Long-Term Survival and Clinicopathological Implications of DNA Mismatch Repair Status in Endometrioid Endometrial Cancers in Hong Kong Chinese Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
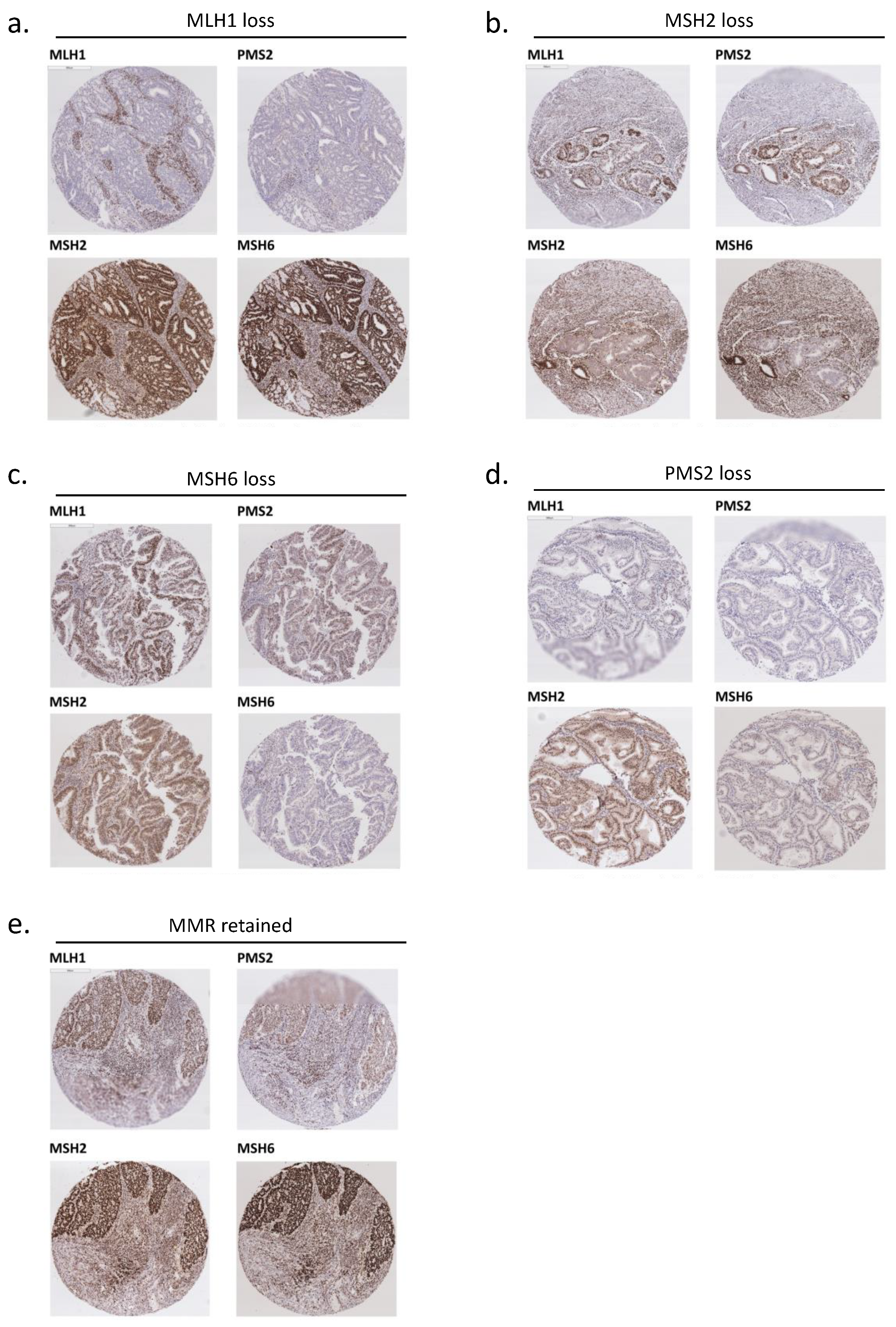
2.2. Mismatch Repair (MMR) Status Analysis with Immunohistochemistry (IHC)
2.3. Statistical Analysis
3. Results
3.1. MMR Protein Expression
3.2. Clinical Characteristics
3.3. Pathological Characteristics
3.4. Survival and Prognosis
4. Discussion
4.1. Personalized Medicine in Endometrial Cancer
4.2. Personalized Medicine in Endometrial Cancer—Adjuvant Treatment
4.3. Personalized Medicine in Endometrial Cancer—Immunotherapy
4.4. Personalized Medicine in Endometrial Cancer—Lynch Syndrome Screening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shikama, A.; Minaguchi, T.; Matsumoto, K.; Akiyama-Abe, A.; Nakamura, Y.; Michikami, H.; Nakao, S.; Sakurai, M.; Ochi, H.; Onuki, M.; et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol. Oncol. 2016, 140, 226–233. [Google Scholar] [CrossRef]
- Nout, R.A.; Bosse, T.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; van Eijk, R.; ter Haar, N.T.; Smit, V.T. Improved risk assessment of endometrial cancer by combined analysis of MSI, PI3K–AKT, Wnt/β-catenin and P53 pathway activation. Gynecol. Oncol. 2012, 126, 466–473. [Google Scholar] [CrossRef]
- Mackay, H.J.; Gallinger, S.; Tsao, M.S.; McLachlin, C.M.; Tu, D.; Keiser, K.; Eisenhauer, E.A.; Oza, A. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: Results from studies of the NCIC Clinical Trials Group (NCIC CTG). Eur. J. Cancer 2010, 46, 1365–1373. [Google Scholar] [CrossRef]
- Li, G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, C.M.; Cohn, D.; Hampel, H.; Frankel, W.L.; Jones, D.; McElroy, J.P.; Suarez, A.A.; Zhao, W.; Chen, W.; Salani, R.; et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecol. Oncol. 2017, 146, 588–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, D.; Soslow, R.A.; Levine, D.A.; Tornos, C.; Chen, S.C.; Hummer, A.J.; Bogomolniy, F.; Olvera, N.; Barakat, R.R.; Boyd, J. Clinicopathologic Significance of Defective DNA Mismatch Repair in Endometrial Carcinoma. J. Clin. Oncol. 2006, 24, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Frankel, W.; Panescu, J.; Lockman, J.; Sotamaa, K.; Fix, D.; Comeras, I.; La Jeunesse, J.; Nakagawa, H.; Westman, J.A.; et al. Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2006, 66, 7810–7817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middha, S.; Zhang, L.; Nafa, K.; Jayakumaran, G.; Wong, D.; Kim, H.R.; Sadowska, J.; Berger, M.F.; Delair, D.F.; Shia, J.; et al. Reliable Pan-Cancer Microsatellite Instability Assessment by Using Targeted Next-Generation Sequencing Data. JCO Precis. Oncol. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Bartley, A.N.; Luthra, R.; Saraiya, D.S.; Urbauer, D.L.; Broaddus, R.R. Identification of Cancer Patients with Lynch Syndrome: Clinically Significant Discordances and Problems in Tissue-Based Mismatch Repair Testing. Cancer Prev. Res. 2012, 5, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Chao, X.; Li, L.; Wu, M.; Ma, S.; Tan, X.; Zhong, S.; Bi, Y.; Lang, J. Comparison of screening strategies for Lynch syndrome in patients with newly diagnosed endometrial cancer: A prospective cohort study in China. Cancer Commun. 2019, 39, 42. [Google Scholar] [CrossRef] [Green Version]
- Bruegl, A.S.; Ring, K.L.; Daniels, M.; Fellman, B.M.; Urbauer, D.L.; Broaddus, R.R. Clinical Challenges Associated with Universal Screening for Lynch Syndrome–Associated Endometrial Cancer. Cancer Prev. Res. 2016, 10, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Wadee, W.G.R. Immunohistochemical mismatch repair deficiency versus PCR microsatellite instability: A tale of two methodologies in endometrial carcinomas. Eur. J. Gynaecol. Oncol. 2020, 41, 952–959. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother. Oncol. 2021, 154, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Nagle, C.M.; O’Mara, T.A.; Tan, Y.; Buchanan, D.D.; Obermair, A.; Blomfield, P.; Quinn, M.A.; Webb, P.M.; Spurdle, A.B.; on behalf of the Australian Endometrial Cancer Study Group. Endometrial cancer risk and survival by tumor MMR status. J. Gynecol. Oncol. 2018, 29, e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvesen, H.B.; Haldorsen, I.S.; Trovik, J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012, 13, e353–e361. [Google Scholar] [CrossRef]
- Ruiz, I.; Martín-Arruti, M.; Lopez-Lopez, E.; Garcia-Orad, A. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol. Oncol. 2014, 134, 20–23. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- An, H.J.; Kim, K.I.; Kim, J.Y.; Shim, J.Y.; Kang, H.; Kim, T.H.; Kim, J.K.; Jeong, J.K.; Lee, S.Y.; Kim, S.J. Microsatellite instability in endometrioid type endometrial adenocarcinoma is associated with poor prognostic indicators. Am. J. Surg. Pathol. 2007, 31, 846–853. [Google Scholar] [CrossRef]
