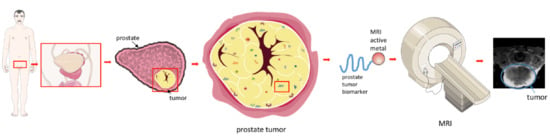
Molecular MR Imaging of Prostate Cancer
Abstract
:1. Introduction
2. Pathophysiology of Prostate Carcinoma
3. Clinical Diagnosis
4. Molecular Magnetic Resonance Imaging (MRI)
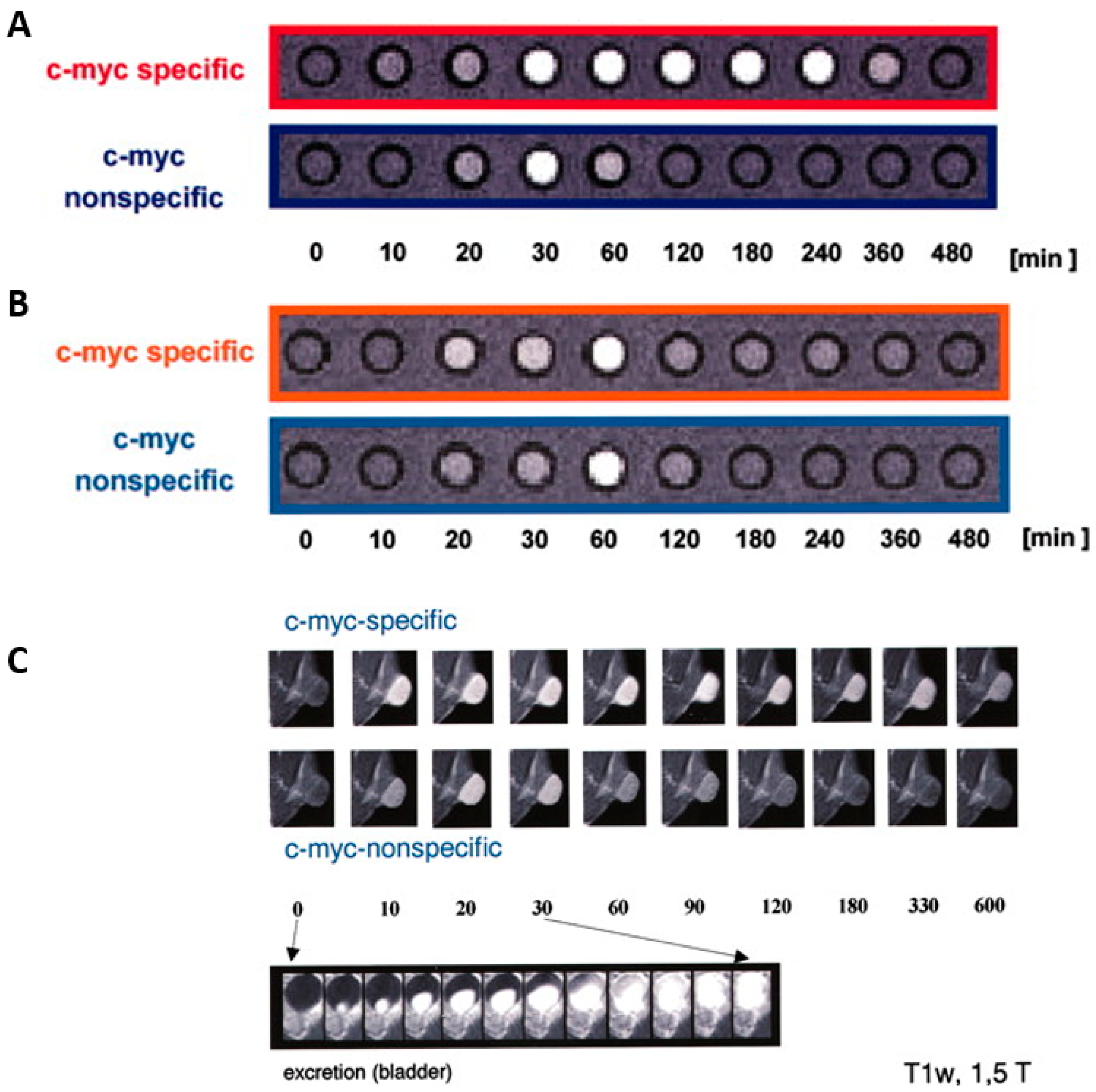
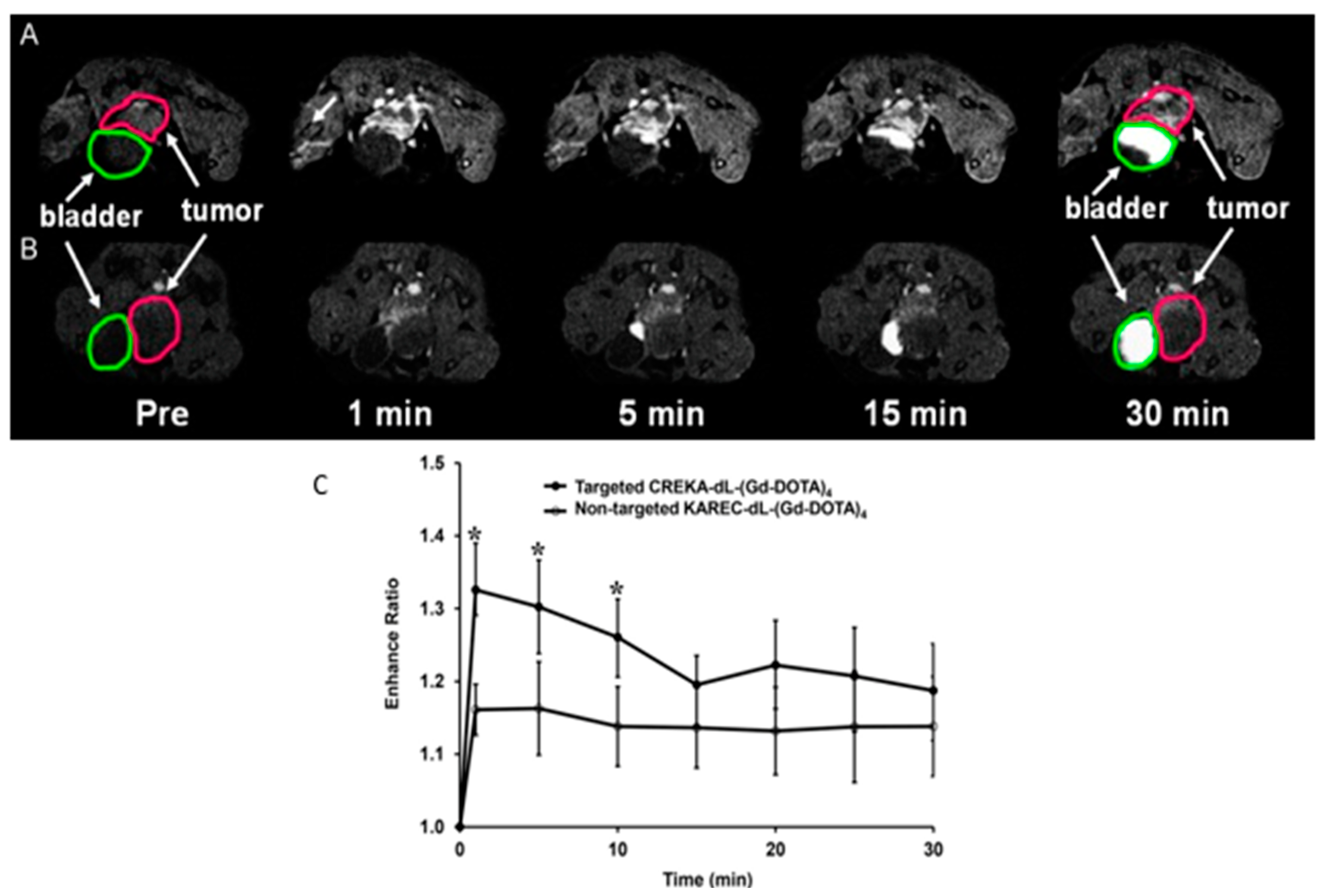
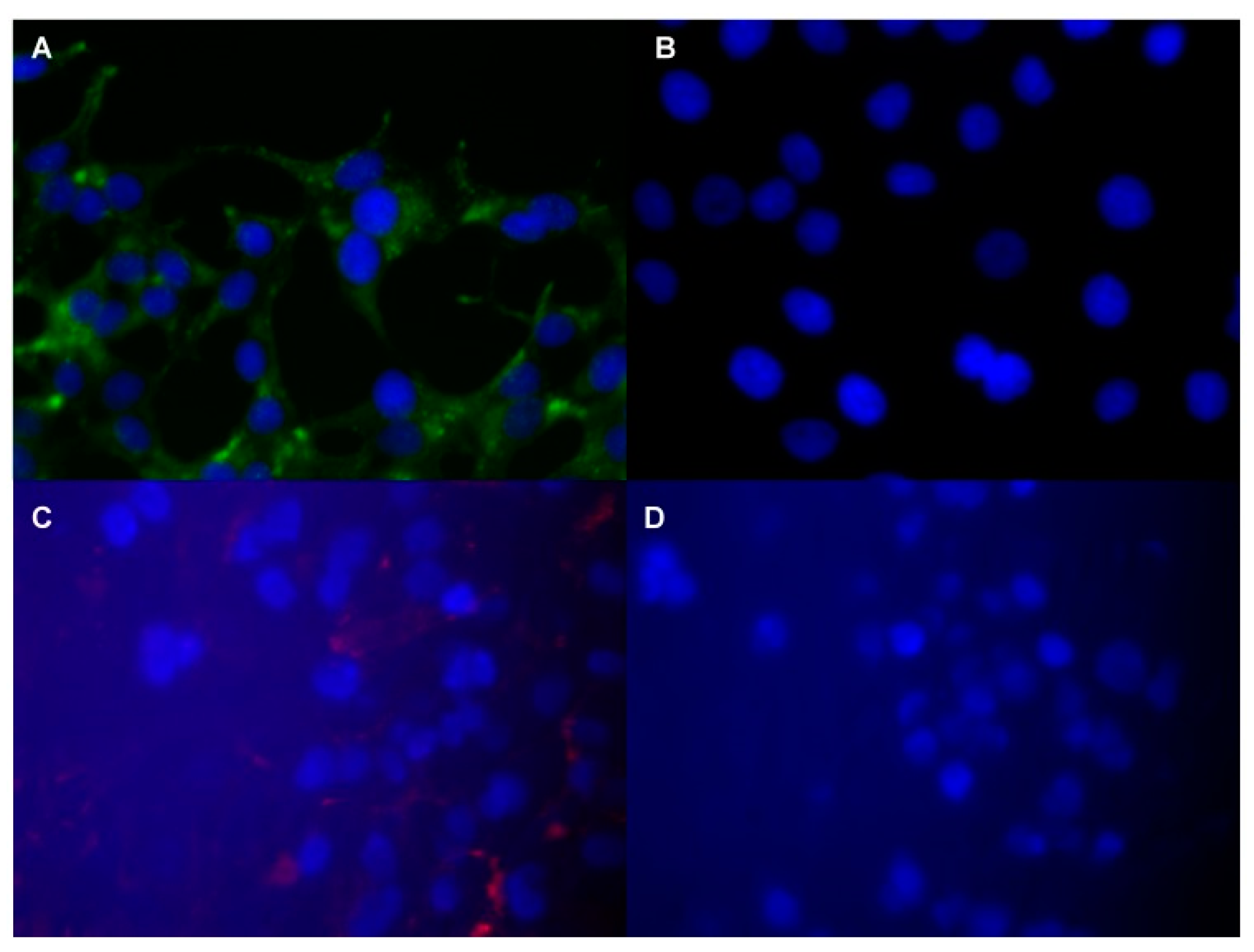
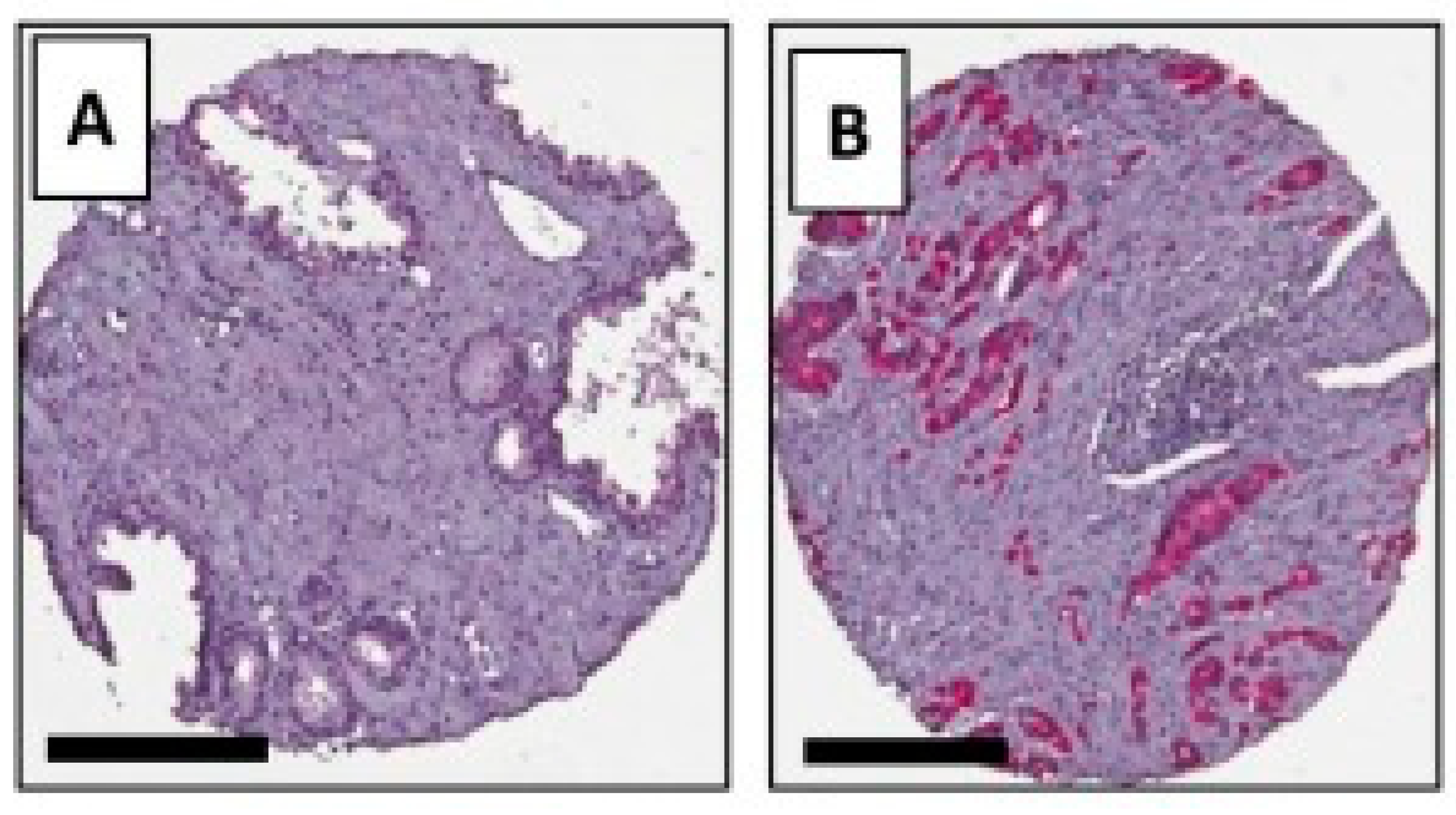
4.1. Peptide-Based Contrast Medium
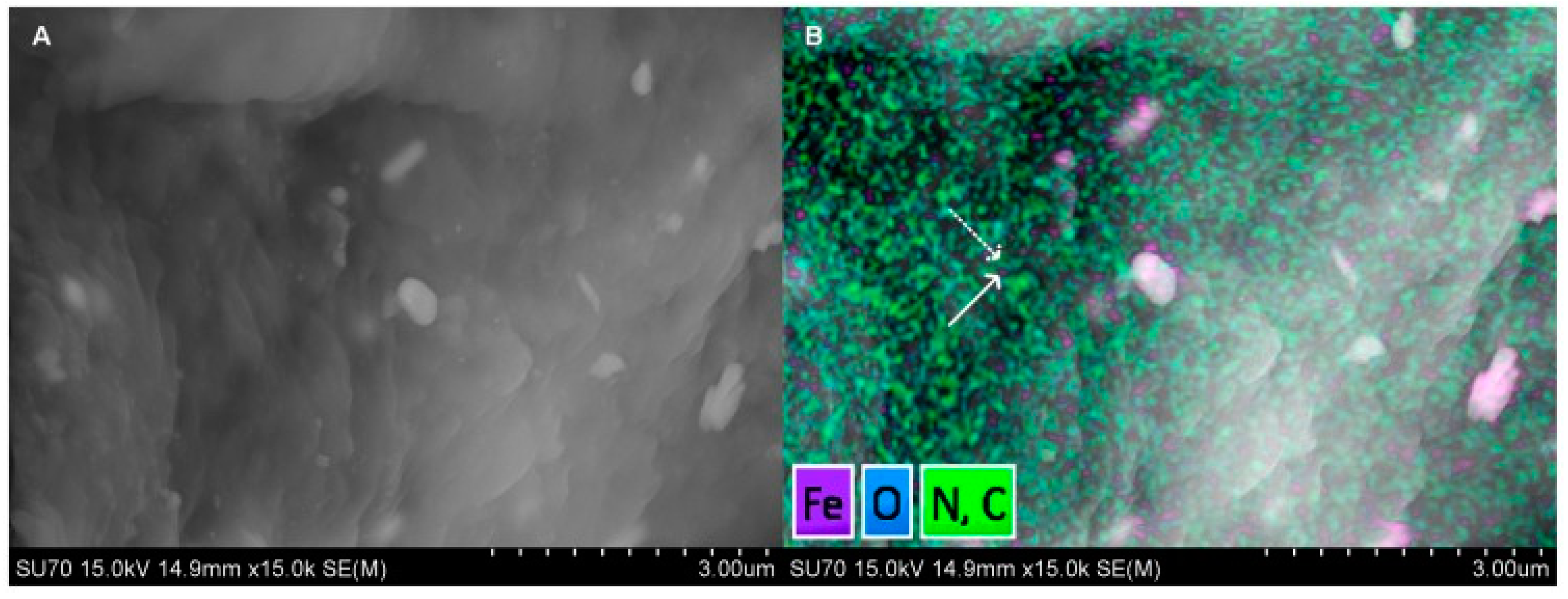
4.2. Iron Oxide Nanoparticle
4.3. New Potential Biomarkers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Forrest, M.S.; Edwards, S.M.; Houlston, R.; Kote-Jarai, Z.; Allen, N.; Knowles, M.A.; Turner, F.; Ardern-Jones, A.; Murkin, A.; Williams, S.; et al. Association between hormonal genetic polymorphisms and early-onset prostate cancer. Prostate Cancer Prostatic Dis. 2005, 8, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Breslow, N.; Chan, C.W.; Dhom, G.; Drury, R.A.; Franks, L.M.; Gellei, B.; Lee, Y.S.; Lundberg, S.; Sparke, B.; Sternby, N.H.; et al. Latent carcinoma of prostate at autopsy in seven areas. Collaborative study organized by the International Agency for Research on Cancer, Lyons, France. Int. J. Cancer 1977, 20, 680–688. [Google Scholar] [CrossRef]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Yatani, R.; Henderson, B.E.; Mack, T.M. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Gary, D.; Steinberg, B.S.C.; Terri, H.; Beaty, B.C.; Walsh, P.C. Family history and the risk of prostate cancer. Prostate 1990, 17, 337–347. [Google Scholar] [CrossRef]
- Ost, P.; Bossi, A.; Decaestecker, K.; De Meerleer, G.; Giannarini, G.; Karnes, R.J.; Roach, M.; Briganti, A. Metastasis-directed Therapy of Regional and Distant Recurrences After Curative Treatment of Prostate Cancer: A Systematic Review of the Literature. Eur. Urol. 2015, 67, 852–863. [Google Scholar] [CrossRef]
- Sharma, S.; Zapatero-Rodríguez, J.; O’Kennedy, R.J. Prostate cancer diagnostics: Clinical challenges and the ongoing need for disruptive and effective diagnostic tools. Biotechnol. Adv. 2017, 35, 135–149. [Google Scholar] [CrossRef]
- Sharma, S. Imaging and intervention in prostate cancer: Current perspectives and future trends. Indian J. Radiol. Imaging 2014, 24, 139–148. [Google Scholar] [CrossRef]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef] [Green Version]
- Green, S.M.; Mostaghel, E.A.; Nelson, P.S. Androgen action and metabolism in prostate cancer. Mol. Cell. Endocrinol. 2012, 360, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Huggins, C.; Hodges, C.V. Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. Cancer Res. 1941, 1, 293–297. [Google Scholar] [CrossRef]
- Imamoto, T.; Suzuki, H.; Yano, M.; Kawamura, K.; Kamiya, N.; Araki, K.; Komiya, A.; Nihei, N.; Naya, Y.; Ichikawa, T. The role of testosterone in the pathogenesis of prostate cancer. Int. J. Urol. 2008, 15, 472–480. [Google Scholar] [CrossRef]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, E.C.; So, A.Y.; Chaivorapol, C.; Haqq, C.M.; Li, H.; Yamamoto, K.R. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007, 21, 2005–2017. [Google Scholar] [CrossRef] [Green Version]
- Michaud, J.E.; Billups, K.L.; Partin, A.W. Testosterone and prostate cancer: An evidence-based review of pathogenesis and oncologic risk. Ther. Adv. Urol. 2015, 7, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhao, D.; Spring, D.J.; Depinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [Green Version]
