New Insights into the Activities of D-Chiro-Inositol: A Narrative Review
Abstract
1. Introduction
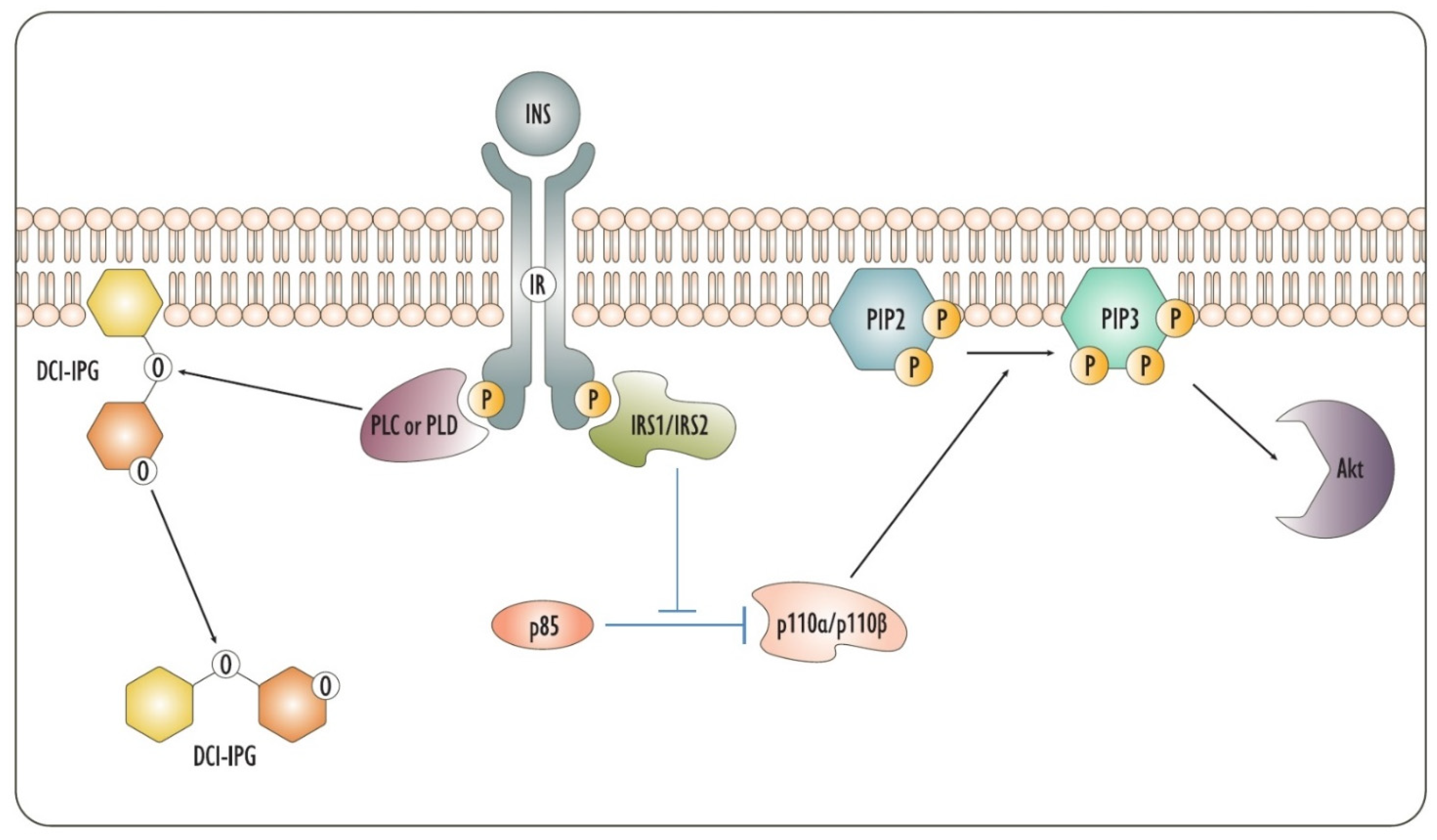
2. Insulin
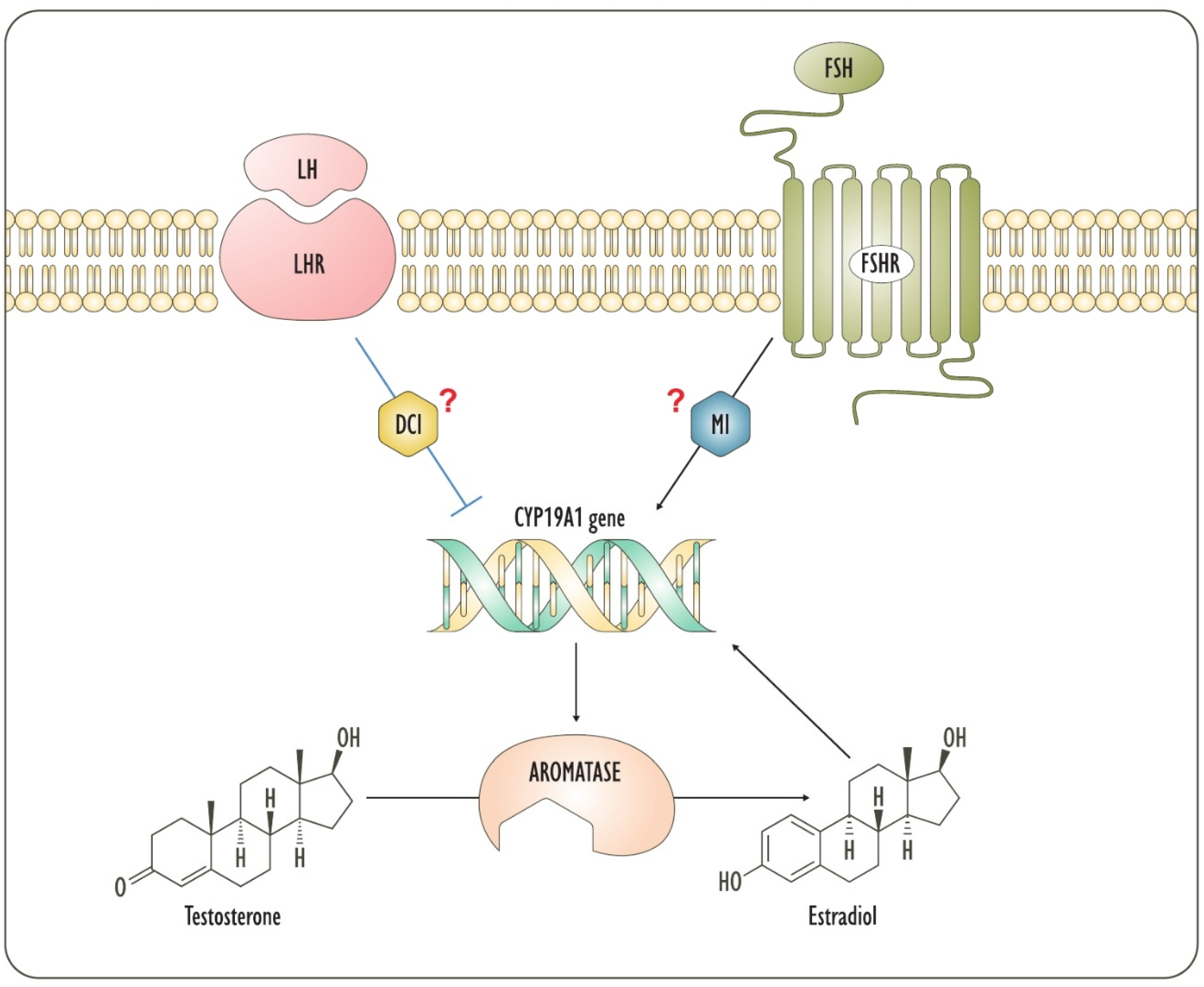
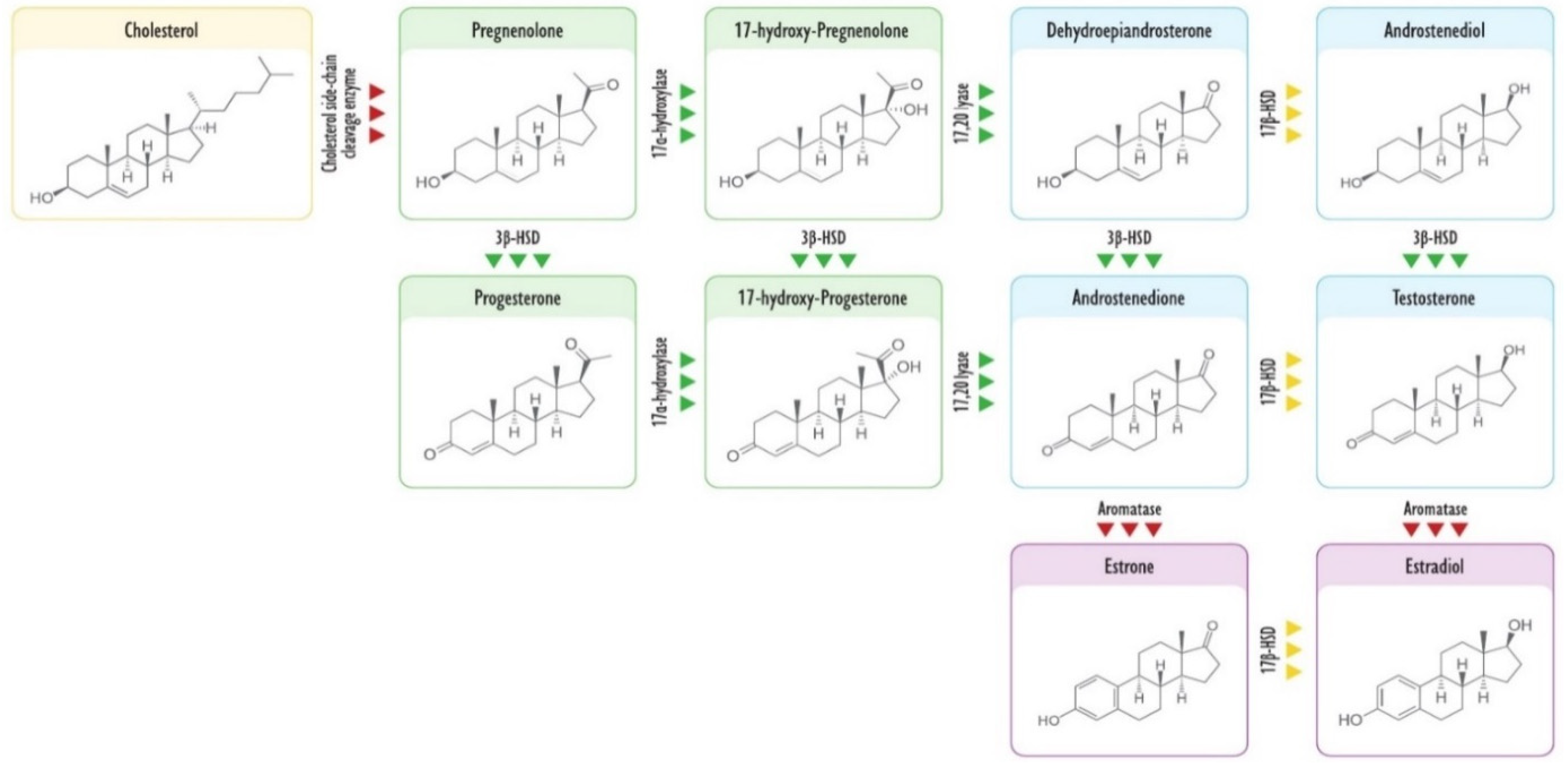
3. Steroidogenesis
4. Integrins
5. Inflammation and Cancer
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef]
- Gambioli, R.; Forte, G.; Aragona, C.; Bevilacqua, A.; Bizzarri, M.; Unfer, V. The use of D-chiro-Inositol in clinical practice. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 438–446. [Google Scholar] [PubMed]
- Sun, T.-H.; Heimark, D.; Nguygen, T.; Nadler, J.L.; Larner, J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem. Biophys. Res. Commun. 2002, 293, 1092–1098. [Google Scholar] [CrossRef]
- Arner, R.J.; Prabhu, K.S.; Thompson, J.T.; Hildenbrandt, G.R.; Liken, A.D.; Reddy, C.C. myo-Inositol oxygenase: Molecular cloning and expression of a unique enzyme that oxidizes myo-inositol and d-chiro-inositol. Biochem. J. 2001, 360, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Chiu, T.T.Y.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The rationale of the myo-inositol and D-chiro-inositol combined treatment for polycystic ovary syndrome. J. Clin. Pharmacol. 2014, 54, 1079–1092. [Google Scholar] [CrossRef]
- Paulick, M.G.; Bertozzi, C.R. The Glycosylphosphatidylinositol Anchor: A Complex Membrane-Anchoring Structure for Proteins. Biochemistry 2008, 47, 6991–7000. [Google Scholar] [CrossRef]
- Gambioli, R.; Forte, G.; Buzzaccarini, G.; Unfer, V.; Laganà, A. Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation. Pharmaceuticals 2021, 14, 504. [Google Scholar] [CrossRef]
- Goel, M.; Azev, V.N.; D’Alarcao, M. The biological activity of structurally defined inositol glycans. Futur. Med. Chem. 2009, 1, 95–118. [Google Scholar] [CrossRef]
- Leroy, C.; Ramos, P.; Cornille, K.; Bonenfant, D.; Fritsch, C.; Voshol, H.; Bentires-Alj, M. Activation of IGF1R/p110β/AKT/mTOR confers resistance to α-specific PI3K inhibition. Breast Cancer Res. 2016, 18, 1–13. [Google Scholar] [CrossRef]
- Matheny, R.W.; Adamo, M.L. PI3K p110α and p110β have differential effects on Akt activation and protection against oxidative stress-induced apoptosis in myoblasts. Cell Death Differ. 2009, 17, 677–688. [Google Scholar] [CrossRef]
- Kayali, A.G.; Eichhorn, J.; Haruta, T.; Morris, A.J.; Nelson, J.G.; Vollenweider, P.; Olefsky, J.M.; Webster, N.J.G. Association of the Insulin Receptor with Phospholipase C-γ (PLCγ) in 3T3-L1 Adipocytes Suggests a Role for PLCγ in Metabolic Signaling by Insulin. J. Biol. Chem. 1998, 273, 13808–13818. [Google Scholar] [CrossRef]
- Eichhorn, J.; Kayali, A.G.; Austin, D.A.; Webster, N.J. Insulin Activates Phospholipase C-γ1 via a PI-3 Kinase Dependent Mechanism in 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 2001, 282, 615–620. [Google Scholar] [CrossRef]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-Chiro-Inositol Glycans in Insulin Signaling and Insulin Resistance. Mol. Med. 2010, 16, 543–552. [Google Scholar] [CrossRef]
