Gravity-Based Flow Efficient Perfusion Culture System for Spheroids Mimicking Liver Inflammation
Abstract
:1. Introduction
2. Material and Methods
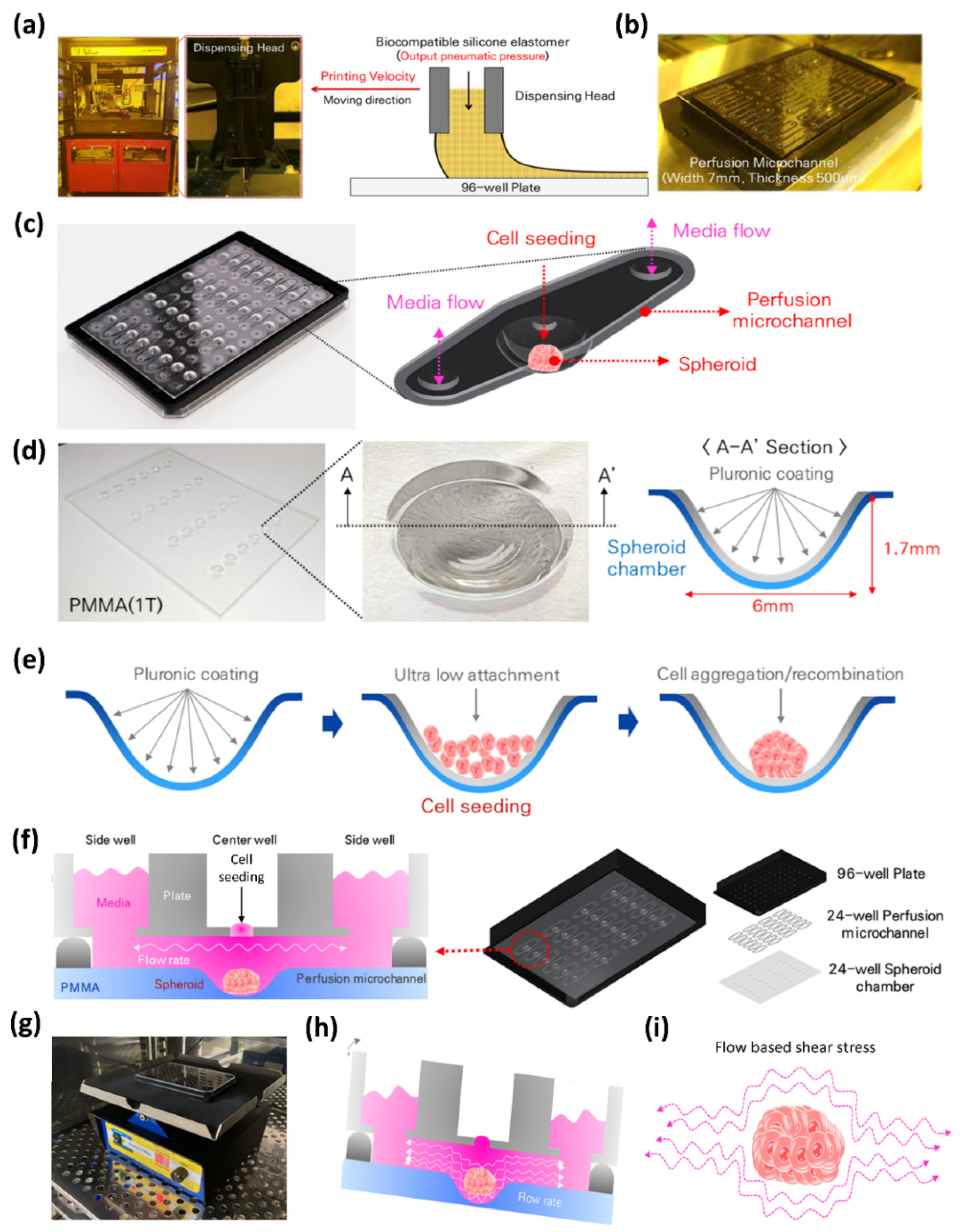
2.1. Fabrication of 24-Well Perfusable Spheroid Plate
2.2. Cell Culture
2.3. Static and Dynamic Culture
2.4. Morphology Assessment of the Spheroids
2.5. Disease Modeling
2.6. ELISA
2.7. Brightfield and Immunofluorescence Microscopy
2.8. Statistical Analysis
3. Results
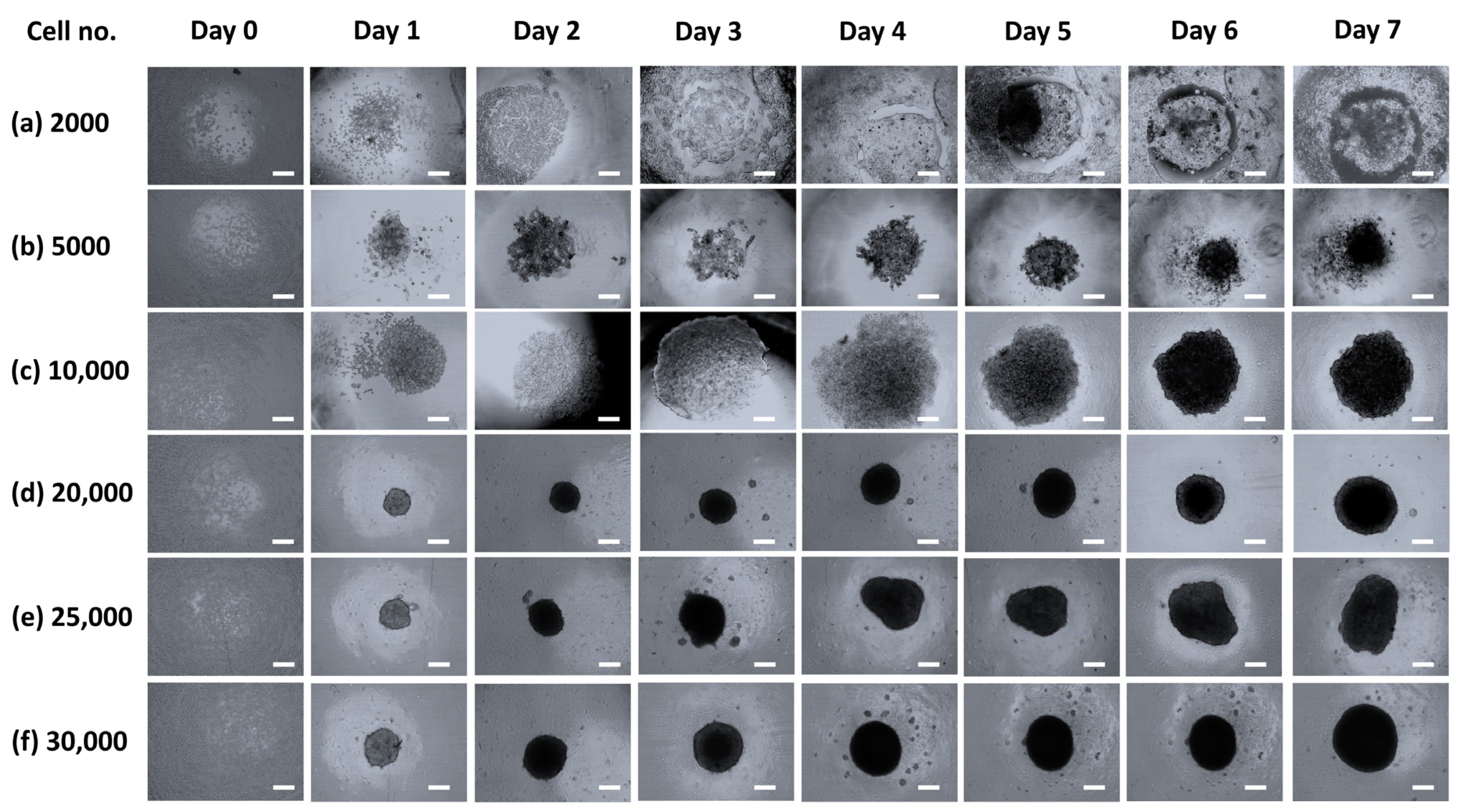
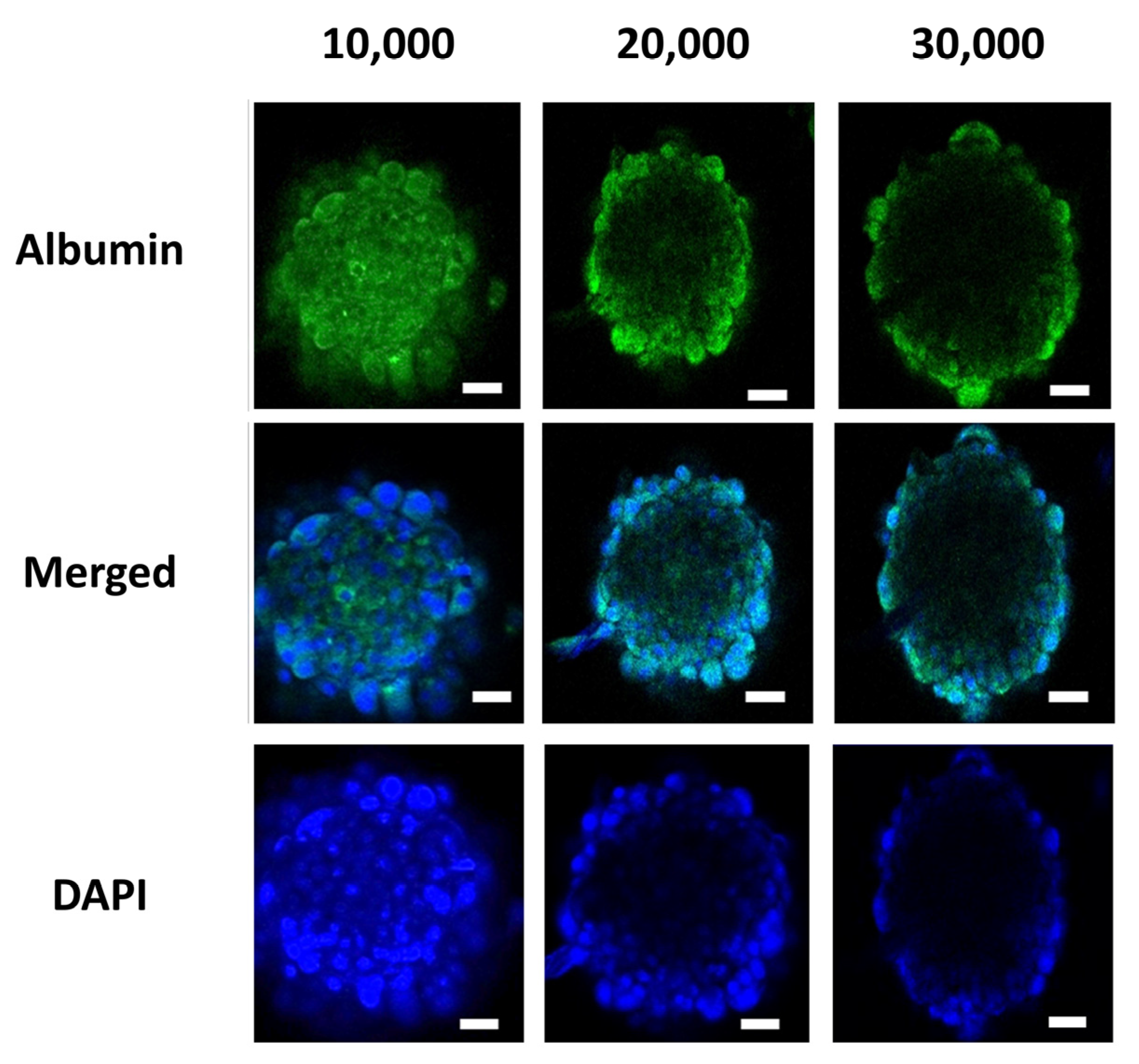
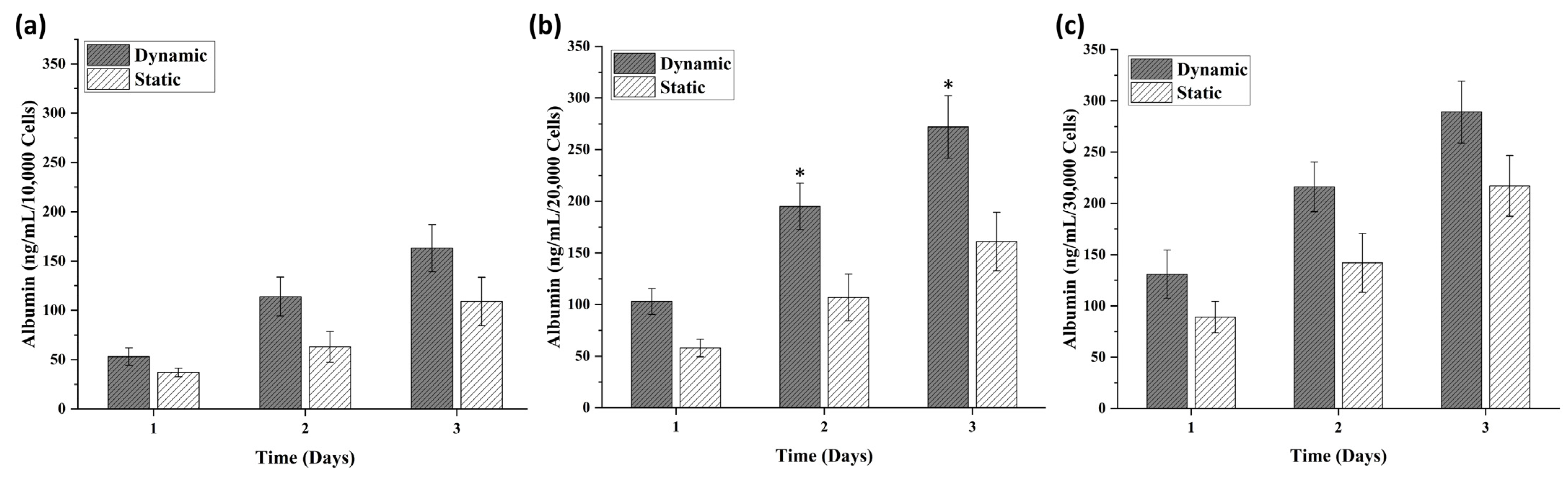
3.1. Spheroid Characterizations and Cell Number Optimization
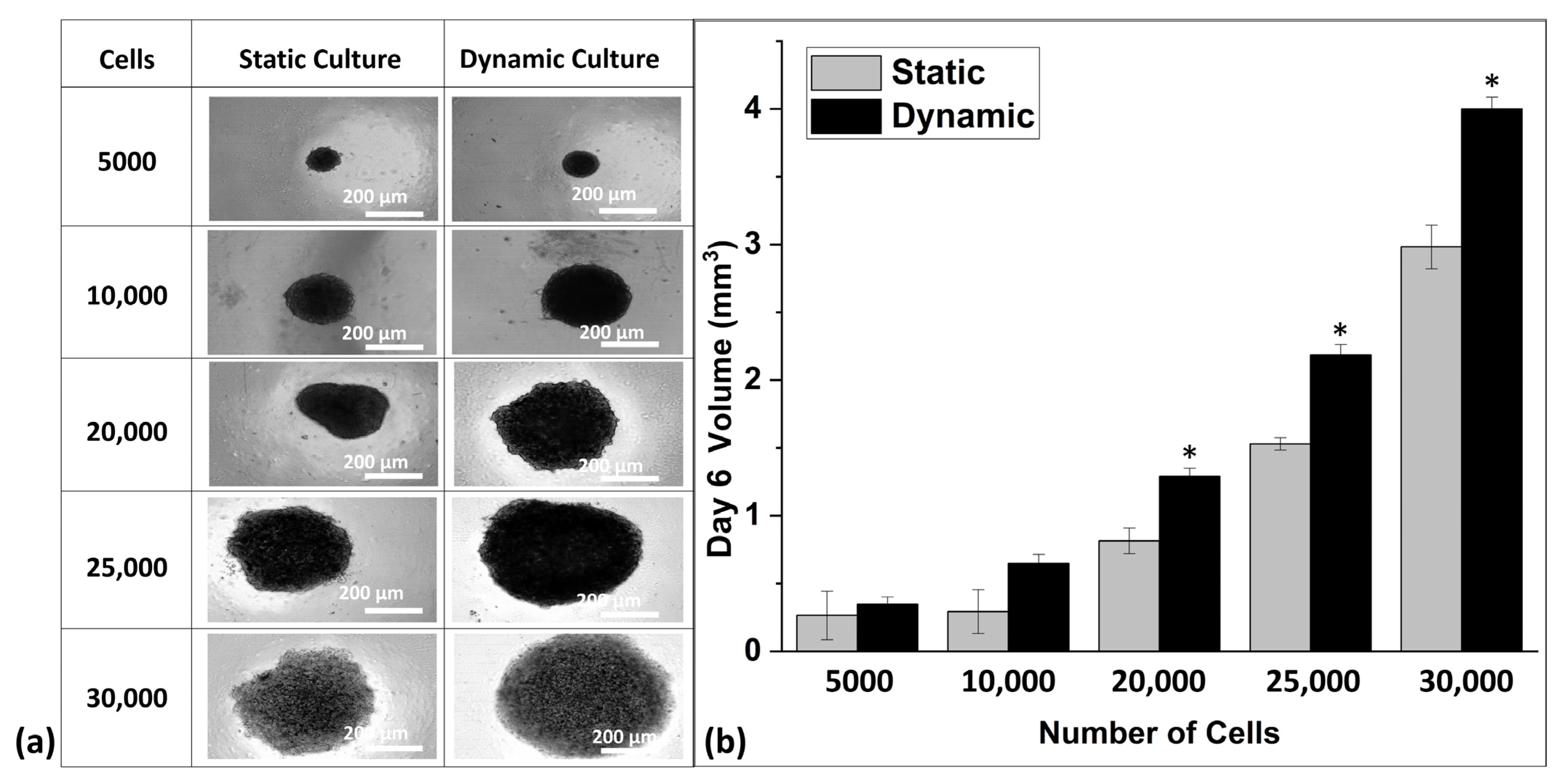
3.2. Comparative Analysis of Spheroids in Static and Dynamic Platforms
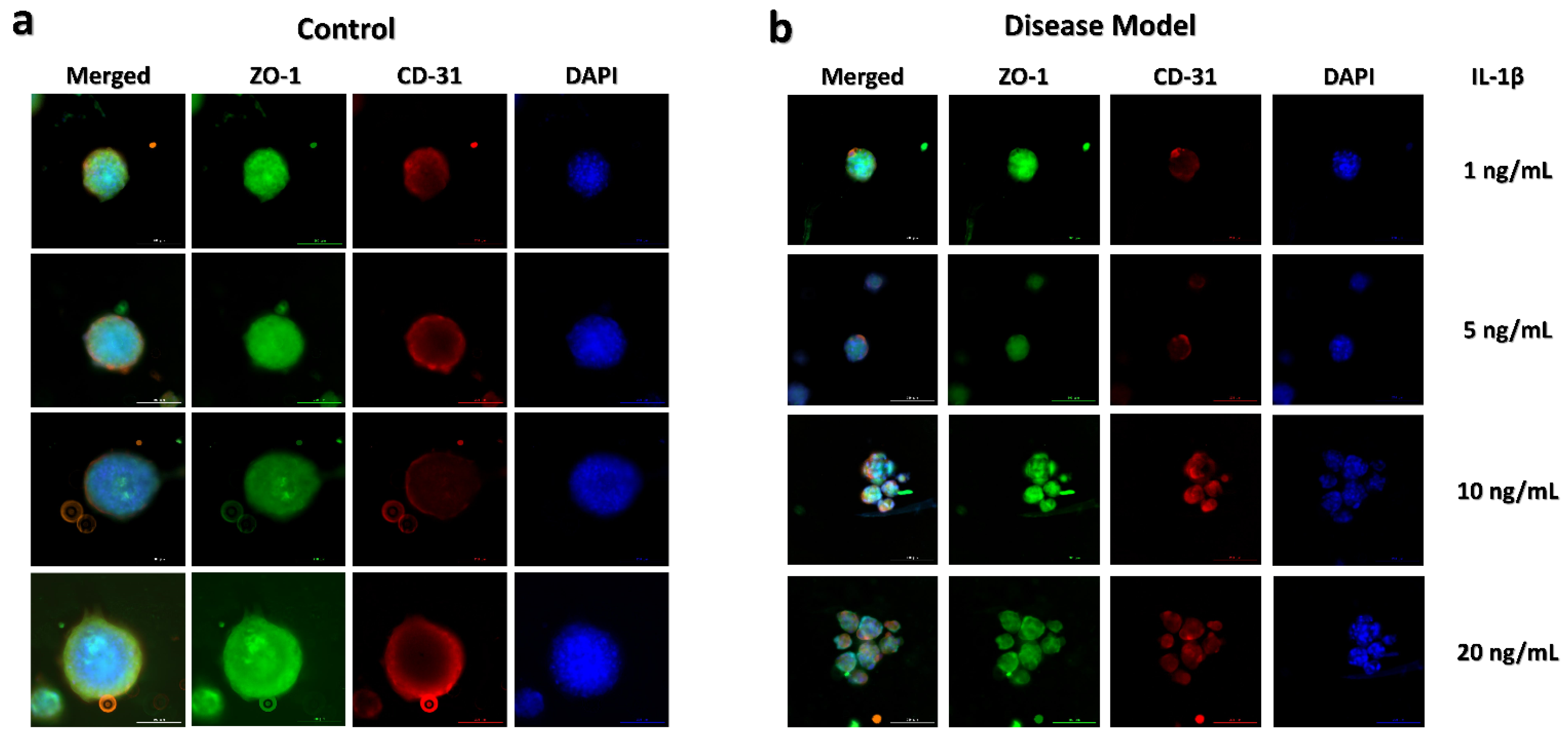
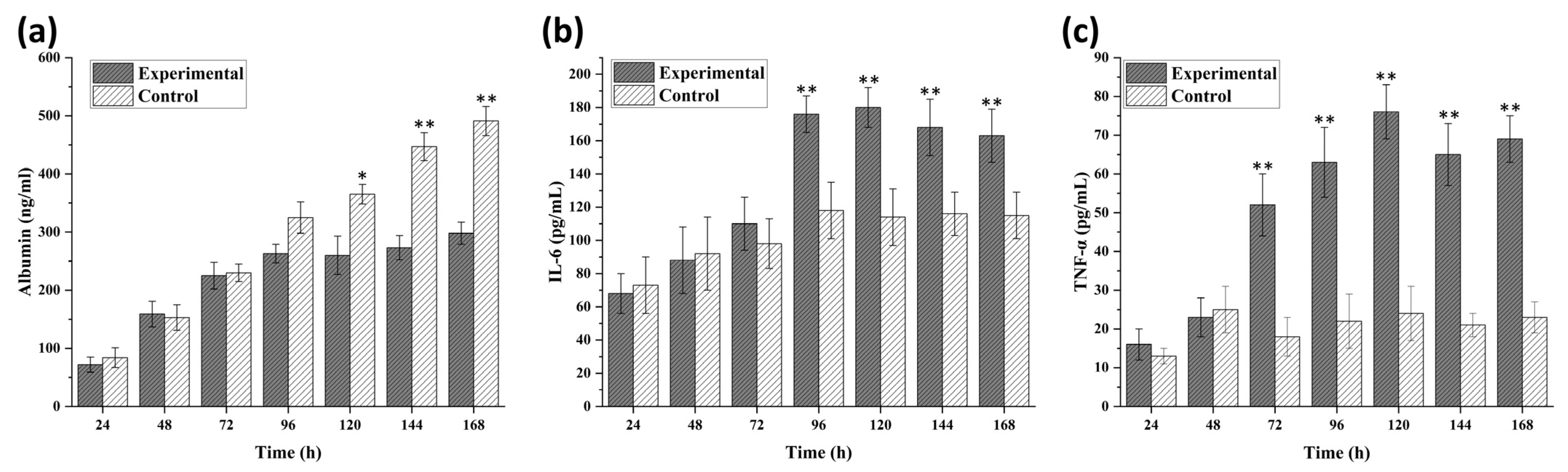
3.3. Disease Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asif, A.; Park, S.H.; Soomro, A.M.; Khalid, M.A.U.; Salih, A.R.C.; Kang, B.; Ahmed, F.; Kim, K.H.; Choi, K.H. Microphysiological system with continuous analysis of albumin for hepatotoxicity modeling and drug screening. J. Ind. Eng. Chem. 2021, 98, 318–326. [Google Scholar] [CrossRef]
- Soomro, A.M.; Khalid, M.A.U.; Shah, I.; wan Kim, S.; Kim, Y.S.; Choi, K.H. Highly stable soft strain sensor based on Gly-KCl filled sinusoidal fluidic channel for wearable and water-proof robotic applications. Smart Mater. Struct. 2020, 29, 025011. [Google Scholar] [CrossRef]