- Van Leenders, G.J.L.H.; Schalken, J.A. Epithelial cell differentiation in the human prostate epithelium: Implications for the pathogenesis and therapy of prostate cancer. Crit. Rev. Oncol. 2003, 46, 3–10. [Google Scholar] [CrossRef]
- Schrecengost, R.; Knudsen, K.E. Molecular Pathogenesis and Progression of Prostate Cancer. Semin. Oncol. 2013, 40, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Wang, S. Prognostic role of tumor-infiltrating CD57-positive lymphocytes in solid tumors: A meta-analysis. Oncotarget 2017, 9, 8111–8119. [Google Scholar] [CrossRef] [Green Version]
- Gurel, B.; Ali, T.Z.; Montgomery, E.A.; Begum, S.; Hicks, J.; Goggins, M.; Eberhart, C.G.; Clark, D.P.; Bieberich, C.J.; Epstein, J.I.; et al. NKX3.1 as a Marker of Prostatic Origin in Metastatic Tumors. Am. J. Surg. Pathol. 2010, 34, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Okada, H.; Tsubura, A.; Okamura, A.; Senzaki, H.; Naka, Y.; Komatz, Y.; Morii, S. Keratin profiles in normal/hyperplastic prostates and prostate carcinoma. Virchows Arch. 1992, 421, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Van Leenders, G.J.L.H.; Gage, W.R.; Hicks, J.L.; Van Balken, B.; Aalders, T.W.; Schalken, J.A.; De Marzo, A.M. Intermediate Cells in Human Prostate Epithelium Are Enriched in Proliferative Inflammatory Atrophy. Am. J. Pathol. 2003, 162, 1529–1537. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.L.; Haffner, M.C.; Esopi, D.M.; Vaghasia, A.M.; Giannico, G.A.; Ross, H.M.; Ghosh, S.; Hicks, J.L.; Zheng, Q.; Sangoi, A.R.; et al. Prostate adenocarcinomas aberrantly expressing p63 are molecularly distinct from usual-type prostatic adenocarcinomas. Mod. Pathol. 2015, 28, 446–456. [Google Scholar]
- Li, W.; Qian, L.; Lin, J.; Huang, G.; Hao, N.; Wei, X.; Wang, W.; Liang, J. CD44 regulates prostate cancer proliferation, invasion and migration via PDK1 and PFKFB4. Oncotarget 2017, 8, 65143–65151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martignano, F.; Gurioli, G.; Salvi, S.; Calistri, D.; Costantini, M.; Gunelli, R.; De Giorgi, U.; Foca, F.; Casadio, V. GSTP1 Methylation and Protein Expression in Prostate Cancer: Diagnostic Implications. Dis. Mark. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.L.; Huang, Y.-F.; You, L.-R.; Chao, N.-C.; Su, F.-Y.; Chang, J.-L.; Chen, C.-M. Conditionally Ablated Pten in Prostate Basal Cells Promotes Basal-to-Luminal Differentiation and Causes Invasive Prostate Cancer in Mice. Am. J. Pathol. 2013, 182, 975–991. [Google Scholar] [CrossRef]
- Song, Z.; Huang, Y.; Zhao, Y.; Ruan, H.; Yang, H.; Cao, Q.; Liu, D.; Zhang, X.; Chen, K. The Identification of Potential Biomarkers and Biological Pathways in Prostate Cancer. J. Cancer 2019, 10, 1398–1408. [Google Scholar] [CrossRef]
- Saha, A.K.; Contreras-Galindo, R.; Niknafs, Y.S.; Iyer, M.; Qin, T.; Padmanabhan, K.; Siddiqui, J.; Palande, M.; Wang, C.; Qian, B.; et al. The role of the histone H3 variant CENPA in prostate cancer. J. Biol. Chem. 2020, 295, 8537–8549. [Google Scholar] [CrossRef]
- Zhang, Z.; Chai, C.; Shen, T.; Li, X.; Ji, J.; Li, C.; Shang, Z.; Niu, Y. Aberrant KIF20A Expression Is Associated with Adverse Clinical Outcome and Promotes Tumor Progression in Prostate Cancer. Dis. Mark. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, W.; Hong, B.; Jiang, X.; Sun, R.; Yan, Q.; Zhang, S.; Lu, M.