Long-Term Pharmacokinetics of Dalbavancin in ABSSSI and Osteoarticular Settings: A Real-Life Outpatient Context
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Enrolment and Treatment
2.2. PK Evaluation
2.3. Pharmacodynamic Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
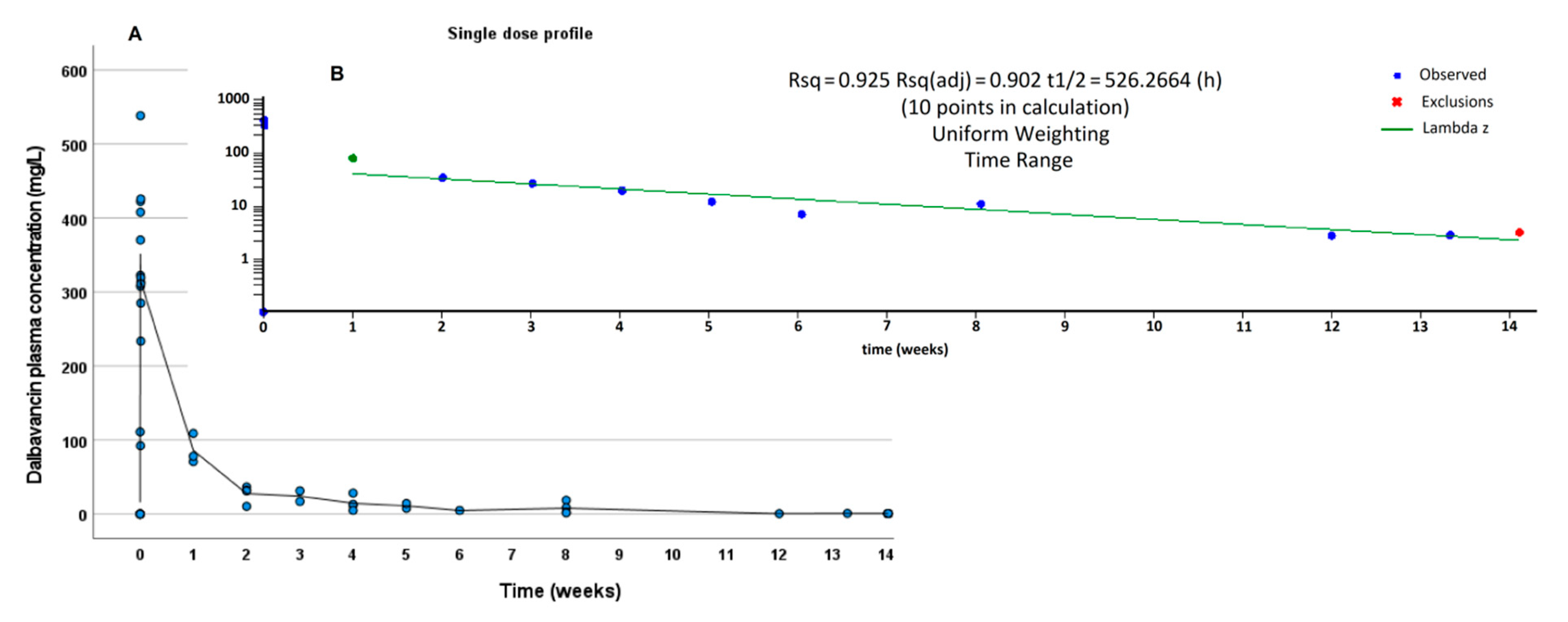
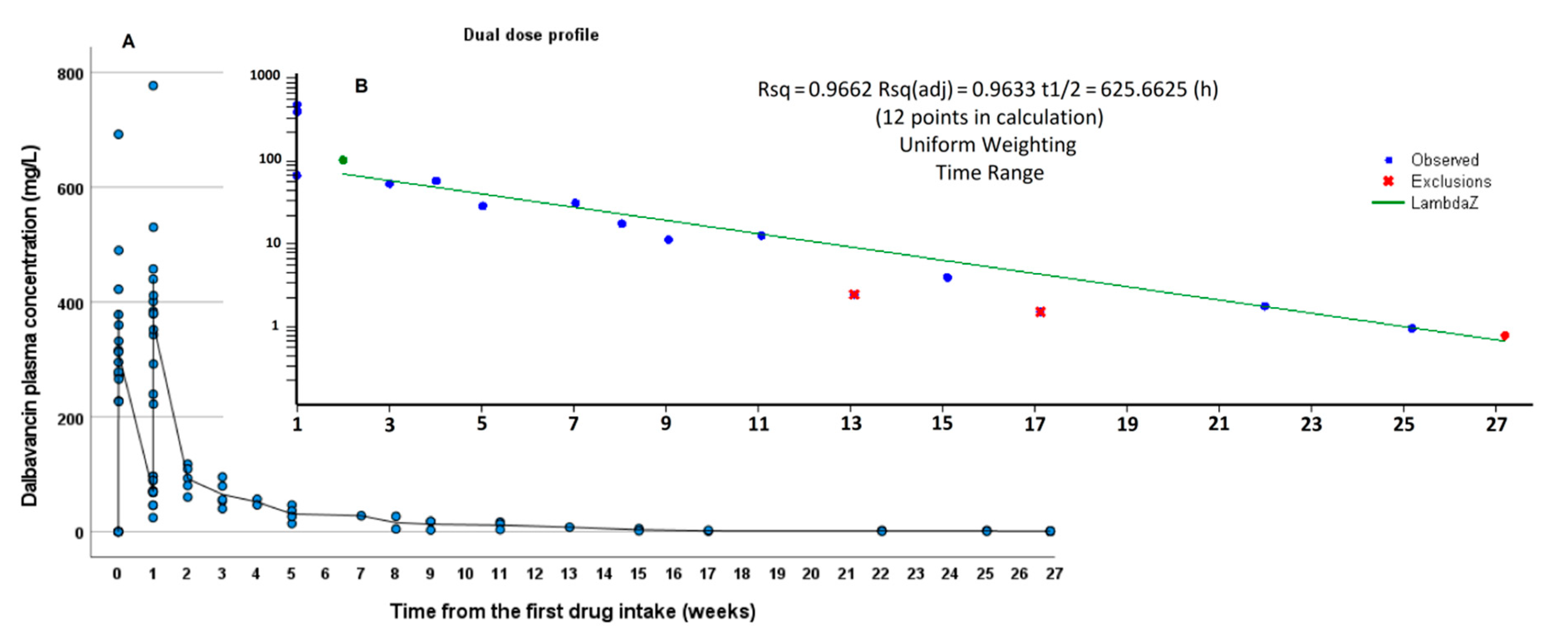
3.2. PK Results
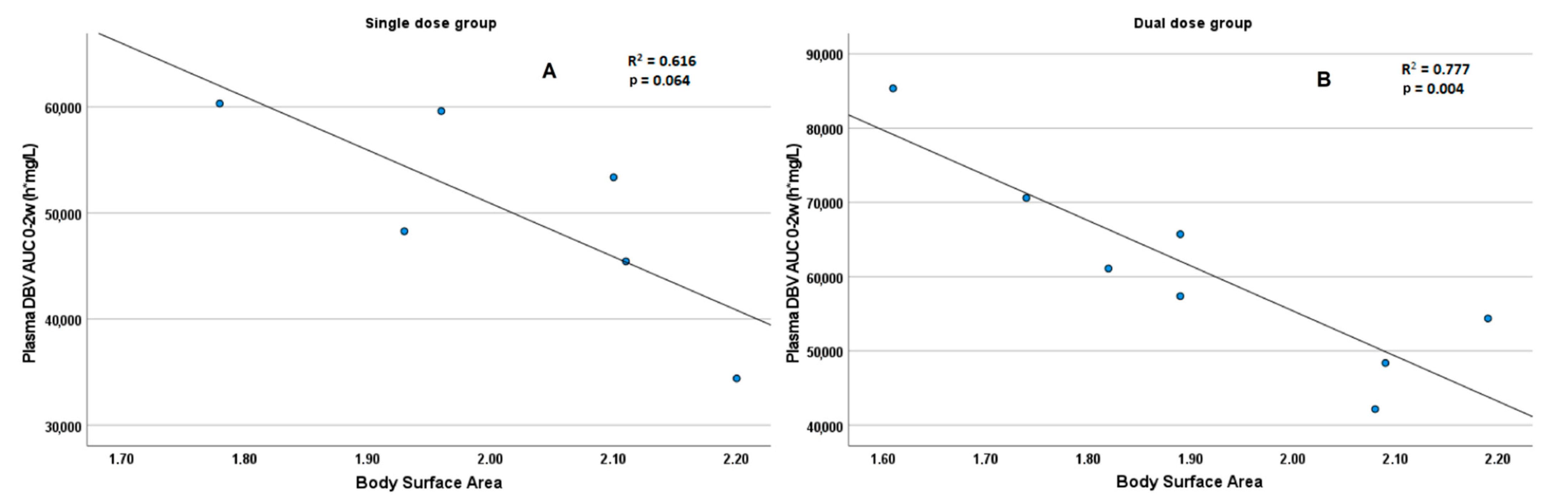
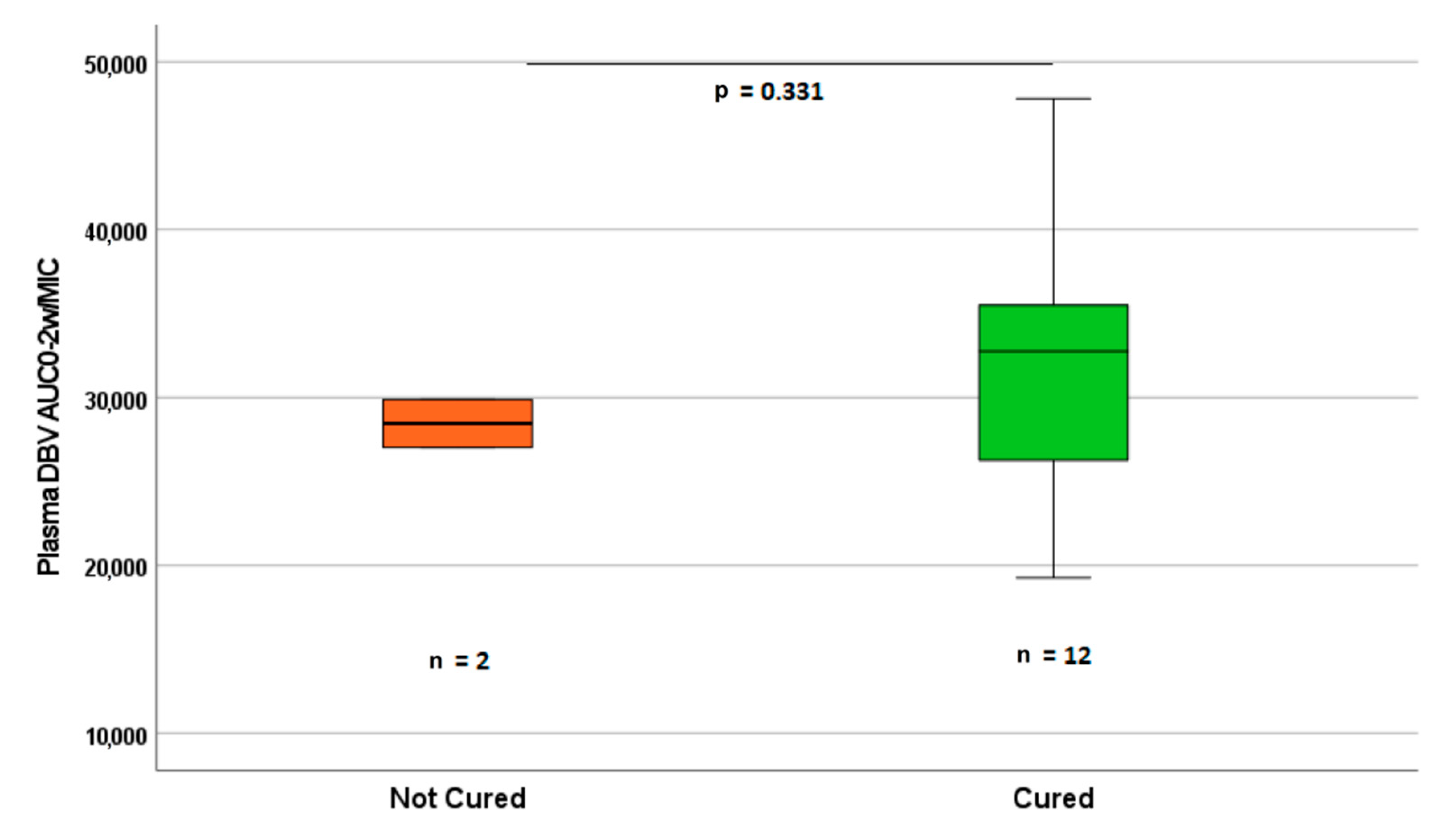
3.3. PK-PD Evaluation
3.4. Clinical Outcome and Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABSSSI | acute bacterial skin and skin-structures infections |
| NCA | non-compartmental analysis |
| MIC | minimum inhibitory concentration |
| AUC | area under the concentration-time curve |
| PK | pharmacokinetics |
| PD | pharmacodynamics |
| BSA | body surface area |
| LLOQ | lower limit of quantification |
| IS | internal standard |
| IQR | interquartile range |
| eGFR | estimated glomerular filtration rate |
| CKD | chronic kidney disease |
| LC-MS | liquid chromatography with mass spectrometry |
| MSSA | methicillin-sensitive Staphylococcus aureus |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MRSE | methicillin-resistant Staphylococcus epidermidis |
| T > MIC | time range of drug concentrations over the minimum inhibitory concentration |
| λz | elimination constant |
References
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0, 2021. Available online: https://eucast.org/clinical_breakpoints/: (accessed on 26 August 2021).
- Klinker, K.P.; Borgert, S.J. Beyond Vancomycin: The Tail of the Lipoglycopeptides. Clin. Ther. 2015, 37, 2619–2636. [Google Scholar] [CrossRef]
