The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients
Abstract
1. Introduction
1.1. General Considerations on Pruritus
1.2. Cancer and Pruritus
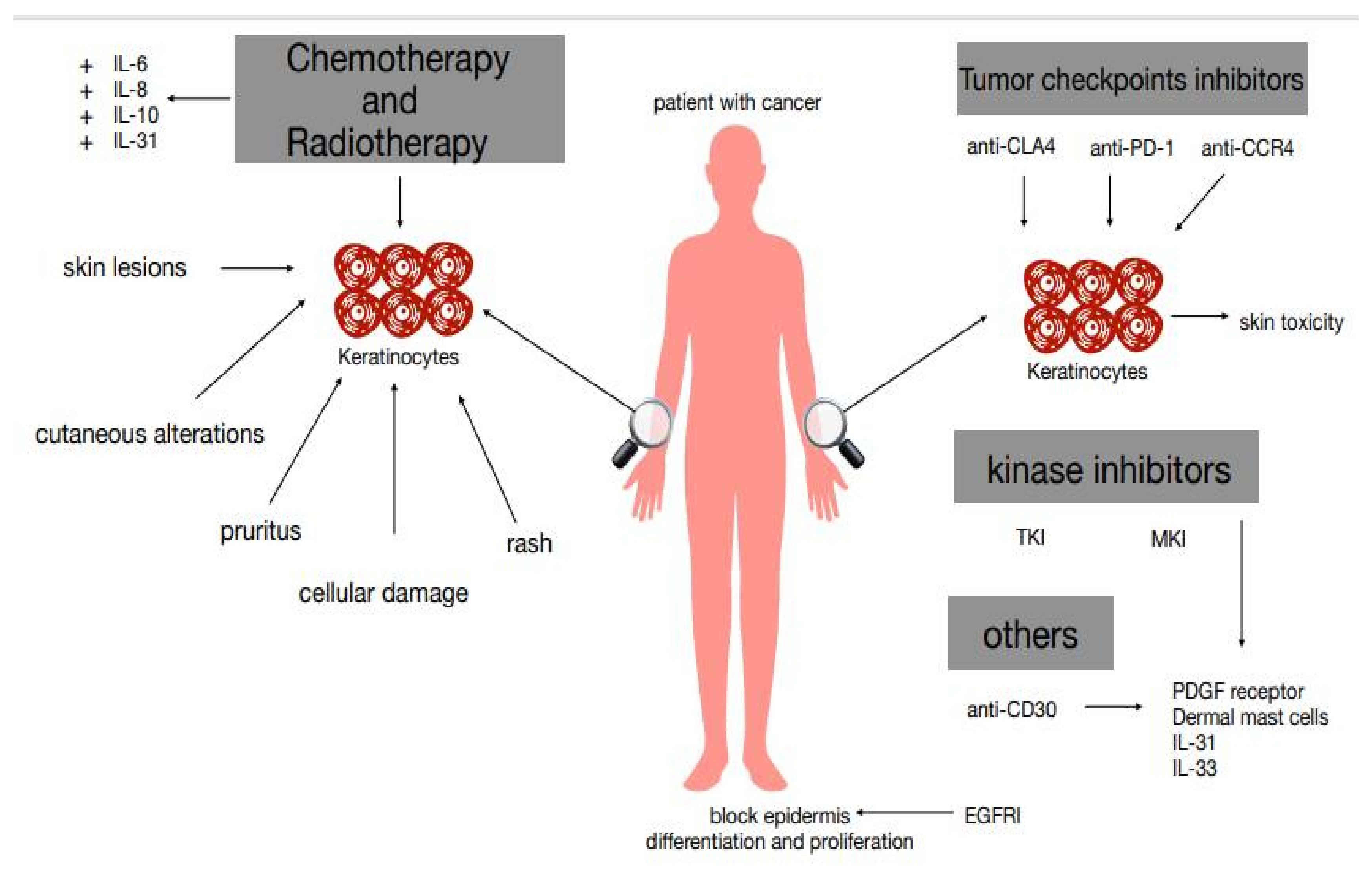
2. Conventional Chemotherapy and Pruritus
3. Immune Checkpoint Inhibitors, Targeted Therapies, and Pruritus
4. Targeted Therapy and Pruritus
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Twycross, R.; Greaves, M.W.; Handwerker, H.; Jones, E.A.; Libretto, S.E.; Szepietowski, J.C.; Zylicz, Z. Itch: Sratching more than the surface. QJM Int. J. Med. 2003, 96, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xian, D.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Pruritus: Progress toward Pathogenesis and Treatment. BioMed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Elberling, J.; Arendt-Nielsen, L. Human Surrogate Models of Histaminergic and Non-histaminergic Itch. Acta Derm. Venereol. 2014, 95, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, G. The Itch-Scratch Cycle: A Review of the Mechanisms. Dermatol. Pract. Concept. 2019, 9, 90–97. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Z.; Surdenikova, L.; Kim, S.; Patel, K.N.; Kim, A.; Ru, F.; Guan, Y.; Weng, H.-J.; Geng, Y.; et al. Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell 2009, 139, 1353–1365. [Google Scholar] [CrossRef]
- Bulca, S.; Bayramgürler, D.; Demirsoy, E.O.; Yavuz, M.; Sikar Aktürk, A.; Bilen, N.; Kıran, R. Comparison of effects of 5 and 10 mg oral desloratadine and levocetirizine on histamine-induced wheal and flare response in healthy volunteers. J. Dermatol. Treat. 2013, 24, 473–476. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Fowler, E.; Yosipovitch, G. Chronic itch management: Therapies beyond those targeting the immune system. Ann. Allergy Asthma Immunol. 2019, 123, 158–165. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, C.-K.; Chen, G.; Han, Q.; Xie, R.-G.; Liu, T.; Ji, R.-R.; Lee, S.-Y. Monoclonal Antibody that Targets a NaV1.7 Channel Voltage Sensor for Pain and Itch Relief. Cell 2014, 157, 1393–1404. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Kahremany, S.; Hofmann, L.; Gruzman, A.; Cohen, G. Advances in Understanding the Initial Steps of Pruritoceptive Itch: How the Itch Hits the Switch. Int. J. Mol. Sci. 2020, 21, 4883. [Google Scholar] [CrossRef]
- Yosipovitch, G. Chronic pruritus: A paraneoplastic sign. Dermatol. Ther. 2010, 23, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediat. Inflamm. 2018, 2018, 3858032. [Google Scholar] [CrossRef] [PubMed]
- Tachi, T.; Teramachi, H.; Tanaka, K.; Asano, S.; Osawa, T.; Kawashima, A.; Yasuda, M.; Mizui, T.; Nakada, T.; Noguchi, Y.; et al. The impact of outpatient chemotherapy-related adverse events on the quality of life of breast cancer patients. PLoS ONE 2015, 10, e0124169. [Google Scholar] [CrossRef] [PubMed]
- Hirose, C.; Fujii, H.; Iihara, H.; Ishihara, M.; Nawa-Nishigaki, M.; Kato-Hayashi, H.; Ohata, K.; Sekiya, K.; Kitahora, M.; Matsuhashi, N.; et al. Real-world data of the association between quality of life using the EuroQol 5 Dimension 5 Level utility value and adverse events for outpatient cancer chemotherapy. Support Care Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.C.; Case, E.C.; Dusza, S.W.; Balagula, Y.; Gordon, J.; West, D.P.; Lacouture, M.E. Impact of dermatologic adverse events on quality of life in 283 cancer patients: A questionnaire study in a dermatology referral clinic. Am. J. Clin. Dermatol. 2013, 14, 327–333. [Google Scholar] [CrossRef]
- Gandhi, M.; Oishi, K.; Zubal, B.; Lacouture, M.E. Unanticipated toxicities from anticancer therapies: Survivors’ perspectives. Support Care Cancer 2010, 18, 1461–1468. [Google Scholar] [CrossRef]
- Fischer, A.; Rosen, A.C.; Ensslin, C.J.; Wu, S.; Lacouture, M.E. Pruritus to anticancer agents targeting the EGFR, BRAF, and CTLA-4. Dermatol. Ther. 2013, 26, 135–148. [Google Scholar] [CrossRef]
- Wohlrab, J.; Bangemann, N.; Kleine-Tebbe, A.; Thill, M.; Kümmel, S.; Grischke, E.M.; Richter, R.; Seite, S.; Lüftner, D. Barrier protective use of skin care to prevent chemotherapy-induced cutaneous symptoms and to maintain quality of life in patients with breast cancer. Breast Cancer 2014, 6, 115–122. [Google Scholar] [CrossRef]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 22, 448. [Google Scholar] [CrossRef]
