Clinical Outcome of RAMPS for Left-Sided Pancreatic Ductal Adenocarcinoma: A Comparison of Anterior RAMPS versus Posterior RAMPS for Patients without Periadrenal Infiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Postoperative Monitoring
2.2. Surgical Techniques
2.2.1. Anterior Radical Antegrade Modular Pancreatosplenectomy
2.2.2. Posterior Radical Antegrade Modular Pancreatosplenectomy
2.3. Comparative Analysis
3. Results
Comparative Analysis of Perioperative, Pathologic, and Survival Outcomes between aRAMPS and pRAMPS Patients without Suspected or Observed Periadrenal Infiltration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, M.F.; Moccia, R.D.; Klimstra, D. Management of adenocarcinoma of the body and tail of the pancreas. Ann. Surg. 1996, 223, 506–511; discussion 511–512. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Drebin, J.A.; Linehan, D. Radical antegrade modular pancreatosplenectomy. Surgery 2003, 133, 521–527. [Google Scholar] [CrossRef]
- Abe, T.; Ohuchida, K.; Miyasaka, Y.; Ohtsuka, T.; Oda, Y.; Nakamura, M. Comparison of Surgical Outcomes Between Radical Antegrade Modular Pancreatosplenectomy (RAMPS) and Standard Retrograde Pancreatosplenectomy (SPRS) for Left-Sided Pancreatic Cancer. World J. Surg. 2016, 40, 2267–2275. [Google Scholar] [CrossRef]
- Kim, E.Y.; You, Y.K.; Kim, D.G.; Hong, T.H. Initial experience with radical antegrade modular pancreatosplenectomy in a single institution. Ann. Surg. Treat Res. 2016, 91, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; You, D.D.; Choi, D.W.; Heo, J.S.; Choi, S.H. Role of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the body and tail of the pancreas. World J. Surg. 2014, 38, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Hinz, U.; Pausch, T.; Fesenbeck, I.; Strobel, O.; Schneider, L.; Fritz, S.; Buchler, M.W. Postoperative pancreatic fistula: We need to redefine grades B and C. Surgery 2016, 159, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Kitagawa, H.; Tajima, H.; Nakagawara, H.; Makino, I.; Miyashita, T.; Terakawa, H.; Nakanuma, S.; Hayashi, H.; Takamura, H.; Ohta, T. A modification of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the left pancreas: Significance of en bloc resection including the anterior renal fascia. World J. Surg. 2014, 38, 2448–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Morchoe, C.C. Lymphatic system of the pancreas. Microsc. Res. Tech. 1997, 37, 456–477. [Google Scholar] [CrossRef]