- Lin, G.; Credito, K.; Ednie, L.M.; Appelbaum, P.C. Antistaphylococcal activity of dalbavancin, an experimental glycopeptide. Antimicrob. Agents Chemother. 2005, 49, 770–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, G.; Smith, K.; Ednie, L.M.; Appelbaum, P.C. Antipneumococcal activity of dalbavancin compared to other agents. Antimicrob. Agents Chemother. 2005, 49, 5182–5184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCurdy, S.P.; Jones, R.N.; Mendes, R.E.; Puttagunta, S.; Dunne, M.W. In Vitro Activity of Dalbavancin against Drug-Resistant Staphylococcus aureus Isolates from a Global Surveillance Program. Antimicrob. Agents Chemother. 2015, 59, 5007–5009. [Google Scholar] [CrossRef][Green Version]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Review of the pharmacokinetics of dalbavancin, a recently approved lipoglycopeptide antibiotic. Infect. Dis. 2017, 49, 483–492. [Google Scholar] [CrossRef]
- Dowell, J.A.; Goldstein, B.P.; Buckwalter, M.; Stogniew, M.; Damle, B. Pharmacokinetic-pharmacodynamic modeling of dalbavancin, a novel glycopeptide antibiotic. J. Clin. Pharmacol. 2008, 48, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.W.; Puttagunta, S.; Sprenger, C.R.; Rubino, C.; Van Wart, S.; Baldassarre, J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob. Agents Chemother. 2015, 59, 1849–1855. [Google Scholar] [CrossRef]
- Cavaleri, M.; Riva, S.; Valagussa, A.; Guanci, M.; Colombo, L.; Dowell, J.; Stogniew, M. Pharmacokinetics and excretion of dalbavancin in the rat. J. Antimicrob. Chemother. 2005, 55 (Suppl. 2), ii31–ii35. [Google Scholar] [CrossRef] [PubMed]
- Leighton, A.; Gottlieb, A.B.; Dorr, M.B.; Jabes, D.; Mosconi, G.; VanSaders, C.; Mroszczak, E.J.; Campbell, K.C.; Kelly, E. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob. Agents Chemother. 2004, 48, 940–945. [Google Scholar] [CrossRef]
- Boucher, H.W.; Wilcox, M.; Talbot, G.H.; Puttagunta, S.; Das, A.F.; Dunne, M.W. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N. Engl. J. Med. 2014, 370, 2169–2179. [Google Scholar] [CrossRef]