-D.; Wang, S.; Zhang, Z.; et al. Upregulation of KIF20A correlates with poor prognosis in gastric cancer. Cancer Manag. Res. 2018, 10, 6205–6216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; He, W.; Shi, Y.; Gu, H.; Li, M.; Liu, Z.; Feng, Y.; Zheng, N.; Xie, C.; Zhang, Y. High Expression of KIF20A Is Associated with Poor Overall Survival and Tumor Progression in Early-Stage Cervical Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0167449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.X.; Duan, Z.; Wang, J.; Sokolov, A.; Xu, J.; Chen, C.Z.; Li, J.J.; Chen, H.W. Kinesin family deregulation coordinated by bromodomain protein ANCCA and histone methyltransferase MLL for breast cancer cell growth, survival, and tamoxifen resistance. Mol. Cancer Res. 2014, 12, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.N.; Xia, S.J. Stroma-epithelium crosstalk in prostate cancer. Asian J. Androl. 2009, 11, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature Cell Biol. 2004, 432, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Moses, H.L. Tumor-stroma interactions. Curr. Opin. Genet. Dev. 2005, 15, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Alkasalias, T.; Moyano-Galceran, L.; Arsenian-Henriksson, M.; Lehti, K. Fibroblasts in the Tumor Microenvironment: Shield or Spear? Int. J. Mol. Sci. 2018, 19, 1532. [Google Scholar] [CrossRef] [Green Version]
- Bonollo, F.; Thalmann, G.N.; Julio, M.K.D.; Karkampouna, S. The Role of Cancer-Associated Fibroblasts in Prostate Cancer Tumorigenesis. Cancers 2020, 12, 1887. [Google Scholar] [CrossRef]
- Lo, C.H.; Lynch, C.C. Multifaceted Roles for Macrophages in Prostate Cancer Skeletal Metastasis. Front. Endocrinol. 2018, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, M.; Masieri, L.; Raspollini, M.R.; Minervini, A.; Mari, A.; Comito, G.; Giannoni, E.; Carini, M.; Chiarugi, P.; Serni, S. The Role of M1 and M2 Macrophages in Prostate Cancer in relation to Extracapsular Tumor Extension and Biochemical Recurrence after Radical Prostatectomy. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.-O.; Andrén, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Cihan, Y.B.; Arslan, A.; Ergul, M.A. Subtypes of White Blood Cells in Patients with Prostate Cancer or Benign Prostatic Hyperplasia and Healthy Individuals. Asian Pac. J. Cancer Prev. 2013, 14, 4779–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhyatma, K.P.; Prapiska, F.F.; Siregar, G.P.; Warli, S.M. Systemic Inflammatory Response in Predicting Prostate Cancer: The Diagnostic Value of Neutrophil-To-Lymphocyte Ratio. Open Access Maced. J. Med. Sci. 2019, 7, 1628–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norrby, K. Mast cells and Angiogenesis. APMIS 2002, 110, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qian, J.; Zeng, F.; Li, S.; Guo, W.; Chen, L.; Li, G.; Zhang, Z.; Wang, Q.J.; Deng, F. Protein kinase Ds promote tumor angiogenesis through mast cell recruitment and expression of angiogenic factors in prostate cancer microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taverna, G.; Giusti, G.; Seveso, M.; Hurle, R.; Colombo, P.; Stifter, S.; Grizzi, F. Mast Cells as a Potential Prognostic Marker in Prostate Cancer. Dis. Mark. 2013, 35, 711–720. [Google Scholar] [CrossRef]
- Johansson, A.; Rudolfsson, S.; Hammarsten, P.; Halin, S.; Pietras, K.; Jones, J.; Stattin, P.; Egevad, L.; Granfors, T.; Wikström, P.; et al. Mast Cells Are Novel Independent Prognostic Markers in Prostate Cancer and Represent a Target for Therapy. Am. J. Pathol. 2010, 177, 1031–1041. [Google Scholar] [CrossRef]
- Georgiou, H.D.; Namdarian, B.; Corcoran, N.M.; Costello, A.J.; Hovens, C.M. Circulating endothelial cells as biomarkers of prostate cancer. Nat. Clin. Pract. Urol. 2008, 5, 445–454. [Google Scholar] [CrossRef]
