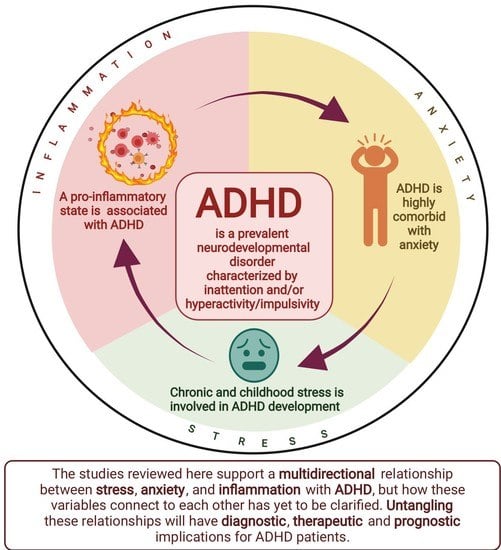
Inflammation, Anxiety, and Stress in Attention-Deficit/Hyperactivity Disorder
Abstract
1. Background
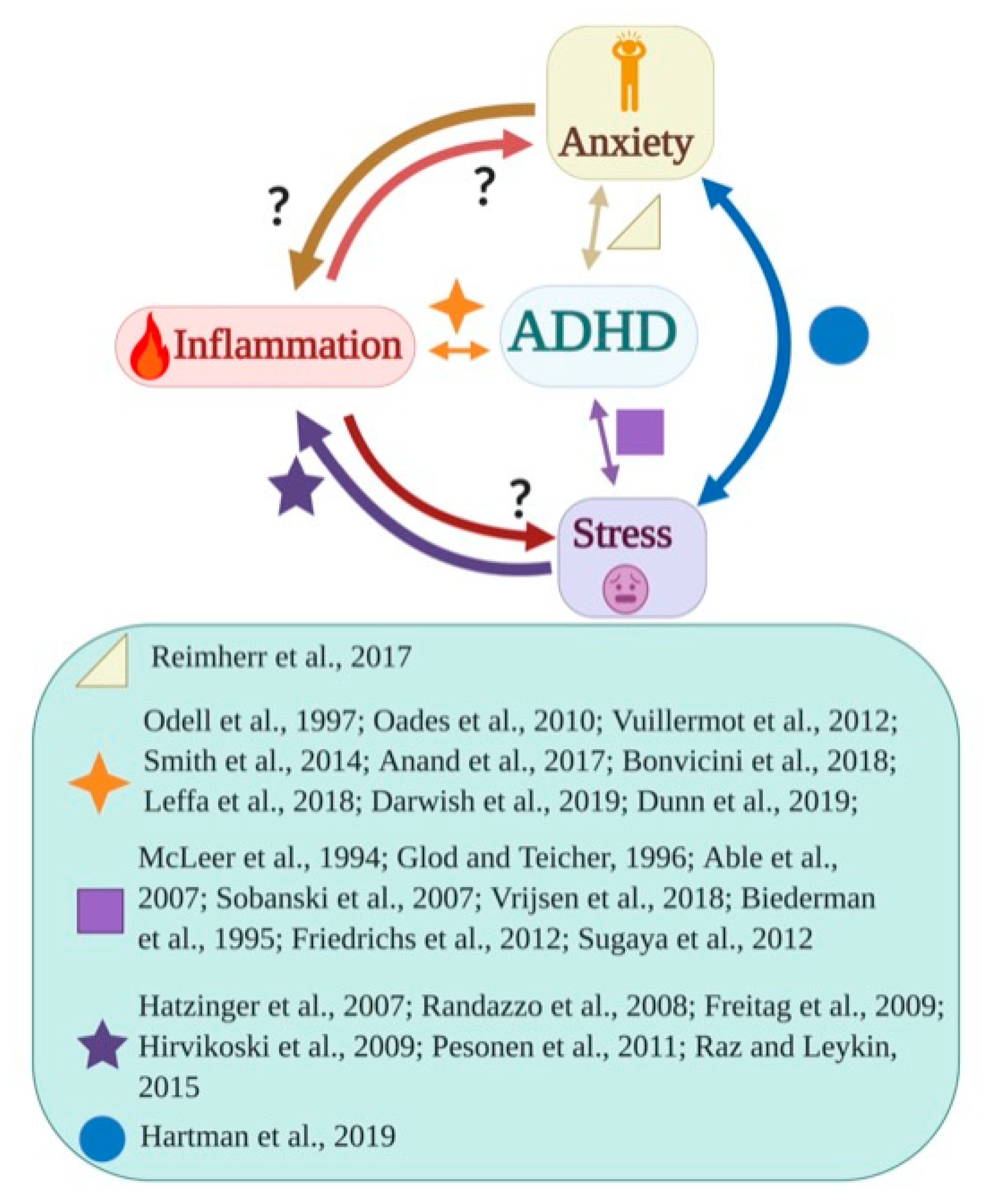
2. Relationship between Stress, Anxiety, and Inflammation
3. Measures of Inflammation in Psychiatric Patients
4. Stress and Inflammation in Patients with ADHD
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsen, F.M.; Tulve, N.S. A systematic review and meta-analysis examining the interrelationships between chemical and non-chemical stressors and inherent characteristics in children with ADHD. Environ. Res. 2019, 180, 108884. [Google Scholar] [CrossRef] [PubMed]
- Agnew-Blais, J.C.; Polanczyk, G.V.; Danese, A.; Wertz, J.; Moffitt, T.; Arseneault, L. Evaluation of the Persistence, Remission, and Emergence of Attention-Deficit/Hyperactivity Disorder in Young Adulthood. JAMA Psychiatry 2016, 73, 713–720. [Google Scholar] [CrossRef]
- Caye, A.; Rocha, T.B.-M.; Anselmi, L.; Murray, J.; Menezes, A.M.B.; Barros, F.C.; Gonçalves, H.; Wehrmeister, F.; Jensen, C.M.; Steinhausen, H.-C.; et al. Attention-Deficit/Hyperactivity Disorder Trajectories From Childhood to Young Adulthood. JAMA Psychiatry 2016, 73, 705–712. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association, Ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Leffa, D.T.; Torres, I.L.; Rohde, L.A. A Review on the Role of Inflammation in Attention-Deficit/Hyperactivity Disorder. Neuroimmunomodulation 2018, 25, 328–333. [Google Scholar] [CrossRef] [PubMed]
- De Zeeuw, E.L.; van Beijsterveldt, T.; Ehli, E.A.; De Geus, E.J.C.; Boomsma, R.I. Attention Deficit Hyperactivity Disorder Symptoms and Low Educational Achievement: Evidence Supporting A Causal Hypothesis. Behav. Genet. 2017, 47, 278–289. [Google Scholar] [CrossRef]
- Jangmo, A.; Stålhandske, A.; Chang, Z.; Chen, Q.; Almqvist, C.; Feldman, I.; Bulik, C.; Lichtenstein, P.; D’Onofrio, B.; Kuja-Halkola, R.; et al. Attention-Deficit/Hyperactivity Disorder, School Performance, and Effect of Medication. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 423–432. [Google Scholar] [CrossRef]
- Cherkasova, M.V.; Roy, A.; Molina, B.S.; Scott, G.; Weiss, G.; Barkley, R.A.; Biederman, J.; Uchida, M.; Hinshaw, S.P.; Owens, E.B.; et al. Review: Adult Outcome as Seen Through Controlled Prospective Follow-up Studies of Children With Attention-Deficit/Hyperactivity Disorder Followed Into Adulthood. J. Am. Acad. Child Adolesc. Psychiatry 2021. [Google Scholar] [CrossRef]
- Xu, G.; Strathearn, L.; Liu, B.; Yang, B.; Bao, W. Twenty-Year Trends in Diagnosed Attention-Deficit/Hyperactivity Disorder Among US Children and Adolescents, 1997–2016. JAMA Netw. Open 2018, 1, e181471. [Google Scholar] [CrossRef]
- Fayyad, J.; De Graaf, R.; Kessler, R.; Alonso, J.; Angermeyer, M.; Demyttenaere, K.; de Girolamo, G.; Haro, J.M.; Karam, E.G.; Lara, C.; et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br. J. Psychiatry 2007, 190, 402–409. [Google Scholar] [CrossRef]
