Tyrosine Kinase Inhibitors Improved Survival of Critically Ill EGFR-Mutant Lung Cancer Patients Undergoing Mechanical Ventilation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Data Collection and Outcome
2.3. Detection of EGFR Mutations
2.4. Statistical Analysis
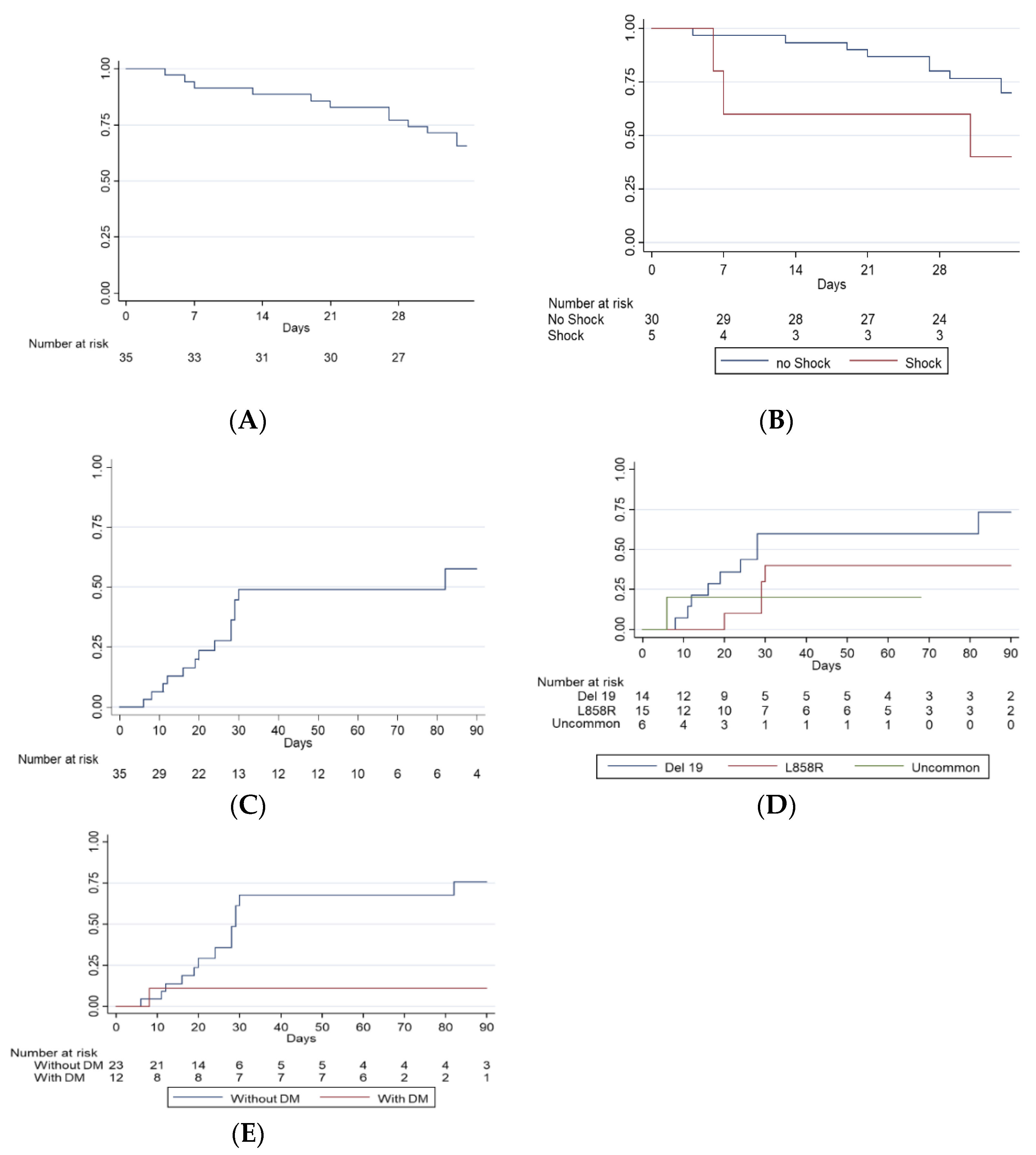
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes in the ICU
3.3. Treatment Toxicity in the ICU
3.4. Patient Deposition after ICU Discharge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, M.; Caruso, P.; Silva, E.; Teles, J.M.; Lobo, S.M.; Friedman, G.; Dal Pizzol, F.; Mello, P.V.; Bozza, F.A.; Silva, U.V.; et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: A prospective multicenter study. Crit. Care Med. 2010, 38, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Moreau, D.; Alberti, C.; Leleu, G.; Adrie, C.; Barboteu, M.; Cottu, P.; Levy, V.; Le Gall, J.R.; Schlemmer, B. Predictors of short-term mortality in critically ill patients with solid malignancies. Intensive Care Med. 2000, 26, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Slatore, C.G.; Cecere, L.M.; Letourneau, J.L.; O’Neil, M.E.; Duckart, J.P.; Wiener, R.S.; Farjah, F.; Cooke, C.R. Intensive care unit outcomes among patients with lung cancer in the surveillance, epidemiology, and end results-medicare registry. J. Clin. Oncol. 2012, 30, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Toffart, A.C.; Timsit, J.F.; Burghi, G.; Irrazabal, C.; Pattison, N.; Tobar, E.; Almeida, B.F.; Silva, U.V.; Azevedo, L.C.; et al. Intensive care in patients with lung cancer: A multinational study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.K.; Soubani, A.O. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur. Respir. J. 2008, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hsia, T.C.; Tu, C.Y.; Chen, H.J. The impact of rescue or maintenance therapy with EGFR TKIs for Stage IIIb-IV non-squamous non-small-cell lung cancer patients requiring mechanical ventilation. BMC Anesth. 2014, 14, 55. [Google Scholar] [CrossRef]
- Benoit, D.D.; Soares, M.; Azoulay, E. Has survival increased in cancer patients admitted to the ICU? We are not sure. Intensive Care Med. 2014, 40, 1576–1579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, Y.C.; Tsai, Y.H.; Huang, C.C.; Hsu, K.H.; Wang, S.W.; Tsao, T.C.; Lin, M.C. Outcome of lung cancer patients with acute respiratory failure requiring mechanical ventilation. Respir. Med. 2004, 98, 43–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inoue, A.; Kobayashi, K.; Usui, K.; Maemondo, M.; Okinaga, S.; Mikami, I.; Ando, M.; Yamazaki, K.; Saijo, Y.; Gemma, A.; et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J. Clin. Oncol. 2009, 27, 1394–1400. [Google Scholar] [CrossRef]