- Zhao, R.; Bei, X.; Yang, B.; Wang, X.; Jiang, C.; Shi, F.; Wang, X.; Zhu, Y.; Jing, Y.; Han, B.; et al. Endothelial cells promote metastasis of prostate cancer by enhancing autophagy. J. Exp. Clin. Cancer Res. 2018, 37, 221. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.L.; Okamoto, O.K. Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kruslin, B.; Ulamec, M.; Tomas, D. Prostate cancer stroma: An important factor in cancer growth and progression. Bosn. J. Basic Med. Sci. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Ohori, M.; Wheeler, T.M.; Scardino, P.T. The New American Joint Committee on Cancer and International Union Against Cancer TNM Classification of Prostate Cancer. Cancer 1994, 74, 104–114. [Google Scholar] [CrossRef]
- Wibmer, A.G.; Burger, I.A.; Sala, E.; Hrical, H.; Weber, W.A.; Vargas, H.A. Molecular Imaging of Prostate Cancer. Radiographics 2016, 36, 142–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaghefi, H.; Magi-Galluzzi, C.; Klein, E.A. Local recurrence of prostate cancer in rectal submucosa after transrectal needle biopsy and radical prostatectomy. Urology 2005, 66, 881.e7–881.e9. [Google Scholar] [CrossRef] [PubMed]
- Lane, Z.; Epstein, J.I.; Ayub, S.; Netto, G.J. Prostatic adenocarcinoma in colorectal biopsy: Clinical and pathologic features. Hum. Pathol. 2008, 39, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Panella, P.; D’Arrigo, L.; Savoca, F.; Pennisi, M.; Aragona, F. Should Men with Serum Prostate-Specific Antigen ≤ 4 ng/mL and Normal Digital Rectal Examination Undergo a Prostate Biopsy? A literature review. Oncology 2006, 70, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gallee, M.P.W.; Visser-de Jong, E.; Van der Korput, J.A.G.M.; van der Kwast, T.H.; Ten Kate, F.J.W.; Schroeder, F.H.; Trapman, J. Variation of prostate-specific antigen expression in different tumour growth patterns present in prostatectomy specimens. Urol. Res. 1990, 18, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Du, Y.; Yang, H.; Huang, Y.; Meng, J.; Xiao, D. Magnetic resonance imaging for prostate cancer clinical application. Chin. J. Cancer Res. 2013, 25, 240–249. [Google Scholar] [CrossRef]
- De Visschere, P.; Oosterlinck, W.; De Meerleer, G.; Villeirs, G. Clinical and imaging tools in the early diagnosis of prostate cancer, a Review. J. Belg. Radiol. 2010, 93, 62. [Google Scholar] [CrossRef] [Green Version]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.A.; Margolis, D.J.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Becker, A.S.; Cornelius, A.; Reiner, C.S.; Stocker, D.; Ulbrich, E.J.; Barth, B.K.; Mortezavi, A.; Eberli, D.; Donati, O.F. Direct comparison of PI-RADS version 2 and version 1 regarding interreader agreement and diagnostic accuracy for the detection of clinically significant prostate cancer. Eur. J. Radiol. 2017, 94, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mangarova, D.B.; Brangsch, J.; Kader, A.; Hamm, B.; Brenner, W.; Makowski, M.R. Correlation between Intraprostatic PSMA Uptake and MRI PI-RADS of [(68)Ga]Ga-PSMA-11 PET/MRI in Patients with Prostate Cancer: Comparison of PI-RADS Version 2.0 and PI-RADS Version 2.1. Cancers 2020, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Asbach, P.; Ro, S.-R.; Aldoj, N.; Snellings, J.; Reiter, R.; Lenk, J.; Köhlitz, T.; Haas, M.; Guo, J.; Hamm, B.; et al. In Vivo Quantification of Water Diffusion, Stiffness, and Tissue Fluidity in Benign Prostatic Hyperplasia and Prostate Cancer. Investig. Radiol. 2020, 55, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Bloch, B.N.; Lenkinski, R.E.; Rofsky, N.M. The role of magnetic resonance imaging (MRI) in prostate cancer imaging and staging at 1.5 and 3 Tesla: The Beth Israel Deaconess Medical Center (BIDMC) approach. Cancer Biomark. 2008, 4, 251–262. [Google Scholar] [CrossRef] [Green Version]
