Anti-Adipogenic Polyacetylene Glycosides from the Florets of Safflower (Carthamus tinctorius)
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
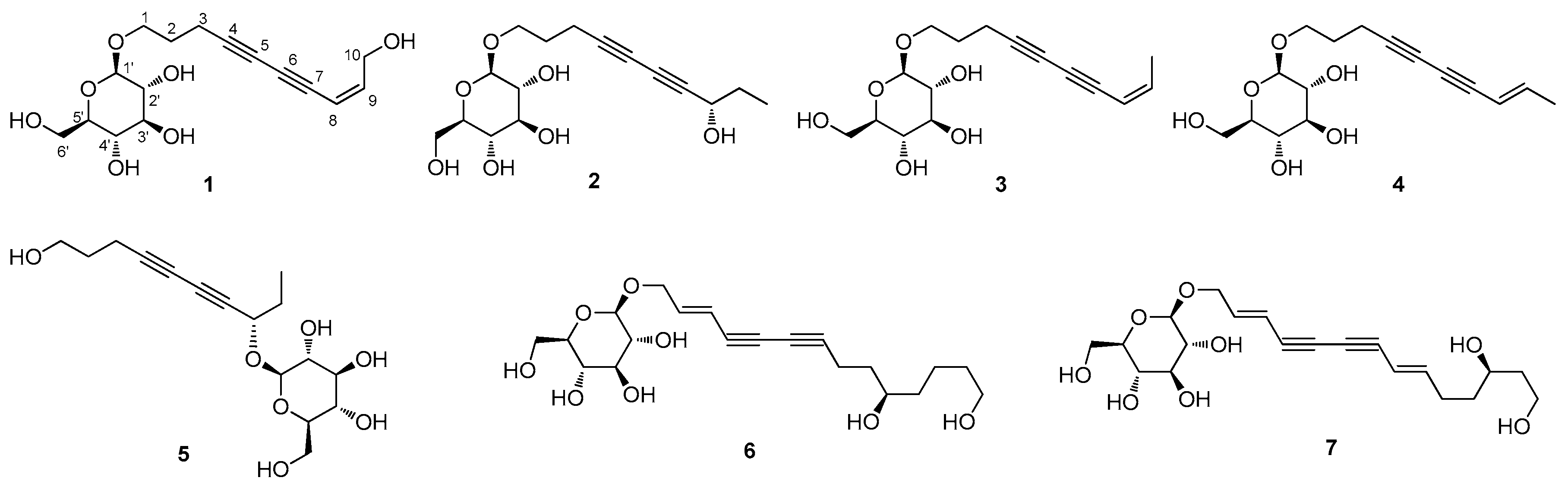
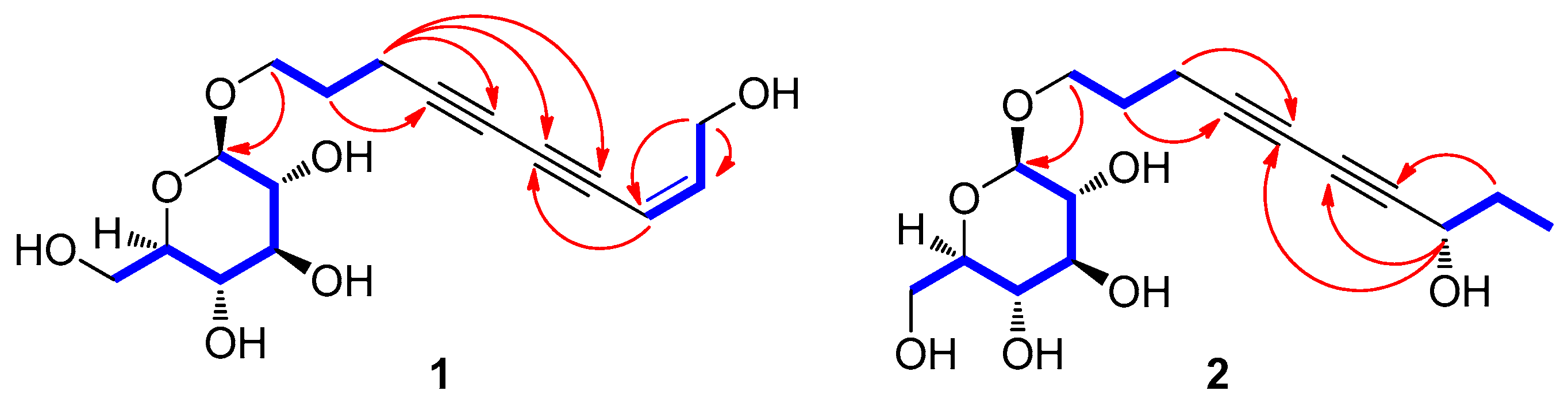
2.3.1. (8Z)-Decaene-4,6-diyne-1,10-diol-1-O-β-d-glucopyranoside (1)
2.3.2. (8S)-Deca-4,6-diyne-1,8-diol-1-O-β-d-glucopyranoside (2)
2.4. Enzymatic Hydrolysis and Absolute Configuration Determination of the Sugar Moiety of 1 and 2
2.5. Cell Culture and Differentiation
2.6. Oil Red O Staining
2.7. Cell Counting
2.8. Western Blotting
2.9. Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation of the Compounds
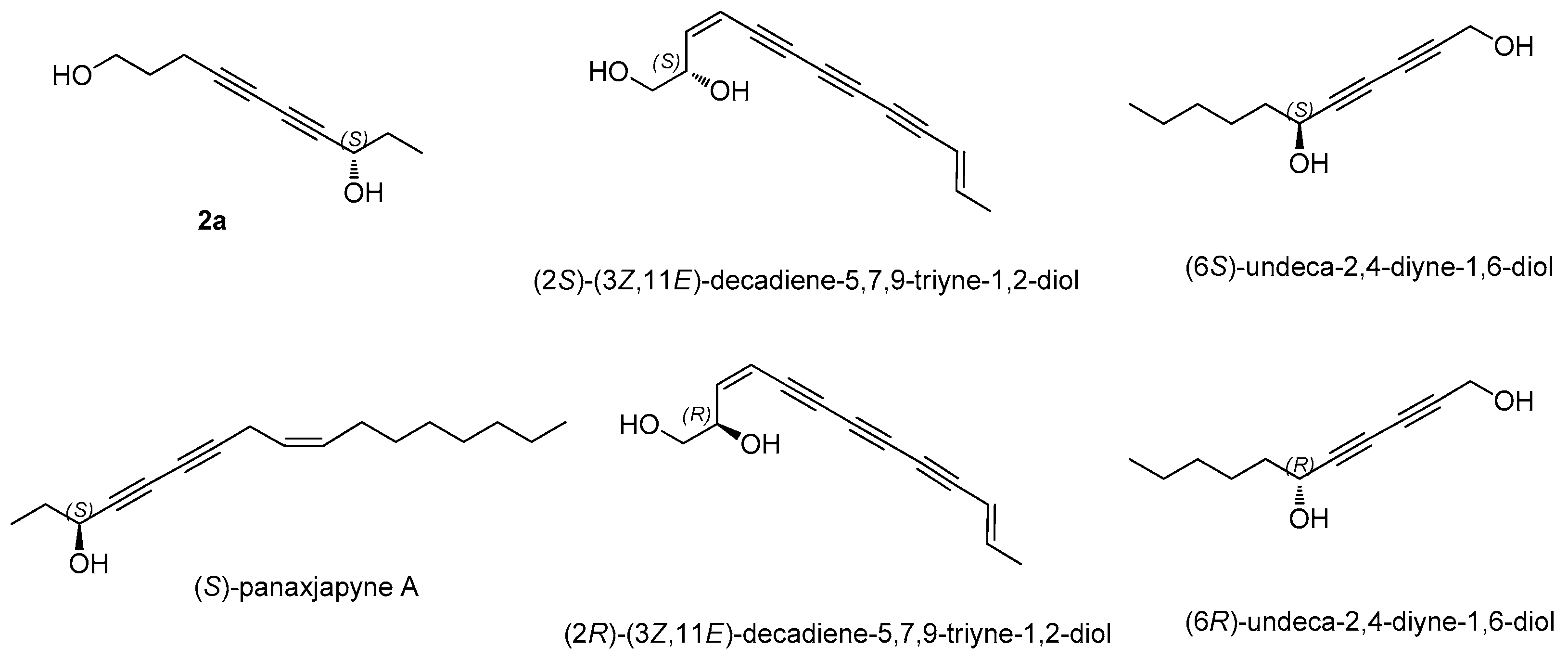
3.2. Structural Elucidation of Isolated Compounds
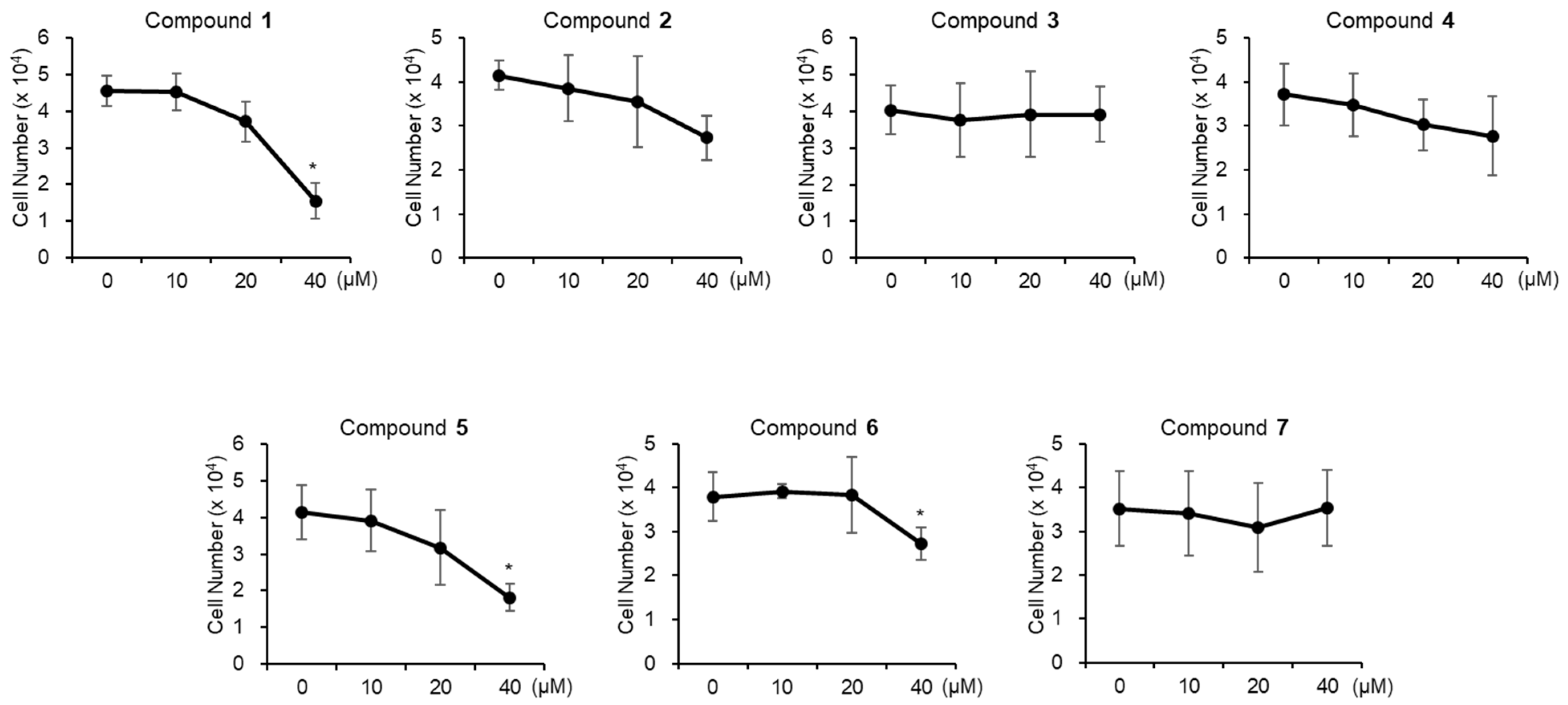
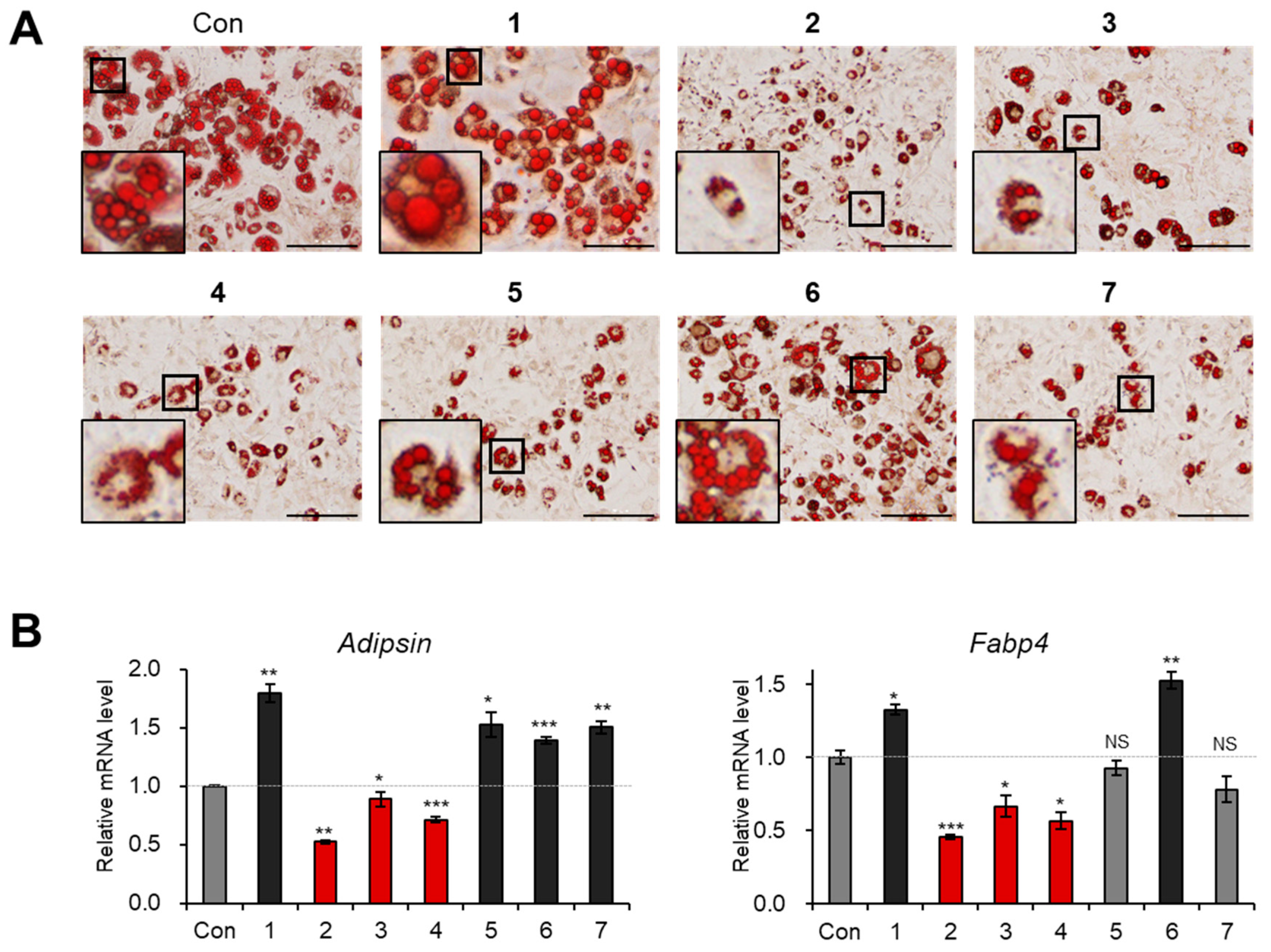
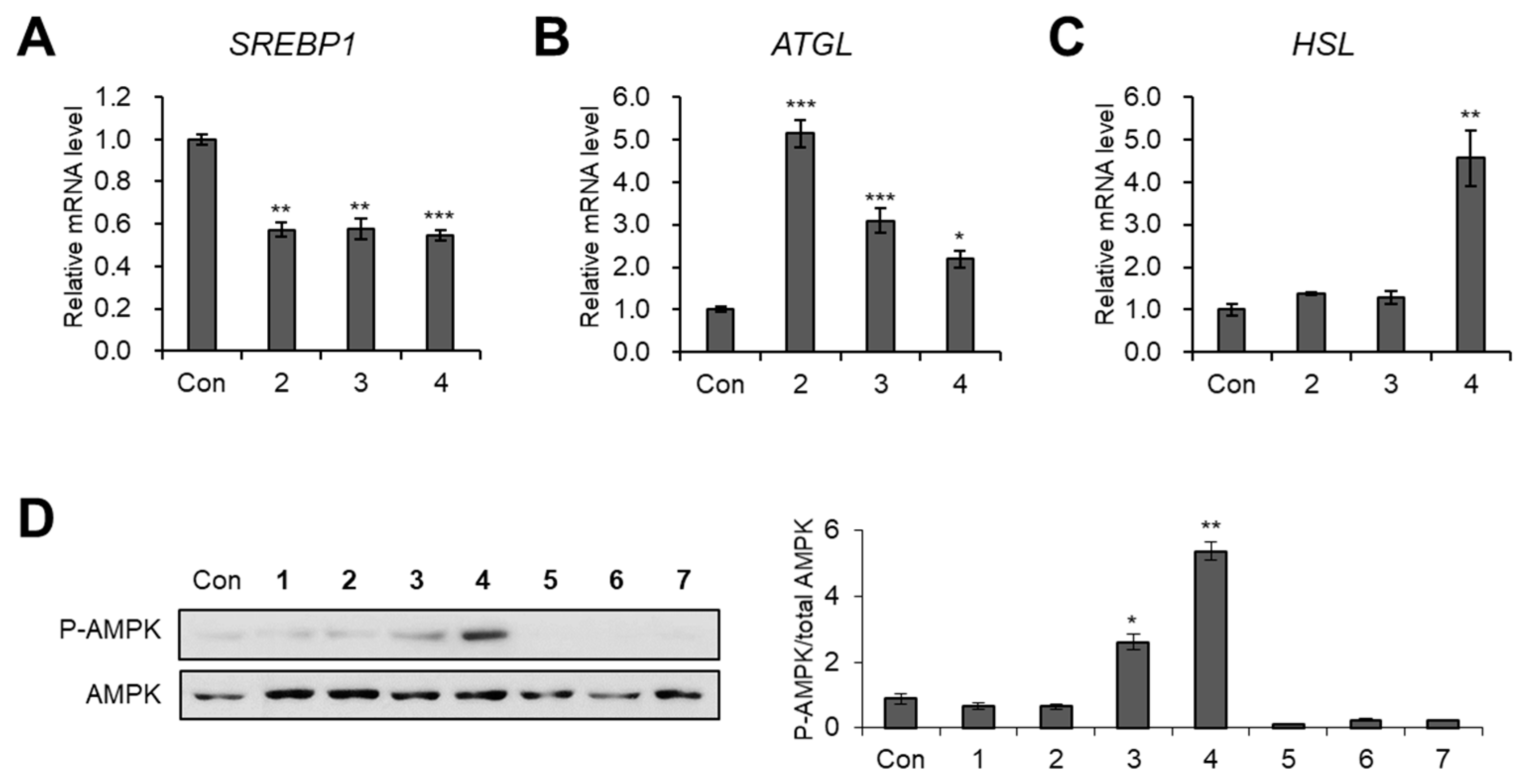
3.3. Evaluation of Biological Activity of the Isolated Compounds
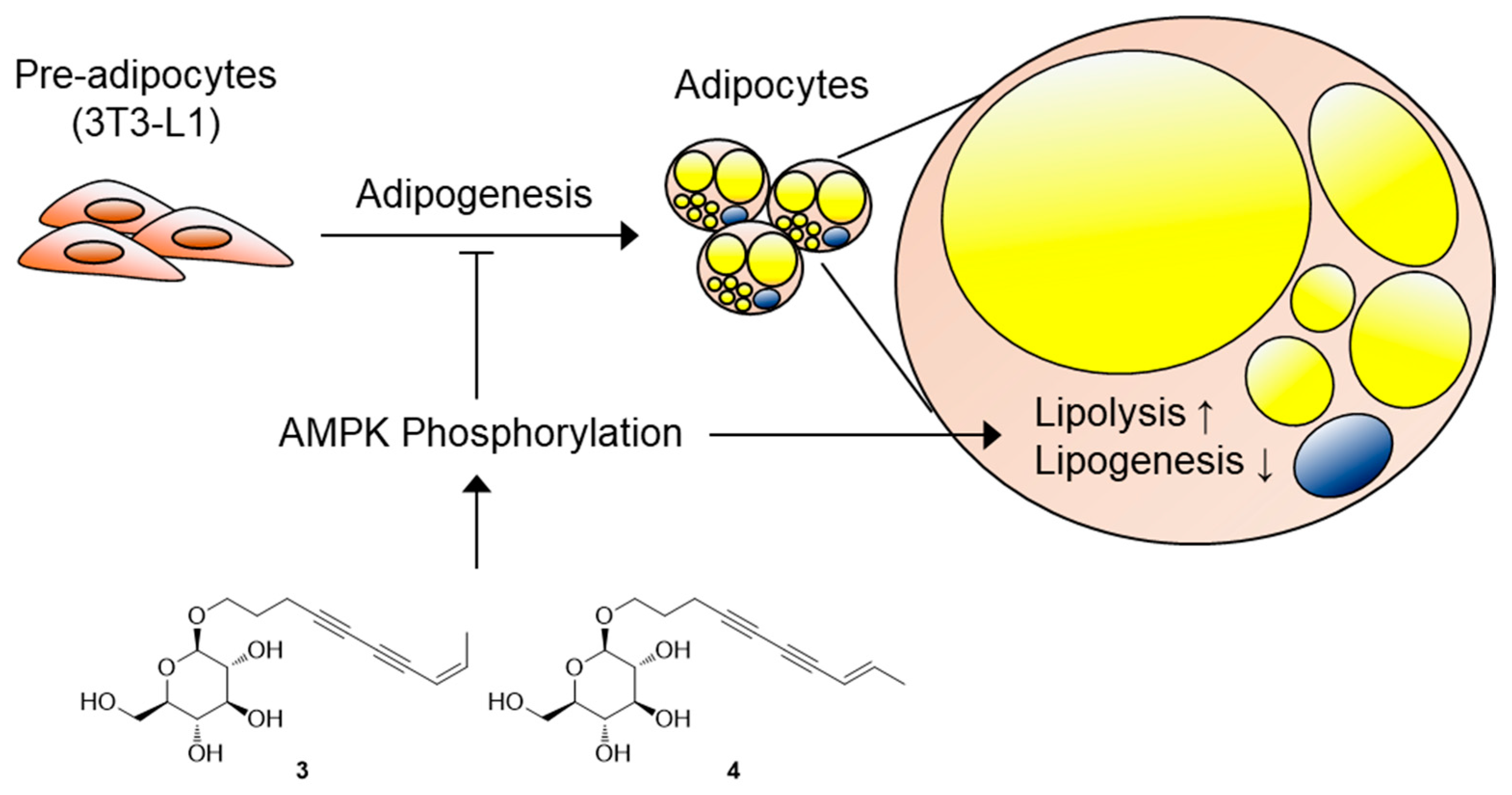
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spiegelman, B.M.; FIier, J.S. Obesity and the Regulation of Energy Balance. Cell 2001, 104, 531–5433. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose Tissue Regulates Insulin Sensitivity: Role of Adipogenesis, De Novo Lipogenesis and Novel Lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Yin, J.G.; Zhang, J.; Wang, X.X.; Wu, M.J.; Liu, F.; Zou, J.D.; Ju, W.Z. Pharmacokinetic Profiles of Hydroxysafflor Yellow a Following Intravenous Administration of Its Pure Preparations in Healthy Chinese Volunteers. J. Ethnopharmacol. 2015, 162, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Dreher, M.C.; Mathre, M.L. Women and Cannabis: Medicine, Science, and Sociology, 1st ed.; Haworth Press: New York, NY, USA, 2003; p. 6. [Google Scholar]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a Better Understanding of Medicinal Uses of Carthamus Tinctorius L. in Traditional Chinese Medicine: A Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shen, Y.; Jiang, J.; Yang, Y.; Feng, Z.; Zhang, P.; Yuan, S.; Hou, Q. New Polyacetylene Glucosides from the Florets of Carthamus Tinctorius and Their Weak Anti-Inflammatory Activities. Carbohydr. Res. 2011, 346, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Lee, Y.J.; Kim, J.D.; Kang, S.N.; Lee, S.K.; Jang, J.Y.; Lee, H.K.; Lim, J.H.; Lee, O.H. Phenolic Composition, Antioxidant Activity and Anti-Adipogenic Effect of Hot Water Extract from Safflower (Carthamus Tinctorius L.) Seed. Nutrients 2013, 5, 4894–4907. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Wang, L.J.; Wang, X.Q.; Pan, H.; Li, N.S.; Yang, H.B.; Jin, M.; Zang, B.X.; Gong, F.Y. Hormone-sensitive Lipase is Involved in the Action of Hydroxysafflor Yellow A (HYSA) Inhibiting Adipogenesis of 3T3-L1 cells. Fitoterapia 2014, 93, 182–188. [Google Scholar] [CrossRef]
- Hwnag, E.Y.; Yu, M.H.; Jung, Y.S.; Lee, S.P.; Shon, J.H.; Lee, S.O. Defatted Safflower Seed Extract Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Improves Lipid Profiles in C57BL/6J ob/ob Mice Fed a High-Fat Diet. Nutr. Res. 2016, 36, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wang, X.; Pan, H.; Wang, L.; Yang, H.; Liu, M.; Zhu, H.; Gong, F. Safflower Yellow and Its Main Component HSYA Alleviate Diet-Induced Obesity in Mice: Possible Involvement of the Increased Antioxidant Enzymes in Liver and Adipose Tissue. Front. Pharmacol. 2020, 11, 482. [Google Scholar] [CrossRef]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.-H.; Kim, S.-H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, E.J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Kung, K.; Kang, K.S.; Yoo, J.E. Estrogenic Activity of Sanguiin H-6 through Activation of Estrogen Receptor α Coactivator-binding Site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.-C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a sterol possessing a 6/5/6/5-fused ring system with a contracted tetrahydrofuran B-ring, from the fruiting bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choi, S.; Yamabe, N.; Kim, K.H.; Kang, K.S. Recent findings on the mechanism of cisplatin-induced renal cytotoxicity. Nat. Prod. Sci. 2020, 26, 28–49. [Google Scholar]
- Lee, S.R.; Kang, H.; Yoo, M.J.; Yu, J.S.; Lee, S.; Yi, S.A.; Beemelmanns, C.; Lee, J.; Kim, K.H. Anti-adipogenic pregnane steroid from a Hydractinia-associated fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Wang, Z.; Wen, Z.; Liu, L.; Zhu, X.; Shen, B.; Yan, X.; Duan, Y.; Huang, Y. Yangpumicins F and G, Enediyne Congeners from Micromonospora yangpuensis DSM 45577. J. Nat. Prod. 2019, 82, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Jiang, J.S.; Feng, Z.M.; Yang, Y.N.; Li, L.; Zang, C.X.; Zhang, P.C. Bioactive Sesquiterpenoid and Polyacetylene Glycosides from Atractylodes Lancea. J. Nat. Prod. 2016, 79, 1567–1575. [Google Scholar] [CrossRef]