- Soubani, A.O.; Ruckdeschel, J.C. The Outcome of Medical Intensive Care for Lung Cancer Patients: The Case for Optimism. J. Thorac. Oncol. 2011, 6, 633–638. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in Previously Treated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 378, 113–125. [Google Scholar] [CrossRef]
- Bosch-Barrera, J.; Sais, E.; Lorencio, C.; Porta, R.; Izquierdo, A.; Menendez, J.A.; Brunet, J.; Sirvent, J.M.; Rosell, R. Successful empirical erlotinib treatment of a mechanically ventilated patient newly diagnosed with metastatic lung adenocarcinoma. Lung Cancer 2014, 86, 102–104. [Google Scholar] [CrossRef]
- Chien, C.R.; Chen, H.J. Lazarus response to treatment of patients with lung cancer and oncogenic mutations in the intensive care unit. J. Thorac. Dis. 2016, 8, E1455–E1461. [Google Scholar] [CrossRef]
- Kerrigan, K.; Shoben, A.; Otterson, G. Treatment of Lung Cancer Patients With Actionable Mutations in the Intensive Care Unit. Clin. Lung Cancer 2016, 17, 523–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dewolf, M.; Dayen, C.; Garoute, C.; Khamis, W.; Fourrier, M.; Rousselle, F.; Sadki, M.; Le Meunier, F.; Suguenot, R.; Lecuyer, E.; et al. Effectiveness of erlotinib in Critical Care Unit in patients with non-small cell lung cancer with EGFR mutation. Rev. Pneumol. Clin. 2017, 73, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Guillon, A.; Reckamp, K.L.; Heuze-Vourc’h, N. Immunotherapy improves the prognosis of lung cancer: Do we have to change intensive care unit admission and triage guidelines? Crit. Care 2017, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Yang, C.Y.; Liao, B.C.; Ho, C.C.; Liao, W.Y.; Chen, K.Y.; Tsai, T.H.; Hsu, C.L.; Hsu, W.H.; Su, K.Y.; et al. Tumor PD-L1 Expression and Clinical Outcomes in Advanced-stage Non-Small Cell Lung Cancer Patients Treated with Nivolumab or Pembrolizumab: Real-World Data in Taiwan. J. Cancer 2018, 9, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Ascierto, P.A.; Darcy, P.K.; Demaria, S.; Eggermont, A.M.M.; Redmond, W.L.; Seliger, B.; Marincola, F.M. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 2017, 81, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Christofi, T.; Baritaki, S.; Falzone, L.; Libra, M.; Zaravinos, A. Current Perspectives in Cancer Immunotherapy. Cancers 2019, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Assi, H.I.; Kamphorst, A.O.; Moukalled, N.M.; Ramalingam, S.S. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 2018, 124, 248–261. [Google Scholar] [CrossRef]
- Soares, M.; Azoulay, E. Critical care management of lung cancer patients to prolong life without prolonging dying. Intensive Care Med. 2009, 35, 2012–2014. [Google Scholar] [CrossRef]