- Asif, A.; Kim, K.H.; Jabbar, F.; Kim, S.; Choi, K.H. Real-time sensors for live monitoring of disease and drug analysis in microfluidic model of proximal tubule. Microfluid. Nanofluidics 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Salih, A.R.C.; Farooqi, H.M.U.; Kim, Y.S.; Lee, S.H.; Choi, K.H. Impact of serum concentration in cell culture media on tight junction proteins within a multiorgan microphysiological system. Microelectron. Eng. 2020, 232, 111405. [Google Scholar] [CrossRef]
- Soomro, A.M.; Jabbar, F.; Ali, M.; Lee, J.-W.; Mun, S.W.; Choi, K.H. All-range flexible and biocompatible humidity sensor based on poly lactic glycolic acid (PLGA) and its application in human breathing for wearable health monitoring. J. Mater. Sci. Mater. Electron. 2019, 30, 9455–9465. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-chip: A fast track for engineered human tissues in drug development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modena, M.M.; Chawla, K.; Misun, P.M.; Hierlemann, A. Smart cell culture systems: Integration of sensors and actuators into microphysiological systems. ACS Chem. Biol. 2018, 13, 1767–1784. [Google Scholar] [CrossRef] [PubMed]
- Chethikkattuveli Salih, A.R.; Hyun, K.; Asif, A.; Soomro, A.M.; Farooqi, H.M.U.; Kim, Y.S.; Kim, K.H.; Lee, J.W.; Huh, D.; Choi, K.H. Extracellular Matrix Optimization for Enhanced Physiological Relevance in Hepatic Tissue-Chips. Polymers 2021, 13, 3016. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Koudan, E.V.; Krokhmal, A.A.; Annenkova, E.A.; Petrov, S.V.; Pereira, F.D.; Karalkin, P.A.; Nezhurina, E.K.; Gryadunova, A.A.; Bulanova, E.A. Biofabrication of a Functional Tubular Construct from Tissue Spheroids Using Magnetoacoustic Levitational Directed Assembly. Adv. Healthc. Mater. 2020, 9, 2000721. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting spheroid function for tissue engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef]
- Bresciani, G.; Hofland, L.J.; Dogan, F.; Giamas, G.; Gagliano, T.; Zatelli, M.C. Evaluation of spheroid 3D culture methods to study a pancreatic neuroendocrine neoplasm cell line. Front. Endocrinol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Cipriano, M.; Freyer, N.; Knöspel, F.; Oliveira, N.G.; Barcia, R.; Cruz, P.E.; Cruz, H.; Castro, M.; Santos, J.M.; Zeilinger, K. Self-assembled 3D spheroids and hollow-fibre bioreactors improve MSC-derived hepatocyte-like cell maturation in vitro. Arch. Toxicol. 2017, 91, 1815–1832. [Google Scholar] [CrossRef]
- Huang, S.-W.; Tzeng, S.-C.; Chen, J.-K.; Sun, J.-S.; Lin, F.-H. A dynamic hanging-drop system for mesenchymal stem cell culture. Int. J. Mol. Sci. 2020, 21, 4298. [Google Scholar] [CrossRef]