- Dunne, M.W.; Puttagunta, S.; Giordano, P.; Krievins, D.; Zelasky, M.; Baldassarre, J. A Randomized Clinical Trial of Single-Dose Versus Weekly Dalbavancin for Treatment of Acute Bacterial Skin and Skin Structure Infection. Clin. Infect. Dis. 2015, 62, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, S.; Krause, R.; Valentin, T.; Prattes, J.; Janata, O.; Lenger, A.; Bellmann-Weiler, R.; Weiss, G.; Zollner-Schwetz, I. Multicenter clinical experience of real life Dalbavancin use in gram-positive infections. Int. J. Infect. Dis. 2019, 81, 210–214. [Google Scholar] [CrossRef]
- Almangour, T.A.; Fletcher, V.; Alessa, M.; Alhifany, A.A.; Tabb, D. Multiple Weekly Dalbavancin Dosing for the Treatment of Native Vertebral Osteomyelitis Caused by Methicillin-Resistant Staphylococcus Aureus: A Case Report. Am. J. Case Rep. 2017, 18, 1315–1319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinh, A.; Duran, C.; Pavese, P.; Khatchatourian, L.; Monnin, B.; Bleibtreu, A.; Denis, E.; Etienne, C.; Rouanes, N.; Mahieu, R.; et al. French national cohort of first use of dalbavancin: A high proportion of off-label use. Int. J. Antimicrob. Agents 2019, 54, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Henao-Martinez, A.F.; Franco-Paredes, C.; Chastain, D.B. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: A systematic review. Int. J. Antimicrob. Agents 2020, 56, 106069. [Google Scholar] [CrossRef]
- Almangour, T.A.; Perry, G.K.; Terriff, C.M.; Alhifany, A.A.; Kaye, K.S. Dalbavancin for the management of gram-positive osteomyelitis: Effectiveness and potential utility. Diagn Microbiol. Infect. Dis. 2018, 93, 213–218. [Google Scholar] [CrossRef]
- Barnea, Y.; Lerner, A.; Aizic, A.; Navon-Venezia, S.; Rachi, E.; Dunne, M.W.; Puttagunta, S.; Carmeli, Y. Efficacy of dalbavancin in the treatment of MRSA rat sternal osteomyelitis with mediastinitis. J. Antimicrob. Chemother. 2015, 71, 460–463. [Google Scholar] [CrossRef]
