Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy
Abstract
1. Introduction
2. Current Limitations of PDT and Nanomedicine
2.1. Advantages and Disadvantages of PDT in Cancer Treatment
2.2. Current Limitations in Clinical Application of Nanomedicine
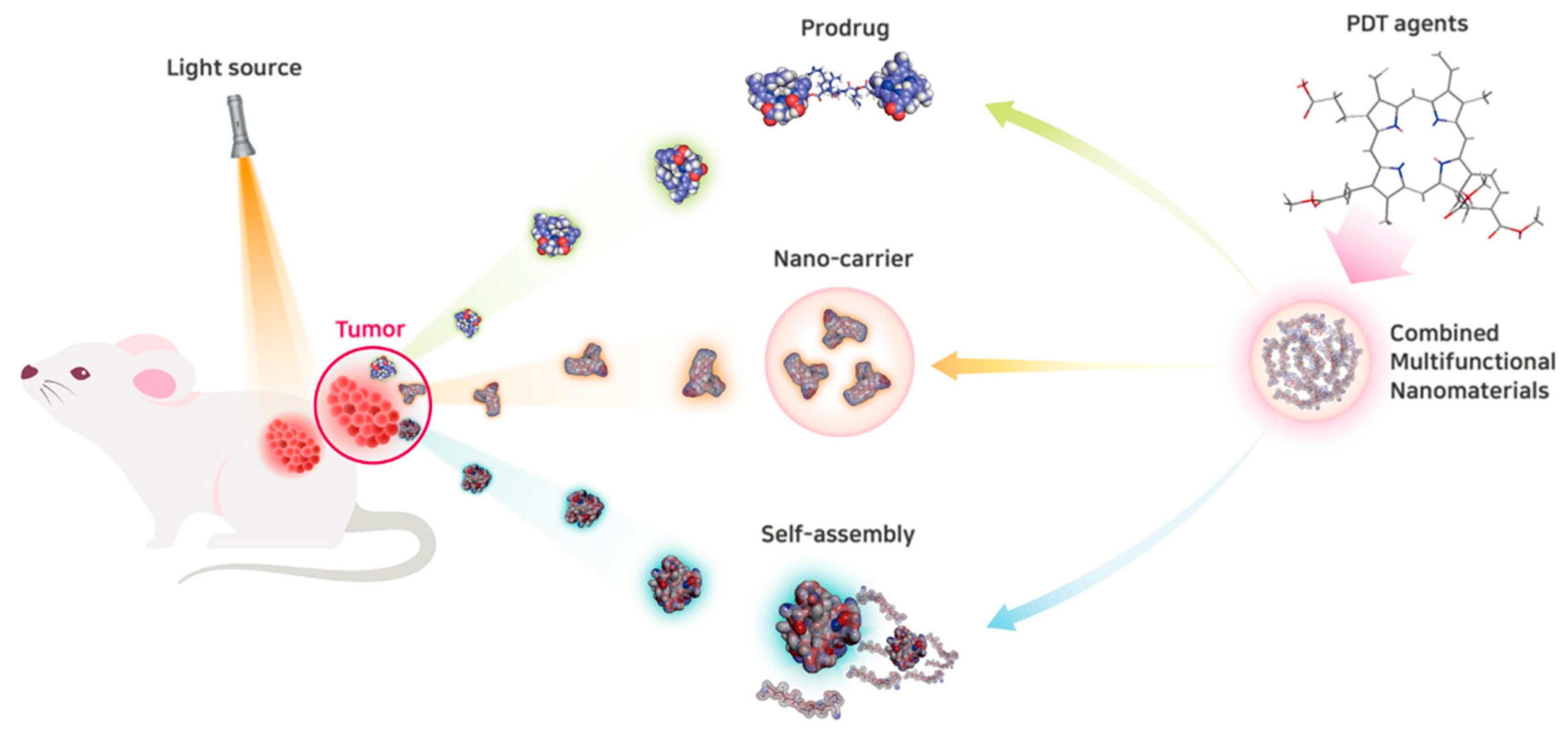
3. Advances in Nanomedicine for PDT to Overcome Current Clinical Limitations
3.1. Advances in Nanocarriers for PDT
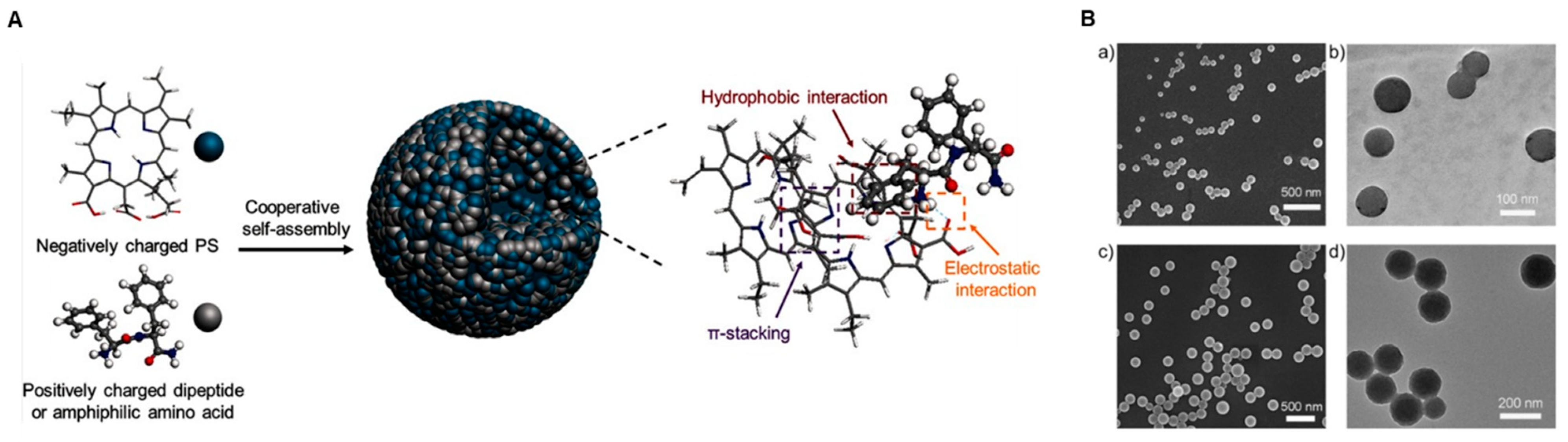
3.2. Self-Assembly of Nanomedicine for PDT
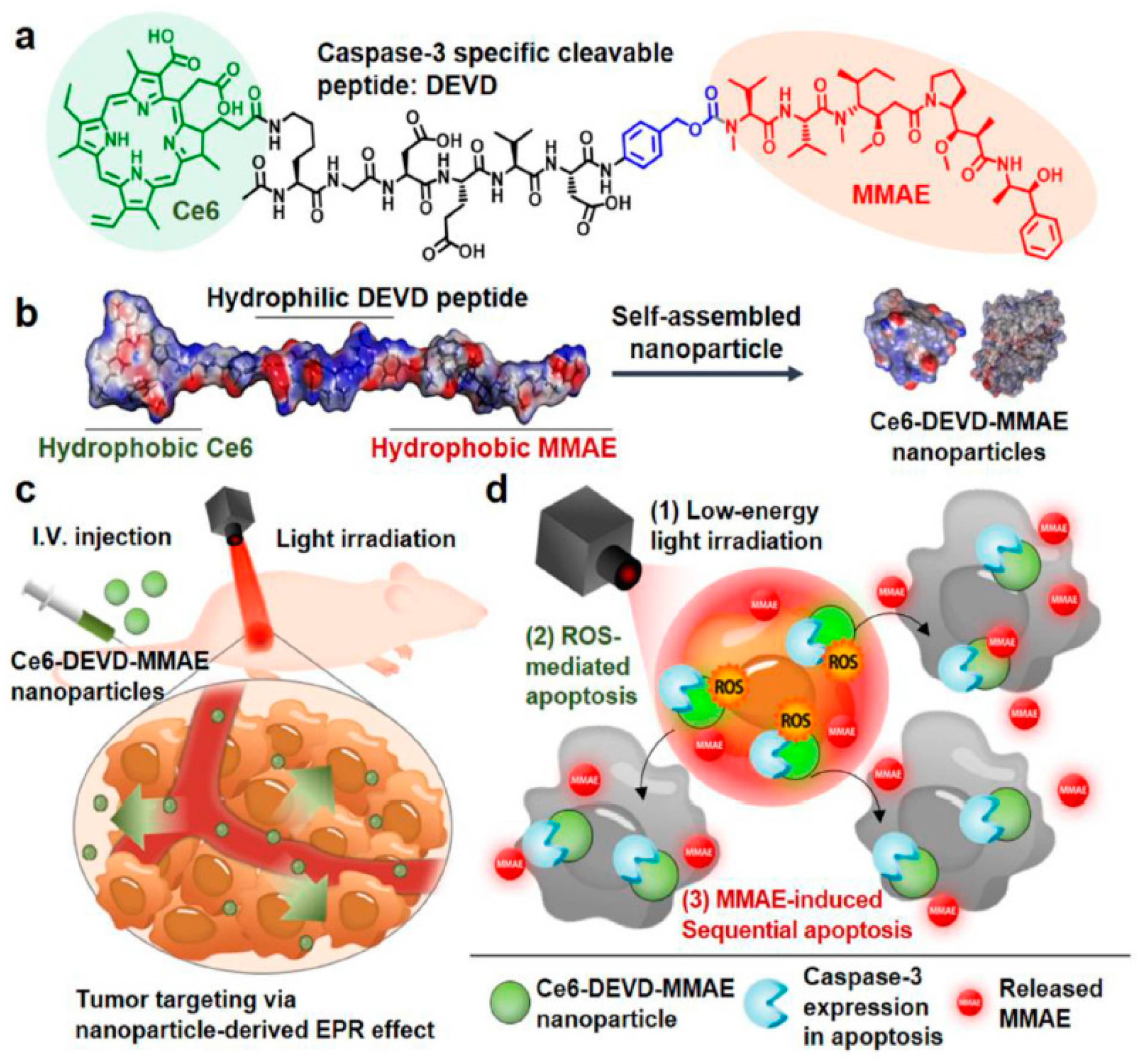
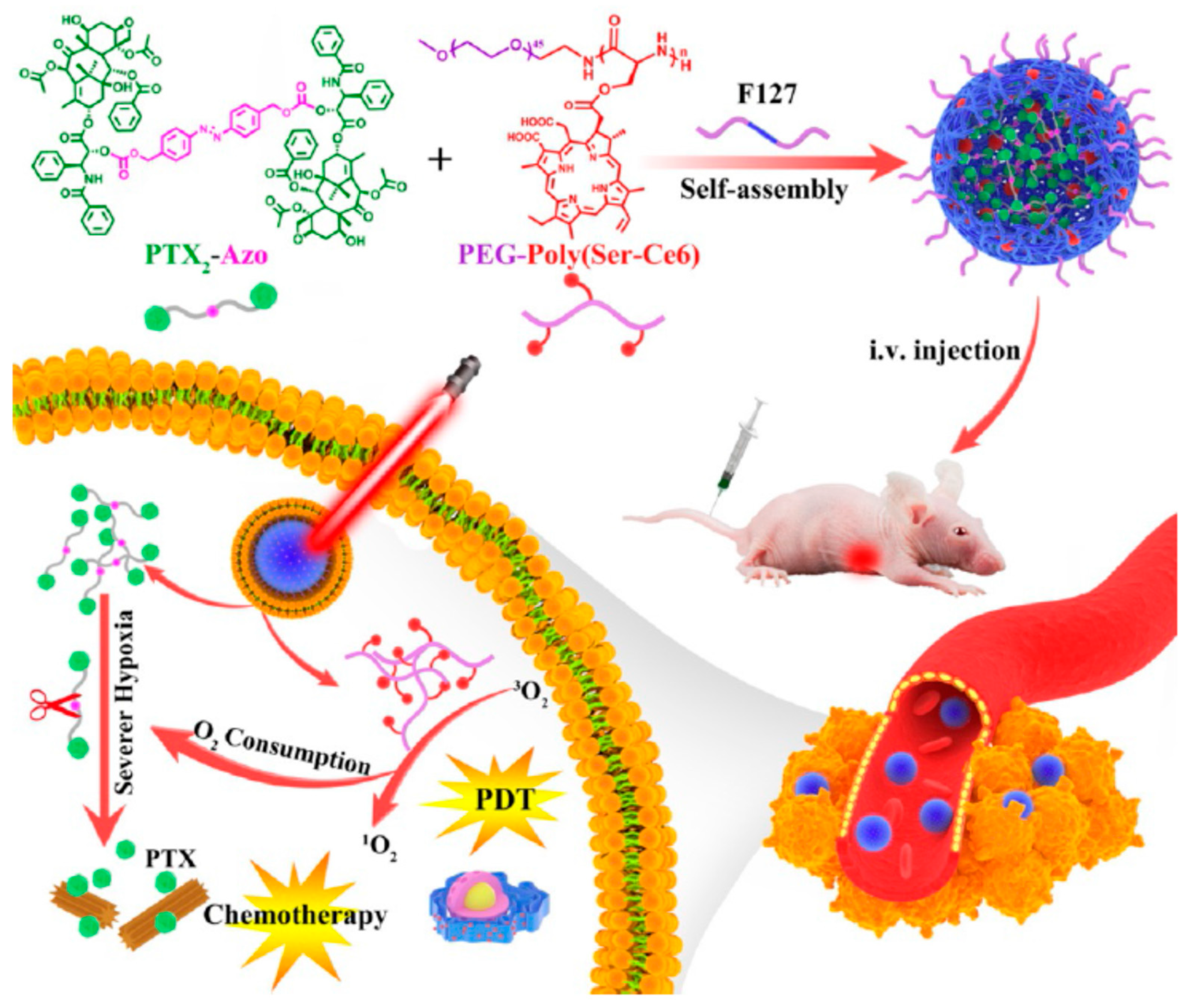
3.3. Stimuli-Responsive Prodrug Nanomedicine for PDT
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wainwright, M. The use of dyes in modern biomedicine. Biotech. Histochem. 2003, 78, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.; Ogilby, P.R. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Chuang, Y.J.; Todd, T.; Zhang, W.; Lin, X.; Niu, G.; Liu, G.; Wang, L.; Pan, Z.; et al. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano 2014, 8, 6004–6013. [Google Scholar] [CrossRef]
- Yoon, J.; Park, J.; Choi, M.; Jong Choi, W.; Choi, C. Application of femtosecond-pulsed lasers for direct optical manipulation of biological functions. Annalen der Physik 2013, 525, 205–214. [Google Scholar] [CrossRef]
- Choi, H.; Choi, M.; Choi, K.; Choi, C. Blockade of vascular endothelial growth factor sensitizes tumor-associated vasculatures to angiolytic therapy with a high-frequency ultrashort pulsed laser. Microvasc. Res. 2011, 82, 141–146. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Choi, M.; Lee, J.; Koh, G.Y.; Kwon, K.; Choi, C. Quantitative analysis of peripheral tissue perfusion using spatiotemporal molecular dynamics. PLoS ONE 2009, 4, e4275. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, H.; Li, M.; Luo, Z.; Zhang, J.; Guo, X.; Cai, K. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials 2018, 157, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.; Choi, K.; Kim, E.; Han, E.C.; Lee, J.; Cha, J.; Ku, T.; Yoon, J.; Park, J.H.; Choi, C. Coloring Brain Tumor with Multi-Potent Micellar Nanoscale Drug Delivery System. In Nanosystems in Engineering and Medicine; International Society for Optics and Photonics: Washington, DC, USA, 2012; p. 85480I. [Google Scholar]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin derivative: A possible aid in the diagnosis and therapy of carcinoma of the bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef]
- Balchum, O.J.; Doiron, D.R.; Huth, G.C. Photoradiation therapy of endobronchial lung cancers employing the photodynamic action of hematoporphyrin derivative. Lasers Surg. Med. 1984, 4, 13–30. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, J.S., Jr.; Williams, T.E., Jr.; Bethel, B.H. Palliation of esophageal malignancy with photodynamic therapy. Ann. Thorac. Surg. 1985, 40, 113–120. [Google Scholar] [CrossRef]