- Fountzilas, E.; Kotoula, V.; Pentheroudakis, G.; Manousou, K.; Polychronidou, G.; Vrettou, E.; Poulios, C.; Papadopoulou, E.; Raptou, G.; Pectasides, E.; et al. Prognostic implications of mismatch repair deficiency in patients with nonmetastatic colorectal and endometrial cancer. ESMO Open 2019, 4, e000474. [Google Scholar] [CrossRef] [Green Version]
- Bilbao, C.; Lara, P.C.; Ramírez, R.; Henríquez-Hernández, L.A.; Rodríguez, G.; Falcón, O.; León, L.; Perucho, M.; Díaz-Chico, B.N.; Díaz-Chico, J.C. Microsatellite Instability Predicts Clinical Outcome in Radiation-Treated Endometrioid Endometrial Cancer. Int. J. Radiat. Oncol. 2010, 76, 9–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Padilla, I.; Romero, N.; Amir, E.; Matias-Guiu, X.; Vilar, E.; Muggia, F.; Garcia-Donas, J. Mismatch repair status and clinical outcome in endometrial cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. 2013, 88, 154–167. [Google Scholar] [CrossRef]
- Resnick, K.E.; Frankel, W.L.; Morrison, C.D.; Fowler, J.M.; Copeland, L.J.; Stephens, J.; Kim, K.H.; Cohn, D.E. Mismatch repair status and outcomes after adjuvant therapy in patients with surgically staged endometrial cancer. Gynecol. Oncol. 2010, 117, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Di Tucci, C.; Capone, C.; Galati, G.; Iacobelli, V.; Schiavi, M.C.; Di Donato, V.; Muzii, L.; Panici, P.B. Immunotherapy in endometrial cancer: New scenarios on the horizon. J. Gynecol. Oncol. 2019, 30, e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, G.S.; Pink, A.; Lee, S.; Han, G.; Morris, D.; Ogilvie, T.; Duggan, M.A.; Köbel, M. MMR deficiency is common in high-grade endometrioid carcinomas and is associated with an unfavorable outcome. Gynecol. Oncol. 2013, 131, 309–314. [Google Scholar] [CrossRef]
- Powell, M. Immunohistochemistry to determine mismatch repair-deficiency in endometrial cancer: The appropriate standard. Ann. Oncol. 2017, 28, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Sarode, V.R.; Robinson, L. Screening for Lynch Syndrome by Immunohistochemistry of Mismatch Repair Proteins: Significance of Indeterminate Result and Correlation With Mutational Studies. Arch. Pathol. Lab. Med. 2019, 143, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Centre for Clinical Research and Biostatistics. Survival Analysis: Comparison of Two Survival Curves—Lachin. Available online: https://www2.ccrb.cuhk.edu.hk/stat/survival/Lachin1981 (accessed on 3 September 2021).