- Andrejak, C.; Terzi, N.; Thielen, S.; Bergot, E.; Zalcman, G.; Charbonneau, P.; Jounieaux, V. Admission of advanced lung cancer patients to intensive care unit: A retrospective study of 76 patients. BMC Cancer 2011, 11, 159. [Google Scholar] [CrossRef]
- Bonomi, M.R.; Smith, C.B.; Mhango, G.; Wisnivesky, J.P. Outcomes of elderly patients with stage IIIB-IV non-small cell lung cancer admitted to the intensive care unit. Lung Cancer 2012, 77, 600–604. [Google Scholar] [CrossRef]
- Chou, K.T.; Chen, C.S.; Su, K.C.; Hung, M.H.; Hsiao, Y.H.; Tseng, C.M.; Chen, Y.M.; Lee, Y.C.; Perng, D.W. Hospital outcomes for patients with stage III and IV lung cancer admitted to the intensive care unit for sepsis-related acute respiratory failure. J. Palliat. Med. 2012, 15, 1234–1239. [Google Scholar] [CrossRef]
- Chang, Y.; Huh, J.W.; Hong, S.B.; Lee, D.H.; Suh, C.; Kim, S.W.; Lim, C.M.; Koh, Y. Outcomes and prognostic factors of patients with lung cancer and pneumonia-induced respiratory failure in a medical intensive care unit: A single-center study. J. Crit. Care 2014, 29, 414–419. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.J.; Cho, Y.J.; Park, J.S.; Kim, J.W.; Chang, H.; Lee, J.O.; Lee, K.W.; Kim, J.H.; Yoon, H.I.; et al. Who should be admitted to the intensive care unit? The outcome of intensive care unit admission in stage IIIB-IV lung cancer patients. Med. Oncol. 2014, 31, 847. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Siegelaar, S.E.; Hickmann, M.; Hoekstra, J.B.; Holleman, F.; DeVries, J.H. The effect of diabetes on mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care 2011, 15, R205. [Google Scholar] [CrossRef]
- Shin, H.J.; Chang, J.S.; Ahn, S.; Kim, T.O.; Park, C.K.; Lim, J.H.; Oh, I.J.; Kim, Y.I.; Lim, S.C.; Kim, Y.C.; et al. Clinical factors associated with weaning failure in patients requiring prolonged mechanical ventilation. J. Thorac. Dis. 2017, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Toffart, A.C.; Dhalluin, X.; Girard, N.; Chouaid, C.; Audigier-Valette, C.; Duruisseaux, M.; Mennecier, B.; Parrot, A.; Fournel, P.; Moro-Sibilot, D.; et al. Patients with advanced lung cancer harboring oncogenic mutations should be admitted to intensive care units. Intensive Care Med. 2015, 41, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Lin, J.W.; Ho, C.C.; Yang, C.Y.; Chang, C.H.; Huang, T.M.; Chen, C.Y.; Chen, K.Y.; Shih, J.Y.; Yu, C.J. Outcomes of cancer therapy administered to treatment-naive lung cancer patients in the intensive care unit. J. Cancer 2017, 8, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Zhou, C.; Hu, C.-P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Lee, S.M.; Khan, I.; Upadhyay, S.; Lewanski, C.; Falk, S.; Skailes, G.; Marshall, E.; Woll, P.J.; Hatton, M.; Lal, R.; et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): A double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2012, 13, 1161–1170. [Google Scholar] [CrossRef]