- Di Pilato, V.; Ceccherini, F.; Sennati, S.; D’Agostino, F.; Arena, F.; D’Atanasio, N.; Di Giorgio, F.P.; Tongiani, S.; Pallecchi, L.; Rossolini, G.M. In vitro time-kill kinetics of dalbavancin against Staphylococcus spp. biofilms over prolonged exposure times. Diagn Microbiol. Infect. Dis. 2019, 96, 114901. [Google Scholar] [CrossRef]
- Solon, E.G.; Dowell, J.A.; Lee, J.; King, S.P.; Damle, B.D. Distribution of radioactivity in bone and related structures following administration of [14C]dalbavancin to New Zealand white rabbits. Antimicrob. Agents Chemother. 2007, 51, 3008–3010. [Google Scholar] [CrossRef]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ziemyte, M.; Rodriguez-Diaz, J.C.; Ventero, M.P.; Mira, A.; Ferrer, M.D. Effect of Dalbavancin on Staphylococcal Biofilms When Administered Alone or in Combination With Biofilm-Detaching Compounds. Front. Microbiol. 2020, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Rappo, U.; Puttagunta, S.; Shevchenko, V.; Shevchenko, A.; Jandourek, A.; Gonzalez, P.L.; Suen, A.; Mas Casullo, V.; Melnick, D.; Miceli, R.; et al. Dalbavancin for the Treatment of Osteomyelitis in Adult Patients: A Randomized Clinical Trial of Efficacy and Safety. Open Forum. Infect. Dis. 2019, 6, ofy331. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Craig, W.A. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob. Agents Chemother. 2007, 51, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Rinaldi, M.; Zamparini, E.; Rossi, N.; Tedeschi, S.; Conti, M.; Pea, F.; Viale, P. Population pharmacokinetics of dalbavancin and dosing consideration for optimal treatment of adult patients with staphylococcal osteoarticular infections. Antimicrob. Agents Chemother. 2021, 65, e02260-20. [Google Scholar] [CrossRef] [PubMed]
- Dorr, M.B.; Jabes, D.; Cavaleri, M.; Dowell, J.; Mosconi, G.; Malabarba, A.; White, R.J.; Henkel, T.J. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J. Antimicrob. Chemother. 2005, 55 (Suppl. 2), ii25–ii30. [Google Scholar] [CrossRef]