- Agha, R.; Kinahan, K.; Bennett, C.L.; Lacouture, M.E. Dermatologic challenges in cancer patients and survivors. Oncology 2007, 21, 1462–1472. [Google Scholar]
- Weisshaar, E. Evidence-based medicine and pruritus. (Abstr. OP4). Acta Derm. Venereol. 2007, 87, 462. [Google Scholar]
- Dunphy, F.R.; Boyd, J.H.; Kim, H.J.; Dunphy, C.H.; Harrison, B.R.; Dunleavy, T.L.; Rodriguez, J.J.; McDonough, E.M.; Minster, J.R.; Hilton, J.G. A phase I report of paclitaxel dose escalation combined with a fixed dose of carboplatin in the treatment of head and neck carcinoma. Cancer 1997, 79, 2016–2023. [Google Scholar] [CrossRef]
- Freilich, R.J.; Seidman, A.D. Pruritis caused by 3-h infusion of high-dose paclitaxel and improvement with tricyclic antidepressants. J. Natl. Cancer Inst. 1995, 87, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Kollmannsberger, C.; Schittenhelm, M.; Honecker, F.; Tillner, J.; Weber, D.; Oechsle, K.; Kanz, L.; Bokemeyer, C.A. A phase I study of the humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody EMD 72000 (matuzumab) in combination with paclitaxel in patients with EGFR-positive advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. 2006, 17, 1007–1013. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Laabs, S.M.; Koehler, M.; Sweetman, R.W.; Preston, A.J.; Di Leo, A.; Gomez, H.L.; Salazar, V.M.; Byrne, J.A.; Koch, K.M.; et al. Analysis of dermatologic events in patients with cancer treated with lapatinib. Breast Cancer Res. Treat. 2009, 114, 485–493. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kawano, I.; Iwase, H. Nab-paclitaxel for the treatment of breast cancer: Efficacy, safety, and approval. Onco Targets Ther. 2011, 4, 123–136. [Google Scholar] [CrossRef]
- Seidman, A.D.; Tiersten, A.; Hudis, C.; Gollub, M.; Barrett, S.; Yao, T.J.; Lepore, J.; Gilewski, T.; Currie, V.; Crown, J.; et al. Phase II trial of paclitaxel by 3-h infusion as initial and salvage chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1995, 13, 2575–2581. [Google Scholar] [CrossRef]
- Tang, L.C.; Wang, B.Y.; Sun, S.; Zhang, J.; Jia, Z.; Lu, Y.H.; Di, G.H.; Shao, Z.M.; Hu, X.C. Higher rate of skin rash in a phase II trial with weekly nanoparticle albumin-bound paclitaxel and cisplatin combination in Chinese breast cancer patients. BMC Cancer 2013, 13, 232. [Google Scholar] [CrossRef]
- Torricelli, R.; Kurer, S.B.; Kroner, T.; Wüthrich, B. Delayed allergic reaction to Chlorambucil (Leukeran). Case report and literature review. Schweiz. Med. Wochenschr. 1995, 125, 1870–1873. [Google Scholar]
- Susser, W.S.; Whitaker-Worth, D.L.; Grant-Kels, J.M. Mucocutaneous reactions to chemotherapy. J. Am. Acad. Dermatol. 1999, 40, 367–398. [Google Scholar] [CrossRef]
- Baack, B.R.; Burgdorf, W.H. Chemotherapy-induced acral erythema. J. Am. Acad. Dermatol. 1991, 24, 457–461. [Google Scholar] [CrossRef]
- Shahab, N.; Haider, S.; Doll, D.C. Vascular toxicity of antineoplastic agents. Semin. Oncol. 2006, 33, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Ruben, B.S.; Yu, W.Y.; Liu, F.; Truong, S.V.; Wang, K.C.; Fox, L.P. Generalized benign cutaneous reaction to cytarabine. J. Am. Acad. Dermatol. 2015, 73, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Castano, E.; Rodrıguez-Peralto, J.L.; Lopez-Rıos, F.; Gómez, C.; Zimmermann, M.; Iglesias Díez, L. Keratinocyte dysplasia: An usual finding after transplantation or chemotherapy. J. Cutan. Pathol. 2002, 29, 579–584. [Google Scholar] [CrossRef]
- Henry, L.B.; Horn, T.D. Chemotherapy and keratinocytes. J. Cutan. Pathol. 2002, 29, 575–578. [Google Scholar] [CrossRef]
- Pietras, K.; Östman, A.; Sjöquist, M.; Buchdunger, E.; Reed, R.K.; Heldin, C.H.; Rubin, K. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001, 61, 2929–2934. [Google Scholar]
- Musolino, C.; Allegra, A.; Mannucci, C.; Russo, S.; Alonci, A.; Maisano, V.; Calapai, G.; Gangemi, S. Possible Role of Interleukin-31/33 Axis in Imatinib Mesylate-Associated Skin Toxicity. Turk. J. Haematol. 2015, 32, 168–171. [Google Scholar] [CrossRef]
- Lacouture, M.E. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat. Rev. Cancer 2006, 6, 803–812. [Google Scholar] [CrossRef]
