Light Cross-Linkable Marine Collagen for Coaxial Printing of a 3D Model of Neuromuscular Junction Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Collagen Extraction and Purification
2.2. Methacrylation of Collagen
2.3. Collagen Hydrogel Formation
2.3.1. Thermally Crosslinked Hydrogels
2.3.2. UV Cross-Linked Hydrogels
2.4. Fourier Transform Infra-Red (FTIR) Spectroscopy
2.5. Circular Dichroism (CD)
2.6. SDS-PAGE
2.7. UV Rheology
2.8. 2D Surface Seeding of Cells
2.9. Cell Encapsulation
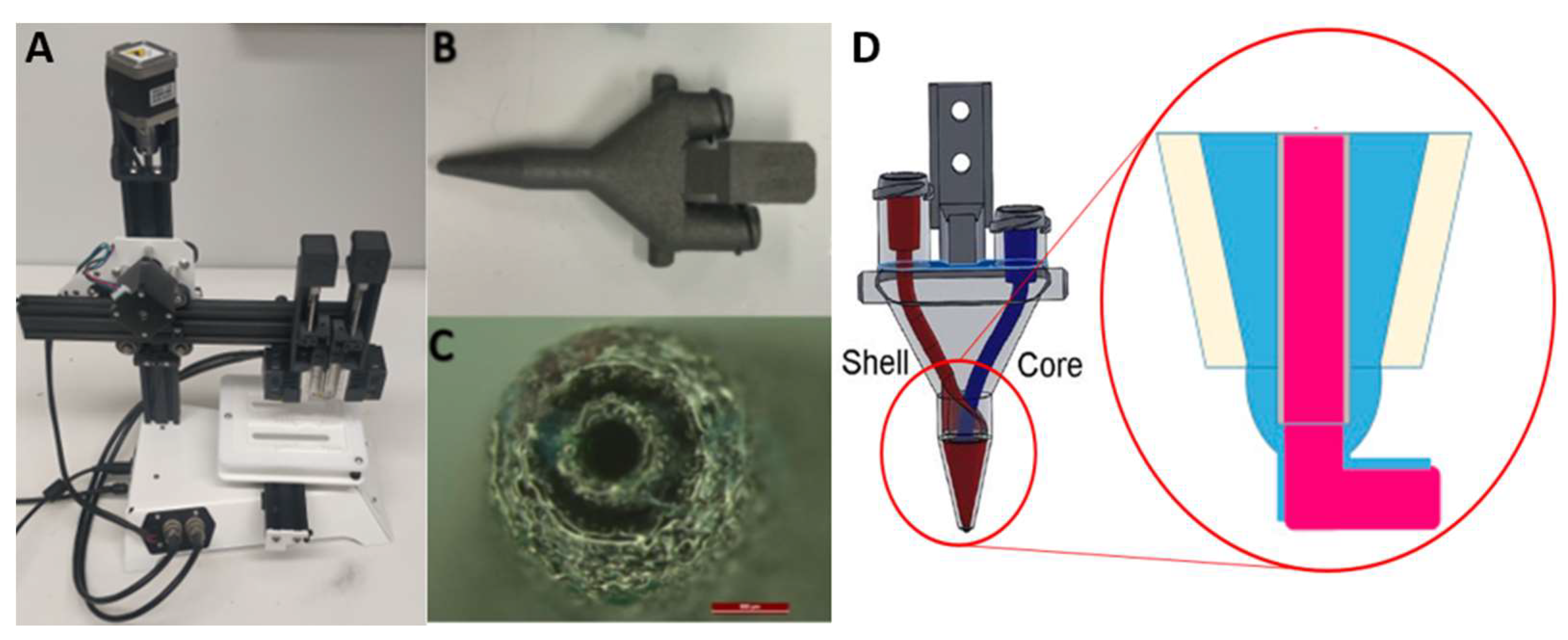
2.10. 3D Bioprinting
2.11. Live-Dead Cell Viability Test
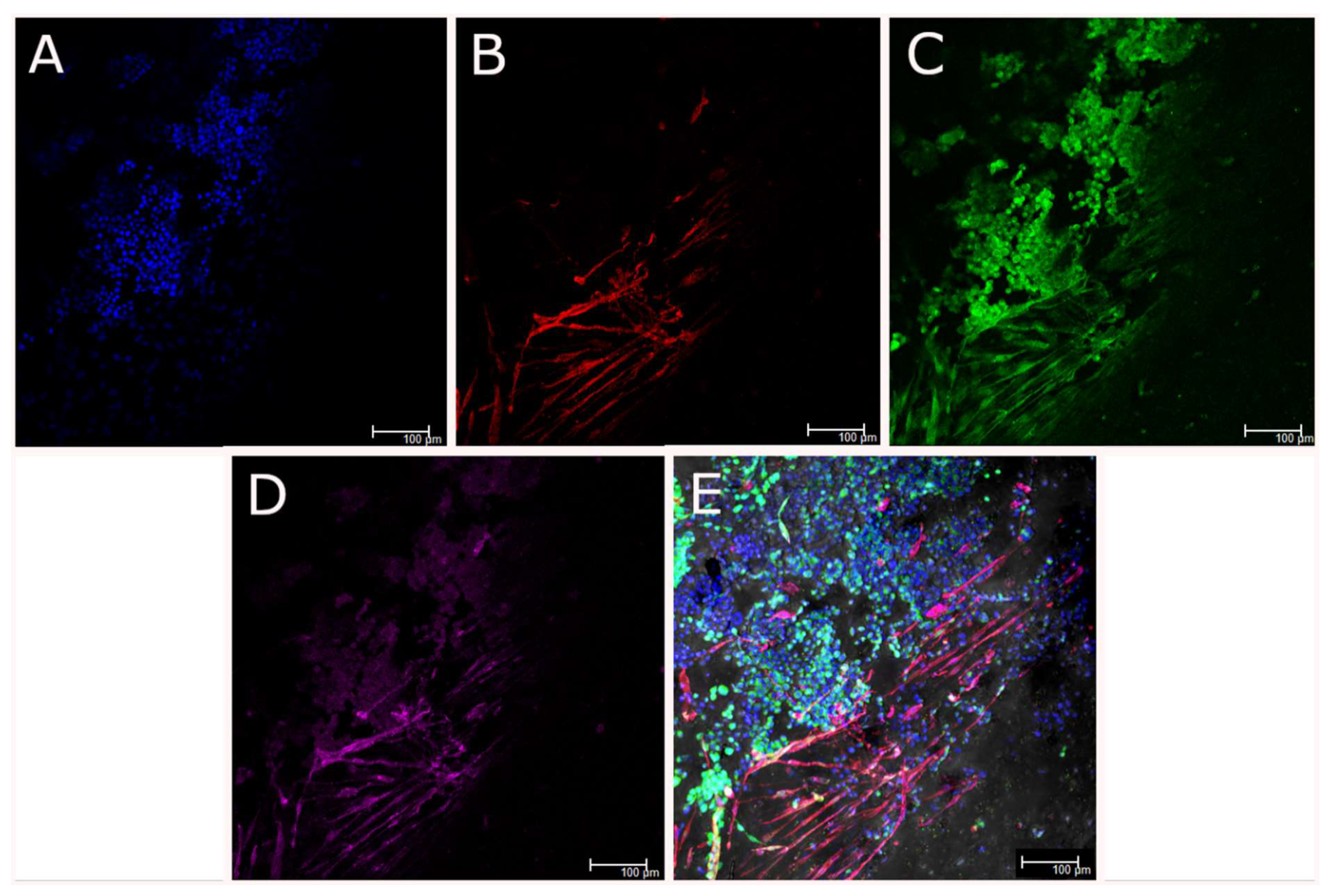