- Franke, B.; on behalf of the International Multicentre persistent ADHD CollaboraTion (IMpACT); Faraone, S.V.; Asherson, P.; Buitelaar, J.; Bau, C.H.D.; Ramos-Quiroga, J.A.; Mick, E.; Grevet, E.H.; Johansson, S.; et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol. Psychiatry 2011, 17, 960–987. [Google Scholar] [CrossRef]
- Smith, A.K.; Mick, E.; Faraone, S.V. Advances in genetic studies of attention-deficit/hyperactivity disorder. Curr. Psychiatry Rep. 2009, 11, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wåhlstedt, C.; Thorell, L.B.; Bohlin, G. Heterogeneity in ADHD: Neuropsychological Pathways, Comorbidity and Symptom Domains. J. Abnorm. Child Psychol. 2008, 37, 551–564. [Google Scholar] [CrossRef]
- Michelini, G.; Kitsune, V.; Vainieri, I.; Hosang, G.M.; Brandeis, D.; Asherson, P.; Kuntsi, J. Shared and Disorder-Specific Event-Related Brain Oscillatory Markers of Attentional Dysfunction in ADHD and Bipolar Disorder. Brain Topogr. 2018, 31, 672–689. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.; Perroud, N.; Weibel, S. Attention Deficit Hyperactivity Disorder And Borderline Personality Disorder In Adults: A Review Of Their Links And Risks. Neuropsychiatr. Dis. Treat. 2019, 15, 3115–3129. [Google Scholar] [CrossRef]
- Arnold, L.E.; Mount, K.; Frazier, T.; Demeter, C.; Youngstrom, E.A.; Fristad, M.A.; Birmaher, B.; Horwitz, S.; Findling, R.L.; Kowatch, R.; et al. Pediatric bipolar disorder and ADHD: Family history comparison in the LAMS clinical sample. J. Affect. Disord. 2012, 141, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.D.; Sugar, C.A.; Walshaw, P.D.; Vasquez, R.E.; Foland-Ross, L.C.; Moody, T.D.; Bookheimer, S.Y.; McGough, J.J.; Altshuler, L.L. Frontostriatal neuroimaging findings differ in patients with bipolar disorder who have or do not have ADHD comorbidity. J. Affect. Disord. 2012, 147, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.A.; Rommelse, N.; Van Der Klugt, C.L.; Wanders, R.B.; Timmerman, M.E. Stress Exposure and the Course of ADHD from Childhood to Young Adulthood: Comorbid Severe Emotion Dysregulation or Mood and Anxiety Problems. J. Clin. Med. 2019, 8, 1824. [Google Scholar] [CrossRef]
- Bayes, A.; Parker, G.; Paris, J. Differential Diagnosis of Bipolar II Disorder and Borderline Personality Disorder. Curr. Psychiatry Rep. 2019, 21, 125. [Google Scholar] [CrossRef]
- Petrovic, P.; Castellanos, F. Top-Down Dysregulation—From ADHD to Emotional Instability. Front. Behav. Neurosci. 2016, 10, 70. [Google Scholar] [CrossRef]
- Beheshti, A.; Chavanon, M.-L.; Christiansen, H. Emotion dysregulation in adults with attention deficit hyperactivity disorder: A meta-analysis. BMC Psychiatry 2020, 20, 120. [Google Scholar] [CrossRef]