- Monastra, G.; Vazquez-Levin, M.; Espinola, M.S.B.; Bilotta, G.; Laganà, A.S.; Unfer, V. D-chiro-inositol, an aromatase down-modulator, increases androgens and reduces estrogens in male volunteers: A pilot study. Basic. Clin. Androl. 2021, 31, 1–17. [Google Scholar] [CrossRef]
- Celentano, C.; Matarrelli, B.; Pavone, G.; Vitacolonna, E.; Mattei, P.A.; Berghella, V.; Liberati, M. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: A randomized controlled trial. J. Matern. Neonatal Med. 2018, 33, 743–751. [Google Scholar] [CrossRef]
- Nestler, J.E.; Jakubowicz, D.J.; Reamer, P.; Gunn, R.D.; Allan, G. Ovulatory and Metabolic Effects of d-Chiro-Inositol in the Polycystic Ovary Syndrome. N. Engl. J. Med. 1999, 340, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J.-P.; Iuorno, M.J.; Jakubowicz, D.J.; Apridonidze, T.; He, N.; Nestler, J.E. Metformin Therapy Increases Insulin-Stimulated Release ofd-Chiro-Inositol-Containing Inositolphosphoglycan Mediator in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 242–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheang, K.I.; Baillargeon, J.-P.; Essah, P.A.; Ostlund, R.E.; Apridonize, T.; Islam, L.; Nestler, J.E. Insulin-stimulated release of d-chiro-inositol–containing inositolphosphoglycan mediator correlates with insulin sensitivity in women with polycystic ovary syndrome. Metabolism 2008, 57, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.; Gamez, G.; Huang, L.C.; Lilley, K.; Luttrell, L. Anti-inositolglycan antibodies selectively block some of the actions of insulin in intact BC3H1 cells. Proc. Natl. Acad. Sci. USA 1990, 87, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Sleight, S.; Wilson, B.; Heimark, D.; Larner, J. Gq/11 is involved in insulin-stimulated inositol phosphoglycan putative mediator generation in rat liver membranes: Co-localization of Gq/11 with the insulin receptor in membrane vesicles. Biochem. Biophys. Res. Commun. 2002, 295, 561–569. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; De Fonseca, F.R. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, S.; Richter, E.A. GLUT4-containing vesicles are released from membranes by phospholipase D cleavage of a GPI anchor. Am. J. Physiol. Metab. 2002, 283, E374–E382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonilla, J.B.; Cid, M.B.; Contreras, F.-X.; Goñi, F.M.; Martín-Lomas, M. Phospholipase Cleavage ofD- andL-chiro-Glycosylphosphoinositides Asymmetrically Incorporated into Liposomal Membranes. Chem.-A Eur. J. 2006, 12, 1513–1528. [Google Scholar] [CrossRef]
- Brautigan, D.L.; Brown, M.; Grindrod, S.; Chinigo, G.; Kruszewski, A.; Lukasik, S.M.; Bushweller, J.H.; Horal, M.; Keller, S.; Tamura, S.; et al. Allosteric Activation of Protein Phosphatase 2C byd-chiro-Inositol−Galactosamine, a Putative Mediator Mimetic of Insulin Action†. Biochemistry 2005, 44, 11067–11073. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Maegawa, H.; Egawa, K.; Ugi, S.; Nishio, Y.; Imamura, T.; Kobayashi, T.; Tamura, S.; Olefsky, J.M.; Kashiwagi, A. Protein Phosphatase-2Cα as a Positive Regulator of Insulin Sensitivity through Direct Activation of Phosphatidylinositol 3-Kinase in 3T3-L1 Adipocytes. J. Biol. Chem. 2004, 279, 22715–22726. [Google Scholar] [CrossRef]