- Das, V.; Fürst, T.; Gurská, S.; Džubák, P.; Hajdúch, M. Reproducibility of uniform spheroid formation in 384-well plates: The effect of medium evaporation. J. Biomol. Screen. 2016, 21, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.-W.; Ahmed, A.R.; Dereli-Korkut, Z.; Wang, S. Microfluidic cell chips for high-throughput drug screening. Bioanalysis 2016, 8, 921–937. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids formation on non-adhesive surfaces by liquid overlay technique: Considerations and practical approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.j.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H. Engineering multi-cellular spheroids for tissue engineering and regenerative medicine. Adv. Healthc. Mater. 2020, 9, 2000608. [Google Scholar] [CrossRef] [PubMed]
- Benien, P.; Swami, A. 3D tumor models: History, advances and future perspectives. Future Oncol. 2014, 10, 1311–1327. [Google Scholar] [CrossRef]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.-T.; Shaegh, S.A.M.; Kashaninejad, N.; Phan, D.-T. Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 2013, 65, 1403–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sun, W.; Kang, L.; Wang, Y.; Zhang, M.; Zhang, H.; Hu, P. Microfluidic co-culture of liver tumor spheroids with stellate cells for the investigation of drug resistance and intercellular interactions. Analyst 2019, 144, 4233–4240. [Google Scholar] [CrossRef] [PubMed]