- Stern, A.; Agnew-Blais, J.; Danese, A.; Fisher, H.L.; Jaffee, S.R.; Matthews, T.; Polanczyk, G.V.; Arseneault, L. Associations between abuse/neglect and ADHD from childhood to young adulthood: A prospective nationally-representative twin study. Child Abus. Negl. 2018, 81, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Gokten, E.S.; Duman, N.S.; Soylu, N.; Uzun, M.E. Effects of attention-deficit/hyperactivity disorder on child abuse and neglect. Child Abus. Negl. 2016, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Milberger, S.; Faraone, S.; Kiely, K.; Guite, J.; Mick, E.; Ablon, J.S.; Warburton, R.; Reed, E.; Davis, S.G. Impact of Adversity on Functioning and Comorbidity in Children with Attention-Deficit Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 1495–1503. [Google Scholar] [CrossRef]
- Friedrichs, B.; Igl, W.; Larsson, H.; Larsson, J.-O. Coexisting Psychiatric Problems and Stressful Life Events in Adults With Symptoms of ADHD—A Large Swedish Population-Based Study of Twins. J. Atten. Disord. 2010, 16, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, L.S.; Hasin, D.S.; Olfson, M.; Lin, K.-H.; Grant, B.F.; Blanco, C. Child physical abuse and adult mental health: A national study. J. Trauma. Stress 2012, 25, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Glod, C.A.; Teicher, M.H. Relationship between Early Abuse, Posttraumatic Stress Disorder, and Activity Levels in Prepubertal Children. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef]
- Vuillermot, S.; Joodmardi, E.; Perlmann, T.; Ögren, S.O.; Feldon, J.; Meyer, U. Prenatal Immune Activation Interacts with Genetic Nurr1 Deficiency in the Development of Attentional Impairments. J. Neurosci. 2012, 32, 436–451. [Google Scholar] [CrossRef]
- Oades, R.D.; Myint, A.-M.; Dauvermann, M.R.; Schimmelmann, B.G.; Schwarz, M.J. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: An exploration of associations of cytokines and kynurenine metabolites with symptoms and attention. Behav. Brain Funct. 2010, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Roso, M.; Armario, A.; Palomar, G.; Corrales, M.; Carrasco, J.; Richarte, V.; Ferrer, R.; Casas, M.; Ramos-Quiroga, J.A. IL-6 and TNF-α in unmedicated adults with ADHD: Relationship to cortisol awakening response. Psychoneuroendocrinology 2017, 79, 67–73. [Google Scholar] [CrossRef]
- Vogel, S.; Bijlenga, D.; Verduijn, J.; Bron, T.; Beekman, A.; Kooij, J.; Penninx, B. Attention-deficit/hyperactivity disorder symptoms and stress-related biomarkers. Psychoneuroendocrinology 2017, 79, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Parent, C.; Pokhvisneva, I.; Filho, E.J.D.M.; O’Donnell, K.J.; Meaney, M.J.; Kee, M.Z.; Thng, G.; Wing, H.; Adler, N.E.; Keeton, V.; et al. Salivary cytokine cluster moderates the association between caregivers perceived stress and emotional functioning in youth. Brain Behav. Immun. 2021, 94, 125–137. [Google Scholar] [CrossRef]
- Daviu, N.; Bruchas, M.R.; Moghaddam, B.; Sandi, C.; Beyeler, A. Neurobiological links between stress and anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef]
- Butler, G. Definitions of Stress. 1993. Available online: https://www.unboundmedicine.com/medline/citation/8199583/Definitions_of_stress_ (accessed on 1 September 2021).