- Jones, R.N.; Farrell, D.J.; Flamm, R.K.; Sader, H.S.; Dunne, M.W.; Mendes, R.E. Surrogate analysis of vancomycin to predict susceptible categorization of dalbavancin. Diagn Microbiol. Infect. Dis. 2015, 82, 73–77. [Google Scholar] [CrossRef]
- EMA. Guideline on Bioanalytical Method Validation. 2011. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (accessed on 28 August 2021).
- FDA. Bioanalytical Method Validation. Guidance for Industry. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf (accessed on 3 August 2018).
- De Nicolò, A.; Cantu, M.; D’Avolio, A. Matrix effect management in liquid chromatography mass spectrometry: The internal standard normalized matrix effect. Bioanalysis 2017, 9, 1093–1105. [Google Scholar] [CrossRef]
- Alebic-Kolbah, T.; Demers, R.; Cojocaru, L. Dalbavancin: Quantification in human plasma and urine by a new improved high performance liquid chromatography-tandem mass spectrometry method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2632–2641. [Google Scholar] [CrossRef]
| N | N. of DBV Doses | Gender | Obesity | eGFR | Diabetes | Indication | Etiology | MIC for Vancom. | Previous Surgery | Previous Antibiotic Therapy | AE | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | F | yes | 86.5 | yes | Chronic osteomyelitis | MSSA | ≤1 | No | Piperacillin-tazobactam + teicoplanin | none | Not Cured |
| 2 | 1 | M | no | 107.5 | no | Septic arthritis | MRSE | ≤1 | Yes | Teicoplanin | none | Cured |
| 3 | 2 | M | no | 53.4 | yes | Spondylodiscitis | MSSA | ≤0.5 | No | Cefazolin, ceftriaxone, daptomycin | none | Cured |
| 4 | 2 | M | no | 139.9 | yes | Spondylodiscitis | MRSE | 2 | No | Teicoplanin | none | Cured |
| 5 | 2 | M | no | 113.8 | no | Chronic osteomyelitis | MRSA | 1 | Yes | Teicoplanin | none | Cured |
| 6 | 1 | M | yes | 75.0 | yes | ABSSSI | - | - | No | Levofloxacin | none | Cured |