- Lauschke, V.M.; Shafagh, R.Z.; Hendriks, D.F.; Ingelman-Sundberg, M. 3D primary hepatocyte culture systems for analyses of liver diseases, drug metabolism, and toxicity: Emerging culture paradigms and applications. Biotechnol. J. 2019, 14, 1800347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, J.E.; Handa, P.; Aouizerat, B.; Wilson, L.; Vemulakonda, L.A.; Yeh, M.M.; Kowdley, K.V.; Network, N.C.R.; Abrams, S.H.; Himes, R. Increased parenchymal damage and steatohepatitis in Caucasian non-alcoholic fatty liver disease patients with common IL 1B and IL 6 polymorphisms. Aliment. Pharmacol. Ther. 2016, 44, 1253–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spahis, S.; Delvin, E.; Borys, J.-M.; Levy, E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Funda Canpolat, K.; Özdolap, Ş.; Sarikaya, S. Pro-inflammatory cytokines and oxidized low-density-lipoprotein in patients with fibromyalgia. Arch. Rheumatol. 2019, 34, 123. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Moschen, A.R. IL-1 cytokine family members and NAFLD: Neglected in metabolic liver inflammation. J. Hepatol. 2011, 55, 960–962. [Google Scholar] [CrossRef] [Green Version]
- Asif, A.; Khalid, M.; Manzoor, S.; Ahmad, H.; Rehman, A.U. Role of purinergic receptors in hepatobiliary carcinoma in Pakistani population: An approach towards proinflammatory role of P2X4 and P2X7 receptors. Purinergic Signal. 2019, 15, 367–374. [Google Scholar] [CrossRef]