2.12. Immunocytochemistry
3. Results
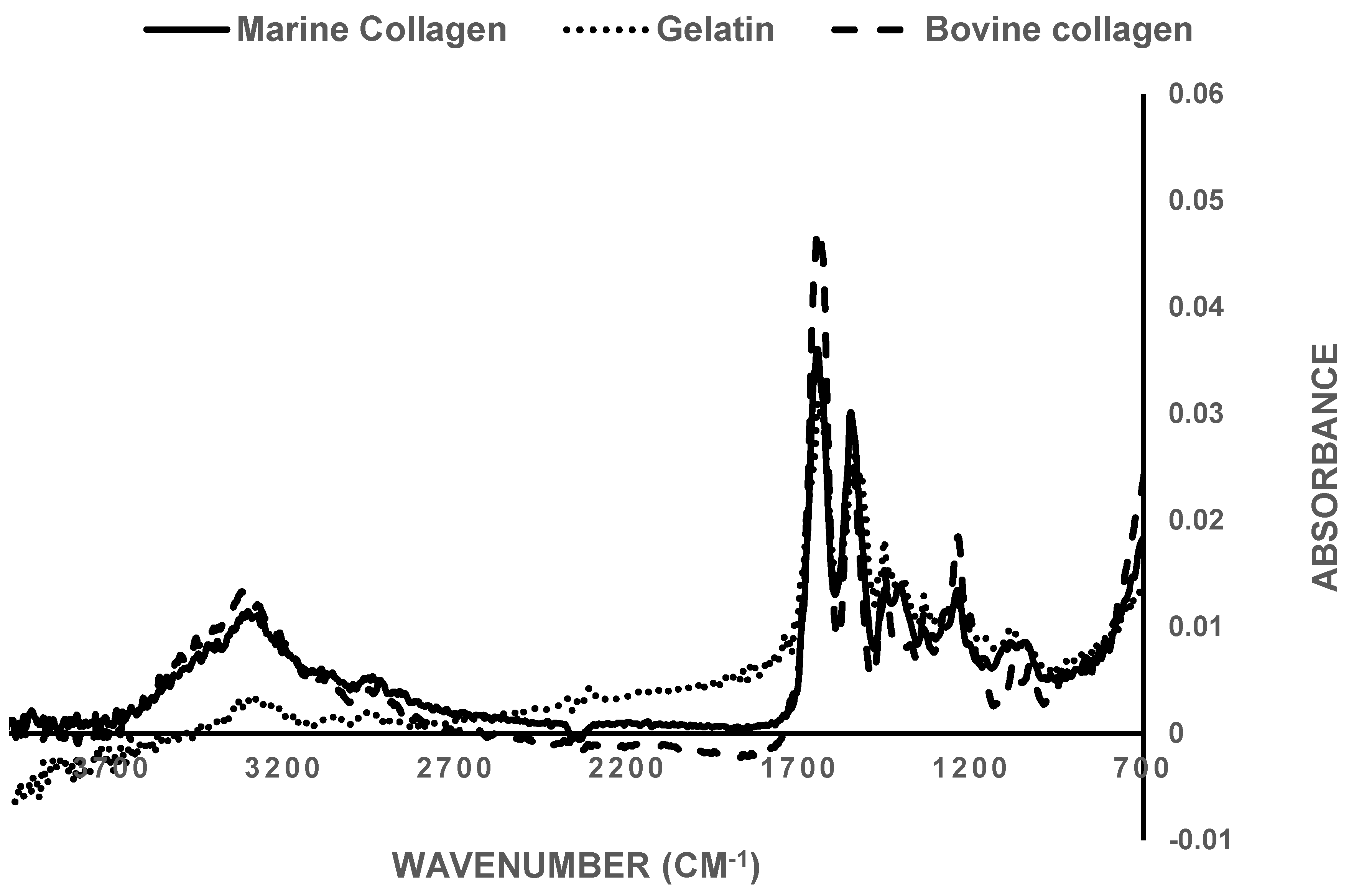
3.1. FTIR Spectroscopy
3.2. Circular Dichroism
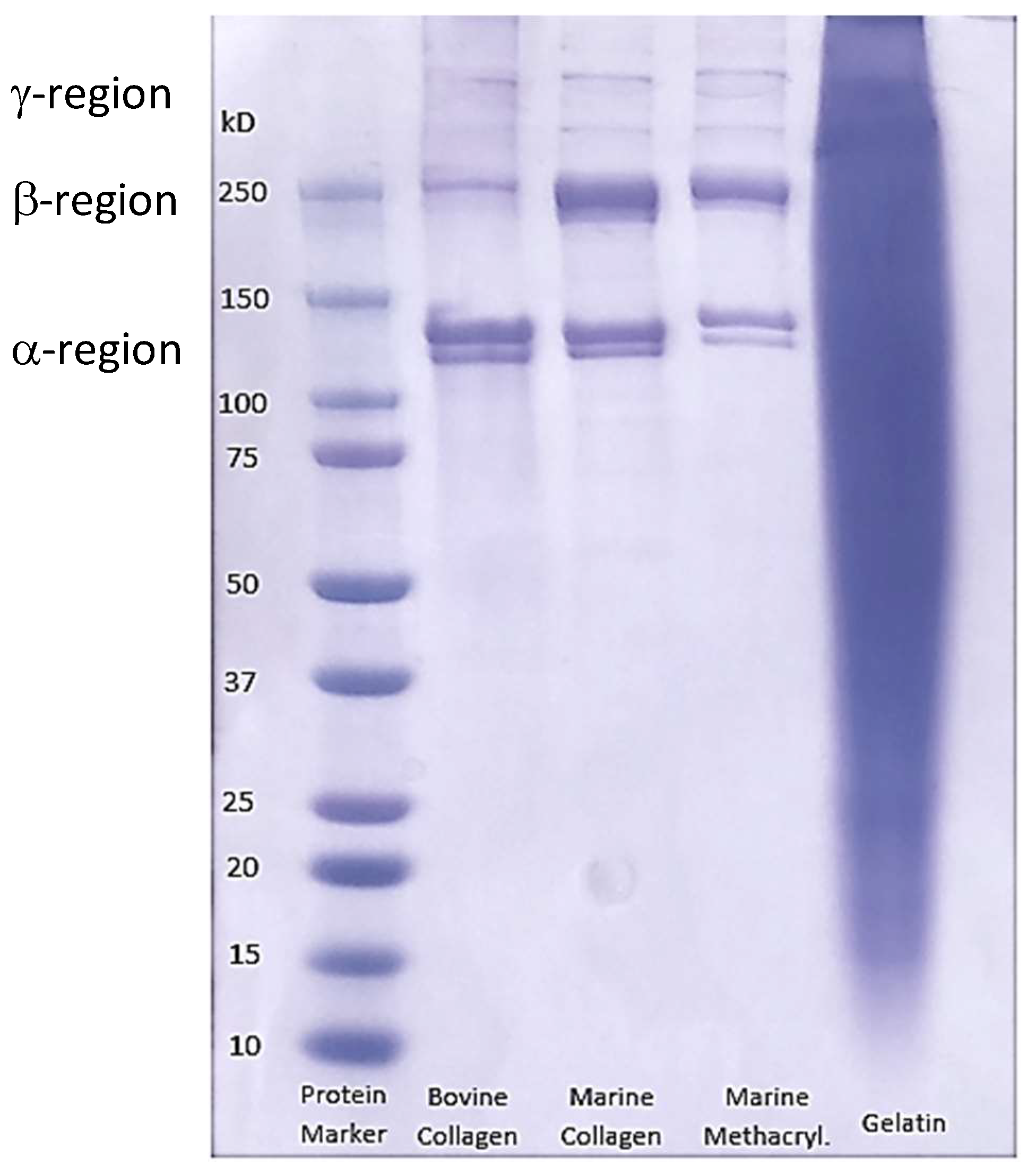