- Mitchem, J.B.; Hamilton, N.; Gao, F.; Hawkins, W.G.; Linehan, D.C.; Strasberg, S.M. Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure. J. Am. Coll. Surg. 2012, 214, 46–52. [Google Scholar] [CrossRef]
- Latorre, M.; Ziparo, V.; Nigri, G.; Balducci, G.; Cavallini, M.; Ramacciato, G. Standard retrograde pancreatosplenectomy versus radical antegrade modular pancreatosplenectomy for body and tail pancreatic adenocarcinoma. Am. Surg. 2013, 79, 1154–1158. [Google Scholar] [CrossRef]
- Sham, J.G.; Guo, S.; Ding, D.; Shao, Z.; Wright, M.; Jing, W.; Yin, L.D.; Zhang, Y.; Gage, M.M.; Zhou, Y.; et al. Radical antegrade modular pancreatosplenectomy versus standard distal pancreatosplenectomy for pancreatic cancer, a dual-institutional analysis. Chin. Clin. Oncol. 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Li, J.; Li, A.; Li, F. Radical antegrade modular pancreatosplenectomy versus standard procedure in the treatment of left-sided pancreatic cancer: A systemic review and meta-analysis. BMC Surg. 2017, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Fengwei, G.; Gong, J.; Xie, Q.; Liu, Y.; Wang, Q.; Lei, Z. Assessement of postoperative long-term survival quality and complications associated with radical antegrade modular pancreatosplenectomy and distal pancreatectomy: A meta-analysis and systematic review. BMC Surg. 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.S.; Stewart, P.M. Corticosteroid insufficiency in acutely ill patients. N. Engl. J. Med. 2003, 348, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, H.; Tanaka, M. Incidence of adrenal involvement and assessing adrenal function in patients with renal cell carcinoma: Is ipsilateral adrenalectomy indispensable during radical nephrectomy? BJU Int. 2005, 95, 526–529. [Google Scholar] [CrossRef]
- Honda, K.; Sone, M.; Tamura, N.; Sonoyama, T.; Taura, D.; Kojima, K.; Fukuda, Y.; Tanaka, S.; Yasuno, S.; Fujii, T.; et al. Adrenal reserve function after unilateral adrenalectomy in patients with primary aldosteronism. J. Hypertens 2013, 31, 2010–2017. [Google Scholar] [CrossRef]
- Yoshiji, S.; Shibue, K.; Fujii, T.; Usui, T.; Hirota, K.; Taura, D.; Inoue, M.; Sone, M.; Yasoda, A.; Inagaki, N. Chronic primary adrenal insufficiency after unilateral adrenonephrectomy: A case report. Medicine 2017, 96, e9091. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Barbosa, G.; Tsinberg, M.; Milas, M.; Siperstein, A.; Berber, E. Unrecognized adrenal insufficiency in patients undergoing laparoscopic adrenalectomy. Surg. Endosc. 2009, 23, 248–254. [Google Scholar] [CrossRef]