- Zawalich, W.S.; Zawalich, K.C. Regulation of insulin secretion by phospholipase C. Am. J. Physiol. Metab. 1996, 271, E409–E416. [Google Scholar] [CrossRef]
- Lazarenko, R.; Geisler, J.; Bayliss, D.; Larner, J.; Li, C. D-chiro-inositol glycan stimulates insulin secretion in pancreatic β cells. Mol. Cell. Endocrinol. 2014, 387, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Filippello, A.; Scamporrino, A.; Di Mauro, S.; Malaguarnera, R.; Di Pino, A.; Scicali, R.; Purrello, F.; Piro, S. Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells. Biomolecules 2020, 10, 1404. [Google Scholar] [CrossRef]
- Lin, X.; Ma, L.; Fitzgerald, R.L.; Ostlund, R.E. Human sodium/inositol cotransporter 2 (SMIT2) transports inositols but not glucose in L6 cells. Arch. Biochem. Biophys. 2009, 481, 197–201. [Google Scholar] [CrossRef]
- Baillargeon, J.-P.; Diamanti-Kandarakis, E.; Ostlund, R.E.; Apridonidze, T.; Iuorno, M.J.; Nestler, J.E. Altered D-Chiro-Inositol Urinary Clearance in Women with Polycystic Ovary Syndrome. Diabetes Care 2006, 29, 300–305. [Google Scholar] [CrossRef]
- Baillargeon, J.-P.; Iuorno, M.J.; Apridonidze, T.; Nestler, J.E. Uncoupling Between Insulin and Release of a d-Chiro-Inositol—Containing Inositolphosphoglycan Mediator of Insulin Action in Obese Women with Polycystic Ovary Syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E.; McGill, J.B.; Herskowitz, I.; Kipnis, D.M.; Santiago, J.V.; Sherman, W.R. D-chiro-inositol metabolism in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1993, 90, 9988–9992. [Google Scholar] [CrossRef] [PubMed]
- Montt-Guevara, M.M.; Finiguerra, M.; Marzi, I.; Fidecicchi, T.; Ferrari, A.; Genazzani, A.D.; Simoncini, T. D-Chiro-Inositol Regulates Insulin Signaling in Human Adipocytes. Front. Endocrinol. 2021, 12, 283. [Google Scholar] [CrossRef]
- Gupta, A.; Jakubowicz, D.; Nestler, J.E. Pioglitazone Therapy Increases Insulin-Stimulated Release of d-Chiro-Inositol-Containing Inositolphosphoglycan Mediator in Women with Polycystic Ovary Syndrome. Metab. Syndr. Relat. Disord. 2016, 14, 391–396. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Marini, G.; Prati, A.; Simoncini, T. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol. Endocrinol. 2014, 30, 438–443. [Google Scholar] [CrossRef]
- Laganà, A.S.; Barbaro, L.; Pizzo, A. Evaluation of ovarian function and metabolic factors in women affected by polycystic ovary syndrome after treatment with d-Chiro-Inositol. Arch. Gynecol. Obstet. 2014, 291, 1181–1186. [Google Scholar] [CrossRef]
- Nordio, M.; Basciani, S.; Camajani, E. The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: Comparison with other ratios. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5512–5521. [Google Scholar]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and hCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, S.; Marinaro, F.; Tondelli, D.; Lui, J.; Xella, S.; Marsella, T.; Tagliasacchi, D.; Argento, C.; Tirelli, A.; Giulini, S.; et al. Modulation of gonadotrophin induced steroidogenic enzymes in granulosa cells by d-chiroinositol. Reprod. Biol. Endocrinol. