Nonalcoholic Fatty Liver Disease and the Kidney: A Review
Abstract
:1. Introduction
1.1. Nonalcoholic Fatty Liver Disease
1.2. Chronic Kidney Disease
2. Materials and Methods
Literature Search, Data Selection and Extraction
3. Results
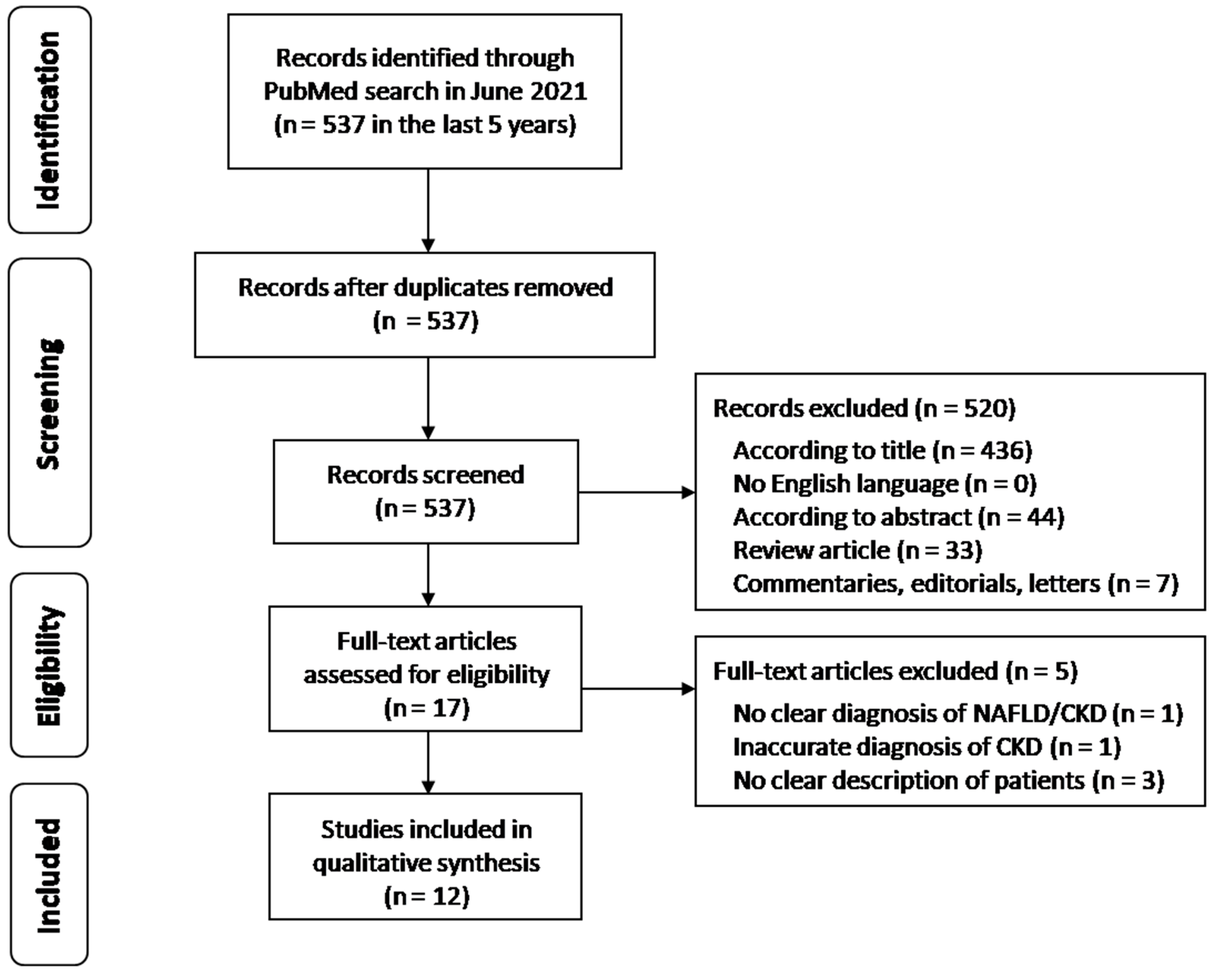
3.1. Study Flow
3.2. Study Characteristics
| Author/Year (Country) | Study Design | Number of Patients | Diagnosis of NAFLD | Diagnosis of CKD | Patient Characteristics | Main Results of the Study |
|---|---|---|---|---|---|---|
| Huh JH et al., 2017 (Korea) [38] | Prospective cohort study | 4761 patients (1808 men and 2953 women) | FLI ≥ 60 | GFR < 60 mL/min/1.73 m2 estimated by CKD-EPI equation | Prevalence of NAFLD was 12.6% (601 patients). 121/601 subjects (20.1%) developed CKD. | NAFLD is associated with CKD independently of traditional risk factors (HR 1.46, 95% CI 1.19–1.79). |
| Sinn DH et al., 2017 (Korea) [30] | Retrospective longitudinal cohort study | 41,430 patients (25,217 men and 16,213 women) | Ultrasound NFS, APRI and FIB-4 score to assess severity of fibrosis | GFR < 60 mL/min/1.73 m2 estimated by CKD-EPI equation | Prevalence of NAFLD was 34.3% (14,223 patients). 691 subjects developed incident CKD. | NAFLD was independently associated with incident CKD (aHR 1.22, 95% CI 1.04–1.43). This association was progressive with increased severity of liver disease (aHR 1.59, 95% CI 1.31–1.93). |
| Wijarnpreecha K et al., 2018 (USA) [31] | Cross-sectional study | 4142 NAFLD patients (1932 men and 2210 women) | Ultrasound NFS, FIB-4 score, APRI and BARD score to assess severity of fibrosis | GFR < 60 mL/min/1.73 m2 estimated by MDRD equation | Prevalence of NAFLD was 100%. 200/4142 subjects (4.8%) developed CKD. | Advanced liver fibrosis, defined by NFS and FIB-4 scores, is associated independently with CKD among individuals with NAFLD. FIB-4 (aHR 2.27, 95% CI 1.05–4.52); NFS (aHR 4.92, 95%CI 2.96–8.15). FIB-4 is the best predictor of an increased risk of prevalent CKD. |
| Ӧnnerhag K et al., 2019 (Sweden) [35] | Retrospective cohort study | 144 NAFLD patients (83 men and 61 women) | Biopsy FIB-4 score, NFS, APRI and BARD score to assess severity of fibrosis | GFR < 60 mL/min/1.73 m2 estimated by CKD-EPI equation | Prevalence of NAFLD was 100%. 47/144 subjects (32.6%) developed CKD. | Intermediate (1.30–2.67) and high (>2.67) FIB-4 scores were independently associated with CKD (aHR 4.77, 95% CI 1.95–11.64; aHR 7.25, 95% CI 2.51–20.94, respectively). Similar results with intermediate and high NFS (aHR 3.31, 95% CI 1.41–7.74; aHR 31.38, 95% CI 7.92–124.38, respectively). |
| Choi JW et al., 2019 (Korea) [40] | Cross-sectional study | 11,836 NAFLD patients (4893 men and 6943 women) | FIB-4 score | GFR < 60 mL/min/1.73 m2 estimated by CKD-EPI equation | Prevalence of NAFLD was 100%. | FIB-4 was an independent predictor of incipient CKD (aOR 1.254, 95% CI 1.034–1.521). |