- Boucher, J.; Olson, L.; Piperdi, B. Preemptive management of dermatologic toxicities asciated with epidermal growth factor receptor inhibitors. Clin. J. Oncol. Nurs. 2011, 15, 501–508. [Google Scholar] [CrossRef]
- Sobańska, K.; Szałek, E.; Grześkowiak, E. Cutaneous toxicity of small-molecular EGFR inhibitors. Farm Współ. 2013, 6, 33–40. [Google Scholar]
- Lynch, T.J., Jr.; Kim, E.S.; Eaby, B.; Garey, J.; West, D.P.; Lacouture, M.E. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: An evolving paradigm in clinical management. Oncologist 2007, 2, 610–621. [Google Scholar] [CrossRef]
- Lacouture, M. Dermatologic Principles and Practice in Oncology: Conditions of the Skin, Hair, and Nails in Cancer Patients; Wiley-Blackwell: New York, NY, USA, 2014. [Google Scholar]
- Mascia, F.; Mariani, V.; Girolomoni, G.; Pastore, S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003, 163, 303. [Google Scholar] [CrossRef]
- Pastore, S.; Mascia, F.; Mariotti, F.; Dattilo, C.; Mariani, V.; Girolomoni, G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J. Immunol. 2005, 174, 5047–5056. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Buhren, B.A.; Homey, B. More on aprepitant for erlotinib-induced pruritus. N. Engl. J. Med. 2011, 364, 486–487. [Google Scholar] [PubMed]
- Lacouture, M.E.; Lai, S.E. The PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) syndrome. Br. J. Dermatol. 2006, 155, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J.; Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 2003, 21, 2787–2799. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Cassinat, B.; Chevret, S.; Turlure, P.; Cambier, N.; Roussel, M.; Bellucci, S.; Grandchamp, B.; Chomienne, C.; Fenaux, P. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008, 112, 3065–3072. [Google Scholar] [CrossRef]
- Phillips, G.S.; Wu, J.; Hellman, M.D.; Postow, M.A.; Rizvi, N.A.; Freites-Martinez, A.; Chan, D.; Dusza, S.; Motzer, R.J.; Rosenberg, J.E.; et al. Treatment outcomes of immune-related cutaneous adverse events. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef] [PubMed]
- Goldinger, S.M.; Stieger, P.; Meier, B.; Micaletto, S.; Contassot, E.; French, L.E.; Dummer, R. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin. Cancer Res. 2016, 22, 4023–4029. [Google Scholar] [CrossRef]
- Yang, J.C.; Gadgeel, S.M.; Sequist, L.V.; Wu, C.L.; Papadimitrakopoulou, V.A.; Su, W.C.; Fiore, J.; Saraf, S.; Raftopoulos, H.; Patnaik, A. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J. Thorac. Oncol. 2019, 14, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Ständer, S.; Szepietowski, J.C. Drug-induced pruritus: A review. Acta Derm. Venereol. 2009, 89, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, B.; Fratto, M.E.; Santini, D.; Tonini, G. Aprepitant against pruritus in patients with solid tumours. Support Care Cancer 2010, 18, 1229–1230. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19 (Suppl. 1), 31–39. [Google Scholar] [CrossRef]
- Salzmann, M.; Marmé, F.; Hassel, J.C. Prophylaxis and Management of Skin Toxicities. Breast Care 2019, 14, 72–77. [Google Scholar] [CrossRef]
- Allegra, A.; Penna, G.; Alonci, A.; Rizzo, V.; Russo, S.; Musolino, C. Nanoparticles in oncology: The new theragnostic molecules. Anticancer Agents Med. Chem. 2011, 11, 669–686. [Google Scholar] [CrossRef]
- Vishnu, P.; Roy, V. nab-paclitaxel: A novel formulation of taxane for treatment of breast cancer. Womens Health 2010, 6, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Feng, F.; Jiang, Z.; Shen, Z.; Yu, S.; Fen, J.; Huang, J.; Yao, Z.; Bhar, P. Superior efficacy of a Cremophor-free albumin-bound paclitaxel compared with solvent-based paclitaxel in Chinese patients with metastatic breast cancer. Asia Pac. J. Clin. Oncol. 2009, 5, 165–174. [Google Scholar] [CrossRef]
- Quesada, J.R.; Gutterman, J.U.; Hersh, E.M. Clinical and immunological study of beta interferon by intramuscular route in patients with metastatic breast cancer. J. Interferon Res. 1982, 2, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Fachinformation. PegIntron. Available online: https://www.ema.europa.eu/en/documents/overview/pegintron-epar-summary-public_it.pdf (accessed on 11 December 2018).