- Trottman, P.; Swett, K.; Shen, P.; Sirintrapun, J. Comparison of standard distal pancreatectomy and splenectomy with radical antegrade modular pancreatosplenectomy. Am. Surg. 2014, 80, 295–300. [Google Scholar] [CrossRef]
- Huo, Z.; Zhai, S.; Wang, Y.; Qian, H.; Tang, X.; Shi, Y.; Weng, Y.; Zhao, S.; Deng, X.; Shen, B. Comparison of Radical Antegrade Modular Pancreatosplenectomy with Standard Retrograde Pancreatosplenectomy for Left-Sided Pancreatic Cancer: A Meta-Analysis and Experience of a Single Center. Med. Sci. Monit. 2019, 25, 4590–4601. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Tajima, H.; Nakagawara, H.; Hayashi, H.; Makino, I.; Takamura, H.; Ninomiya, I.; Fushida, S.; Kayahara, M.; Ohta, T.; et al. The retropancreatic fusion fascia acts as a barrier against infiltration by pancreatic carcinoma. Mol. Clin. Oncol. 2013, 1, 418–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | aRAMPS (n = 112) | pRAMPS (n = 224) | p-Value | |
|---|---|---|---|---|
| Age, years (±SD) | Mean | 61.8 (±10.3) | 62.7 (±9.5) | 0.426 |
| Sex, n (%) | Female | 44 (39.3) | 112 (50.0) | 0.063 |
| Male | 68 (60.7) | 112 (50.0) | ||
| BMI, kg/m2 (±SD) | Mean | 23.9 (±2.8) | 23.6 (±2.9) | 0.462 |
| ASA score, n (%) | I | 8 (7.1) | 17 (7.6) | 0.281 |
| II | 95 (84.8) | 198 (88.4) | ||
| III | 9 (8.0) | 9 (4.0) | ||
| CA 19-9, n (%) | Normal | 47 (42.0) | 93 (41.5) | 0.727 |
| Increased | 60 (53.6) | 129 (57.6) | ||
| NA | 5 (4.5) | 2 (0.1) | ||
| CEA, n (%) | Normal | 86 (76.8) | 180 (80.4) | 0.687 |
| Increased | 20 (17.9) | 37 (16.5) | ||
| NA | 6 (5.4) | 5 (2.2) | ||
| mGPS, n (%) | 0 | 95 (84.8) | 174 (77.7) | 0.188 |
| 1-2 | 5 (4.5) | 18 (8.0) | ||
| NA | 12 (10.7) | 32 (14.3) | ||
| Clinical tumor size, cm (±SD) | Mean | 2.8 (±1.4) | 3.3 (±1.7) | 0.021 |
| Tumor location | Neck | 2 (1.8) | 1 (0.4) | 0.317 |
| Body | 54 (48.2) | 121 (54.0) | ||
| Tail | 56 (50.0) | 102 (45.5) | ||
| Periadrenal infiltration | Yes | 6 (5.4) | 67 (29.9) | <0.001 |
| No | 106 (94.6) | 157 (70.1) | ||
| Neoadjuvant, n (%) | Yes | 8 (7.1) | 36 (16.1) | 0.022 |
| No | 104 (92.9) | 188 (83.9) |
| Variables | aRAMPS (n = 112) | pRAMPS (n = 224) | p-Value | |