- Hayata, Y.; Kato, H.; Okitsu, H.; Kawaguchi, M.; Konaka, C. Photodynamic therapy with hematoporphyrin derivative in cancer of the upper gastrointestinal tract. Semin. Surg. Oncol. 1985, 1, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Moon, H.T.; Park, J.-Y.; Heo, Y.J.; Choi, Y.; Tran, T.H.; Lee, Y.-k.; Kim, S.Y.; Huh, K.M. Heparin-based self-assembled nanoparticles for photodynamic therapy. Macromol. Res. 2011, 19, 487–494. [Google Scholar] [CrossRef]
- Stranadko, E.; Shabarov, V.; Riabov, M.; Duvansky, V. Photodynamic therapy in the treatment of esophageal cancer. Endoscopy 2020, 52, OP339. [Google Scholar]
- Doustvandi, M.A.; Mohammadnejad, F.; Mansoori, B.; Tajalli, H.; Mohammadi, A.; Mokhtarzadeh, A.; Baghbani, E.; Khaze, V.; Hajiasgharzadeh, K.; Moghaddam, M.M. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn. Ther. 2019, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Doko, M.; Jurin, M.; Svarc, A.; Tonkovic, G.; Ledinsky, M. The introduction of photodynamic therapy for tumorous patients in Croatia based on our experimental experiences and clinical approaches of the other groups. Coll Antropol. 1998, 22, 315–319. [Google Scholar] [PubMed]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the barriers of light penetration: Strategies, perspectives and possibilities for photodynamic therapy. Theranostics 2016, 6, 2458. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy and the development of metal-based photosensitisers. Met. Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photoch. Photobio. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Paul, S.; Heng, P.W.; Chan, L.W. Implications of Photophysical and Physicochemical Factors on Successful Application of Photodynamic Therapy. Curr. Pharm. Des. 2017, 23, 6194–6205. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Inoue, K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int. J. Urol. 2017, 24, 97–101. [Google Scholar] [CrossRef]
- Schuitmaker, H.J.; Barthen, E.; Keunen, J.E.; Wolff-Rouendaal, D.M. Evaluation of Photodynamically Induced Damage to Healthy Eye Tissues of Rabbits Using the Second-Generation Photosensitizers Bacteriochlorin a and Mthpc. In Photochemotherapy of Cancer and Other Diseases; International Society for Optics and Photonics: Washington, DC, USA, 1999; pp. 52–58. [Google Scholar]