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Nestler, J.E.; Romero, G.; Huang, L.C.; Zhang, C.; Larner, J. Insulin Mediators Are the Signal Transduction System Responsible for Insulin’s Actions on Human Placental Steroidogenesis*. Endocrinology 1991, 129, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Niiyama, S.; Huth, A.; Kissling, S.; Happle, R. 17α-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Exp. Dermatol. 2002, 11, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, C.; Zapata, L.E.C.; Arevalo, M.-A.; Garcia-Segura, L.; Cambiasso, M.J. Regulation of aromatase expression in the anterior amygdala of the developing mouse brain depends on ERβ and sex chromosome complement. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.L.; Richards, J.S. Regulation of Cytochrome P450 Aromatase Messenger Ribonucleic Acid and Activity by Steroids and Gonadotropins in Rat Granulosa Cells *. Endocrinology 1991, 129, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Haltia, U.-M.; Pihlajoki, M.; Andersson, N.; Mäkinen, L.; Tapper, J.; Cervera, A.; Horlings, H.M.; Turpeinen, U.; Anttonen, M.; Bützow, R.; et al. Functional Profiling of FSH and Estradiol in Ovarian Granulosa Cell Tumors. J. Endocr. Soc. 2020, 4, bvaa034. [Google Scholar] [CrossRef]
- Marino, M.; Distefano, E.; Trentalance, A.; Smith, C. Estradiol-induced IP3 mediates the estrogen receptor activity expressed in human cells. Mol. Cell. Endocrinol. 2001, 182, 19–26. [Google Scholar] [CrossRef]
- Szatkowski, C.; Parys, J.B.; Ouadid-Ahidouch, H.; Matifat, F. Inositol 1,4,5-trisphosphate-induced Ca2+ signalling is involved in estradiol-induced breast cancer epithelial cell growth. Mol. Cancer 2010, 9, 1–13. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Wu, W.; Strom, A.M.; Warner, M.; Gustafsson, J.-Å. Estrogen receptor β regulates AKT activity through up-regulation of INPP4B and inhibits migration of prostate cancer cell line PC-3. Proc. Natl. Acad. Sci. USA 2020, 117, 26347–26355. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.O.; Lucas, T.F.G.; Porto, C.S.; Abdalla, F.M.F. Estrogen receptor ESR1 regulates the phospholipase C-inositol phosphate signaling in the hippocampus from rats in proestrous and estrous phases. Steroids 2013, 78, 8–14. [Google Scholar] [CrossRef]
- Stocco, C. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids 2008, 73, 473–487. [Google Scholar] [CrossRef]
- Fuhrmeister, I.P.; Branchini, G.; Pimentel, A.M.; Ferreira, G.D.; Capp, E.; Brum, I.S.; Corleta, H.V.E. Human Granulosa Cells: Insulin and Insulin-Like Growth Factor-1 Receptors and Aromatase Expression Modulation by Metformin. Gynecol. Obstet. Investig. 2014, 77, 156–162. [Google Scholar] [CrossRef]
- Fitzpatrick, S.L.; Carlone, D.L.; Robker, R.; Richards, J.S. Expression of aromatase in the ovary: Down-regulation of mRNA by the ovulatory luteinizing hormone surge. Steroids 1997, 62, 197–206. [Google Scholar] [CrossRef]
- McNatty, K.P.; Makris, A.; Osathanondh, R.; Ryan, K.J. Effects of luteinizing hormone on steroidogenesis by thecal tissue from human ovarian follicles in vitro. Steroids 1980, 36, 53–63. [Google Scholar] [CrossRef]