|---|---|---|---|---|
| Operation time, minutes (±SD) | Mean | 204 (±61) | 228 (±63) | 0.001 |
| Operation type | Minimal invasive | 80 (71.4) | 122 (54.5) | 0.003 |
| Open | 32 (28.6) | 102 (45.5) | ||
| Concurrent vessel resection, n (%) | Yes | 6 (5.4) | 30 (13.4) | 0.025 |
| No | 106 (94.6) | 194 (86.6) | ||
| Concurrent resection of other organs, n (%) | Yes | 19 (17.0) | 49 (21.9) | 0.291 |
| No | 93 (83.0) | 175 (78.1) | ||
| Hospital stay after operation, days(±SD) | Mean | 10.9 (±6.5) | 11.5 (±7.0) | 0.422 |
| Complications grade +, n (%) | No | 86 (76.8) | 164 (73.5) | 0.97 |
| Grade I-II | 12 (10.7) | 39 (17.5) | ||
| Grade III-IV | 14 (12.5) | 20 (9.0) | ||
| POPF ++, n (%) + | No or | 97 (86.6) | 195 (87.1) | 0.909 |
| Biochemical leakage | ||||
| Grade B or C | 15 (13.4) | 29 (12.9) | ||
| 90-day mortality, n (%) | Yes | 1 (0.9) | 1 (0.4) | 1 |
| No | 111 (99.1) | 223 (99.6) | ||
| Adjuvant | No | 26 (23.2) | 64 (28.6) | 0.522 |
| CTx | 68 (60.7) | 123 (54.9) | ||
| CCRTx | 18 (16.1) | 35 (15.6) | ||
| RT | 0 (0.0) | 2 (0.9) | ||
| Interval between surgery and adjuvant treatment, days (±SD) | Mean | 42.5 (±21.5) | 44.1 (±28.6) | 0.673 |
| First line adjuvant chemotherapy regimen | Fluoropyrimidine | 32 (37.6) | 64 (40.5) | 0.235 |
| Gemcitabine based | 46 (53.5) | 60 (38.0) | ||
| FOLFIRONOX | 1 (1.2) | 11 (7.0) | ||
| Others | 7 (8.2) | 23 (14.6) |
| Variables | aRAMPS (n = 112) | pRAMPS (n = 224) | p-Value | |
|---|---|---|---|---|
| Pathologic tumor size, cm (±SD) | Mean | 3.4 (±1.6) | 3.8 (±1.8) | 0.128 |
| T stage (AJCC 8th), n (%) | T1 | 20 (17.9) | 27 (12.1) | 0.173 |
| T2 | 58 (51.8) | 118 (52.7) | ||
| T3 | 33 (29.5) | 77 (34.4) | ||
| T4 | 1 (0.9) | 2 (0.9) | ||
| N stage (AJCC 8th), n (%) | N0 | 50 (44.6) | 83 (37.2) | 0.263 |
| N1 | 48 (42.9) | 109 (48.9) | ||
| N2 | 14 (12.5) | 31 (13.9) | ||
| Staging (AJCC 8th) +, n (%) | IA | 14 (12.5) | 15 (6.7) | 0.078 |
| IB | 21 (18.8) | 44 (19.6) | ||
| IIA | 12 (10.7) | 19 (8.5) | ||
| IIB | 48 (42.9) | 100 (44.6) | ||
| III | 14 (12.5) | 29 (12.9) | ||
| IV | 3 (2.7) | 16 (7.1) | ||
| NA | 0 (0.0) | 1 (0.4) | ||
| Differentiation | WD | 11 (9.8) | 15 (6.7) | 0.817 |
| MD | 80 (71.4) | 170 (75.9) | ||
| PD | 16 (14.3) | 27 (12.1) | ||
| Others | 3 (2.7) | 7 (3.1) | ||
| NA | 2 (1.8) | 5 (2.2) | ||
| Peripancreatic infiltration, n (%) | Yes | 106 (94.6) | 210 (93.8) | 0.744 |
| No | 6 (5.4) | 14 (6.3) | ||