- Ray, A.; Gulati, K.; Rai, N. Stress, Anxiety, and Immunomodulation. Vitam. Horm. 2017, 103, 1–25. [Google Scholar] [CrossRef]
- Morris, L.S.; McCall, J.G.; Charney, D.S.; Murrough, J.W. The role of the locus coeruleus in the generation of pathological anxiety. Brain Neurosci. Adv. 2020, 4, 2398212820930321. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Heinz, A.; Hermann, D.; Smolka, M.; Rieks, M.; Gräf, K.-J.; Pöhlau, D.; Kuhn, W.; Bauer, M. Effects of acute psychological stress on adhesion molecules, interleukins and sex hormones: Implications for coronary heart disease. Psychopharmacology 2003, 165, 111–117. [Google Scholar] [CrossRef]
- Slavish, D.; Graham-Engeland, J.E.; Smyth, J.; Engeland, C.G. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 2014, 44, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Preacher, K.J.; MacCallum, R.C.; Atkinson, C.; Malarkey, W.B.; Glaser, R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA 2003, 100, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Francis, J.; Beltz, T.G.; Felder, R.; Johnson, A.K. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol. Behav. 2005, 84, 697–706. [Google Scholar] [CrossRef]
- Hodes, G.; Pfau, M.L.; Leboeuf, M.; Golden, S.; Christoffel, D.; Bregman, D.; Rebusi, N.; Heshmati, M.; Aleyasin, H.; Warren, B.L.; et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA 2014, 111, 16136–16141. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.E.; O’Toole, M.S.; Spaeth, P.E.; Lekander, M.; Mennin, D.S. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depress. Anxiety 2018, 35, 1081–1094. [Google Scholar] [CrossRef]
- Bellavance, M.-A.; Rivest, S. The HPA–Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Kuzminskaite, E.; Lamers, F.; Giltay, E.J.; Penninx, B.W. An integrated approach to understand biological stress system dysregulation across depressive and anxiety disorders. J. Affect. Disord. 2021, 283, 139–146. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Pangelinan, M.; Björnholm, L.; Klasnja, A.; Leemans, A.; Drakesmith, M.; Evans, C.; Barker, E.; Paus, T. Associations between prenatal, childhood, and adolescent stress and variations in white-matter properties in young men. NeuroImage 2018, 182, 389–397. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Hear. Fail. Rev. 2021, 1–19. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2019, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.S.; Saccaro, L.F.; Galgani, A.; Busceti, C.L.; Biagioni, F.; Frati, A.; Fornai, F. The role of Locus Coeruleus in neuroinflammation occurring in Alzheimer’s disease. Brain Res. Bull. 2019, 153, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.S.; Saccaro, L.F.; Busceti, C.L.; Biagioni, F.; Fornai, F. Epilepsy and Alzheimer’s Disease: Potential mechanisms for an association. Brain Res. Bull. 2020, 160, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, J.A.; Settipane, R.A. Asthma, Allergy, and Psychiatric Disease. Allergy Asthma Proc. 2015, 36, 415–417. [Google Scholar] [CrossRef]
- Dubois, T.; Reynaert, C.; Jacques, D.; Lepiece, B.; Patigny, P.; Zdanowicz, N. Immunity and Psychiatric Disorders: Variabilities of Immunity Biomarkers Are They Specific? Psychiatr. Danub. 2018, 30, S447–S451. [Google Scholar]
- Kramer, N.E.; Cosgrove, V.E.; Dunlap, K.; Subramaniapillai, M.; McIntyre, R.S.; Suppes, T. A clinical model for identifying an inflammatory phenotype in mood disorders. J. Psychiatr. Res. 2019, 113, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Chen, M.-H.; Jeng, M.-J.; Hsu, J.-W.; Tsai, S.-J.; Bai, Y.-M.; Hung, G.-Y.; Yen, H.-J.; Chen, T.-J.; Su, T.-P. Longitudinal association between early atopic dermatitis and subsequent attention-deficit or autistic disorder. Medicine 2016, 95, e5005. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yu, Y.-H.; Fu, M.-L.; Yeh, W.-T.; Hsu, J.-L.; Yang, Y.-H.; Chen, W.J.; Chiang, B.-L.; Pan, W.-H. Attention deficit–hyperactivity disorder is associated with allergic symptoms and low levels of hemoglobin and serotonin. Sci. Rep. 2018, 8, 10229. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, A.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef]
- Krahel, A.; Paszynska, E.; Slopien, A.; Gawriolek, M.; Otulakowska-Skrzynska, J.; Rzatowski, S.; Hernik, A.; Hanć, T.; Bryl, E.; Szczesniewska, P.; et al. Stress/Immune Biomarkers in Saliva among Children with ADHD Status. Int. J. Environ. Res. Public Health 2021, 18, 769. [Google Scholar] [CrossRef] [PubMed]