- Schröer, A.; Köster, F.; Fischer, D.; Dubitscher, R.M.; Woll-Hermann, A.; Diedrich, K.; Friedrich, M.; Salehin, D. Immunohistochemistry of DNA mismatch repair enzyme MSH2 is not correlated with prognostic data from endometrial carcinomas. Anticancer. Res. 2009, 29, 4833–4837. [Google Scholar]
- Ribic, C.M.; Sargent, D.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor Microsatellite-Instability Status as a Predictor of Benefit from Fluorouracil-Based Adjuvant Chemotherapy for Colon Cancer. New Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Takano, M.; Miyamoto, M.; Sasaki, N.; Goto, T.; Tsuda, H.; Furuya, K. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J. Gynecol. Oncol. 2015, 26, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi33–vi38. [Google Scholar] [CrossRef]
- Sundar, S.; Balega, J.; Crosbie, E.; Drake, A.; Edmondson, R.; Fotopoulou, C.; Gallos, I.; Ganesan, R.; Gupta, J.; Johnson, N.; et al. BGCS uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 71–97. [Google Scholar] [CrossRef] [Green Version]
- Cavaliere, A.; Perelli, F.; Zaami, S.; Piergentili, R.; Mattei, A.; Vizzielli, G.; Scambia, G.; Straface, G.; Restaino, S.; Signore, F. Towards Personalized Medicine: Non-Coding RNAs and Endometrial Cancer. Heal. 2021, 9, 965. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Koh, W.-J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 170–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (accessed on 4 April 2021).
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, R.; Nakayama, K.; Nakamura, K.; Yamashita, H.; Ishibashi, T.; Ishikawa, M.; Minamoto, T.; Razia, S.; Ishikawa, N.; Otsuki, Y.; et al. Dedifferentiated Endometrial Carcinoma Could be A Target for Immune Checkpoint Inhibitors (Anti PD-1/PD-L1 Antibodies). Int. J. Mol. Sci. 2019, 20, 3744. [Google Scholar] [CrossRef] [Green Version]
- Kastrinos, F.; Uno, H.; Ukaegbu, C.; Alvero, C.; McFarland, A.; Yurgelun, M.B.; Kulke, M.H.; Schrag, D.; Meyerhardt, J.A.; Fuchs, C.S.; et al. Development and Validation of the PREMM5 Model for Comprehensive Risk Assessment of Lynch Syndrome. J. Clin. Oncol. 2017, 35, 2165–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adar, T.; Rodgers, L.H.; Shannon, K.M.; Yoshida, M.; Ma, T.; Mattia, A.; Lauwers, G.Y.; Iafrate, A.J.; Hartford, N.M.; Oliva, E.; et al. Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer 2018, 124, 3145–3153. [Google Scholar] [CrossRef] [Green Version]

| MMR Status | Number [Percentage] |
|---|---|
| MMR Retained | 162/238 [62.1%] |
MMR Loss
| 43/238 [16.5%]
|
MMR Indetermined
| 33/238 [12.6%]
|
| Clinical Parameters | MMR Deficient (n = 43) | MMR Proficient (n = 162) | p-Value |
|---|---|---|---|
| Age | Mean 57.9 (SD 11.29) | Mean 53.6 (SD 12.29) | 0.04 |
| Parity (missing n = 62) | Mean 2.33 (SD 2.19) | Mean 1.82 (SD 1.49) | 0.14 |
Parity
| (missing n = 13)
| (missing n = 49)
| |
| Menopause | 22/40 (55%) | 76/152 (50%) | 0.7 |
| Colorectal cancer | 3/43 (7%) | 4/162 (2.5%) | 0.16 |
Operation
|
|
| 0.08 |
| Bilateral salpingoophorectomy | 43/43 (100%) | 153/162 (94.4%) | 0.25 |
| Adjuvant vault radiotherapy | 10/43 (23.3%) | 24/162 (14.8%) | 0.28 |
| Adjuvant pelvic radiotherapy | 18/43 (41.9%) | 38/162 (23.5%) | 0.03 |