- Jo, M.S.; Yu, J.S.; Lee, J.C.; Lee, S.; Cho, Y.C.; Park, H.J.; Kim, K.H. Lobatamunsolides A-C, Norlignans From the Roots of Pueraria lobata and Their Nitric Oxide Inhibitory Activities in Macrophages. Biomolecules 2019, 9, 755. [Google Scholar] [CrossRef]
- Lee, K.; Seo, Y.-J.; Song, J.-H.; Chei, S.; Lee, B.-Y. Ginsenoside Rg1 promotes browning by inducing UCP1 expression and mitochondrial activity in 3T3-L1 and subcutaneous white adipocytes. J. Ginseng Res. 2019, 43, 589–599. [Google Scholar] [CrossRef]
- Yi, S.A.; Lee, J.; Park, S.K.; Kim, J.Y.; Park, J.W.; Lee, M.G.; Nam, K.H.; Park, J.H.; Oh, H.; Kim, S.; et al. Fermented ginseng extract, BST204, disturbs adipogenesis of mesenchymal stem cells through inhibition of S6 kinase 1 signaling. J. Ginseng Res. 2020, 44, 58–66. [Google Scholar] [CrossRef]
- Li, S.; Kuang, H.X.; Okada, Y.; Okuyama, T. New Acetylenic Glucosides from Bidens Bipinnata LINNE. Chem. Pharm. Bull. 2004, 52, 439–440. [Google Scholar] [CrossRef]
- Lie, Y.; Xue, J.; Han, H.; Yuan, T. Polyacetylenes from the florets of Carthamus tinctorius and their cytotoxicity. Phytochem. Lett. 2018, 23, 168–171. [Google Scholar] [CrossRef]