| Sun DQ et al., 2019 (China) [36] | Cross-sectional study | 217 NAFLD patients (171 men and 46 women) | Biopsy | GFR < 60 mL/min/1.73 m2 estimated by CKD-EPI equation | Prevalence of NAFLD was 100%. 47/217 subjects had CKD (21.6%). | PNPLA3 GG genotype was significantly associated with an increased risk of CKD in the whole population (aOR 3.42, 95% CI 1.07–10.85). |
| Liu HW et al., 2020 (Taiwan) [34] | Cross-sectional study | 37,825 patients (13,288 men and 24,537 women) | Ultrasound | GFR < 60 mL/min/1.73 m2 estimated by CKD-EPI equation | Prevalence of NAFLD was 61.3% (23,209/37,825 patients). 4071/23,209 (17.5%) subjects developed incident CKD. | NAFLD was significantly associated with CKD (aOR 1.13, 95% CI 1.04–1.23). Prevalence of CKD increased with increasing severity of NAFLD (aOR 1.17, 95% CI 1.03–1.33). |
| Akahane T et al., 2020 (Japan) [32] | Cross-sectional study | 3725 patients (1751 men and 1974 women) | Ultrasound | GFR < 60 mL/min/1.73 m2 estimated by MDRD Japanese equation | Prevalence of NAFLD was 31% (1154/3725 patients). 146/1154 (12.6%) subjects developed incident CKD. After propensity score matching, 138/1097 (12.6%) NAFLD patients developed CKD. | CKD prevalence was significantly higher in NAFLD patients only before propensity score matching. However, obesity (OR 2.10, 95% CI 1.40–3.17), hypertension (OR 1.50, 95% CI 1.02–2.22), and hyperuricemia (OR 2.41, 95% CI 1.54–3.79), were independent risk factors for CKD in patients with NAFLD. |
| An JN et al., 2020 (Korea) [37] | Prospective cohort study | 455 NAFLD patients (221 men and 234 women) | Biopsy | GFR < 60 mL/min/1.73 m2 estimated by MDRD equation | Prevalence of NAFLD was 100%. 15/455 (3.3%) subjects developed incident CKD. | Risk of renal outcomes increased (aHR 5.63, 95% CI 1.81–17.51) with increased severity of portal inflammation. Patients with a higher score for portal inflammation (score 3–4) were found to be more prone to renal function deterioration, with a 7.7-fold increase in the risk of renal outcomes. |
| Chen PC et al., 2020 (Taiwan) [33] | Cross-sectional study | 13,255 NAFLD patients (8710 men and 4545 women) | Ultrasound FLI and NFS to assess severity of fibrosis | GFR < 60 mL/min/1.73 m2 estimated by MDRD equation | Prevalence of NAFLD was 100%. 3195/13,255 (24.1%) developed CKD. | CKD patients had higher FLI and NFS than those without CKD. When subjects were stratified by NFS, CKD was found in 40.52% of patients with an NFS > 0.676. NFS > 0.676 was related to CKD with an OR of 2.266, 95% CI 1.560–3.291, and an aOR of 2.284, 95% CI 1.513–3.448. |
| Kaps L et al., 2020 (Germany) [41] | Retrospective cohort study | 48,057 NAFLD patients (25,422 men and 22,635 women) | International Classification of Diseases, 10th revision (ICD-10) | International Classification of Diseases, 10th revision (ICD-10) | Prevalence of NAFLD was 100%. 8218/48,057 (17.1%) of patients were diagnosed with CKD. | Independent association of NAFLD with emerging CKD (HR 1.58, 95% CI 1.51–1.66). |