- Degen, A.; Weichenthal, M.; Ugurel, S.; Trefzer, U.; Kilian, K.; Garbe, C.; Egberts, F.; Poppe, L.M.; Hauschild, A.; Gutzmer, R. Cutaneous side effects of combined therapy with sorafenib and pegylated interferon alpha-2b in metastatic melanoma (phase II DeCOG trial). J. Dtsch. Dermatol. Ges. 2013, 11, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Lazarus, H.M.; Hines, J.D.; Herzig, R.H. High-dose cytosine arabinoside in previously treated patients with poor-prognosis non-Hodgkin’s lymphoma. Cancer 1985, 56, 1493–1496. [Google Scholar] [CrossRef]
- Cassileth, P.A. Adult acute nonlymphocytic leukemia. Med. Clin. N. Am. 1984, 68, 675–695. [Google Scholar] [CrossRef]
- Rohatiner, A.; Slevin, M.L.; Dhaliwal, H.S.; Malpas, J.S.; Lister, T.A. High-dose cytosine arabinoside: Response to therapy in acute leukemia and non-Hodgkin’s lymphoma. Cancer Chemother. Pharmacol. 1984, 12, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Bolwell, B.J.; Cassileth, P.A.; Gale, R.P. High dose cytarabine: A review. Leukemia 1988, 2, 253–260. [Google Scholar]
- Richards, C.; Wujcik, D. Cutaneous toxicity associated with high-dose cytosine arabinoside. Oncol. Nurs. Forum 1992, 19, 1191–1195. [Google Scholar]
- Whitlock, J.A.; Wells, R.J.; Hord, J.D.; Janco, R.L.; Greer, J.P.; Gay, J.C.; Edwards, J.R.; McCurley, T.L.; Lukens, J.N. High-dose cytosine arabinoside and etoposide: An effective regimen without anthracyclines for refractory childhood acute non-lymphocytic leukemia. Leukemia 1997, 11, 185–189. [Google Scholar] [CrossRef]
- Constantin, D.; Widmann, C. ASH2L drives proliferation and sensitivity to bleomycin and other genotoxins in Hodgkin’s lymphoma and testicular cancer cells. Cell Death Dis. 2020, 11, 1019. [Google Scholar] [CrossRef]
- Chen, J.; Stubbe, J. Bleomycin: Towards better therapeutics. Nat. Rev. Cancer 2005, 5, 102–112. [Google Scholar] [CrossRef]
- Yamamoto, T. Bleomycin and the skin. Br. J. Dermatol. 2006, 155, 869–875. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Testori, A.A.E.; Chiellino, S.; van Akkooi, A.C.J. Adjuvant Therapy for Melanoma: Past, Current, and Future Developments. Cancers 2020, 12, 1994. [Google Scholar] [CrossRef]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bahr, O.; Eigentler, T.K.; Grimm, M.O.; Grunwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat. Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Yang, Q. Risk of dermatologic and mucosal adverse events associated with PD-1/PD-L1 inhibitors in cancer patients: A meta-analysis of randomized controlled trials. Medicine 2019, 98, e15731. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; You, Z.; Jiang, L.; Weng, Y.; Shi, Q.; Du, L.; Yan, S. A large scale meta analysis identifies common adverse events with checkpoint inhibitors vs chemotherapy in melanoma patients. Int. Immunopharmacol. 2019, 74, 105691. [Google Scholar] [CrossRef]
- Berner, F.; Bomze, D.; Diem, S.; Ali, O.H.; Fässler, M.; Ring, S.; Niederer, R.; Ackermann, C.J.; Baumgaertner, P.; Pikor, N.; et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1043–1047. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; El Halawani, H.; Fouad, M. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: A meta-analysis. Future Oncol. 2015, 11, 2471–2484. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Razak, A.R.; Bedard, P.L.; Siu, L.L.; Hansen, A.R. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann. Oncol. 2015, 9, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and class specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 10, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Innao, V.; Musolino, C.; Allegra, A. Immune checkpoint inhibitors in multiple myeloma: A review of the literature. Pathol. Res. Pract. 2020, 216, 153114. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Chen, Y.; Song, S.Y.; Wang, T.J.; Ji, W.J.; Li, S.W.; Liu, N.; Yan, C.X. Immune related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front. Pharmacol. 2017, 18, 730. [Google Scholar] [CrossRef] [PubMed]
- Garrett, N.F.M.D.S.; da Costa, A.C.C.; Damiani, G.; Vasques, C.I. Patients with lung cancer undergoing immune checkpoint inhibitors: A meta-analysis of dermatological toxicities. Crit. Rev. Oncol. Hematol. 2020, 152, 102983. [Google Scholar] [CrossRef]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016, 28, 254–263. [Google Scholar] [CrossRef]