- Khalid, M.; Manzoor, S.; Ahmad, H.; Asif, A.; Bangash, T.A.; Latif, A.; Jaleel, S. Purinoceptor expression in hepatocellular virus (HCV)-induced and non-HCV hepatocellular carcinoma: An insight into the proviral role of the P2X4 receptor. Mol. Biol. Rep. 2018, 45, 2625–2630. [Google Scholar] [CrossRef]
- Tukov, F.F.; Luyendyk, J.P.; Ganey, P.E.; Roth, R.A. The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol. Sci. 2007, 100, 267–280. [Google Scholar] [CrossRef]
- Lacour, S.; Gautier, J.-C.; Pallardy, M.; Roberts, R. Cytokines as potential biomarkers of liver toxicity. Cancer Biomark. 2005, 1, 29–39. [Google Scholar] [CrossRef]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Off. J. Am. Coll. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef]
- Hong, F.; Radaeva, S.; Pan, H.N.; Tian, Z.; Veech, R.; Gao, B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology 2004, 40, 933–941. [Google Scholar] [CrossRef]
- Gieling, R.G.; Wallace, K.; Han, Y.-P. Interleukin-1 participates in the progression from liver injury to fibrosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, G1324–G1331. [Google Scholar] [CrossRef]
- Brunet-Maheu, J.M.; Fernandes, J.C.; de Lacerda, C.A.; Shi, Q.; Benderdour, M.; Lavigne, P. Pluronic F-127 as a cell carrier for bone tissue engineering. J. Biomater. Appl. 2009, 24, 275–287. [Google Scholar] [CrossRef]
