of the Bladder in Dogs and Humans. Biomedicines 2021, 9, 1472. https://doi.org/10.3390/biomedicines9101472
Tsamouri MM, Steele TM, Mudryj M, Kent MS, Ghosh PM. Comparative Cancer Cell Signaling in Muscle-Invasive Urothelial Carcinoma of the Bladder in Dogs and Humans. Biomedicines. 2021; 9(10):1472. https://doi.org/10.3390/biomedicines9101472
Chicago/Turabian StyleTsamouri, Maria Malvina, Thomas M. Steele, Maria Mudryj, Michael S. Kent, and Paramita M. Ghosh. 2021. "Comparative Cancer Cell Signaling in Muscle-Invasive Urothelial Carcinoma of the Bladder in Dogs and Humans" Biomedicines 9, no. 10: 1472. https://doi.org/10.3390/biomedicines9101472
APA StyleTsamouri, M. M., Steele, T. M., Mudryj, M., Kent, M. S., & Ghosh, P. M. (2021). Comparative Cancer Cell Signaling in Muscle-Invasive Urothelial Carcinoma of the Bladder in Dogs and Humans. Biomedicines, 9(10), 1472. https://doi.org/10.3390/biomedicines9101472