- Rapoport, B.L.; van Eeden, R.; Sibaud, V.; Epstein, J.B.; Klastersky, J.; Aapro, M.; Moodley, D. Supportive care for patients undergoing immunotherapy. Support Care Cancer 2017, 25, 3017–3030. [Google Scholar] [CrossRef]
- Larkin, J.; Lao, C.D.; Urba, W.J.; McDermott, D.F.; Horak, C.; Jiang, J.; Wolchok, J.D. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: A pooled analysis of 4 clinical trials. JAMA Oncol. 2015, 1, 433–440. [Google Scholar] [CrossRef]
- McDermott, D.F.; Sosman, J.A.; Sznol, M.; Massard, C.; Gordon, M.S.; Hamid, O.; Powderly, J.D.; Infante, J.R.; Fassò, M.; Wang, Y.V.; et al. Atezolizumab, an anti-programmed deathligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 2016, 34, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef]
- Kamińska-Winciorek, G.; Cybulska-Stopa, B.; Lugowska, I.; Ziobro, M.; Rutkowski, P. Principles of prophylactic and therapeutic management of skin toxicity during treatment with checkpoint inhibitors. Postep. Dermatol. Alergol. 2019, 36, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Aso, M.; Toi, Y.; Sugisaka, J.; Aiba, T.; Kawana, S.; Saito, R.; Ogasawara, T.; Tsurumi, K.; Ono, K.; Shimizu, H.; et al. Association between skin reaction and clinical benefit in patients treated with anti-programmed cell death 1 monotherapy for advanced non-small cell lung Cancer. Oncologist 2019, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Lunghi, A.; Cortesi, E.; Turci, D.; Signorelli, D.; Stati, V.; Melotti, B.; Ricciuti, B.; Frassoldati, A.; Romano, G.; et al. Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: The Italian NSCLC expanded access program. Lung Cancer 2020, 140, 59–64. [Google Scholar] [CrossRef]
- Dupont, R.; Berard, E.; Puisset, F.; Comont, T.; Delord, J.P.; Guimbaud, R.; Meyer, N.; Mazieres, J.; Alric, L. The prognostic impact of immune-related adverse events during anti-PD1 treatment in melanoma and non-small-cell lung cancer: A real-life retrospective study. Oncoimmunology 2019, 9, 1682383. [Google Scholar] [CrossRef]
- Cortelini, A.; Chiari, R.; Ricciuti, B.; Metro, G.; Perrone, F.; Tiseo, M.; Bersanelli, M.; Bordi, P.; Santini, D.; Giusti, R.; et al. Correlations between the immune-related adverse events Spectrum and efficacy of Anti-PD1 immunotherapy in NSCLC patients. Clin. Lung Cancer 2019, 20, 237–247. [Google Scholar] [CrossRef]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of immune-related adverse events with nivolumab efficacy in non-small-Cell lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef]
- Teraoka, S.; Fujimoto, D.; Morimoto, T.; Kawachi, H.; Ito, M.; Sato, Y.; Nagata, K.; Nakagawa, A.; Otsuka, K.; Uehara, K.; et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung Cancer patients treated with nivolumab: A prospective cohort study. J. Thorac. Oncol. 2017, 12, 1798–1805. [Google Scholar] [CrossRef]
- Imai, K.; Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 2006, 6, 714–727. [Google Scholar] [CrossRef]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar]
- Balagula, Y.; Lacouture, M.E.; Cotliar, J.A. Dermatologic toxicities of targeted anticancer therapies. J. Support Oncol. 2010, 8, 149–161. [Google Scholar] [PubMed]
- Ebata, T. Drug-induced itch management. Curr. Probl. Dermatol. 2016, 50, 155–163. [Google Scholar] [PubMed]
- Santoni, M.; Conti, A.; Andrikou, K.; Bittoni, A.; Lanese, A.; Pistelli, M.; Pantano, F.; Vincenzi, B.; Armento, G.; Massari, F.; et al. Risk of pruritus in cancer patients treated with biological therapies: A systematic review and meta-analysis of clinical trials. Crit. Rev. Oncol. Hematol. 2015, 96, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.D.; McCarten, K.M.; Pei, Q.; Spira, M.; Metzger, M.L.; Drachtman, R.A.; Horton, T.M.; Bush, R.; Blaney, S.M.; Weigel, B.J.; et al. Brentuximab vedotin with gemcitabine for paediatric and young adult patients with relapsed or refractory Hodgkin’s lymphoma (AHOD1221): A Children’s Oncology Group, multicentre single-arm, phase 1-2 trial. Lancet Oncol. 2018, 19, 1229–1238. [Google Scholar] [CrossRef]
- Fachinformation. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nexavar (accessed on 18 December 2019).