- Wu, Z.; Hjort, K. Surface modification of PDMS by gradient-induced migration of embedded Pluronic. Lab A Chip 2009, 9, 1500–1503. [Google Scholar] [CrossRef]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a cell encapsulation material: Utilization of membrane-stabilizing agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef]
- Bauer, S.; Huldt, C.W.; Kanebratt, K.P.; Durieux, I.; Gunne, D.; Andersson, S.; Ewart, L.; Haynes, W.G.; Maschmeyer, I.; Winter, A. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ji, S.T.; Yong, U.; Das, S.; Jang, W.B.; Ahn, G.; Kwon, S.-M.; Jang, J. 3D bioprinted tissue-specific spheroidal multicellular microarchitectures for advanced cell therapy. Biofabrication 2021, 13, 045017. [Google Scholar] [CrossRef] [PubMed]
- Baze, A.; Parmentier, C.; Hendriks, D.F.; Hurrell, T.; Heyd, B.; Bachellier, P.; Schuster, C.; Ingelman-Sundberg, M.; Richert, L. Three-dimensional spheroid primary human hepatocytes in monoculture and coculture with nonparenchymal cells. Tissue Eng. Part C Methods 2018, 24, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Brophy, C.M.; Luebke-Wheeler, J.L.; Amiot, B.P.; Khan, H.; Remmel, R.P.; Rinaldo, P.; Nyberg, S.L. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology 2009, 49, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [Green Version]