| Lymphovascular invasion, n (%) | Yes | 58 (51.8) | 127 (56.7) | 0.394 |
| No | 54 (48.2) | 97 (43.3) | ||
| Perineural invasion, n (%) | Yes | 88 (78.6) | 186 (83.0) | 0.32 |
| No | 24 (21.4) | 38 (17.0) | ||
| Number of harvested lymph nodes, n (±SD) | Mean | 14.8 (±8.9) | 16.9 (±9.7) | 0.059 |
| Number of positive lymph nodes, n (±SD) | Mean | 1.4 (±1.9) | 1.7 (±2.1) | 0.272 |
| Positive lymph node ratio, % (±SD) | Mean | 11.2 (±15.0) | 10.7 (±13.3) | 0.771 |
| Resection margin ++, n (%) | R0 | 86 (76.8) | 173 (77.2) | 0.927 |
| R1 | 26 (23.2) | 51 (22.8) | ||
| Location of margin involved, n (%) | Pancreas margin | 1 (3.8) | 1 (2.0) | 0.115 |
| Tangential margin | 23 (88.5) | 50 (98.0) | ||
| Both | 2 (7.7 | 0 (0.0) | ||
| Pathologic adrenal gland invasion, n (%) | Yes | 13 (5.8) | NA | |
| No | 211 (94.2) |
| Variables | aRAMPS (n = 106) | pRAMPS (n = 157) | p-Value | |
|---|---|---|---|---|
| Age, years (±SD) | Mean | 62.1 (±10.3) | 62.1 (±9.2) | 0.999 |
| Sex, n (%) | Female | 44 (41.5) | 82 (52.2) | 0.088 |
| Male | 62 (58.5) | 75 (47.8) | ||
| BMI, kg/m2 (±SD) | Mean | 23.9 (±2.9) | 23.8 (±2.9) | 0.888 |
| ASA score, n (%) | I | 8 (7.5) | 13 (8.3) | 0.276 |
| II | 90 (84.9) | 139 (88.5) | ||
| III | 8 (7.5) | 5 (3.2) | ||
| CA 19-9, n (%) | Normal | 44 (41.5) | 67 (42.7) | 0.888 |
| Increased | 57 (53.8) | 90 (57.3) | ||
| NA | 5 (4.7) | 0 (0.0) | ||
| CEA, n (%) | Normal | 83 (78.3) | 128 (81.5) | 0.89 |
| Increased | 17 (16.0) | 25 (15.9) | ||
| NA | 6 (5.7) | 4 (2.5) | ||
| mGPS, n (%) | 0 | 90 (84.9) | 126 (80.3) | 0.567 |
| 1-2 | 4 (3.8) | 9 (5.7) | ||
| NA | 12 (11.3) | 22 (14.0) | ||
| Clinical tumor size, cm (±SD) | Mean | 2.8 (±1.4) | 2.9 (±1.3) | 0.778 |
| Tumor location | Neck | 2 (1.9) | 1 (0.6) | 0.102 |
| Body | 53 (50.0) | 99 (63.1) | ||
| Tail | 51 (48.1) | 57 (36.3) | ||
| Neoadjuvant, n (%) | Yes | 8 (7.5) | 23 (14.6) | 0.08 |
| No | 98 (92.5) | 134 (85.4) |
| Variables | aRAMPS (n = 106) | pRAMPS (n = 157) | p-Value | |
|---|---|---|---|---|
| Operation time, minutes (±SD) | Mean | 202 (±61) | 226 (±62) | 0.002 |
| Operation type | Minimally invasive | 75 (70.8) | 93 (59.2) | 0.056 |
| Open | 31 (29.2) | 64 (40.8) | ||
| Concurrent vessel resection, n (%) | Yes | 6 (5.7) | 23 (14.6) | 0.022 |
| No | 100 (94.3) | 134 (85.4) | ||
| Concurrent resection of other organs, n (%) | Yes | 16 (15.1) | 25 (15.9) | 0.856 |