- Scott, L.C.; White, J.D.; Reid, R.; Cowie, F. Management of skin toxicity related to the use of imatinib mesylate (STI571, Glivec trade mark) for advanced stage gastrointestinal stromal tumours. Sarcoma 2005, 9, 157–160. [Google Scholar] [CrossRef]
- Le Nouail, P.; Viseux, V.; Chaby, G.; Billet, A.; Denoeux, J.P.; Lok, C. Drug reaction with eosinophilia and systemic symptoms (DRESS) following imatinib therapy. Ann. Dermatol. Venereol. 2006, 133, 686–688. [Google Scholar] [CrossRef]
- Oosterom, A.T.; van Judson, I.R.; Verweij, J.; Stroobants, S.; Dumez, H.; Sciot, R.; Van Glabbeke, M.; Dimitrijevic, S.; Nielsen, O.S. Update of phase I study of imatinib (STI5721) in advanced soft tissue sarcoma and gastrointestinal stromal tumors: A report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer 2002, 38, 83–87. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; Fletcher, J.A.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Verweij, J.; Oosterom, A.; van Blay, J.Y.; Judson, I.; Rodenhuis, S.; Radford, J.; Le Cesne, A.; Hogendoorn, P.C.; di Paola, E.D.; Brown, M.; et al. Imatinib mesylate (STI-571 Glivec®, Gleevec™) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft tissue sarcomas that are unselected for a molecular target: Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur. J. Cancer 2003, 39, 2006–2011. [Google Scholar]
- Verweij, J.; Casali, P.G.; Zalcberg, J.; Le Cesne, A.; Reichardt, P.; Blay, J.Y.; Issels, R.; Oosterom, A.; van Hogendoorn, P.C.; Van Glabbeke, M.; et al. Progression-free survival in gastrointestinal stromal tumors with high-dose imatinib: Randomised trial. Lancet 2004, 364, 1127–1134. [Google Scholar] [CrossRef]
- Ugurel, S.; Hildenbrand, R.; Dippel, E.; Hochhaus, A.; Schadendorf, D. Dose-dependent severe cutaneous reactions to imatinib. Br. J. Cancer 2003, 88, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zeng, S.; Metcalfe, D.D.; Akin, C.; Dimitrijevic, S.; Butterfield, J.H.; McMahon, G.; Longley, B.J. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood 2002, 99, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Sadeghi, P.; Pinter-Brown, L.C.; Yashar, S.; Chiu, M.W. Cutaneous side effects of epidermal growth factor receptor inhibitors: Clinical presentation, pathogenesis, and management. J. Am. Acad. Dermatol. 2007, 56, 317–326. [Google Scholar] [CrossRef]
- Bianchini, D.; Jayanth, A.; Chua, Y.J.; Cunningham, D. Epidermal growth factor receptor inhibitor-related skin toxicity: Mechanisms treatment, and its potential role as a predictive marker. Clin. Colorectal. Cancer 2008, 7, 33–43. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Dunn, E.F.; Harari, P.M. Understanding resistance to EGFR inhibitors—Impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010, 7, 493–507. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stańczyk, M.; Klajnert-Maculewicz, B. Przeciwciało monoklonalne trastuzumab i dendrymery w terapii celowanej raka piersi. Postep. Hig. Med. Dosw. 2015, 69, 1313–1324. [Google Scholar] [CrossRef]
- Ziemska, J.; Solecka, J. Tyrosine kinase, aurora kinase and leucine aminopeptidase as attractive drug targets in anticancer therapy—Characterisation of their inhibitors. Rocz. Panstw. Zakl. Hig. 2016, 67, 329–342. [Google Scholar]
- Macdonald, J.B.; Macdonald, B.; Golitz, L.E.; LoRusso, P.; Sekulic, A. Cutaneous adverse effects of targeted therapies. Part I: Inhibitors of the cellular membrane. J. Am. Acad. Dermatol. 2015, 72, 203–218. [Google Scholar] [CrossRef]
- Wang, S.; Cang, S.; Liu, D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 34. [Google Scholar] [CrossRef]
- Pirker, R. Third-generation epidermal growth factor receptor tyrosine kinase inhibitors in advanced non small cell lung cancer. Curr. Opin. Oncol. 2016, 28, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.H. Second-generation irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): A better mousetrap? A review of the clinical evidence. Crit. Rev. Oncol. Hematol. 2012, 83, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.C.; Lin, C.C.; Yang, J.C.H. Second and third-generation EGFR-TKIs in advanced nonsmall cell lung cancer. Curr. Opin. Oncol. 2015, 27, 94–100. [Google Scholar] [CrossRef]
- Peus, D.; Hamacher, L.; Pittelkow, M.R. EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J. Investig. Dermatol. 1997, 109, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Lulli, D.; Girolomoni, G. Epidermal growth factor receptor signalling in keratinocyte biology: Implications for skin toxicity of tyrosine kinase inhibitors. Arch. Toxicol. 2014, 88, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Blaydon, D.C.; Biancheri, P.; Di, W.L.; Plagnol, V.; Cabral, R.M.; Brooke, M.A.; van Heel, D.A.; Ruschendorf, F.; Toynbee, M.; Walne, A.; et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N. Engl. J. Med. 2011, 365, 1502–1508. [Google Scholar] [CrossRef]