- Sharma, S.K.; Mroz, P.; Dai, T.; Huang, Y.Y.; Denis, T.G.S.; Hamblin, M.R. Photodynamic therapy for cancer and for infections: What is the difference? Isr. J. Chem. 2012, 52, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Moan, J. Singlet oxygen in photosensitization. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Engbrecht, B.W.; Menon, C.; Kachur, A.V.; Hahn, S.M.; Fraker, D.L. Photofrin-mediated photodynamic therapy induces vascular occlusion and apoptosis in a human sarcoma xenograft model. Cancer Res. 1999, 59, 4334–4342. [Google Scholar] [PubMed]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 11522–11531. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-O.; Ha, K.-S. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 295, pp. 139–174. [Google Scholar]
- Li, Y.; Zhang, Y.; Wang, W. Phototriggered targeting of nanocarriers for drug delivery. Nano Res. 2018, 11, 5424–5438. [Google Scholar] [CrossRef]
- Mustafa, F.H.; Jaafar, M.S.; Ismail, A.H.; Houssein, H.A. The Effect of Laser Wavelength in Photodynamic Therapy and Phototherapy for Superficial Skin Diseases. In Proceedings of the 2011 IEEE International Conference on Imaging Systems and Techniques, Penang, Malaysia, 17–18 May 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 232–236. [Google Scholar]
- Shin, T.H.; Cheon, J. Synergism of Nanomaterials with Physical Stimuli for Biology and Medicine. Acc. Chem. Res. 2017, 50, 567–572. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; What is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Cong, H.L.; Hu, H.; Xu, F.J. Rational design and latest advances of codelivery systems for cancer therapy. Mater. Today Bio 2020, 7, 100056. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Zamboni, W.C. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist 2008, 13, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Caron, W.P.; Morgan, K.P.; Zamboni, B.A.; Zamboni, W.C. A review of study designs and outcomes of phase I clinical studies of nanoparticle agents compared with small-molecule anticancer agents. Clin. Cancer Res. 2013, 19, 3309–3315. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258. [Google Scholar] [CrossRef]
- Stirland, D.L.; Matsumoto, Y.; Toh, K.; Kataoka, K.; Bae, Y.H. Analyzing spatiotemporal distribution of uniquely fluorescent nanoparticles in xenograft tumors. J. Control. Release 2016, 227, 38–44. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Ping, Q.; Yeo, Y. Quantitative assessment of nanoparticle biodistribution by fluorescence imaging, revisited. ACS Nano 2018, 12, 6458–6468. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Yang, C.; Fu, Y.; Huang, C.; Hu, D.; Zhou, K.; Hao, Y.; Chu, B.; Yang, Y.; Qian, Z. Chlorin e6 and CRISPR-Cas9 dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy. Biomaterials 2020, 255, 120194. [Google Scholar] [CrossRef]