- De Bazelaire, C.; Calmon, R.; Thomassin-Naggara, I.; Brunon, C.; Hamy, A.-S.; Fournier, L.S.; Balvay, D.; Espié, M.; Siauve, N.; Clément, O.; et al. Accuracy of perfusion MRI with high spatial but low temporal resolution to assess invasive breast cancer response to neoadjuvant chemotherapy: A retrospective study. BMC Cancer 2011, 11, 361. [Google Scholar] [CrossRef] [Green Version]
- Pinker, K.; Grabner, G.; Bogner, W.; Gruber, S.; Szomolanyi, P.; Trattnig, S.; Heinz-Peer, G.; Weber, M.; Fitzal, F.; Pluschnig, U.; et al. A Combined High Temporal and High Spatial Resolution 3 Tesla MR Imaging Protocol for the Assessment of Breast Lesions. Investig. Radiol. 2009, 44, 553–558. [Google Scholar] [CrossRef]
- Khawaja, A.Z.; Cassidy, D.B.; Al Shakarchi, J.; McGrogan, D.G.; Inston, N.G.; Jones, R.G. Revisiting the risks of MRI with Gadolinium based contrast agents—Review of literature and guidelines. Insights Imaging 2015, 6, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Clough, T.J.; Jiang, L.; Wong, K.L.; Long, N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Moon, W.J. Gadolinium Deposition in the Brain: Current Updates. Korean J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef]
- Robert, P.; Frenzel, T.; Factor, C.; Jost, G.; Rasschaert, M.; Schuetz, G.; Fretellier, N.; Boyken, J.; Idée, J.-M.; Pietsch, H. Methodological Aspects for Preclinical Evaluation of Gadolinium Presence in Brain Tissue. Investig. Radiol. 2018, 53, 499–517. [Google Scholar] [CrossRef]
- Swaminathan, S.; High, W.; Ranville, J.; Horn, T.; Hiatt, K.; Thomas, M.; Brown, H.; Shah, S. Cardiac and vascular metal deposition with high mortality in nephrogenic systemic fibrosis. Kidney Int. 2008, 73, 1413–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Hesse, B.; Roman, M.; Stier, D.; Castillo-Michel, H.; Cotte, M.; Suuronen, J.P.; Lagrange, A.; Radbruch, H.; Paul, F.; et al. Increased Retention of Gadolinium in the Inflamed Brain After Repeated Administration of Gadopentetate Dimeglumine: A Proof-of-Concept Study in Mice Combining ICP-MS and Micro- and Nano-SR-XRF. Investig. Radiol. 2019, 54, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Wiginton, C.D.; Kelly, B.; Oto, A.; Jesse, M.; Aristimuno, P.; Ernst, R.; Chaljub, G. Gadolinium-Based Contrast Exposure, Nephrogenic Systemic Fibrosis, and Gadolinium Detection in Tissue. Am. J. Roentgenol. 2008, 190, 1060–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho, M.; Ramalho, J.; Burke, L.M.; Semelka, R.C. Gadolinium Retention and Toxicity—An Update. Adv. Chronic Kidney Dis. 2017, 24, 138–146. [Google Scholar] [CrossRef]
- Kay, J.; Bazari, H.; Avery, L.L.; Koreishi, A.F. Case 6-2008: A 46-Year-Old Woman with Renal Failure and Stiffness of the Joints and Skin. N. Engl. J. Med. 2008, 358, 827–838. [Google Scholar] [CrossRef]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magnet. Reson. Imaging: An. Off. J. Int. Soc. Magnet. Reson. Med. 2009, 30, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Sieber, M.A.; Lengsfeld, P.; Frenzel, T.; Golfier, S.; Schmitt-Willich, H.; Siegmund, F.; Walter, J.; Weinmann, H.-J.; Pietsch, H. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur. Radiol. 2008, 18, 2164–2173. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, A.H.; Zhao, Y.; Farooq, Z.; Prince, M. Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology 2018, 286, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Sage, E.H. Regulation of interactions between cells and extracellular matrix: A command performance on several stages. J. Clin. Investig. 2001, 107, 781–783. [Google Scholar] [CrossRef] [Green Version]