- Fang, Z.; Wills, M. Asymmetric Reduction of Diynones and the Total Synthesis of (S)-panaxjapyne A. Org. Lett. 2014, 16, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, D.; Liang, Y.; Xin, G.; Huang, B.Z.; Huang, W. Novel Polyacetylenes From Coreopsis Tinctoria Nutt. J. Asian Nat. Prod. Res. 2015, 17, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Yun, J.H.; Kang, K.; Nho, C.W.; Shin, D. Identification of Dialkyl Diacetylene Diols with Potent Cancer Chemopreventive Activity. Bioorg. Med. Chem. Lett. 2015, 25, 4020–4023. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yu, D.Q. Lignan and acetylenic glycosides from Aster auriculatus. Phytochemistry 1998, 48, 711–717. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferré, P.J. Functions of AMP-activated Protein Kinase in Adipose Tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Du, S.; Deng, Y.; Yuan, H.; Sun, Y. Safflower Yellow B Protects Brain Against Cerebral Ischemia Reperfusion Injury Through AMPK/NF-kB Pathway. Evid. Based Complement. Altern. Med. 2019, 2019, 7219740. [Google Scholar] [CrossRef]
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 68.9, CH2 | 3.98, dt (10.0, 6.0) 3.67 dt (10.0, 6.0) | 69.1, CH2 | 3.95, dt (10.0, 6.0) 3.64 dt (10.0, 6.0) |