| Takahashi S et al., 2021 (Japan) [39] | Cross-sectional study | 14,163 patients (9077 men and 5086 women) | FLI | GFR < 60 mL/min/1.73 m2 estimated by MDRD Japanese equation | 2195/14,163 (15.5%) of patients developed CKD. | Higher FLI levels were independently associated with deterioration of renal function (aHR 1.33, 95% CI 1.16–1.54 in males; aHR 1.33, 95% CI 1.08–1.63 in females). |
3.3. Main Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morelli, M.C.; Rendina, M.; La Manna, G.; Alessandria, C.; Pasulo, L.; Lenci, I.; Bhoori, S.; Messa, P.; Biancone, L.; Gesualdo, L.; et al. Position paper on liver and kidney diseases from the Italian Association for the Study of Liver (AISF), in collaboration with the Italian Society of Nephrology (SIN). Dig. Liver Dis. 2021, 53 (Suppl. 2), S49–S86. [Google Scholar] [CrossRef]
- Cornec-Le Gall, E.; Torres, V.E.; Harris, P.C. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J. Am. Soc. Nephrol. 2018, 29, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Fabrizi, F.; Donato, F.M.; Messa, P. Association between hepatitis C virus and chronic kidney disease: A systematic review and meta-analysis. Ann. Hepatol. 2018, 17, 364–391. [Google Scholar] [CrossRef]
- Fabrizi, F.; Donato, F.M.; Messa, P. Association between hepatitis B virus and chronic kidney disease: A systematic review and meta-analysis. Ann. Hepatol. 2017, 16, 21–47. [Google Scholar] [CrossRef]
- Varga, Z.V.; Matyas, C.; Paloczi, J.; Pacher, P. Alcohol misuse and kidney injury: Epidemiological evidence and potential mechanisms. Alcohol Res. 2017, 38, 283–288. [Google Scholar] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta-analysis. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef]
- Wong, W.K.; Chan, W.K. Nonalcoholic fatty liver disease: A global perspective. Clin. Ther. 2021, 43, 473–499. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Angelico, F.; Del Ben, M.; Conti, R.; Francioso, S.; Feole, K.; Maccioni, D.; Antonini, T.M.; Alessandri, C. Nonalcoholic fatty liver syndrome: A hepatic consequence of common metabolic diseases. J. Gastroenterol. Hepatol. 2003, 18, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Angelico, F.; Del Ben, M.; Conti, R.; Francioso, S.; Feole, K.; Fiorello, S.; Cavallo, M.G.; Zalunardo, B.; Lirussi, F.; Alessandri, C.; et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2005, 90, 1578–1582. [Google Scholar] [CrossRef]
- Del Ben, M.; Baratta, F.; Polimeni, L.; Angelico, F. Non-alcoholic fatty liver disease and cardiovascular disease: Epidemiological, clinical and pathophysiological evidences. Intern. Emerg. Med. 2012, 7 (Suppl. 3), S291–S296. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Angelico, F.; Balla, A.; Paganini, A.M.; Cocomello, N.; Ferro, D.; Violi, F.; Sanyal, A.J.; Del Ben, M. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin. Gastroenterol. Hepatol. 2020, 18, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Umbro, I.; Fabiani, V.; Fabiani, M.; Angelico, F.; Del Ben, M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J. Gastroenterol. 2020, 26, 2669–2681. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef]