| 7 | 1 | F | no | 62.2 | no | ABSSSI | MRSE | ≤2 | No | Amoxicillin/clav. | none | Cured |
| 8 | 1 | M | no | 121.1 | no | Septic arthritis | MRSA | 2 | Yes | Daptomycin | none | Not Cured |
| 9 | 1 | M | no | 69.9 | no | ABSSSI | - | - | No | Amoxicillin | none | Cured |
| 10 | 2 | M | no | 68.6 | no | Prosthetic infection | MRSA | 1 | Yes | Teicoplanin + rifampin | none | Cured |
| 11 | 2 | M | no | 131.0 | yes | Chronic osteomyelitis | MRSA | ≤1 | No | Amoxicillin/clav., Vancomycin, daptomycin, cefazolin | none | Cured |
| 12 | 2 | M | no | 82.5 | no | Septic arthritis and spondylodiscitis | MRSA | 1 | Yes | Daptomycin | none | Cured |
| 13 | 2 | M | no | 142.0 | no | Chronic osteomyelitis | MSSA | ≤0.5 | No | Ceftriaxone, daptomycin + ceftarolin | none | Cured |
| 14 | 2 | F | no | 91.0 | no | Septic arthritis | Streptococcus dysgalactiae | ≤0.5 | No | Ceftriaxone | none | Cured |
| PK Parameters | Single Dose | Dual Dose (First Dose) | Dual Dose (Second Dose) |
|---|---|---|---|
| C max (mg/L) | 390.1 | 359.0 | 431.2 |
| Observed AUC0-1w (h × mg/L) | 27,230 | 25,110 | 32,902 |
| Observed AUC0-2w (h × mg/L) | 35,647 | n.a. | 45,658 |
| Observed AUC0-∞ (h × mg/L) | 54,666 | n.a. | 91,086 |
| Dual dose AUC0-2wtotal (h × mg/L) | n.a. | n.a. | 58,012 |
| Dual-dose AUC0-∞total (h × mg/L) | n.a. | n.a. | 116,196 |
| Terminalt1/2 (h) | 526 | n.a. | 626 |
| Pt # | N of Doses | Previous Dose | Last Dose Parameters | Overall Exposure | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obs.AUC0-1w (h × mg/L) | LastCmax (mg/L) | Obs. AUC0-1w (h × mg/L) | Obs. AUC0-2w (h × mg/L) | Obs. AUC0-∞ (h × mg/L) | Tlast (w) | % ext.AUC | Term.t1/2 (h) | TotalAUC0-2w (h × mg/L) | TotalAUC0-∞ (h × mg/L) | ||
| 1 | 1 | n.a. | 315.5 | 27,134 | 37,539 | 84,972 | 14 | 7.9 | 860 | n.a. | n.a. |
| 2 | 1 | n.a. | 307.9 | 23,059 | 31,353 | 41,917 | 8 | 25.0 | 225 | n.a. | n.a. |
| 3 | 2 | 22,786 | 384.1 | 32,426 | 48,940 | 107,603 | 27 | 1.2 | 671 | 55,212 | 130,389 |