- Kraynak, T.E.; Marsland, A.L.; Wager, T.D.; Gianaros, P.J. Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neurosci. Biobehav. Rev. 2018, 94, 76–92. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Cole, S.W. Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci. 2012, 15, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.; Tracey, K.J. Neural regulation of immunity: Molecular mechanisms and clinical translation. Nat. Neurosci. 2017, 20, 156–166. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology 2011, 37, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test’—A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Kennedy, P.J.; Dockray, S.; Cryan, J.F.; Dinan, T.; Clarke, G. The Trier Social Stress Test: Principles and practice. Neurobiol. Stress 2016, 6, 113–126. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Schommer, N.C.; Hellhammer, D.H.; Kirschbaum, C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 2004, 29, 983–992. [Google Scholar] [CrossRef]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.-A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2018, 25, 1301–1311. [Google Scholar] [CrossRef]
- Saccaro, L.; Schilliger, Z.; Dayer, A.; Perroud, N.; Piguet, C. Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: A narrative review. Neurosci. Biobehav. Rev. 2021, 127, 184–192. [Google Scholar] [CrossRef] [PubMed]
- McLeer, S.V.; Callaghan, M.; Henry, D.; Wallen, J. Psychiatric Disorders in Sexually Abused Children. J. Am. Acad. Child Adolesc. Psychiatry 1994, 33, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Able, S.L.; Johnston, J.A.; Adler, L.; Swindle, R.W. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol. Med. 2006, 37, 97–107. [Google Scholar] [CrossRef]
- Sobanski, E.; Brüggemann, D.; Alm, B.; Kern, S.; Deschner, M.; Schubert, T.; Philipsen, A.; Rietschel, M. Psychiatric comorbidity and functional impairment in a clinically referred sample of adults with attention-deficit/hyperactivity disorder (ADHD). Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 371–377. [Google Scholar] [CrossRef]
- Vrijsen, J.N.; Tendolkar, I.; Onnink, M.; Hoogman, M.; Schene, A.H.; Fernández, G.; Van Oostrom, I.; Franke, B. ADHD symptoms in healthy adults are associated with stressful life events and negative memory bias. ADHD Atten. Deficit Hyperact. Disord. 2017, 10, 151–160. [Google Scholar] [CrossRef]
- Grizenko, N.; Fortier, M.-E.; Zadorozny, C.; Thakur, G.; Schmitz, N.; Duval, R.; Joober, R. Maternal Stress during Pregnancy, ADHD Symptomatology in Children and Genotype: Gene-Environment Interaction. J. Can. Acad. Child Adolesc. Psychiatry 2012, 21, 9–15. [Google Scholar]
- Okano, L.; Ji, Y.; Riley, A.W.; Wang, X. Maternal psychosocial stress and children’s ADHD diagnosis: A prospective birth cohort study. J. Psychosom. Obstet. Gynecol. 2018, 40, 217–225. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Adeyemo, B.O.; Biederman, J.; Zafonte, R.; Kagan, E.; Spencer, T.J.; Uchida, M.; Kenworthy, T.; Spencer, A.E.; Faraone, S. Mild Traumatic Brain Injury and ADHD. J. Atten. Disord. 2014, 18, 576–584. [Google Scholar] [CrossRef]
- Grigorian, A.; Nahmias, J.; Dolich, M.; Barrios, C.; Schubl, S.D.; Sheehan, B.; Lekawa, M. Increased risk of head injury in pediatric patients with attention deficit hyperactivity disorder. J. Child Adolesc. Psychiatr. Nurs. 2019, 32, 171–176. [Google Scholar] [CrossRef]
- Chasle, V.; Riffaud, L.; Longuet, R.; Martineau-Curt, M.; Collet, Y.; Le Fournier, L.; Pladys, P. Mild head injury and attention deficit hyperactivity disorder in children. Child’s Nerv. Syst. 2016, 32, 2357–2361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reimherr, F.W.; Marchant, B.K.; Gift, T.E.; Steans, T.A. ADHD and Anxiety: Clinical Significance and Treatment Implications. Curr. Psychiatry Rep. 2017, 19, 109. [Google Scholar] [CrossRef]
- Anand, D.; Colpo, G.D.; Zeni, G.; Zeni, C.P.; Teixeira, A.L. Attention-Deficit/Hyperactivity Disorder And Inflammation: What Does Current Knowledge Tell Us? A Systematic Review. Front. Psychiatry 2017, 8, 228. [Google Scholar] [CrossRef]
- Martins-Silva, T.; Vaz, J.; Hutz, M.H.; Salatino-Oliveira, A.; Genro, J.P.; Hartwig, F.P.; Moreira-Maia, C.R.; Rohde, L.A.; Borges, M.C.; Tovo-Rodrigues, L. Assessing causality in the association between attention-deficit/hyperactivity disorder and obesity: A Mendelian randomization study. Int. J. Obes. 2019, 43, 2500–2508. [Google Scholar] [CrossRef]
- Wootton, R.E.; Jones, H.J.; Sallis, H.M. Mendelian randomisation for psychiatry: How does it work, and what can it tell us? Mol. Psychiatry 2021, 1–5. [Google Scholar] [CrossRef]
- Welsh, P.; Polisecki, E.; Robertson, M.; Jahn, S.; Buckley, B.M.; De Craen, A.J.M.; Ford, I.; Jukema, J.W.; Macfarlane, P.W.; Packard, C.J.; et al. Unraveling the Directional Link between Adiposity and Inflammation: A Bidirectional Mendelian Randomization Approach. J. Clin. Endocrinol. Metab. 2010, 95, 93–99. [Google Scholar] [CrossRef]
- Darwish, A.; Elgohary, T.M.; Nosair, N.A. Serum Interleukin-6 Level in Children with Attention-Deficit Hyperactivity Disorder (ADHD). J. Child Neurol. 2018, 34, 61–67. [Google Scholar] [CrossRef]
- Donfrancesco, R.; Nativio, P.; Di Benedetto, A.; Villa, M.P.; Andriola, E.; Melegari, M.G.; Cipriano, E.; Di Trani, M. Anti-Yo Antibodies in Children With ADHD: First Results About Serum Cytokines. J. Atten. Disord. 2016, 24, 1497–1502. [Google Scholar] [CrossRef]