- Chung, C.H.; Lu, K.Y.; Lee, W.C.; Hsu, W.J.; Lee, W.F.; Dai, J.Z.; Shueng, P.W.; Lin, C.W.; Mi, F.L. Fucoidan-based, tumor-activated nanoplatform for overcoming hypoxia and enhancing photodynamic therapy and antitumor immunity. Biomaterials 2020, 257, 120227. [Google Scholar] [CrossRef]
- Sheng, S.; Liu, F.; Lin, L.; Yan, N.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J. Control. Release 2020, 328, 631–639. [Google Scholar] [CrossRef]
- Uthaman, S.; Pillarisetti, S.; Mathew, A.P.; Kim, Y.; Bae, W.K.; Huh, K.M.; Park, I.K. Long circulating photoactivable nanomicelles with tumor localized activation and ROS triggered self-accelerating drug release for enhanced locoregional chemo-photodynamic therapy. Biomaterials 2020, 232, 119702. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.; Choi, Y. Theranostic nanoparticles for enzyme-activatable fluorescence imaging and photodynamic/chemo dual therapy of triple-negative breast cancer. Quant. Imaging Med. Surg. 2015, 5, 656–664. [Google Scholar] [PubMed]
- Luo, Y.; Wu, H.; Feng, C.; Xiao, K.; Yang, X.; Liu, Q.; Lin, T.Y.; Zhang, H.; Walton, J.H.; Ajena, Y.; et al. “One-Pot” Fabrication of Highly Versatile and Biocompatible Poly(vinyl alcohol)-porphyrin-based Nanotheranostics. Theranostics 2017, 7, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, Y.; Wang, X.; Yang, W.; Bai, F.; Zhang, B.; Alarid, L.; Bian, K.; Fan, H. pH-Dependent Assembly of Porphyrin-Silica Nanocomposites and Their Application in Targeted Photodynamic Therapy. Nano Lett. 2017, 17, 6916–6921. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Mohwald, H.; Yan, X. Simple Peptide-Tuned Self-Assembly of Photosensitizers towards Anticancer Photodynamic Therapy. Angew Chem. Int. Ed. Engl. 2016, 55, 3036–3039. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Gao, Q.; Dong, X.; Yin, W.; Gu, Z.; Gan, Z.; Zhao, Y.; Yin, M. A Size-Reducible Nanodrug with an Aggregation-Enhanced Photodynamic Effect for Deep Chemo-Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 11384–11388. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Kang, M.; Song, N.; Wang, D.; Tang, B.Z. Boosting the photodynamic therapy efficiency by using stimuli-responsive and AIE-featured nanoparticles. Biomaterials 2020, 232, 119749. [Google Scholar] [CrossRef]
- Cho, M.H.; Li, Y.; Lo, P.C.; Lee, H.R.; Choi, Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nano Micro Lett. 2020, 12, 47. [Google Scholar] [CrossRef]
- Um, W.; Park, J.; Ko, H.; Lim, S.; Yoon, H.Y.; Shim, M.K.; Lee, S.; Ko, Y.J.; Kim, M.J.; Park, J.H.; et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials 2019, 224, 119494. [Google Scholar] [CrossRef]