| 4 | 2 | 24,984 | 379.5 | 40,791 | 60,347 | 115,735 | 27 | 0.8 | 703 | 65,775 | 140,719 |
| 5 | 2 | 28,150 | 401.3 | 32,937 | 41,579 | - | 2 | - | - | 61,087 | - |
| 6 | 1 | n.a. | 422.4 | 28,701 | 39,157 | 56,314 | 13 | 0.5 | 614 | n.a. | n.a. |
| 7 | 1 | n.a. | 538.2 | 39,338 | 53,086 | 78,307 | 14 | 1.0 | 441 | n.a. | n.a. |
| 8 | 1 | n.a. | 322.5 | 29,196 | 38,910 | - | 2 | - | - | n.a. | n.a. |
| 9 | 1 | n.a. | 407.8 | 32,585 | 43,276 | - | 4 | - | - | n.a. | n.a. |
| 10 | 2 | 24,543 | 239.5 | 24,554 | 37,039 | 74,069 | 25 | 0.4 | 640 | 49,097 | 98612 |
| 11 | 2 | 16,705 | 370.2 | 25,457 | 30,090 | 35,552 | 17 | 1.2 | 310 | 42,162 | 52,257 |
| 12 | 2 | 25,955 | 457.8 | 28,414 | 47,210 | 68,816 | 27 | 1.0 | 647 | 53,871 | 94,273 |
| 13 | 2 | 28,413 | 440.4 | 32,685 | 42,411 | 56,544 | 9 | 14.2 | 628 | 61,098 | 84,957 |
| 14 | 2 | 40,035 | 776.9 | 45,322 | 58,713 | 100,922 | 25 | 0.7 | 463 | 85,357 | 140,957 |
| PT Code | Protein-Binding Corrected PK/PD Parameters | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Last DoseCmax/MIC | AUC/MIC | T > MIC (w) | |||||||
| MIC = 0.125 mg/L | MIC = 0.250 mg/L | AUC0-1w/MIC (First Dose) | AUC0-2w/MIC (Total) | MIC = 0.125 mg/L | MIC = 0.250 mg/L | ||||
| MIC = 0.125 mg/L | MIC = 0.250 mg/L | MIC = 0.125 mg/L | MIC = 0.250 mg/L | ||||||
| 1 | 177 | 88 | 15,195 | 7598 | 21,022 | 10,511 | 24.6 | 19.5 | Not cured |
| 2 | 172 | 86 | 12,913 | 6457 | 17,558 | 8779 | 7.9 | 6.6 | Cured |
| 3 | 215 | 108 | 12,760 | 6380 | 30,919 | 15,459 | 20.5 | 16.6 | Cured |
| 4 | 213 | 106 | 13,991 | 6996 | 36,834 | 18,417 | 20.6 | 16.4 | Cured |
| 5 | 225 | 112 | 15,764 | 7882 | 34,209 | 17,104 | - | - | Cured |
| 6 | 237 | 118 | 16,073 | 8036 | 21,928 | 10,964 | 9.8 | 6.1 | Cured |
| 7 | 301 | 151 | 22,029 | 11,015 | 29,728 | 14,864 | 14.0 | 11.4 | Cured |
| 8 | 181 | 90 | 16,350 | 8175 | 21,790 | 10,895 | - | - | Not cured |