- Brady-Kalnay, S.M. Molecular mechanisms of cancer cell-cell interactions: Cell-cell adhesion-dependent signaling in the tumor microenvironment. Cell Adhes. Migrat. 2012, 6, 344–345. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, K.; Szczylik, C.; Bielecka, Z.F.; Bartnik, E.; Porta, C.; Lian, F.; Czarnecka, A.M. The role of the cell-cell interactions in cancer progression. J. Cell. Mol. Med. 2015, 19, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungefroren, H.; Sebens, S.; Seidl, D.; Lehnert, H.; Hass, R. Interaction of tumor cells with the microenvironment. Cell Commun. Signal. 2011, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerardinis, R.; Thompson, C.B. Cellular metabolism and disease: What do metabolic outliers teach us? Cell 2012, 148, 1132–1144. [Google Scholar] [CrossRef] [Green Version]
- Fadaka, A.O.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Rinaldi, G.; Rossi, M.; Fendt, S.M. Metabolic interactions in cancer: Cellular metabolism at the interface between the microenvironment, the cancer cell phenotype and the epigenetic landscape. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1397. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.W.L.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2016, 14, 169–186. [Google Scholar] [CrossRef]
- Yang, J.J.; Yang, J.; Wei, L.; Zurkiya, O.; Yang, W.; Li, S.; Zou, J.; Zhou, Y.; Maniccia, A.L.W.; Mao, H.; et al. Rational Design of Protein-Based MRI Contrast Agents. J. Am. Chem. Soc. 2008, 130, 9260–9267. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Xie, J.; Chen, X. Peptide-Based Probes for Targeted Molecular Imaging. Biochemistry 2010, 49, 1364–1376. [Google Scholar] [CrossRef] [Green Version]
- Yoo, B.; Pagel, M.D. An overview of responsive MRI contrast agents for molecular imaging. Front. Biosci. 2008, 13, 1733–1752. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Li, S.; Yang, J.; Ye, Y.; Zou, J.; Wang, L.; Long, R.; Zurkiya, O.; Zhao, T.; Johnson, J.; et al. Protein-based MRI contrast agents for molecular imaging of prostate cancer. Mol. Imaging Biol. 2011, 13, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, F.; Qiao, J.; Xue, S.; Yang, H.; Patel, A.; Wei, L.; Hekmatyar, K.; Salarian, M.; Grossniklaus, H.E.; Liu, Z.-R.; et al. GRPR-targeted Protein Contrast Agents for Molecular Imaging of Receptor Expression in Cancers by MRI. Sci. Rep. 2015, 5, 16214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelio, D.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Elshafae, S.M.; Hassan, B.B.; Supsavhad, W.; Dirksen, W.P.; Camiener, R.Y.; Ding, H.; Tweedle, M.F.; Rosol, T.J. Gastrin-releasing peptide receptor (GRPr) promotes EMT, growth, and invasion in canine prostate cancer. Prostate 2016, 76, 796–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, F.; Salarian, M.; Xue, S.; Qiao, J.; Feng, J.; Tan, S.; Patel, A.; Li, X.; Mamouni, K.; Hekmatyar, K.; et al. Prostate-specific membrane antigen targeted protein contrast agents for molecular imaging of prostate cancer by MRI. Nanoscale 2016, 8, 12668–12682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckl, S.; Pipkorn, R.; Waldeck, W.; Spring, H.; Jenne, J.; Von Der Lieth, C.-W.; Corban-Wilhelm, H.; Debus, J.; Braun, K. Intracellular visualization of prostate cancer using magnetic resonance imaging. Cancer Res. 2003, 63, 4766–4772. [Google Scholar]