- Toto, M.; Margari, F.; Simone, M.; Craig, F.; Petruzzelli, M.G.; Tafuri, S.; Margari, L. Antibasal Ganglia Antibodies and Antistreptolysin O in Noncomorbid ADHD. J. Atten. Disord. 2012, 19, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, F.; Donfrancesco, R.; Nativio, P.; Pascale, E.; Di Trani, M.; Patti, A.M.; Vulcano, A.; Gozzo, P.; Villa, M.P. Anti-Purkinje cell antibody as a biological marker in attention deficit/hyperactivity disorder: A pilot study. J. Neuroimmunol. 2013, 258, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Chua, R.X.Y.; Tay, M.J.Y.; Ooi, D.S.Q.; Siah, K.T.H.; Tham, E.H.; Shek, L.P.-C.; Meaney, M.J.; Broekman, B.F.P.; Loo, E.X.L. Understanding the Link Between Allergy and Neurodevelopmental Disorders: A Current Review of Factors and Mechanisms. Front. Neurol. 2021, 11. [Google Scholar] [CrossRef]
- Miyazaki, C.; Koyama, M.; Ota, E.; Swa, T.; Mlunde, L.B.; Amiya, R.M.; Tachibana, Y.; Yamamoto-Hanada, K.; Mori, R. Allergic diseases in children with attention deficit hyperactivity disorder: A systematic review and meta-analysis. BMC Psychiatry 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Muskens, J.B.; Velders, F.P.; Staal, W.G. Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: A systematic review. Eur. Child Adolesc. Psychiatry 2017, 26, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- van der Schans, J.; Çiçek, R.; de Vries, T.W.; Hak, E.; Hoekstra, P.J. Association of atopic diseases and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. Neurosci. Biobehav. Rev. 2017, 74, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bull-Larsen, S.; Mohajeri, M.H. The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 2019, 11, 2805. [Google Scholar] [CrossRef]
- Bonvicini, C.; Faraone, S.V.; Scassellati, C. Common and specific genes and peripheral biomarkers in children and adults with attention-deficit/hyperactivity disorder. World J. Biol. Psychiatry 2017, 19, 80–100. [Google Scholar] [CrossRef]
- Zayats, T.; Athanasiu, L.; Sonderby, I.; Djurovic, S.; Westlye, L.T.; Tamnes, C.K.; Fladby, T.; Aase, H.; Zeiner, P.; Reichborn-Kjennerud, T.; et al. Genome-Wide Analysis of Attention Deficit Hyperactivity Disorder in Norway. PLoS ONE 2015, 10, e0122501. [Google Scholar] [CrossRef]
- Odell, D.; Warren, R.P.; Warren, L.; Burger, R.A.; Maciulis, A. Association of Genes within the Major Histocompatibility Complex with Attention Deficit Hyperactivity Disorder. Neuropsychobiology 1997, 35, 181–186. [Google Scholar] [CrossRef]
- Smith, T.F.; Anastopoulos, A.D.; Garrett, M.E.; Arias-Vasquez, A.; Franke, B.; Oades, R.D.; Sonuga-Barke, E.; Asherson, P.; Gill, M.; Buitelaar, J.K.; et al. Angiogenic, neurotrophic, and inflammatory system SNPs moderate the association between birth weight and ADHD symptom severity. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 691–704. [Google Scholar] [CrossRef]
- Nielsen, P.R.; Benros, M.; Dalsgaard, S. Associations Between Autoimmune Diseases and Attention-Deficit/Hyperactivity Disorder: A Nationwide Study. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 234–240.e1. [Google Scholar] [CrossRef]
- Instanes, J.T.; Halmøy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsøyr, K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol. Psychiatry 2017, 81, 452–459. [Google Scholar] [CrossRef]
- Fetene, D.M.; Betts, K.; Alati, R. Mechanisms in Endocrinology: Maternal thyroid dysfunction during pregnancy and behavioural and psychiatric disorders of children: A systematic review. Eur. J. Endocrinol. 2017, 177, R261–R273. [Google Scholar] [CrossRef]
- Hirvikoski, T.; Lindholm, T.; Nordenström, A.; Nordström, A.-L.; Lajic, S. High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder). Horm. Behav. 2009, 55, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Raz, S.; Leykin, D. Psychological and cortisol reactivity to experimentally induced stress in adults with ADHD. Psychoneuroendocrinology 2015, 60, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Hatzinger, M.; Brand, S.; Perren, S.; von Wyl, A.; von Klitzing, K.; Holsboer-Trachsler, E. Hypothalamic–pituitary–adrenocortical (HPA) activity in kindergarten children: Importance of gender and associations with behavioral/emotional difficulties. J. Psychiatr. Res. 2007, 41, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.L.; Coogan, A.N.; Siddiqui, A.; Donev, R.; Thome, J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol. Psychiatry 2011, 17, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Roso, M.; Palomar, G.; Ferrer, R.; Real, A.; Nogueira, M.; Corrales, M.; Casas, M.; Ramos-Quiroga, J.A. Cortisol Response to Stress in Adults with Attention Deficit Hyperactivity Disorder. Int. J. Neuropsychopharmacol. 2015, 18, pyv027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freitag, C.M.; Hänig, S.; Palmason, H.; Meyer, J.; Wüst, S.; Seitz, C. Cortisol awakening response in healthy children and children with ADHD: Impact of comorbid disorders and psychosocial risk factors. Psychoneuroendocrinology 2009, 34, 1019–1028. [Google Scholar] [CrossRef]