| No | 90 (84.9) | 132 (84.1) | ||
| Hospital stay after operation, days (±SD) | Mean | 10.8 (±6.6) | 11.0 (±6.0) | 0.786 |
| Complications grade +, n (%) | No | 81 (76.4) | 114 (72.6) | 0.782 |
| Grade I-II | 12 (11.3) | 26 (16.6) | ||
| Grade III-IV | 13 (12.3) | 17 (10.8) | ||
| POPF ++, n (%) + | No or | 92 (86.8) | 135 (86.0) | 0.852 |
| Biochemical leakage | ||||
| Grade B or C | 14 (13.2) | 22 (14.0) | ||
| 90-day mortality, n (%) | Yes | 1 (0.9) | 0 (0.0) | 0.403 |
| No | 105 (99.1) | 157 (100.0) | ||
| Adjuvant | No | 25 (23.6) | 41 (26.1) | 0.647 |
| CTx | 64 (60.4) | 91 (58.0) | ||
| CCRTx | 17 (16.0) | 23 (14.6) | ||
| RT | 0 (0.0) | 2 (1.3) | ||
| Interval between surgery and adjuvant treatment, days (±SD) | Mean | 42.9 (±21.7) | 43.7 (±32.8) | 0.867 |
| First line adjuvant chemotherapy regimen | Fluoropyrimidine | 30 (37.0) | 46(40.4) | 0.125 |
| Gemcitabine based | 44 (54.3) | 41 (36.0) | ||
| FOLFIRONOX | 1 (1.2) | 7 (6.1) | ||
| Others | 6 (7.4) | 20 (17.5) |
| Variables | aRAMPS (n = 106) | pRAMPS (n = 157) | p-Value | |
|---|---|---|---|---|
| Pathologic tumor size, cm (±SD) | Mean | 3.4 (±1.7) | 3.4 (±1.5) | 0.909 |
| T stage (AJCC 8th), n (%) | T1 | 20 (18.9) | 22 (14.0) | 0.694 |
| T2 | 55 (51.9) | 91 (58.0) | ||
| T3 | 30 (28.3) | 43 (27.4) | ||
| T4 | 1 (0.9) | 1 (0.6) | ||
| N stage (AJCC 8th), n (%) | N0 | 46 (43.4) | 61 (39.1) | 0.725 |
| N1 | 47 (44.3) | 78 (50.0) | ||
| N2 | 13 (12.3) | 17 (10.9) | ||
| Staging (AJCC 8th) +, n (%) | IA | 14 (13.2) | 12 (7.6) | 0.314 |
| IB | 19 (17.9) | 35 (22.3) | ||
| IIA | 11 (10.4) | 11 (7.0) | ||
| IIB | 47 (44.3) | 72 (45.9) | ||
| III | 13 (12.3) | 18 (11.5) | ||
| IV | 2 (1.9) | 8 (5.1) | ||
| NA | 0 (0.0) | 1 (0.6) | ||
| Differentiation | WD | 11 (10.4) | 13 (8.3) | 0.558 |
| MD | 75 (70.8) | 123 (78.3) | ||
| PD | 15 (14.2) | 16 (10.2) | ||
| Others | 3 (2.8) | 2 (1.3) | ||
| NA | 2 (1.9) | 3 (1.9) | ||
| Peripancreatic infiltration, n (%) | Yes | 100 (94.3) | 147 (93.6) | 0.813 |
| No | 6 (5.7) | 10 (6.4) | ||
| Lymphovascular invasion, n (%) | Yes | 54 (50.9) | 87 (55.4) | 0.476 |
| No | 52 (49.1) | 70 (44.6) | ||
| Perineural invasion, n (%) | Yes | 82 (77.4) | 127 (80.9) | 0.487 |
| No | 24 (22.6) | 30 (19.1) | ||
| Number of harvested lymph nodes, n (±SD) | Mean | 15.1 (±9.0) | 17.0 (±9.9) | 0.118 |
| Number of positive lymph nodes, n (±SD) | Mean | 1.4 (±1.8) | 1.5 (±2.0) | 0.675 |