- Campbell, W.W.; Ostlund, J.R.E.; Joseph, L.J.; Farrell, P.A.; Evans, W.J. Relationships of Plasma C-Peptide and Gender to the Urinary Excretion of Inositols in Older People. Horm. Metab. Res. 2001, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, G.; Unfer, V.; Roseff, S. The D-chiro-inositol paradox in the ovary. Fertil. Steril. 2011, 95, 2515–2516. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; De Vargas, A.F.; Brik, C.; Quintero, N.; Medina, F. Insulin Stimulates Testosterone Biosynthesis by Human Thecal Cells from Women with Polycystic Ovary Syndrome by Activating Its Own Receptor and Using Inositolglycan Mediators as the Signal Transduction System 1. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar] [CrossRef]
- Munir, I.; Yen, H.-W.; Geller, D.H.; Torbati, D.; Bierden, R.M.; Weitsman, S.R.; Agarwal, S.K.; Magoffin, D.A. Insulin Augmentation of 17α-Hydroxylase Activity Is Mediated by Phosphatidyl Inositol 3-Kinase but not Extracellular Signal-Regulated Kinase-1/2 in Human Ovarian Theca Cells. Endocrinol. 2004, 145, 175–183. [Google Scholar] [CrossRef]
- Ueshiba, H.; Shimizu, Y.; Hiroi, N.; Yakushiji, F.; Shimojo, M.; Tsuboi, K.; Miyachi, Y. Decreased steroidogenic enzyme 17,20-lyase and increased 17-hydroxylase activities in type 2 diabetes mellitus. Eur. J. Endocrinol. 2002, 146, 375–380. [Google Scholar] [CrossRef]
- Heimark, D.; McAllister, J.; Larner, J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr. J. 2014, 61, 111–117. [Google Scholar] [CrossRef]
- Jakimiuk, A.J.; Weitsman, S.R.; Navab, A.; Magoffin, D.A. Luteinizing Hormone Receptor, Steroidogenesis Acute Regulatory Protein, and Steroidogenic Enzyme Messenger Ribonucleic Acids Are Overexpressed in Thecal and Granulosa Cells from Polycystic Ovaries 1. J. Clin. Endocrinol. Metab. 2001, 86, 1318–1323. [Google Scholar] [CrossRef][Green Version]
- Nestler, J.E.; Jakubowicz, D.J. Decreases in Ovarian Cytochrome P450c17α Activity and Serum Free Testosterone after Reduction of Insulin Secretion in Polycystic Ovary Syndrome. N. Engl. J. Med. 1996, 335, 617–623. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Dragotto, J.; Lucarelli, M.; Di Emidio, G.; Monastra, G.; Tatone, C. High Doses of D-Chiro-Inositol Alone Induce a PCO-Like Syndrome and Other Alterations in Mouse Ovaries. Int. J. Mol. Sci. 2021, 22, 5691. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, W.-Y.; Heo, J.H.; Huh, J.; Kim, G.; Lee, K.-P.; Kwun, H.-J.; Shin, H.-J.; Baek, I.-J.; Hong, E.-J. Progesterone increases blood glucose via hepatic progesterone receptor membrane component 1 under limited or impaired action of insulin. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Tan, T.-W.; Tsai, T.-H.; Chen, C.-C.; Hsieh, T.-F.; Lee, S.-S.; Liu, H.-H.; Chen, W.-C.; Tang, C.-H. D-pinitol Inhibits Prostate Cancer Metastasis through Inhibition of αVβ3 Integrin by Modulating FAK, c-Src and NF-κB Pathways. Int. J. Mol. Sci. 2013, 14, 9790–9802. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Elangbam, C.S.; Qualls, C.W.; Dahlgren, R.R. Cell Adhesion Molecules—Update. Vet. Pathol. 1997, 34, 61–73. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Wang, J.; Bäcker, T.; Krueger, M.; Zamani, S.; Rosowski, S.; Gruber, T.; Onogi, Y.; Feuchtinger, A.; Schulz, T.J.; et al. Active integrins regulate white adipose tissue insulin sensitivity and brown fat thermogenesis. Mol. Metab. 2021, 45, 101147. [Google Scholar] [CrossRef]