| 9 | 228 | 114 | 18,248 | 9124 | 24,235 | 12,117 | - | - | Cured |
| 10 | 134 | 67 | 13,744 | 6872 | 27,494 | 13,747 | 12.2 | 9.0 | Cured |
| 11 | 207 | 104 | 14,256 | 7128 | 23,611 | 11,805 | 7.7 | 6.0 | Cured |
| 12 | 256 | 178 | 15,912 | 7956 | 30,168 | 15,084 | 13.7 | 9.7 | Cured |
| 13 | 247 | 124 | 18,304 | 9152 | 34,215 | 17,107 | 13.6 | 7.9 | Cured |
| 14 | 435 | 218 | 25,380 | 12,690 | 47,800 | 23,900 | 18.7 | 15.8 | Cured |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nicolò, A.; Stroffolini, G.; Antonucci, M.; Mula, J.; De Vivo, E.D.; Cusato, J.; Palermiti, A.; Cariti, G.; Di Perri, G.; Corcione, S.; et al. Long-Term Pharmacokinetics of Dalbavancin in ABSSSI and Osteoarticular Settings: A Real-Life Outpatient Context. Biomedicines 2021, 9, 1288. https://doi.org/10.3390/biomedicines9101288
De Nicolò A, Stroffolini G, Antonucci M, Mula J, De Vivo ED, Cusato J, Palermiti A, Cariti G, Di Perri G, Corcione S, et al. Long-Term Pharmacokinetics of Dalbavancin in ABSSSI and Osteoarticular Settings: A Real-Life Outpatient Context. Biomedicines. 2021; 9(10):1288. https://doi.org/10.3390/biomedicines9101288
Chicago/Turabian StyleDe Nicolò, Amedeo, Giacomo Stroffolini, Miriam Antonucci, Jacopo Mula, Elisa Delia De Vivo, Jessica Cusato, Alice Palermiti, Giuseppe Cariti, Giovanni Di Perri, Silvia Corcione, and et al. 2021. "Long-Term Pharmacokinetics of Dalbavancin in ABSSSI and Osteoarticular Settings: A Real-Life Outpatient Context" Biomedicines 9, no. 10: 1288. https://doi.org/10.3390/biomedicines9101288
APA StyleDe Nicolò, A., Stroffolini, G., Antonucci, M., Mula, J., De Vivo, E. D., Cusato, J., Palermiti, A., Cariti, G., Di Perri, G., Corcione, S., De Rosa, F. G., & D’Avolio, A. (2021). Long-Term Pharmacokinetics of Dalbavancin in ABSSSI and Osteoarticular Settings: A Real-Life Outpatient Context. Biomedicines, 9(10), 1288. https://doi.org/10.3390/biomedicines9101288