- Kudoh, S.; Kato, H.; Nishiwaki, Y.; Fukuoka, M.; Nakata, K.; Ichinose, Y.; Tsuboi, M.; Yokota, S.; Nakagawa, K.; Suga, M.; et al. Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case-control study. Am. J. Respir. Crit. Care Med. 2008, 177, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Oxnard, G.R.; Sima, C.S.; Kris, M.G.; Miller, V.A.; Riely, G.J. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6298–6303. [Google Scholar] [CrossRef]
- Chen, H.J.; Yan, H.H.; Yang, J.J.; Chen, Z.H.; Su, J.; Zhang, X.C.; Wu, Y.L. Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non-small cell lung cancer. Pathol. Oncol. Res. POR 2013, 19, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, H.; Ono, A.; Shukuya, T.; Tsuya, A.; Nakamura, Y.; Kenmotsu, H.; Naito, T.; Murakami, H.; Endo, M.; Nakajima, T.; et al. Disease flare after gefitinib discontinuation. Respir. Investig. 2015, 53, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hanna, N.; Johnson, D.; Jr, S.B.; Biermann, W.A.; Brahmer, J.; Ellis, P.M.; Giaccone, G.; Hesketh, P.J.; Jaiyesimi, I.; et al. Case for Stopping Targeted Therapy When Lung Cancer Progresses on Treatment in Hospice-Eligible Patients. J. Oncol. Pract. 2017, 13, 780–783. [Google Scholar] [CrossRef]
- Lemiale, V.; Meert, A.P.; Vincent, F.; Darmon, M.; Bauer, P.R.; Van de Louw, A.; Azoulay, E. Severe toxicity from checkpoint protein inhibitors: What intensive care physicians need to know? Ann. Intensive Care 2019, 9, 25. [Google Scholar] [CrossRef]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef]
- Lee, C.K.; Man, J.; Lord, S.; Links, M.; Gebski, V.; Mok, T.; Yang, J.C. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J. Thorac. Oncol. 2017, 12, 403–407. [Google Scholar] [CrossRef] [PubMed]


| EGFR Mutation (n = 35) | ||
|---|---|---|
| Gender (Male/Female) | 12 (34%) | 23 (66%) |
| Age (median, range) | 73 (67–79) | |
| Smokers (n, %) | 8 | 23% |
| APACHE II score | 25 (22–28) | |
| Stage IV (n, %) | 34 | 97% |
| Interval between cancer diagnosis and ICU admission (days) | 134 (6–546) | |
| Histology | ||
| Adenocarcinoma (11 with histological subclassification) (n, %) | 34 | 97% |
| Acinar (n, %) | 6/11 | 54.5% |
| Papillary (n, %) | 2/11 | 18.2% |
| Solid (n, %) | 1/11 | 9.1% |
| Mucinous (n, %) | 0/11 | 0% |
| Poorly differentiated (n, %) | 2/11 | 18.2% |
| Sarcomatoid carcinoma (n, %) | 1 | 3% |
| Comorbidity | ||
| DM (n, %) | 12 | 34% |
| HTN (n, %) | 16 | 46% |
| COPD (n, %) | 6 | 17% |