- Imeraj, L.; Antrop, I.; Roeyers, H.; Swanson, J.; Deschepper, E.; Bal, S.; Deboutte, D. Time-of-day effects in arousal: Disrupted diurnal cortisol profiles in children with ADHD. J. Child Psychol. Psychiatry 2012, 53, 782–789. [Google Scholar] [CrossRef]
- Pesonen, A.-K.; Kajantie, E.; Jones, A.; Pyhälä, R.; Lahti, J.; Heinonen, K.; Eriksson, J.G.; Strandberg, T.E.; Räikkönen, K. Symptoms of attention deficit hyperactivity disorder in children are associated with cortisol responses to psychosocial stress but not with daily cortisol levels. J. Psychiatr. Res. 2011, 45, 1471–1476. [Google Scholar] [CrossRef]
- Randazzo, W.T.; Dockray, S.; Susman, E.J. The Stress Response in Adolescents with Inattentive Type ADHD Symptoms. Child Psychiatry Hum. Dev. 2007, 39, 27–38. [Google Scholar] [CrossRef]
- Vreeburg, S.A.; Kruijtzer, B.P.; van Pelt, J.; van Dyck, R.; DeRijk, R.H.; Hoogendijk, W.J.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology 2009, 34, 1109–1120. [Google Scholar] [CrossRef]
- Bijlenga, D.; Van Der Heijden, K.B.; Breuk, M.; Van Someren, E.J.W.; Lie, M.E.H.; Boonstra, A.M.; Swaab, H.J.T.; Kooij, J.J.S. Associations Between Sleep Characteristics, Seasonal Depressive Symptoms, Lifestyle, and ADHD Symptoms in Adults. J. Atten. Disord. 2011, 17, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Dolsen, M.R.; Crosswell, A.D.; Prather, A.A. Links Between Stress, Sleep, and Inflammation: Are There Sex Differences? Curr. Psychiatry Rep. 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2015, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Roso, M.; Ramos-Quiroga, J.A.; Ribases, M.; Sanchez-Mora, C.; Palomar, G.; Valero, S.; Bosch, R.; Casas, M. Decreased serum levels of brain-derived neurotrophic factor in adults with attention-deficit hyperactivity disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, H.; Cui, L.; Chu, S.; Chen, N. The progress of chemokines and chemokine receptors in autism spectrum disorders. Brain Res. Bull. 2021, 174, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Lee, B.K.; Brynge, M.; Sjöqvist, H.; Dalman, C.; Karlsson, H. Neonatal Levels of Acute Phase Proteins and Risk of Autism Spectrum Disorder. Biol. Psychiatry 2020, 89, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Jaini, R.; Wolf, M.R.; Yu, Q.; King, A.T.; Frazier, T.W.; Eng, C. Maternal genetics influences fetal neurodevelopment and postnatal autism spectrum disorder-like phenotype by modulating in-utero immunosuppression. Transl. Psychiatry 2021, 11, 348. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Block, C.L.; Bolton, J.; Hanamsagar, R.; Tran, P.K. Beyond infection—Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2017, 299, 241–251. [Google Scholar] [CrossRef]
- Wang, M.; Saudino, K.J. Emotion Regulation and Stress. J. Adult Dev. 2011, 18, 95–103. [Google Scholar] [CrossRef]
- Ly, V.; Bottelier, M.; Hoekstra, P.J.; Vasquez, A.A.; Buitelaar, J.K.; Rommelse, N.N. Elimination diets’ efficacy and mechanisms in attention deficit hyperactivity disorder and autism spectrum disorder. Eur. Child Adolesc. Psychiatry 2017, 26, 1067–1079. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.; Brandeis, D.; Cortese, S.; Daley, D.; Ferrin, M.; Holtmann, M.; Stevenson, J.; Danckaerts, M.; Van Der Oord, S.; Döpfner, M.; et al. Nonpharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological Treatments. Am. J. Psychiatry 2013, 170, 275–289. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.-T.; Chiang, Y.-J.; Chen, H.-T.; Pariante, C.M. High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 303. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette Smoking and Variations in Systemic Immune and Inflammation Markers. J. Natl. Cancer Inst. 2014, 106, dju294. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Gofita, E.; Costa, C.; Teodoro, M.; Briguglio, G.; Nikitovic, D.; Tzanakakis, G.; Tsatsakis, A.; Wilks, M.; Spandidos, D.; et al. Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases (Review). Int. J. Mol. Med. 2016, 38, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.; Carlson, J.M.; Cha, J.; Rubin, D. The fine line between ‘brave’ and ‘reckless’: Amygdala reactivity and regulation predict recognition of risk. NeuroImage 2014, 103, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Findings | Study Type | References | |
|---|---|---|---|
| ADHD patients, including children, may have altered levels of immunological markers, such as: - increased cytokines (e.g., IL10 and IL6, although contrasting results exist on IL6) - auto-antibodies (e.g., anti-basal ganglia antibodies and anti-Yo antibodies targeting Purkinje cells) | Case-control observational study | Darwish et al.2019 [86] | |
| Comparative observational studies | Donfrancesco et al., 2016 [87] Toto et al., 2015 [88] Corominas-Roso et al., 2017 [89] Passarelli et al., 2013 [90] | ||
| ADHD is associated with autoimmune, atopic, and inflammatory disorders (e.g., diabetes, psoriasis, asthma, allergic rhinitis and conjunctivitis, atopic dermatitis/eczema) | Review | Chua et al., 2021 [91] | |