3.3. SDS-PAGE Electrophoresis
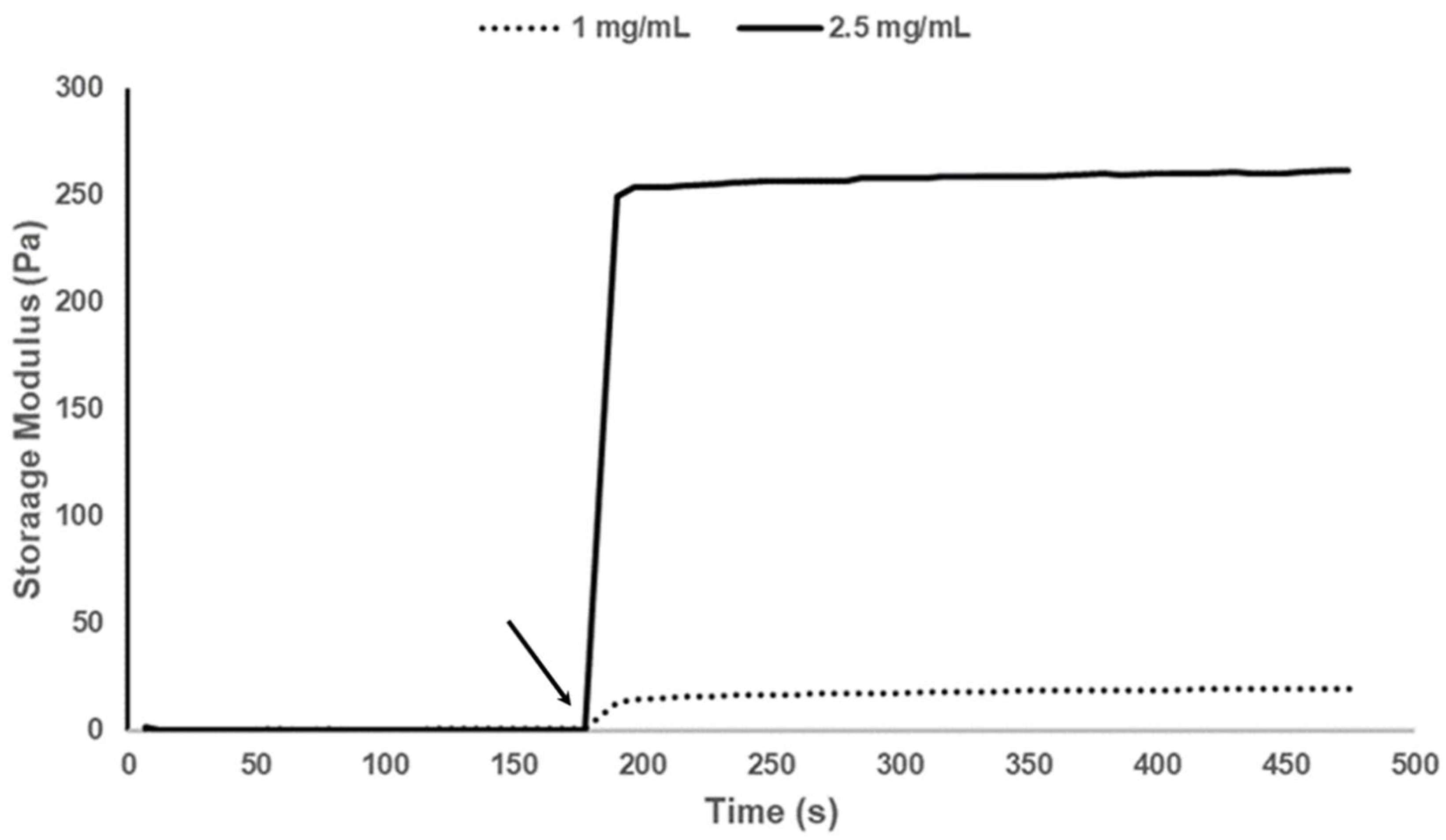
3.4. Rheology
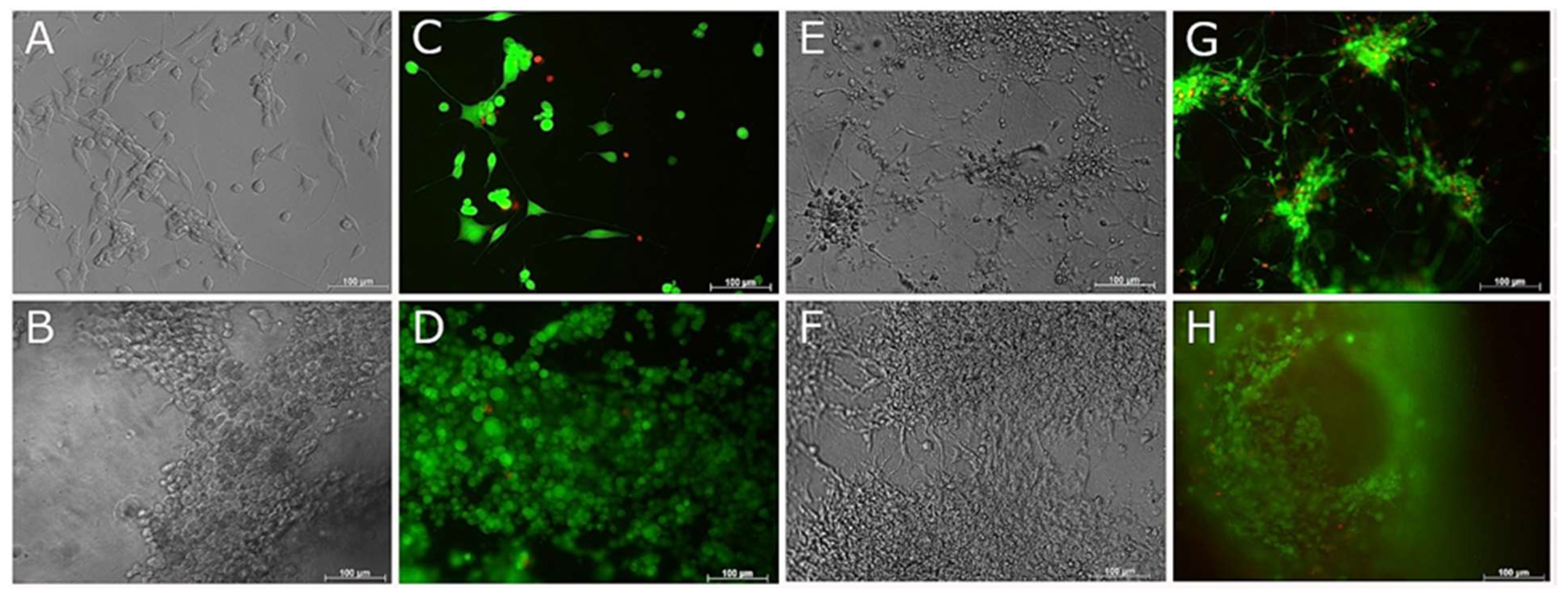