| CAD/HF (n, %) | 3 | 9% |
| CKD (n, %) | 3 | 9% |
| Reason for ICU admission | ||
| Pneumonia (n, %) | 28 | 80% |
| Shock (n, %) | 6 | 17% |
| Cardiac-related (n, %) | 1 | 3% |
| Neurological deficit (n, %) | 3 | 9% |
| Operation (n, %) | 1 | 3% |
| Type of EGFR mutation | ||
| L858R (n, %) | 15 | 43% |
| Deletion 19 (n, %) | 14 | 40% |
| Uncommon (n, %) | 6 | 17% |
| Metastatic site | ||
| Lung-to-lung (n, %) | 20 | 57% |
| Pleura (n, %) | 25 | 71% |
| Pericardial effusion (n, %) | 4 | 11% |
| Bone (n, %) | 17 | 49% |
| Brain (n, %) | 8 | 23% |
| Liver (n, %) | 8 | 23% |
| EGFR-TKI treatment | ||
| Gefitinib (n, %) | 22 | 63% |
| Erlotinib (n, %) | 11 | 31% |
| Afatinib (n, %) | 1 | 3% |
| Osimertinib (n, %) | 1 | 3% |
| Adverse events | ||
| Interstitial pneumonitis (n, %) | 2 | 6% |
| Diarrhea (n, %) | 2 | 6% |
| Hepatitis (n, %) | 1 | 3% |
| Skin toxicity (n, %) | 4 | 11% |
| Outcome | ||
| ICU 28-day-survival rate (n, %) | 27 | 77% |
| Overall survival (days) | 67 (31–320) | |
| Successful weaning from ventilator (n, %) | 15 | 43% |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | p Value | ||
| Demographic factors | ||||
| Age | 1.070 (0.993–1.153) | 0.074 | 1.090 (0.990–1.199) | 0.078 |
| APACHE II | 0.555 (0.117–2.634) | 0.459 | 0.982 (0.834–1.157) | 0.830 |
| Gender (male vs. female) | 1.054 (0.934–1.189) | 0.397 | ||
| Brain metastasis | 0.476 (0.087–2.593) | 0.391 | ||
| Liver metastasis | 1.051 (0.171–6.462) | 0.958 | ||
| EGFR mutation (based on Deletion 19) | ||||
| L8585R | 0.688 (0.124–3.786) | 0.667 | ||
| Uncommon | 0.375 (0.042–3.355) | 0.380 | ||
| Comorbidity | ||||
| COPD | 0.167 (0.023–1.232) | 0.079 | 0.139 (0.011–1.764) | 0.128 |
| CAD/HF | 0.667 (0.053–8.372) | 0.753 | ||
| DM | 0.294 (0.061–1.423) | 0.128 | ||
| Reason for ICU admission | ||||
| Shock | 0.167 (0.023–1.232) | 0.079 | 0.017 (0.000–0.629) | 0.027 |
| Pneumonia | 0.277 (0.029–2.637) | 0.264 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | p Value | ||
| Demographic factors | ||||
| Age | 1.019 (0.920–1.046) | 0.559 | 0.900 (0.791–1.026) | 0.112 |
| APACHE II | 1.017 (0.915–1.130) | 0.759 | 0.931 (0.777–1.116) | 0.440 |
| Gender (male vs. female) | 1.875 (0.453–7.758) | 0.386 | ||
| Brain metastasis | 0.873 (0.172–4.429) | 0.870 | ||
| Liver metastasis | 0.873 (0.172–4.429) | 0.870 | ||
| EGFR mutation (based on Deletion 19) | ||||
| L8585R | 0.242 (0.052–1.133) | 0.072 | 0.014 (0.000–0.450) | 0.016 |
| Uncommon | 0.167 (0.015–1.879) | 0.147 | 0.032 (0.001–1.358) | 0.072 |
| Comorbidity | ||||
| COPD | 1.000 (0.145–6.907) | 1.000 | ||
| CAD/HF | 0.731 (0.033–3.284) | 0.806 | ||
| DM | 0.070 (0.008–0.635) | 0.018 | 0.014 (0.000–0.416) | 0.014 |
| Reason for ICU admission | ||||