- Lessey, B.A.; Castelbaum, A.J.; Buck, C.A.; Lei, Y.; Yowell, C.W.; Sun, J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil. Steril. 1994, 62, 497–506. [Google Scholar] [CrossRef]
- Apparao, K.; Lovely, L.P.; Gui, Y.; Lininger, R.A.; Lessey, B.A. Elevated Endometrial Androgen Receptor Expression in Women with Polycystic Ovarian Syndrome1. Biol. Reprod. 2002, 66, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.A.; Bazer, F.W.; Burghardt, R. Spatial and Temporal Analyses of Integrin and Muc-1 Expression in Porcine Uterine Epithelium and Trophectoderm in Vivo1. Biol. Reprod. 1996, 55, 1098–1106. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Forbes, K.; Carver, J.; Aplin, J.D. The role of the osteopontin–integrin αvβ3 interaction at implantation: Functional analysis using three different in vitro models. Hum. Reprod. 2014, 29, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, X.; Yang, J. Integrin αvβ3 Is Essential for Maintenance of Decidua Tissue Homeostasis and of Natural Killer Cell Immune Tolerance During Pregnancy. Reprod. Sci. 2018, 25, 1424–1430. [Google Scholar] [CrossRef]
- Germeyer, A.; Savaris, R.F.; Jauckus, J.; Lessey, B. Endometrial beta3 Integrin profile reflects endometrial receptivity defects in women with unexplained recurrent pregnancy loss. Reprod. Biol. Endocrinol. 2014, 12, 1–5. [Google Scholar] [CrossRef]
- Elnaggar, A.; Farag, A.H.; Gaber, M.E.; Hafeez, M.A.; Ali, M.S.; Atef, A.M. AlphaVBeta3 Integrin expression within uterine endometrium in unexplained infertility: A prospective cohort study. BMC Women’s Health 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Lessey, B.A.; Castelbaum, A.J.; Sawin, S.W.; Buck, C.A.; Schinnar, R.; Bilker, W.; Strom, B.L. Aberrant integrin expression in the endometrium of women with endometriosis. J. Clin. Endocrinol. Metab. 1994, 79, 643–649. [Google Scholar] [CrossRef]
- Kimmins, S.; Lim, H.C.; Parent, J.; Fortier, M.A.; MacLaren, L.A. The effects of estrogen and progesterone on prostaglandins and integrin beta 3 (β3) subunit expression in primary cultures of bovine endometrial cells. Domest. Anim. Endocrinol. 2003, 25, 141–154. [Google Scholar] [CrossRef]
- Lessey, B.A. Two pathways of progesterone action in the human endometrium: Implications for implantation and contraception. Steroids 2003, 68, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Hills, F.A.; Abrahams, V.M.; González-Timón, B.; Francis, J.; Cloke, B.; Hinkson, L.; Rai, R.; Mor, G.; Regan, L.; Sullivan, M.; et al. Heparin prevents programmed cell death in human trophoblast. Mol. Hum. Reprod. 2006, 12, 237–243. [Google Scholar] [CrossRef]