- Argiris, A.; Lee, S.C.; Feinstein, T.; Thomas, S.; Branstetter, B.F., 4th; Seethala, R.; Wang, L.; Gooding, W.; Grandis, J.R.; Ferris, R.L. Serum biomarkers as potential predictors of antitumor activity of cetuximab-containing therapy for locally advanced head and neck cancer. Oral. Oncol. 2011, 47, 961–966. [Google Scholar] [CrossRef]
- Kimura, H.; Kasahara, K.; Sekijima, M.; Tamura, T.; Nishio, K. Plasma MIP-1beta levels and skin toxicity in Japanese non-small cell lung cancer patients treated with the EGFR-targeted tyrosine kinase inhibitor, gefitinib. Lung Cancer 2005, 50, 393. [Google Scholar] [CrossRef]
- Kanazawa, S.; Yamaguchi, K.; Kinoshita, Y.; Komiyama, Y.; Muramatsu, M.; Nomura, S. Elevation of soluble interleukin-2 receptor in patients with non-small cell lung cancer treated with gefitinib. J. Cancer Res. Clin. Oncol. 2006, 132, 719–725. [Google Scholar] [CrossRef]
- Gangemi, S.; Franchina, T.; Minciullo, P.L.; Profita, M.; Zanghì, M.; David, A.; Kennez, I.; Adamo, V. IL-33/IL-31 axis: A new pathological mechanisms for EGFR tyrosine kinase inhibitors-associated skin toxicity. J. Cell Biochem. 2013, 114, 2673–2676. [Google Scholar] [CrossRef]
- Reguiai, Z.; Bachet, J.B.; Bachmeyer, C.; Peuvrel, L.; Beylot-Barry, M.; Bezier, M.; Boucher, E.; Chevelle, C.; Colin, P.; Guimbaud, R.; et al. Management of cutaneous adverse events induced by anti-EGFR (epidermal growth factor receptor): A French interdisciplinary therapeutic algorithm. Support Care Cancer 2012, 20, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Anadkat, M.J.; Bensadoun, R.J.; Bryce, J.; Chan, A.; Epstein, J.B.; Eaby-Sandy, B.; Murphy, B.A. MASCC Skin Toxicity Study Group. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011, 19, 1079–1095. [Google Scholar] [CrossRef]
- Osio, A.; Mateus, C.; Soria, J.C.; Massard, C.; Malka, D.; Boige, V.; Besse, B.; Robert, C. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br. J. Dermatol. 2009, 161, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ensslin, C.J.; Rosen, A.C.; Wu, S.; Lacouture, M.E. Pruritus in patients treated with targeted cancer therapies: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 2013, 69, 708–720. [Google Scholar] [CrossRef]
- Swain, S.M.; Kim, S.B.; Cortes, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2- positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Ma, L.; Wu, Z.; Wang, Y. Meta-analysis of dermatological toxicities associated with sorafenib. Clin. Exp. Dermatol. 2011, 36, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomized, open-label, phase 3 trial. Lancet Oncol. 2014, 10, 1065–1075. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Chiang, T.Y.; Hsu, H.C.; Jane, S.W.; Chen, S.C. EGFRI-associated health-related quality of life by severity of skin toxicity in metastatic colorectal cancer patients receiving epidermal growth factor receptor inhibitor target therapy. Support Care Cancer 2020, 28, 4771–4779. [Google Scholar] [CrossRef]
- Annunziata, M.C.; De Stefano, A.; Fabbrocini, G.; Leo, S.; Marchetti, P.; Romano, M.C.; Romano, I. Current Recommendations and Novel Strategies for the Management of Skin Toxicities Related to Anti-EGFR Therapies in Patients with Metastatic Colorectal Cancer. Clin. Drug Investig. 2019, 39, 825–834. [Google Scholar] [CrossRef]
- Holcmann, M.; Sibilia, M. Mechanisms underlying skin disorders induced by EGFR inhibitors. Mol. Cell Oncol. 2015, 2, e1004969. [Google Scholar] [CrossRef] [PubMed]
- Mittmann, N.; Seung, S.J. Rash rates with EGFR inhibitors: Meta-analysis. Curr. Oncol. 2011, 18, e54–e63. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; Lee, J.; Lim, J.; Park, J.S.; Kim, M.; Kim, T.Y.; Kim, T.M.; Lee, K.H.; Keam, B.; Han, S.W.; et al. Pruritus in Patients Under Targeted Anticancer Therapy: A Multidimensional Analysis Using the 5-D Itch Scale. Acta Derm. Venereol. 2019, 99, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Beech, J.; Germetaki, T.; Judge, M.; Paton, N.; Collins, J.; Garbutt, A.; Braun, M.; Fenwick, J.; Saunders, M.P. Management and grading of EGFR inhibitor-induced cutaneous toxicity. Future Oncol. 2018, 14, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, R.D.; Deplanque, G.; Komatsu, Y.; Kobayashi, Y.; Ocvirk, J.; Racca, P.; Guenther, S.; Zhang, J.; Lacouture, M.E.; Jatoi, A. Recommendations for the prophylactic management of skin reactions induced by epidermal growth factor receptor inhibitors in patients with solid tumors. Oncologist 2016, 21, 1483–1491. [Google Scholar] [CrossRef]
- Kozuki, T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn. J. Clin. Oncol. 2016, 46, 291–298. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Anadkat, M.; Jatoi, A.; Garawin, T.; Bohac, C.; Mitchell, E. Dermatologic toxicity occurring during anti-EGFR monoclonal inhibitor therapy in patients with metastatic colorectal cancer: A systematic review. Clin. Colorectal. Cancer 2018, 17, 85–96. [Google Scholar] [CrossRef]