- Baratta, F.; Ferro, D.; Pastori, D.; Colantoni, A.; Cocomello, N.; Coronati, M.; Angelico, F.; Del Ben, M. Open issues in the transition from NAFLD to MAFLD: The experience of the plinio study. Int. J. Environ. Res. Public Health 2021, 18, 8993. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Wong, G.L.; Woo, J.; Abrigo, J.M.; Chan, C.K.; Shu, S.S.; Leung, J.K.; Chim, A.M.; Kong, A.P.; Lui, G.C.; et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin. Gastroenterol. Hepatol. 2021, 19, 2161–2171. [Google Scholar] [CrossRef]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New insights into the pathogenesis of non-alcoholic fatty liver disease: Gut-derived lipopolysaccharides and oxidative stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef]
- Carpino, G.; Pastori, D.; Baratta, F.; Overi, D.; Labbadia, G.; Polimeni, L.; Di Costanzo, A.; Pannitteri, G.; Carnevale, R.; Del Ben, M.; et al. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: A possible role for oxidative stress. Sci. Rep. 2017, 7, 15756. [Google Scholar] [CrossRef]
- Del Ben, M.; Polimeni, L.; Brancorsini, M.; Di Costanzo, A.; D’Erasmo, L.; Baratta, F.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Violi, F.; et al. Non-alcoholic fatty liver disease, metabolic syndrome and patatin-like phospholipase domain-containing protein3 gene variants. Eur. J. Intern. Med. 2014, 25, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Martín-Mateos, R.; Albillos, A. The role of the gut-liver axis in metabolic dysfunction-associated fatty liver disease. Front. Immunol. 2021, 12, 660179. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Fraser, S.D.S. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol. Dial. Transplant. 2017, 32 (Suppl. S2), ii121–ii128. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national Disability-Adjusted Life-Years (DALYs) for 359 diseases and injuries and Healthy Life Expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [Green Version]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Cassader, M.; Cohney, S.; Pinach, S.; Saba, F.; Gambino, R. Emerging liver-kidney interactions in nonalcoholic fatty liver disease. Trends Mol. Med. 2015, 21, 645–662. [Google Scholar] [CrossRef]
- Sinn, D.H.; Kang, D.; Jang, H.R.; Gu, S.; Cho, S.J.; Paik, S.W.; Ryu, S.; Chang, Y.; Lazo, M.; Guallar, E.; et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J. Hepatol. 2017, 67, 1274–1280. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Thongprayoon, C.; Scribani, M.; Ungprasert, P.; Cheungpasitporn, W. Noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver in USA. Eur. J. Gastroenterol. Hepatol. 2018, 30, 404–410. [Google Scholar] [CrossRef]