- Liu, H.; Radisky, D.C.; Yang, D.; Xu, R.; Radisky, E.; Bissell, M.J.; Bishop, J.M. MYC suppresses cancer metastasis by direct transcriptional silencing of αv and β3 integrin subunits. Nat. Cell Biol. 2012, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Fujita, T.; Kajiya, M.; Takeda, K.; Shiba, H.; Kawaguchi, H.; Kurihara, H. Brain-derived neurotrophic factor induces migration of endothelial cells through a TrkB-ERK-integrin αVβ3-FAK cascade. J. Cell. Physiol. 2012, 227, 2123–2129. [Google Scholar] [CrossRef]
- Douma, S.; van Laar, T.; Zevenhoven, J.; Meuwissen, R.; Van Garderen, E.; Peeper, D.S. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nat. Cell Biol. 2004, 430, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Qiu, H.; Yang, T.; Luo, X.; Zhang, H.; Wan, X. Upregulation of TrkB Promotes Epithelial-Mesenchymal Transition and Anoikis Resistance in Endometrial Carcinoma. PLoS ONE 2013, 8, e70616. [Google Scholar] [CrossRef]
- Li, T.; Yu, Y.; Song, Y.; Li, X.; Lan, D.; Zhang, P.; Xiao, Y.; Xing, Y. Activation of BDNF/TrkB pathway promotes prostate cancer progression via induction of epithelial-mesenchymal transition and anoikis resistance. FASEB J. 2020, 34, 9087–9101. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.G.; Al-Shahrour, F.; Hartwell, K.A.; Chu, L.P.; Järås, M.; Puram, R.V.; Puissant, A.; Callahan, K.P.; Ashton, J.; McConkey, M.E.; et al. In Vivo RNAi Screening Identifies a Leukemia-Specific Dependence on Integrin Beta 3 Signaling. Cancer Cell 2013, 24, 45–58. [Google Scholar] [CrossRef]
- Seguin, L.; Kato, S.; Franovic, A.; Camargo, M.F.; Lesperance, J.; Elliott, K.C.; Yebra, M.; Mielgo, A.; Lowy, A.M.; Husain, H.; et al. An integrin β3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 2014, 16, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-κB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef]
- Choi, M.-S.; Lee, W.-H.; Kwon, E.-Y.; Kang, M.A.; Lee, M.-K.; Park, Y.B.; Jeon, S.-M. Effects of Soy Pinitol on the Pro-Inflammatory Cytokines and Scavenger Receptors in Oxidized Low-Density Lipoprotein-Treated THP-1 Macrophages. J. Med. Food 2007, 10, 594–601. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, I.D.; Jeong, Y.-I.; Lee, C.-M.; Shin, Y.K.; Lee, S.-Y.; Suh, D.-S.; Yoon, M.-S.; Lee, K.-S.; Choi, Y.H.; et al. d-pinitol inhibits Th1 polarization via the suppression of dendritic cells. Int. Immunopharmacol. 2007, 7, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, C.-M.; Jeong, Y.-I.; Jung, I.D.; Kim, B.-H.; Seong, E.-Y.; Kim, J.-I.; Choi, I.-W.; Chung, H.Y.; Park, Y.-M. d-pinitol regulates Th1/Th2 balance via suppressing Th2 immune response in ovalbumin-induced asthma. FEBS Lett. 2006, 581, 57–64. [Google Scholar] [CrossRef]
- Fortis-Barrera, Á.; Aguilar, F.A.; Banderas-Dorantes, T.; Diaz-Flores, M.; Román-Ramos, R.; Cruz, M.; García-Macedo, R. C ucurbita ficifolia Bouché (Cucurbitaceae) and D-chiro-inositol modulate the redox state and inflammation in 3T3-L1 adipocytes. J. Pharm. Pharmacol. 2013, 65, 1563–1576. [Google Scholar] [CrossRef]