- Porock, D. Factors influencing the severity of radiation skin and oralmucosal reactions: Development of a conceptual framework. Eur. J. Cancer Care 2002, 11, 33–43. [Google Scholar]
- Bentzen, S.M.; Saunders, M.I.; Dische, S.; Bond, S.J. Radiotherapy-related early morbidity in head and neck cancer: Quantitative clinical radiobiology as deduced from the CHART trial. Radiother. Oncol. 2001, 60, 123–135. [Google Scholar] [CrossRef]
- Wong, A.S.C.; Soo, R.A.; Lu, J.J.; Loh, K.S.; Tan, K.S.; Hsieh, W.S.; Shakespeare, T.P.; Chua, E.T.; Lim, H.L.; Goh, B.C. Paclitaxel, 5-fluorouracil and hydroxyurea concurrent with radiation in locally advanced nasopharyngeal carcinoma. Ann. Oncol. 2006, 17, 1152–1157. [Google Scholar] [CrossRef]
- Pryor, D.I.; Porceddu, S.V.; Burmeister, B.H.; Guminski, A.; Thomson, D.B.; Shepherdson, K.; Poulsen, M. Enhanced toxicity with concurrent Cetuximab and radiotherapy in head and neck cancer. Radiother. Oncol. 2009, 90, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Russi, E.G.; Moretto, F.; Rampino, M.; Benasso, M.; Bacigalupo, A.; De Sanctis, V.; Numico, G.; Bossi, P.; Buglione, M.; Lombardo, A.; et al. Acute skin toxicity management in head and neck cancer patients treated with radiotherapy and chemotherapy or EGFR inhibitors: Literature review and consensus. Crit. Rev. Oncol. Hematol. 2015, 96, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.J.; Yang, J.I.; Kim, B.K.; Cho, K.H. Clinical differentiation of acute cutaneous graft-versus-host disease from drug hypersensitivity reactions. J. Am. Acad. Dermatol. 2011, 65, 726–732. [Google Scholar] [CrossRef]
- Klager, S.; Lacouture, M.E.; Hannum, M.; Devlin, S.M.; Maloy, M.; Pulitzer, M.; Jakubowski, A.A.; Markova, A. Drugs as a Frequent Cause of Acute Rash in Patients after CD34+-Selected Peripheral Blood Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Vallely, J.J.; Hudson, K.E.; Locke, S.C.; Wolf, S.P.; Samsa, G.P.; Abernethy, A.P.; LeBlanc, T.W. Pruritus in patients with solid tumors: An overlooked supportive care need. Support Care Cancer 2019, 27, 3897–3904. [Google Scholar] [CrossRef]
- Penna, G.; Allegra, A.; Romeo, G.; Alonci, A.; Cannavò, A.; Russo, S.; D’Angelo, A.; Petrungaro, A.; Musolino, C. Severe dermatologic adverse reactions after exposure to lenalidomide in multiple myeloma patients with a positive HLA-DRB1*1501 and HLA-DQB1*0602. Acta Oncol. 2012, 51, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Imbesi, S.; Allegra, A.; Calapai, G.; Musolino, C.; Gangemi, S. Cutaneous adverse reactions to lenalidomide. Allergol. Immunopathol. 2015, 43, 88–91. [Google Scholar] [CrossRef] [PubMed]
| Drug | Mechanisms | Characteristics | Ref. |
|---|---|---|---|
| Paclitaxel | Skin lesions | Acute pruritus | [23,24,25,26] |
| Nab-paclitaxel | Skin lesions induced by albumin constituents | [24,25,27,28,29] | |
| Chlorambucil | Delayed reaction | [30] | |
| Cytarabine | Immune reactions Epithelial toxicity Changes in keratinocyte activities | [31,32,33,34,35,36] | |
| Imatinib | Inhibition of PDGF receptor on dermal mast cells Release of Il-33 and IL-31 | [37] [38] | |
| Epidermal Growth Factor Receptor inhibitors | Alteration of epidermal homeostasis Effect on keratinocyte apoptosis Increased release of chemokines | Acute pruritus | [39,40] [41,42] [43,44,45] |
| Erlotinib | Increase of mast cells Changes in keratinocyte activities Release of IL-1, TNF, IL-8 | Acute pruritus | [46] [39] [47,48] |
| Lapatinib | Unidentified mechanism | Acute pruritus | [49] |
| Immune checkpoint inhibitors | Release of inflammatory cytokine (IL6, IL-10) | Acute pruritus | [50] |
| Anti-PD1 | Release of perforin1, granzyme B, CXCL9, CXCL10, CXCL11 | [51] | |
| Pembrolizumab | Chronic pruritus | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, A.; Di Salvo, E.; Casciaro, M.; Musolino, C.; Pioggia, G.; Gangemi, S. The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients. Biomedicines 2021, 9, 2. https://doi.org/10.3390/biomedicines9010002
Allegra A, Di Salvo E, Casciaro M, Musolino C, Pioggia G, Gangemi S. The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients. Biomedicines. 2021; 9(1):2. https://doi.org/10.3390/biomedicines9010002
Chicago/Turabian StyleAllegra, Alessandro, Eleonora Di Salvo, Marco Casciaro, Caterina Musolino, Giovanni Pioggia, and Sebastiano Gangemi. 2021. "The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients" Biomedicines 9, no. 1: 2. https://doi.org/10.3390/biomedicines9010002
APA StyleAllegra, A., Di Salvo, E., Casciaro, M., Musolino, C., Pioggia, G., & Gangemi, S. (2021). The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients. Biomedicines, 9(1), 2. https://doi.org/10.3390/biomedicines9010002