- Wu, X.; Yu, G.; Lindner, D.; Brady-Kalnay, S.M.; Zhang, Q.; Lu, Z.-R. Peptide targeted high-resolution molecular imaging of prostate cancer with MRI. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 525. [Google Scholar]
- Tan, M.; Burden-Gulley, S.M.; Li, W.; Wu, X.; Lindner, D.; Brady-Kalnay, S.M.; Gulani, V.; Lu, Z.-R. MR molecular imaging of prostate cancer with a peptide-targeted contrast agent in a mouse orthotopic prostate cancer model. Pharm. Res. 2012, 29, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Petrini, I.; Barachini, S.; Carnicelli, V.; Galimberti, S.; Modeo, L.; Boni, R.; Sollini, M.; Erba, P.A. ED-B bronectin expression is a marker of epithelial-mesenchymal transition in translational oncology. Oncotarget 2017, 8, 4914–4921. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Han, Z.; Roelle, S.; Desanto, A.; Sabatelle, R.; Schur, R.; Lu, Z.-R. Synthesis and Assessment of Peptide Gd–DOTA Conjugates Targeting Extradomain B Fibronectin for Magnetic Resonance Molecular Imaging of Prostate Cancer. Mol. Pharm. 2017, 14, 3906–3915. [Google Scholar] [CrossRef]
- Stoll, G.; Bendszus, M. New approaches to neuroimaging of central nervous system inflammation. Curr. Opin. Neurol. 2010, 23, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Hamm, B.; Brenner, W.; Makowski, M.R. Lesion-to-background ratio threshold value of SUVmax of simultaneous [(68)Ga]Ga-PSMA-11 PET/MRI imaging in patients with prostate cancer. Insights Imaging 2020, 11, 137. [Google Scholar] [CrossRef]
- Minner, S.; Wittmer, C.; Graefen, M.; Salomon, G.; Steuber, T.; Haese, A.; Huland, H.; Bokemeyer, C.; Yekebas, E.; Dierlamm, J.; et al. High level PSMA expression is associated with early psa recurrence in surgically treated prostate cancer. Prostate 2011, 71, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.E.; Sheehan, C.; Fisher, H.A.G.; Kaufman, R.P.; Kaur, P.; Gray, K.; Webb, I.; Gray, G.S.; Mosher, R.; Kallakury, B.V.S. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 6357–6362. [Google Scholar] [PubMed]
- Bates, D.; Abraham, S.; Campbell, M.; Zehbe, I.; Curiel, L. Development and characterization of an antibody-labeled super-paramagnetic iron oxide contrast agent targeting prostate cancer cells for magnetic resonance imaging. PLoS ONE 2014, 9, e97220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaittanis, C.; Bolaender, A.; Yoo, B.; Shah, N.; Ouerfelli, O.; Grimm, J. Targetable Clinical Nanoparticles for Precision Cancer Therapy Based on Disease-Specific Molecular Inflection Points. Nano Lett. 2017, 17, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, Y.; Chen, Y.; Liu, W.; Jiang, J.; Guan, W.; Zhang, Z.; Duan, Y. In Vivo Molecular MRI Imaging of Prostate Cancer by Targeting PSMA with Polypeptide-Labeled Superparamagnetic Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 9573–9587. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Chinnaiyan, A.; Kleer, C.; Lucas, P.; Rubin, M. Alpha-Methylacyl-CoA Racemase. Am. J. Surg. Pathol. 2002, 26, 926–931. [Google Scholar] [CrossRef]