| 2 | 29.5, CH2 | 1.87, m | 29.6, CH2 | 1.83, m |
| 3 | 16.5, CH2 | 2.52, t (7.0) | 16.3, CH2 | 2.44, t (7.0) |
| 4 | 85.9, C | 81.4, C | ||
| 5 | 65.5, C | 65.6, C | ||
| 6 | 80.3, C | 70.1, C | ||
| 7 | 71.4, C | 78.0, C | ||
| 8 | 109.3, CH | 5.61, d (11.0) | 64.0, CH | 4.27, d (6.5) |
| 9 | 146.6, CH | 6.20, dt (11.0, 6.5) | 31.7, CH2 | 1.67, m |
| 10 | 60.8, CH2 | 4.32, d (6.5) | 9.6, CH3 | 0.98, t (7.5) |
| 1′ | 104.2, CH | 4.28, d (8.0) | 104.5, CH | 4.25, d (8.0) |
| 2′ | 74.9, CH | 3.19, dd (9.0, 8.0) | 74.9, CH | 3.16, dd (9.0, 8.0) |
| 3′ | 77.7, CH | 3.28, m | 77.7, CH | 3.28, m |
| 4′ | 71.3, CH | 3.29, m | 71.3, CH | 3.29, m |
| 5′ | 77.8, CH | 3.36, m | 77.8, CH | 3.35, m |
| 6′ | 62.5, CH2 | 3.89, dd (12.0, 2.0) 3.69, dd (12.0, 5.5) | 62.6, CH2 | 3.86, dd (12.0, 2.0) 3.67, dd (12.0, 5.5) |
| Gene | Forward | Reverse |
|---|---|---|
| β-Actin | 5′-ACGGCCAGGTCATCACTATTG-3’ | 5′-TGGATGCCACAGGATTCCA-3′ |
| Adipsin | 5′-CATGCTCGGCCCTACATG-3’ | 5′-CACAGAGTCGTCATCCGTCAC-3′ |
| Fabp4 | 5′-AAGGTGAAGAGCATCATAACCCT-3’ | 5′-TCACGCCTTTCATAACACATTCC-3′ |
| SREBP1 | 5′-AACGTCACTTCCAGCTAGAC-3’ | 5′-CCACTAAGGTGCCTACAGAGC-3′ |
| ATGL | 5′-TTCACCATCCGCTTGTTGGAG-3’ | 5′-AGATGGTCACCCAATTTCCTC-3′ |