| Systematic reviews and Meta-analyses | Miyazaki et al., 2017 [92] Muskens et al., 2017 [93] van der Schans et al., 2017 [94] | ||
| Microbiome-dependent systemic inflammation might affect neurodevelopment and predispose to ADHD | Reviews | Bull-Larsen and Hasan Mohajeri, 2019 [95] Chua et al., 2021 [91] | |
| Genetic polymorphisms in inflammation-related genes (e.g., antioxidant enzymes like superoxide dismutase, cytokine-related genes, and major histocompatibility complex) have been associated with ADHD | Systematic review | Bonvicini et al., 2018 [96] | |
| GWA analysis | Zayats et al., 2015 [97] | ||
| Original papers | Odell et al., 1997 [98] Smith et al., 2014 [99] | ||
| Maternal pro-inflammatory factors | Maternal autoimmune, atopic, and inflammatory disorders (e.g., multiple sclerosis, type 1 diabetes, asthma, autoimmune thyroiditis) may increase the risk for ADHD in the offspring | Cohort epidemiological study | Nielsen et al., 2017 [100] |
| Population-based nested case-control study | Instanes et al., 2017 [101] | ||
| Systematic reviews | Fetene et al., 2017 [102] Han et al., 2021 [77] | ||
| Stress during pregnancy increases the risk of ADHD in the offspring | Case-control study (intra-familial matched subject pairs) | Grizenko et al., 2012 [75] | |
| Prospective birth cohort study | Okano et al., 2018 [76] | ||
| Systematic review | Han et al., 2021 [77] | ||
| Maternal obesity, pre-eclampsia, smoking (before and during pregnancy), and low socioeconomic status increases the risk of ADHD in the offspring | Systematic review | Han et al., 2021 [77] | |
| Findings | Study Design | Type of Marker | References |
|---|---|---|---|
| Elevated levels of IL16 and IL13 in ADHD children, independent of anxiety symptoms. | Cross-sectional study | Serum cytokines: IL1β, IL2, IL6, IL16, IL13, TNF-α, IFN-γ. | Oades et al., 2010 [103] |
| No association between ADHD symptomatology and IL6, CRP, or TNF-α. Loss of the association between HPA axis dysregulation and ADHD symptoms after adjusting for anxiety and depression. | Cohort study, cross-sectional | Plasma cytokines: IL6, CRP, TNF-α HPA-axis activity: Cortisol awakening response Dexamethasone suppression test Evening cortisol | Vogel et al., 2017 [104] |
| Children with higher salivary pro-inflammatory cytokine levels showed significantly stronger associations between ADHD symptoms and their caregivers’ perceived life stress scale scores. | Prospective Cohort study | Salivary cytokines: IL-6, IL-1β, IL-8, clustered into low, average, and high cytokine cluster groups by hierarchical cluster analysis | Parent et al., 2021 [59] |
| In 44 ADHD patients of the inattentive subtype, there was a negative correlation between the level of IL6 & TNF-α and cortisol awakening response | Cross-sectional study | Serum cytokines: IL6 & TNF-α Salivary cortisol awakening response | Corominas-Roso et al., 2017 [89] |
| In 102 healthy children, hyperactivity and impulsivity were associated with higher basal and acute stress-related HPA axis activity in boys but not in girls. | Prospective study | Salivary cortisol awakening response, salivary cortisol response to stress | Hatzinger et al., 2007 [107] |
| Among all participants, impulsivity levels correlated with high post-stress cortisol concentration, which was more common in ADHD patients. Higher post-stress cortisol was also associated with symptoms of depression and anxiety, which may be possible confounders | Cross-sectional study | Diurnal salivary cortisol and salivary cortisol in response to laboratory stress | Tatja Hirvikoski., 2009 [105] |
| Past and current environmental stressors were associated with higher cortisol awakening responses in ADHD children | Comparative study | Salivary cortisol awakening response | Freitag et al., 2009 [110] |
| Cortisol response to acute experimental stress was found to be higher in ADHD adults than in controls | Cross-sectional study | Salivary cortisol in response to laboratory stress | Raz, 2015 [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saccaro, L.F.; Schilliger, Z.; Perroud, N.; Piguet, C. Inflammation, Anxiety, and Stress in Attention-Deficit/Hyperactivity Disorder. Biomedicines 2021, 9, 1313. https://doi.org/10.3390/biomedicines9101313
Saccaro LF, Schilliger Z, Perroud N, Piguet C. Inflammation, Anxiety, and Stress in Attention-Deficit/Hyperactivity Disorder. Biomedicines. 2021; 9(10):1313. https://doi.org/10.3390/biomedicines9101313
Chicago/Turabian StyleSaccaro, Luigi F., Zoé Schilliger, Nader Perroud, and Camille Piguet. 2021. "Inflammation, Anxiety, and Stress in Attention-Deficit/Hyperactivity Disorder" Biomedicines 9, no. 10: 1313. https://doi.org/10.3390/biomedicines9101313
APA StyleSaccaro, L. F., Schilliger, Z., Perroud, N., & Piguet, C. (2021). Inflammation, Anxiety, and Stress in Attention-Deficit/Hyperactivity Disorder. Biomedicines, 9(10), 1313. https://doi.org/10.3390/biomedicines9101313