| Adjuvant chemotherapy | 3/43 (7%) | 12/162 (7.4%) | 0.61 |
Stage
|
|
| |
| 0.01 | |||
| 0.05 | |||
| Pathological Parameters | MMR Deficient (n = 43) | MMR Proficient (n = 162) | p-Value |
|---|---|---|---|
| Uterine tumour size (cm) | Mean 3.39 (SD 2.26) | Mean 2.46 (SD 1.89) | 0.01 |
Grade
|
|
| 0.01 |
| Deep myometrial invasion | 13/43 (30.2%) | 28/162 (17.3%) | 0.09 |
| Cervical invasion | 11/43 (25.6%) | 20/162 (12.3%) | 0.06 |
| Lymphovascular space invasion (LVSI) (missing n = 88) | 10/26 (38.5%) | 17/91 (18.7%) | 0.07 |
| Pelvic lymph node involved | 2/12 (16.7%) (Not performed n = 31) | 3/46 (6.5%) (Not performed n = 116) | 0.59 |
| Para-aortic lymph node involved | 2/10 (20%) (Not performed n = 33) | 3/26 (11.5%) (Not performed n =136) | 0.43 |
| Survival Parameters | MMR Deficient (n = 43) | MMR Proficient (n = 162) | p-Value |
|---|---|---|---|
| 5 years overall survival | 39/43 (90.7%) (Loss to follow up n = 0) | 145/157 (92.4%) (Loss to follow up n = 5) | 0.46 |
| 10 years overall survival | 26/34 (76.5%) (Loss to follow up n = 9) | 117/134 (87.3%) (Loss to follow up n = 28) | 0.19 |
| 5 years disease-specific overall survival | 36/39 (92.3%) (Loss to follow up n = 0) | 134/139 (96.4%) (Loss to follow up n = 5) | 0.38 |
| 10 years disease-specific overall survival | 24/30 (80%) (Loss to follow up n = 9) | 110/116 (94.8%) (Loss to follow up n = 28) | 0.02 |
| 5 years disease-free survival | 39/43 (90.7%) (Loss to follow up n = 0) | 144/157 (91.7%) (Loss to follow up n = 5) | 0.52 |
| 10 years disease-free survival | 25/34 (73.5%) (Loss to follow up n = 9) | 117/134 (87.3%) (Loss to follow up n = 28) | 0.05 |
| Disease recurrence | 7/43 (16.3%) | 1/162 (4.3%) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.S.; Li, J.J.X.; Chow, C.; Chan, R.C.K.; Kwan, J.S.H.; Lau, T.S.; To, K.F.; Yim, S.F.; Yeung, S.Y.; Kwong, J. Long-Term Survival and Clinicopathological Implications of DNA Mismatch Repair Status in Endometrioid Endometrial Cancers in Hong Kong Chinese Women. Biomedicines 2021, 9, 1385. https://doi.org/10.3390/biomedicines9101385
Lee JHS, Li JJX, Chow C, Chan RCK, Kwan JSH, Lau TS, To KF, Yim SF, Yeung SY, Kwong J. Long-Term Survival and Clinicopathological Implications of DNA Mismatch Repair Status in Endometrioid Endometrial Cancers in Hong Kong Chinese Women. Biomedicines. 2021; 9(10):1385. https://doi.org/10.3390/biomedicines9101385
Chicago/Turabian StyleLee, Jacqueline Ho Sze, Joshua Jing Xi Li, Chit Chow, Ronald Cheong Kin Chan, Johnny Sheung Him Kwan, Tat San Lau, Ka Fai To, So Fan Yim, Suet Ying Yeung, and Joseph Kwong. 2021. "Long-Term Survival and Clinicopathological Implications of DNA Mismatch Repair Status in Endometrioid Endometrial Cancers in Hong Kong Chinese Women" Biomedicines 9, no. 10: 1385. https://doi.org/10.3390/biomedicines9101385
APA StyleLee, J. H. S., Li, J. J. X., Chow, C., Chan, R. C. K., Kwan, J. S. H., Lau, T. S., To, K. F., Yim, S. F., Yeung, S. Y., & Kwong, J. (2021). Long-Term Survival and Clinicopathological Implications of DNA Mismatch Repair Status in Endometrioid Endometrial Cancers in Hong Kong Chinese Women. Biomedicines, 9(10), 1385. https://doi.org/10.3390/biomedicines9101385