- Kunjara, S.; Wang, D.Y.; McLean, P.; Greenbaum, A.; Rademacher, T.W. Inositol Phosphoglycans and the Regulation of the Secretion of Leptin: In Vitro Effects on Leptin Release from Adipocytes and the Relationship to Obesity. Mol. Genet. Metab. 2000, 70, 61–68. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.; Li, Y.; Peng, Q.; Gao, J.; Liu, B.; Wang, M. d-Chiro inositol ameliorates endothelial dysfunction via inhibition of oxidative stress and mitochondrial fission. Mol. Nutr. Food Res. 2017, 61, 1600710. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; De La Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- You, H.J.; Oh, D.-K.; Ji, G.E. Anticancerogenic effect of a novel chiroinositol-containing polysaccharide fromBifidobacterium bifidumBGN4. FEMS Microbiol. Lett. 2004, 240, 131–136. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Park, J.-S.; Hwang, B.Y.; Lee, C.-K.; Song, S. Inhibitory Effect of D-chiro-inositol on Both Growth and Recurrence of Breast Tumor from MDA-MB-231 Cancer Cells. Nat. Prod. Sci. 2017, 23, 35–39. [Google Scholar] [CrossRef]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Haribabu, L.; Nishigaki, I.; Balasubramanian, M.P. D-Pinitol Promotes Apoptosis in MCF-7 Cells via Induction of p53 and Bax and Inhibition of Bcl-2 and NF-κB. Asian Pac. J. Cancer Prev. 2014, 15, 1757–1762. [Google Scholar] [CrossRef]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Ganesh, M.K.; Balasubramanian, M.P.; Nishigaki, I. d-pinitol mitigates tumor growth by modulating interleukins and hormones and induces apoptosis in rat breast carcinogenesis through inhibition of NF-κB. J. Physiol. Biochem. 2015, 71, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Shi, K.; Yang, Y.; Gu, X.; Tan, W.; Wang, Q.; Gao, X.; Veeraraghavan, V.P.; Mohan, S.K.; Jin, S. D-Pinitol treatment induced the apoptosis in human leukemia MOLT-4 cells by improved apoptotic signaling pathway. Saudi J. Biol. Sci. 2020, 27, 2134–2138. [Google Scholar] [CrossRef]
| Upregulated Genes | Downregulated Genes | Downregulated Genes | ||
|---|---|---|---|---|
| DCI | NRF-2 | Integrin β3 | Pinitol | Integrin β3 |
| TNF-α | TNF-α | |||
| IL-6 | IL-8 | |||
| Leptin | NF-κB | |||
| NOX4 | c-Src | |||
| c-myc | ||||
| COX-2 | ||||
| Bcl-2 | ||||
| Bcl-xL | ||||
| VEGF | ||||
| MMP-9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambioli, R.; Montanino Oliva, M.; Nordio, M.; Chiefari, A.; Puliani, G.; Unfer, V. New Insights into the Activities of D-Chiro-Inositol: A Narrative Review. Biomedicines 2021, 9, 1378. https://doi.org/10.3390/biomedicines9101378
Gambioli R, Montanino Oliva M, Nordio M, Chiefari A, Puliani G, Unfer V. New Insights into the Activities of D-Chiro-Inositol: A Narrative Review. Biomedicines. 2021; 9(10):1378. https://doi.org/10.3390/biomedicines9101378
Chicago/Turabian StyleGambioli, Riccardo, Mario Montanino Oliva, Maurizio Nordio, Alfonsina Chiefari, Giulia Puliani, and Vittorio Unfer. 2021. "New Insights into the Activities of D-Chiro-Inositol: A Narrative Review" Biomedicines 9, no. 10: 1378. https://doi.org/10.3390/biomedicines9101378
APA StyleGambioli, R., Montanino Oliva, M., Nordio, M., Chiefari, A., Puliani, G., & Unfer, V. (2021). New Insights into the Activities of D-Chiro-Inositol: A Narrative Review. Biomedicines, 9(10), 1378. https://doi.org/10.3390/biomedicines9101378