| Positive lymph node ratio, % (±SD) | Mean | 11.0 (±13.9) | 9.6 (±11.5) | 0.414 |
| Resection margin ++, n (%) | R0 | 82 (77.4) | 127 (80.9) | 0.487 |
| R1 | 24 (22.6) | 30 (19.1) | ||
| Location of margin involved, n (%) | Pancreas margin | 1 (4.2) | 1 (3.3) | 0.519 |
| Tangential margin | 22 (91.7) | 29 (96.7) | ||
| Both | 1 (4.2) | 0 (0.0) | ||
| Pathologic adrenal gland invasion, n (%) | Yes | 1 (0.6) | NA | |
| No | 156 (99.4) |
| Variables | aRAMPS (n = 106) | pRAMPS (n = 157) | p-Value | |
|---|---|---|---|---|
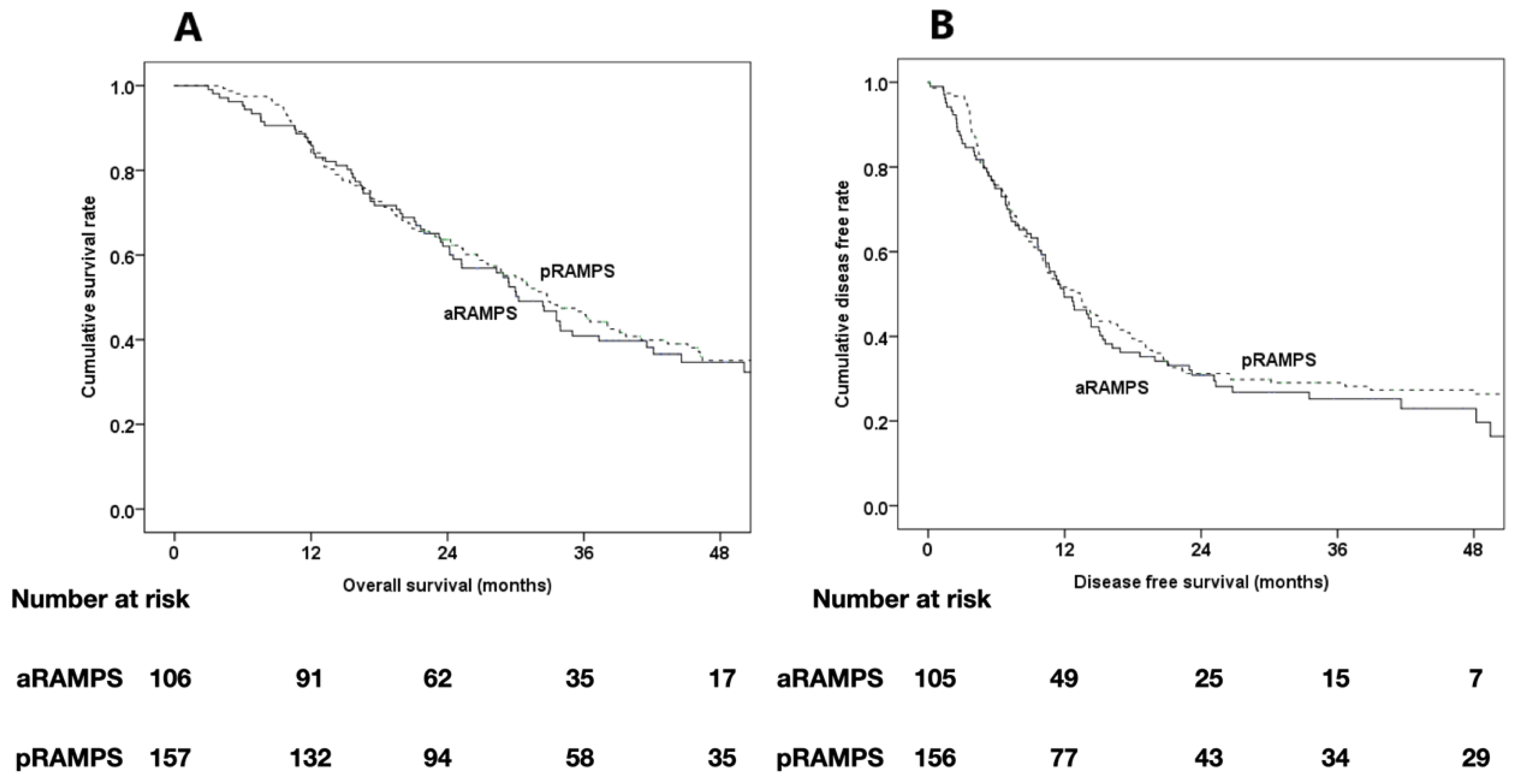
| Time to recurrence, months | Median | 12 | 13.3 | 0.534 |
| Site of recurrence | Local | 10 (13.0) | 24 (20.9) | 0.463 |
| Systemic | 35 (45.5) | 50 (43.5) | ||
| Local + systemic | 10 (13.0) | 16 (13.9) | ||
| Peritoneal carcinomatosis | 22 (28.6) | 25 (21.7) | ||
| Median overall survival, months | Median | 30.3 | 32.8 | 0.318 |
| 4-year survival rate, (%) | 34.7 | 35.1 | 0.947 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Park, Y.; Jun, E.; Lee, W.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Kim, S.C. Clinical Outcome of RAMPS for Left-Sided Pancreatic Ductal Adenocarcinoma: A Comparison of Anterior RAMPS versus Posterior RAMPS for Patients without Periadrenal Infiltration. Biomedicines 2021, 9, 1291. https://doi.org/10.3390/biomedicines9101291
Kwon J, Park Y, Jun E, Lee W, Song KB, Lee JH, Hwang DW, Kim SC. Clinical Outcome of RAMPS for Left-Sided Pancreatic Ductal Adenocarcinoma: A Comparison of Anterior RAMPS versus Posterior RAMPS for Patients without Periadrenal Infiltration. Biomedicines. 2021; 9(10):1291. https://doi.org/10.3390/biomedicines9101291
Chicago/Turabian StyleKwon, Jaewoo, Yejong Park, Eunsung Jun, Woohyung Lee, Ki Byung Song, Jae Hoon Lee, Dae Wook Hwang, and Song Cheol Kim. 2021. "Clinical Outcome of RAMPS for Left-Sided Pancreatic Ductal Adenocarcinoma: A Comparison of Anterior RAMPS versus Posterior RAMPS for Patients without Periadrenal Infiltration" Biomedicines 9, no. 10: 1291. https://doi.org/10.3390/biomedicines9101291
APA StyleKwon, J., Park, Y., Jun, E., Lee, W., Song, K. B., Lee, J. H., Hwang, D. W., & Kim, S. C. (2021). Clinical Outcome of RAMPS for Left-Sided Pancreatic Ductal Adenocarcinoma: A Comparison of Anterior RAMPS versus Posterior RAMPS for Patients without Periadrenal Infiltration. Biomedicines, 9(10), 1291. https://doi.org/10.3390/biomedicines9101291