| Shock | 0.327 (0.033–3.284) | 0.342 | ||
| Pneumonia | 2.014 (0.363–11.187) | 0.423 |
| EGFR Mutation (n = 35) | ||
|---|---|---|
| Weaning Success | Weaning Failure | |
| CT image (n = 16) | ||
| CR/PR | 12 | 4 |
| SD/PD | 0 | 0 |
| Chest radiography (n = 19) | ||
| Improve | 3 | 0 |
| Stable/Deteriorate | 0 | 16 |
| Studies | Patient Population | Treatment | Outcomes |
|---|---|---|---|
| The present study | EGFR mutation: 35, EGFR wild-type: 28 | All received EGFR-TKI | EGFR mutation vs. wild-type: 28-day ICU survival rate: 77% vs. 50%, p = 0.025 Median overall survival: 67 vs. 28 days, p = 0.01 Rate of weaning from MV: 43% vs. 25%, p = 0.14 |
| Hsia et al. [6] | n = 83 (EGFR: 6) Respiratory failure | EGFR-TKI: 23 (6 with confirmed EGFR mutation) | Rate of weaning from MV: Standard care vs. EGFR-TKI: 18% vs. 22%, p = 0.81 |
| Toffart AC et al. [35] | n = 14 (EGFR:5, ALK: 8, ROS1: 1) Respiratory failure (MV: 9, NIPPV: 4) | All received TKI | ICU survival rate 57% Median overall survival: 91 days Longer late survival versus histological control: HR 0.12, p = 0.002 |
| Kerrigan et al. [17] | n = 9 (EGFR: 3, ALK: 3, ROS1: 1, MET: 1, unknown: 1) Respiratory failure (MV: 6, NIPPV: 3) | EGFR: Erlotinib: 3 ALK: Crizotinib: 1, Ceritinib: 1, erlotinib 1 ROS1: Crizotinib: 1 MET: Crizotinib: 1 Unknown: Erlotinib: 1 | Rate of weaning from MV: 3 of 9 (33%) ICU mortality rate: 56% |
| Chen et al. [36] | n = 72 (EGFR was confirmed in only 1 case) | EGFR-TKI: 24 (1 with confirmed EGFR mutation) | ICU survival was better in patients receiving chemotherapy or EGFR-TKI vs. BSC (p = 0.011) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-H.; Yang, C.-Y.; Shih, J.-Y.; Yu, C.-J. Tyrosine Kinase Inhibitors Improved Survival of Critically Ill EGFR-Mutant Lung Cancer Patients Undergoing Mechanical Ventilation. Biomedicines 2021, 9, 1416. https://doi.org/10.3390/biomedicines9101416
Lee I-H, Yang C-Y, Shih J-Y, Yu C-J. Tyrosine Kinase Inhibitors Improved Survival of Critically Ill EGFR-Mutant Lung Cancer Patients Undergoing Mechanical Ventilation. Biomedicines. 2021; 9(10):1416. https://doi.org/10.3390/biomedicines9101416
Chicago/Turabian StyleLee, I-Hsien, Ching-Yao Yang, Jin-Yuan Shih, and Chong-Jen Yu. 2021. "Tyrosine Kinase Inhibitors Improved Survival of Critically Ill EGFR-Mutant Lung Cancer Patients Undergoing Mechanical Ventilation" Biomedicines 9, no. 10: 1416. https://doi.org/10.3390/biomedicines9101416
APA StyleLee, I.-H., Yang, C.-Y., Shih, J.-Y., & Yu, C.-J. (2021). Tyrosine Kinase Inhibitors Improved Survival of Critically Ill EGFR-Mutant Lung Cancer Patients Undergoing Mechanical Ventilation. Biomedicines, 9(10), 1416. https://doi.org/10.3390/biomedicines9101416