- Kim, Y.; Uthaman, S.; Pillarisetti, S.; Noh, K.; Huh, K.M.; Park, I.K. Bioactivatable reactive oxygen species-sensitive nanoparticulate system for chemo-photodynamic therapy. Acta Biomater. 2020, 108, 273–284. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, X.; Fu, T.; Li, K.; He, Y.; Luo, Z.; Dai, L.; Zeng, R.; Cai, K. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, X.; Xia, R.; Liu, S.; Pei, Q.; Chen, G.; Xie, Z.; Jing, X. A paclitaxel prodrug activatable by irradiation in a hypoxic microenvironment. Angew. Chem. Int. Ed. Engl. 2020, 59, 23198–23205. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, X.; Bian, Q.; Zhang, F.; Wu, H.; Wang, H.; Gao, J. Photosensitizer-stabilized self-assembling nanoparticles potentiate chemo/photodynamic efficacy of patient-derived melanoma. J. Control. Release 2020, 328, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Guo, J.; Liu, X.; Song, H.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Cascade of reactive oxygen species generation by polyprodrug for combinational photodynamic therapy. Biomaterials 2020, 255, 120210. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.P.; Zhang, Z.; Cai, W.L.; Flores, A.D. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- Choi, K.Y.; Silvestre, O.F.; Huang, X.; Hida, N.; Liu, G.; Ho, D.N.; Lee, S.; Lee, S.W.; Hong, J.I.; Chen, X. A nanoparticle formula for delivering siRNA or miRNAs to tumor cells in cell culture and in vivo. Nat. Protoc. 2014, 9, 1900–1915. [Google Scholar] [CrossRef]
- Cohen, Z.R.; Ramishetti, S.; Peshes-Yaloz, N.; Goldsmith, M.; Wohl, A.; Zibly, Z.; Peer, D. Localized RNAi Therapeutics of Chemoresistant Grade IV Glioma Using Hyaluronan-Grafted Lipid-Based Nanoparticles. ACS Nano 2015, 9, 1581–1591. [Google Scholar] [CrossRef]
- Shamay, Y.; Elkabets, M.; Li, H.; Shah, J.; Brook, S.; Wang, F.; Adler, K.; Baut, E.; Scaltriti, M.; Jena, P.V. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci. Transl. Med. 2016, 8, 345ra87. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Li, R.; Hsu, C.-H.; Lin, C.-W.; Chou, S.-C.; Tsai, M.-L.; Mi, F.-L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yu, Q.; Xue, H.; Cheng, G.; Jiang, S. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew. Chem. Int. Ed. Engl. 2010, 49, 3771–3776. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Ji, F.; Zhang, J.; Wang, L.; Sun, H.; Chen, S. Biocompatible long-circulating star carboxybetaine polymers. J. Mater. Chem. B 2015, 3, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Goda, T.; Miyahara, Y.; Ishihara, K. Phospholipid-mimicking cell-penetrating polymers: Principles and applications. J. Mater. Chem. B 2020, 8, 7633–7641. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Koo, H.; Choi, K.Y.; Lee, S.J.; Kim, K.; Kwon, I.C.; Leary, J.F.; Park, K.; Yuk, S.H.; Park, J.H.; et al. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials 2012, 33, 3980–3989. [Google Scholar] [CrossRef]