- Akahane, T.; Akahane, M.; Namisaki, T.; Kaji, K.; Moriya, K.; Kawaratani, H.; Takaya, H.; Sawada, Y.; Shimozato, N.; Fujinaga, Y.; et al. Association between non-alcoholic fatty liver disease and chronic kidney disease: A cross-sectional study. J. Clin. Med. 2020, 9, 1635. [Google Scholar] [CrossRef]
- Chen, P.C.; Kao, W.Y.; Cheng, Y.L.; Wang, Y.J.; Hou, M.C.; Wu, J.C.; Su, C.W. The correlation between fatty liver disease and chronic kidney disease. J. Formos. Med. Assoc. 2020, 119 Pt 1, 42–50. [Google Scholar] [CrossRef]
- Liu, H.W.; Liu, J.S.; Kuo, K.L. Association of nonalcoholic fatty liver and chronic kidney disease: An analysis of 37,825 cases from health checkup center in Taiwan. Tzu Chi Med. J. 2019, 32, 65–69. [Google Scholar]
- Önnerhag, K.; Hartman, H.; Nilsson, P.M.; Lindgren, S. Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD). Scand. J. Gastroenterol. 2019, 54, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.Q.; Zheng, K.I.; Xu, G.; Ma, H.L.; Zhang, H.Y.; Pan, X.Y.; Zhu, P.W.; Wang, X.D.; Targher, G.; Byrne, C.D.; et al. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020, 40, 107–119. [Google Scholar] [CrossRef] [PubMed]
- An, J.N.; Joo, S.K.; Koo, B.K.; Kim, J.H.; Oh, S.; Kim, W. Portal inflammation predicts renal dysfunction in patients with nonalcoholic fatty liver disease. Hepatol. Int. 2020, 14, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Kim, J.Y.; Choi, E.; Kim, J.S.; Chang, Y.; Sung, K.C. The fatty liver index as a predictor of incident chronic kidney disease in a 10-year prospective cohort study. PLoS ONE 2017, 12, e0180951. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tanaka, M.; Furuhashi, M.; Moniwa, N.; Koyama, M.; Higashiura, Y.; Osanami, A.; Gocho, Y.; Ohnishi, H.; Numata, K.; et al. Fatty liver index is independently associated with deterioration of renal function during a 10-year period in healthy subjects. Sci. Rep. 2021, 11, 8606. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, C.H.; Park, J.S. Comparison of laboratory indices of non-alcoholic fatty liver disease for the detection of incipient kidney dysfunction. PeerJ 2019, 7, e6524. [Google Scholar] [CrossRef] [Green Version]
- Kaps, L.; Labenz, C.; Galle, P.R.; Weinmann-Menke, J.; Kostev, K.; Schattenberg, J.M. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. United Eur. Gastroenterol. J. 2020, 8, 942–948. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: Revisited after a decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [Green Version]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Renal Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Carpino, G.; Oliveira, F.L.; Panera, N.; Nobili, V.; Gaudio, E. The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediat. Inflamm. 2017, 2017, 8162421. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettiga, A.; Fiorio, F.; Di Marco, F.; Trevisani, F.; Romani, A.; Porrini, E.; Salonia, A.; Montorsi, F.; Vago, R. The modern western diet rich in advanced glycation end-products (AGEs): An overview of its impact on obesity and early progression of renal pathology. Nutrients 2019, 11, 1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jegatheesan, P.; De Bandt, J.P. Fructose and NAFLD: The multifaceted aspects of fructose metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, R.; Iuculano, F.; Pallini, G.; Fargion, S.; Fracanzani, A.L. Nutrients, genetic factors, and their interaction in non-alcoholic fatty liver disease and cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 8761. [Google Scholar] [CrossRef] [PubMed]
- Brymora, A.; Flisiński, M.; Johnson, R.J.; Goszka, G.; Stefańska, A.; Manitius, J. Low-fructose diet lowers blood pressure and inflammation in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to mediterranean diet and non-alcoholic fatty liver disease: Effect on insulin resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Bartimoccia, S.; Cammisotto, V.; Cocomello, N.; Colantoni, A.; Nocella, C.; Carnevale, R.; Ferro, D.; Angelico, F.; et al. Poor adherence to mediterranean diet and serum lipopolysaccharide are associated with oxidative stress in patients with non-alcoholic fatty liver disease. Nutrients 2020, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Tanaka, T.; Nangaku, M. Hypoxia as a key player in the AKI-to-CKD transition. Am. J. Physiol. Renal Physiol. 2014, 307, F1187–F1195. [Google Scholar] [CrossRef] [Green Version]
- Cosola, C.; Rocchetti, M.T.; Sabatino, A.; Fiaccadori, E.; Di Iorio, B.R.; Gesualdo, L. Microbiota issue in CKD: How promising are gut-targeted approaches? J. Nephrol. 2019, 32, 27–37. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Alisi, A.; Mosca, A.; Panera, N.; Byrne, C.D.; Nobili, V. Relationship between PNPLA3 rs738409 polymorphism and decreased kidney function in children with NAFLD. Hepatology 2019, 70, 142–153. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umbro, I.; Baratta, F.; Angelico, F.; Del Ben, M. Nonalcoholic Fatty Liver Disease and the Kidney: A Review. Biomedicines 2021, 9, 1370. https://doi.org/10.3390/biomedicines9101370
Umbro I, Baratta F, Angelico F, Del Ben M. Nonalcoholic Fatty Liver Disease and the Kidney: A Review. Biomedicines. 2021; 9(10):1370. https://doi.org/10.3390/biomedicines9101370
Chicago/Turabian StyleUmbro, Ilaria, Francesco Baratta, Francesco Angelico, and Maria Del Ben. 2021. "Nonalcoholic Fatty Liver Disease and the Kidney: A Review" Biomedicines 9, no. 10: 1370. https://doi.org/10.3390/biomedicines9101370
APA StyleUmbro, I., Baratta, F., Angelico, F., & Del Ben, M. (2021). Nonalcoholic Fatty Liver Disease and the Kidney: A Review. Biomedicines, 9(10), 1370. https://doi.org/10.3390/biomedicines9101370