- Masiello, T.; Dhall, A.; Hemachandra, L.; Tokranova, N.; Melendez, J.A.; Castracane, J. A dynamic culture method to produce ovarian cancer spheroids under physiologically-relevant shear stress. Cells 2018, 7, 277. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Asif, A.; Chethikkattuveli Salih, A.R.; Lee, J.-W.; Hyun, K.-N.; Choi, K.-H. Gravity-Based Flow Efficient Perfusion Culture System for Spheroids Mimicking Liver Inflammation. Biomedicines 2021, 9, 1369. https://doi.org/10.3390/biomedicines9101369
Kim Y-S, Asif A, Chethikkattuveli Salih AR, Lee J-W, Hyun K-N, Choi K-H. Gravity-Based Flow Efficient Perfusion Culture System for Spheroids Mimicking Liver Inflammation. Biomedicines. 2021; 9(10):1369. https://doi.org/10.3390/biomedicines9101369
Chicago/Turabian StyleKim, Young-Su, Arun Asif, Abdul Rahim Chethikkattuveli Salih, Jae-Wook Lee, Ki-Nam Hyun, and Kyung-Hyun Choi. 2021. "Gravity-Based Flow Efficient Perfusion Culture System for Spheroids Mimicking Liver Inflammation" Biomedicines 9, no. 10: 1369. https://doi.org/10.3390/biomedicines9101369
APA StyleKim, Y.-S., Asif, A., Chethikkattuveli Salih, A. R., Lee, J.-W., Hyun, K.-N., & Choi, K.-H. (2021). Gravity-Based Flow Efficient Perfusion Culture System for Spheroids Mimicking Liver Inflammation. Biomedicines, 9(10), 1369. https://doi.org/10.3390/biomedicines9101369