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-Assembled Peptide- and Protein-Based Nanomaterials for Antitumor Photodynamic and Photothermal Therapy. Adv. Mater. 2017, 29, 1605021. [Google Scholar] [CrossRef]
- Li, S.K.; Zou, Q.L.; Xing, R.R.; Govindaraju, T.; Fakhrullin, R.; Yan, X.H. Peptide-modulated self-assembly as a versatile strategy for tumor supramolecular nanotheranostics. Theranostics 2019, 9, 3249–3261. [Google Scholar] [CrossRef]
- Shim, M.K.; Yoon, H.Y.; Lee, S.; Jo, M.K.; Park, J.; Kim, J.H.; Jeong, S.Y.; Kwon, I.C.; Kim, K. Caspase-3/-7-Specific Metabolic Precursor for Bioorthogonal Tracking of Tumor Apoptosis. Sci. Rep. 2017, 7, 16635. [Google Scholar] [CrossRef]
- Baldea, I.; Giurgiu, L.; Teacoe, I.D.; Olteanu, D.E.; Olteanu, F.C.; Clichici, S.; Filip, G.A. Photodynamic Therapy in Melanoma-Where do we Stand? Curr. Med. Chem. 2018, 25, 5540–5563. [Google Scholar] [CrossRef] [PubMed]
- Chizenga, E.P.; Chandran, R.; Abrahamse, H. Photodynamic therapy of cervical cancer by eradication of cervical cancer cells and cervical cancer stem cells. Oncotarget 2019, 10, 4380–4396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhou, L.; Lu, J.; Jiang, B.; Liu, C.; Guo, J. Photodynamic therapy of pancreatic cancer: Where have we come from and where are we going? Photodiagnosis Photodyn. Ther. 2020, 31, 101876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yamazaki, K.; Zhang, D.; Li, Y.; Su, M.; Xie, Q.; Chen, Y.; Bai, M. Minimally Invasive Intraperitoneal Photodynamic Therapy Using a New Soft Robot System. In Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XXIX; International Society for Optics and Photonics: Washington, DC, USA, 2020; p. 112200D. [Google Scholar]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Hwang, H.S.; Cherukula, K.; Bang, Y.J.; Vijayan, V.; Moon, M.J.; Thiruppathi, J.; Puth, S.; Jeong, Y.Y.; Park, I.K.; Lee, S.E.; et al. Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression. Cells 2020, 9, 2432. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- Li, X.; Jeon, Y.H.; Kwon, N.; Park, J.G.; Guo, T.; Kim, H.R.; Huang, J.D.; Lee, D.S.; Yoon, J. In Vivo-assembled phthalocyanine/albumin supramolecular complexes combined with a hypoxia-activated prodrug for enhanced photodynamic immunotherapy of cancer. Biomaterials 2021, 266, 120430. [Google Scholar] [CrossRef]
- Xiao, M.; Fan, J.; Li, M.; Xu, F.; Zhao, X.; Xi, D.; Ma, H.; Li, Y.; Du, J.; Sun, W. A photosensitizer-inhibitor conjugate for photodynamic therapy with simultaneous inhibition of treatment escape pathways. Biomaterials 2020, 257, 120262. [Google Scholar] [CrossRef]