- Humphrey, P.A. Histopathology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapovalova, M.; Davydova, J.; Henzler, C.; Daniel, M.; Dehm, S.M.; Warlick, C.A.; LeBeau, A.M. Exploiting the transcriptional specificity of the alpha-methylacylCoA racemase AMACR promoter for the molecular imaging of prostate cancer. Oncotarget 2018, 9, 36693–36704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homma, I.; Torigoe, T.; Hirohashi, Y.; Kitamura, H.; Sato, E.; Masumori, N.; Tamura, Y.; Tsukamoto, T.; Sato, N. Aberrant expression and potency as a cancer immunotherapy target of alpha-methylacyl-coenzyme A racemase in prostate cancer. J. Transl. Med. 2009, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.; Gu, Z.; Watabe, T.; Thomas, G.; Szigeti, K.; Davis, E.; Wahl, M.; Nisitani, S.; Yamashiro, J.; Le Beau, M.M.; et al. Prostate stem cell antigen: A cell surface marker overexpressed in prostate cancer. Proc. Natl. Acad. Sci. USA 1998, 95, 1735–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazaris, E.; Alexios, T. Molecular pathways in prostate cancer. Nephro-Urol. Mon. 2013, 5, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Thomas, G.; Yamashiro, J.; Shintaku, I.P.; Dorey, F.J.; Raitano, A.B.; Witte, O.N.; Said, J.W.; Loda, M.; Reiter, R.E. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 2000, 19, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Ono, H.; Sakamoto, H.; Yoshida, T.; Saeki, N. Prostate stem cell antigen is expressed in normal and malignant human brain tissues. Oncol. Lett. 2018, 15, 3081–3084. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhu, Z.; Luo, L.; Wang, J.; Liu, Y.; Deng, Y.; Zhou, M.; Zhao, Z. The Correlation between PSCA Expression and Neuroendocrine Differentiation in Prostate Cancer. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef]

| T | Characteristics | ||
| T1 | Clinically asymptotic, non-palpable tumor | ||
| T1a | Histological analysis of prostate tissue with < 5% tumorous tissue | ||
| T1b | Histological analysis of prostate tissue with >5% tumorous tissue | ||
| T1c | Tumor detection by punch biopsy, elevated PSA level | ||
| T2 | Palpable tumor, localized in the prostate | ||
| T2a | Maximal half tumor flap tumorous | ||
| T2b | More than half of a prostate lobe is tumorous | ||
| T2c | Both prostate lobes affected | ||
| T3 | Volume of tumor over the prostate capsule | ||
| T3a | Tumor spread on one or both sides beyond the prostate capsule, seminal vesicles not affected | ||
| T3b | Extension into the seminal vesicle | ||
| T4 | Tumor is fixed and/ or adjacent tissue involved | ||
| N | Characteristics1 | M | Characteristics |
| N0 | No regional lymph nodes | M0 | No metastasis |
| N1 | Regional lymph nodes are existing | M1 | Metastasis are existing |
| NX | Regional lymph nodes cannot be identified | M1a | No regional lymph nodes |
| M1b | In bones | ||
| M1c | Other areas | ||
| MX | Metastasis cannot be identified | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kader, A.; Brangsch, J.; Kaufmann, J.O.; Zhao, J.; Mangarova, D.B.; Moeckel, J.; Adams, L.C.; Sack, I.; Taupitz, M.; Hamm, B.; et al. Molecular MR Imaging of Prostate Cancer. Biomedicines 2021, 9, 1. https://doi.org/10.3390/biomedicines9010001
Kader A, Brangsch J, Kaufmann JO, Zhao J, Mangarova DB, Moeckel J, Adams LC, Sack I, Taupitz M, Hamm B, et al. Molecular MR Imaging of Prostate Cancer. Biomedicines. 2021; 9(1):1. https://doi.org/10.3390/biomedicines9010001
Chicago/Turabian StyleKader, Avan, Julia Brangsch, Jan O. Kaufmann, Jing Zhao, Dilyana B. Mangarova, Jana Moeckel, Lisa C. Adams, Ingolf Sack, Matthias Taupitz, Bernd Hamm, and et al. 2021. "Molecular MR Imaging of Prostate Cancer" Biomedicines 9, no. 1: 1. https://doi.org/10.3390/biomedicines9010001
APA StyleKader, A., Brangsch, J., Kaufmann, J. O., Zhao, J., Mangarova, D. B., Moeckel, J., Adams, L. C., Sack, I., Taupitz, M., Hamm, B., & Makowski, M. R. (2021). Molecular MR Imaging of Prostate Cancer. Biomedicines, 9(1), 1. https://doi.org/10.3390/biomedicines9010001