| HSL | 5′-CACAAAGGCTGCTTCTACGG-3’ | 5′-GGAGAGAGTCTGCAGGAACG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.C.; Yi, S.A.; Lee, B.S.; Yu, J.S.; Kim, J.-C.; Pang, C.; Jang, T.S.; Lee, J.; Kim, K.H. Anti-Adipogenic Polyacetylene Glycosides from the Florets of Safflower (Carthamus tinctorius). Biomedicines 2021, 9, 91. https://doi.org/10.3390/biomedicines9010091
Baek SC, Yi SA, Lee BS, Yu JS, Kim J-C, Pang C, Jang TS, Lee J, Kim KH. Anti-Adipogenic Polyacetylene Glycosides from the Florets of Safflower (Carthamus tinctorius). Biomedicines. 2021; 9(1):91. https://doi.org/10.3390/biomedicines9010091
Chicago/Turabian StyleBaek, Su Cheol, Sang Ah Yi, Bum Soo Lee, Jae Sik Yu, Jin-Chul Kim, Changhyun Pang, Tae Su Jang, Jaecheol Lee, and Ki Hyun Kim. 2021. "Anti-Adipogenic Polyacetylene Glycosides from the Florets of Safflower (Carthamus tinctorius)" Biomedicines 9, no. 1: 91. https://doi.org/10.3390/biomedicines9010091
APA StyleBaek, S. C., Yi, S. A., Lee, B. S., Yu, J. S., Kim, J.-C., Pang, C., Jang, T. S., Lee, J., & Kim, K. H. (2021). Anti-Adipogenic Polyacetylene Glycosides from the Florets of Safflower (Carthamus tinctorius). Biomedicines, 9(1), 91. https://doi.org/10.3390/biomedicines9010091