- Uthaman, S.; Kim, Y.; Lee, J.Y.; Pillarisetti, S.; Huh, K.M.; Park, I.K. Self-Quenched Polysaccharide Nanoparticles with a Reactive Oxygen Species-Sensitive Cascade for Enhanced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 28004–28013. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.; Choi, Y. Photosensitizer-complexed polypyrrole nanoparticles for activatable fluorescence imaging and photodynamic therapy. J. Mater. Chem. B 2016, 4, 7545–7548. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, C.J.; Gao, M.; Zhang, R.; Tang, B.Z.; Liu, B. Specific light-up bioprobe with aggregation-induced emission and activatable photoactivity for the targeted and image-guided photodynamic ablation of cancer cells. Angew. Chem. Int. Ed. Engl. 2015, 54, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Hsieh, M.-C.; Lin, J.-C.; Chang, T.-C. Selective photodynamic therapy based on aggregation-induced emission enhancement of fluorescent organic nanoparticles. Biomaterials 2012, 33, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hu, F.; Kong, D.; Ding, D.; Liu, B. Chemiluminescence-guided cancer therapy using a chemiexcited photosensitizer. Chem 2017, 3, 991–1007. [Google Scholar] [CrossRef]
- Gu, B.; Wu, W.; Xu, G.; Feng, G.; Yin, F.; Chong, P.H.J.; Qu, J.; Yong, K.T.; Liu, B. Precise two-photon photodynamic therapy using an efficient photosensitizer with aggregation-induced emission characteristics. Adv. Mater. 2017, 29, 1701076. [Google Scholar] [CrossRef]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef]

| Photosensitizer | Other Name (s) | Indications | Clinical Trial |
|---|---|---|---|
| Photofrin | Porfimer sodium | Esophageal cancer, endobronchial cancer, high-grade dysplasia in Barrett’s esophagus | FDA-approved PDT drug |
| Photofrin | Porfimer sodium | Clinical trials (phases I–II) for various cancers | NCT00054002 NCT00003788 NCT00118222 NCT00322699 (Completed 1) |
| Temoporfin | Foscan | Head and neck cancer | - |
| Temoporfin | Foscan | Clinical trials (phase II) | NCT00003856 (Unknown 2) |
| 5-Aminolevulinic acid | 5-ALA | Malignant gliomas | For the guiding agent, not therapeutics |
| Talaporfin | Mono-l-aspartyl chlorin e6, Laserphyrin | Lung cancer | Approved in Japan |
| Verteporfin | Visudyne | Age-related macular degeneration, subfoveal choroidal neovascularization | Clinical trials for macular degeneration (NCT02081339) |
| Class | Photosensitizer | Nanomaterial | Highlight | Year | Reference |
|---|---|---|---|---|---|
| Nanocarrier | Chlorin e6 (Ce6) | Hyaluronic acid (HA)–based nanomaterials | CRISPR–Cas9 system targeting the Ptpn2 gene | 2020 | [52] |
| Nanocarrier | Verteporfin | Dendrimer–fucoidan nanocomplex | Sensitive to glutathione-targeted P-selectin | 2020 | [53] |
| Nanocarrier | Ce6 | HA shielding could endow nanoplatform | Peroxidase mimic metal–organic framework | 2020 | [54] |
| Nanocarrier | Pheophorbide A (PhA) | Polyethylene glycol (PEG)–stearamine | Long-circulating, photodynamic/chemo dual therapy | 2020 | [55] |
| Self-assembly | Ce6 | mPEG-grafted HA | Photodynamic/chemo dual therapy | 2015 | [56] |
| Self-assembly | Porphyrin | Polyvinyl alcohol | One-pot fabrication, theranostics | 2017 | [57] |
| Self-assembly | Porphyrin | Silica | pH-Dependent assembly of porphyrin–silica nanocomposites | 2017 | [58] |
| Self-assembly | Ce6 | Peptide conjugate | Dipeptide and Ce6 conjugate | 2016 | [59] |
| Self-assembly | Indocyanine | Pentamethine indocyanine | Indocyanine, camptothecin, RGD peptide | 2018 | [60] |
| Self-assembly | MeTTMN | mPEG-SS-OH and cinnamic acid conjugate | Photosensitizers with aggregation-induced emission characteristics | 2020 | [61] |
| Self-assembly | Ce6 | Fucoidan | Theranostic nanogel | 2020 | [62] |
| Prodrug | Ce6 | DEVD–monomethyl auristatin E conjugate | Caspase 3-cleavable photosensitizer–drug conjugate | 2019 | [63] |
| Prodrug | PhA | PEG–doxorubicin conjugate | Reactive oxygen species (ROS)-sensitive nanoparticles for photodynamic/chemo dual therapy | 2020 | [64] |
| Prodrug | IR780 | PEG with further modification of internalizing RGD peptide | Camptothecin prodrug for blood–brain barrier penetration | 2020 | [65] |
| Prodrug | Ce6 | Dimeric paclitaxel encapsulated by a PEGylated peptide copolymer | Prodrug activatable via irradiation in a hypoxic microenvironment | 2020 | [66] |
| Prodrug | Ce6 | Dimeric cabazitaxel | Cabazitaxel prodrug for photodynamic/chemo dual therapy against melanoma | 2020 | [67] |
| Prodrug | PhA | Polygalactose-co-poly-cinnamaldehyde | ROS generation by polyprodrug for combinational therapy | 2020 | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, Y.-K.; Park, I.-K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. https://doi.org/10.3390/biomedicines9010085
Park J, Lee Y-K, Park I-K, Hwang SR. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines. 2021; 9(1):85. https://doi.org/10.3390/biomedicines9010085
Chicago/Turabian StylePark, Jooho, Yong-Kyu Lee, In-Kyu Park, and Seung Rim Hwang. 2021. "Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy" Biomedicines 9, no. 1: 85. https://doi.org/10.3390/biomedicines9010085
APA StylePark, J., Lee, Y.-K., Park, I.-K., & Hwang, S. R. (2021). Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines, 9(1), 85. https://doi.org/10.3390/biomedicines9010085