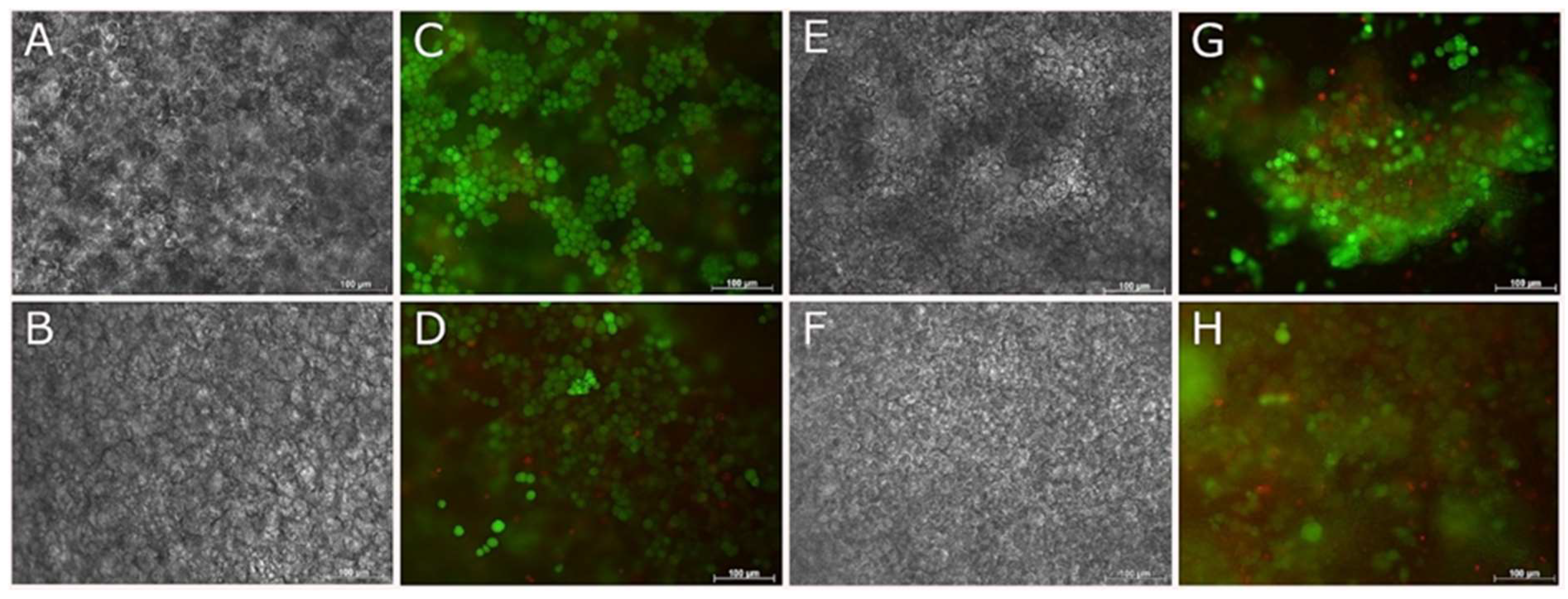
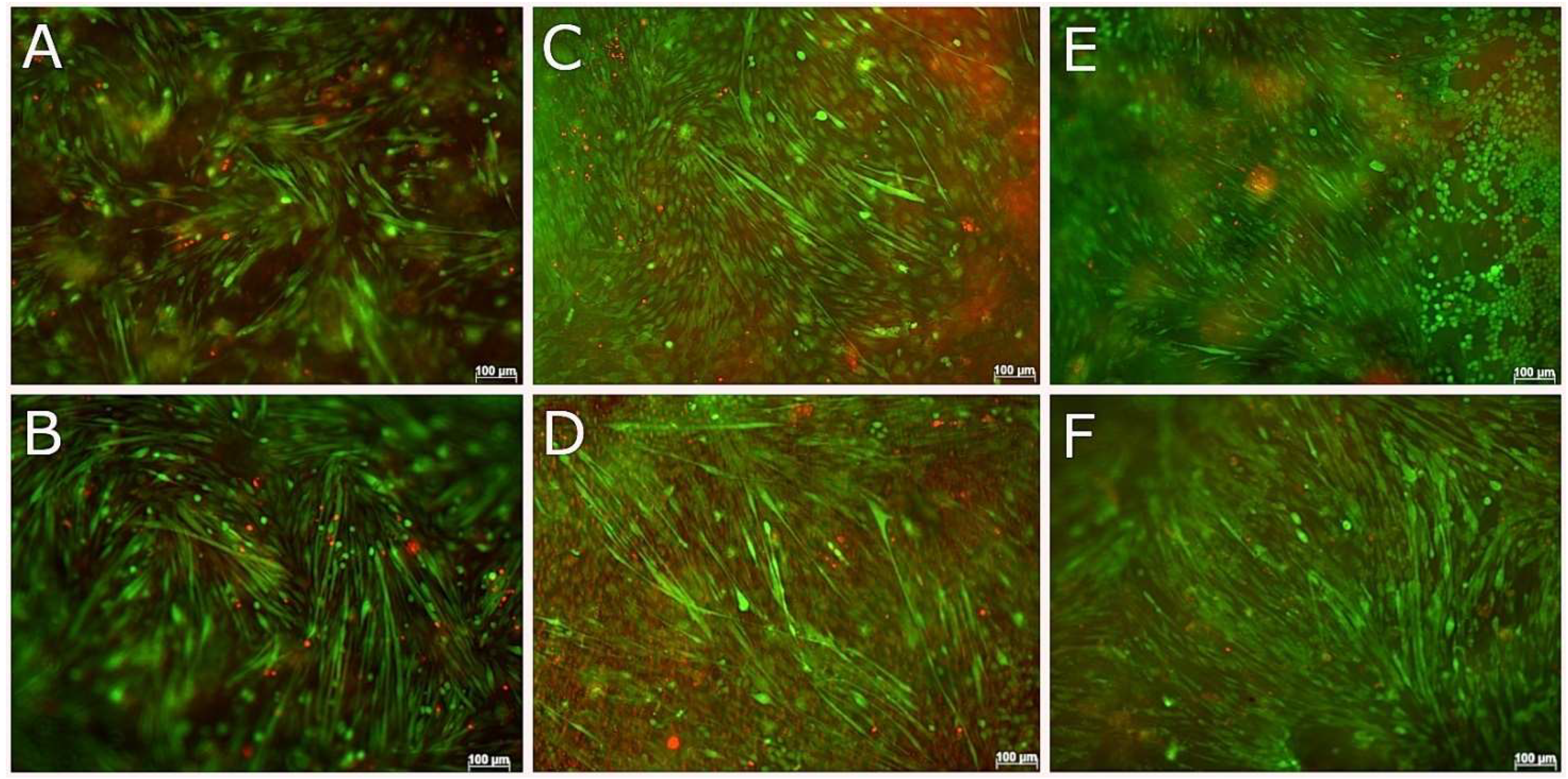
3.5. Compatibility of Neural Cells with Marine Collagen
3.6. Live and Dead Staining
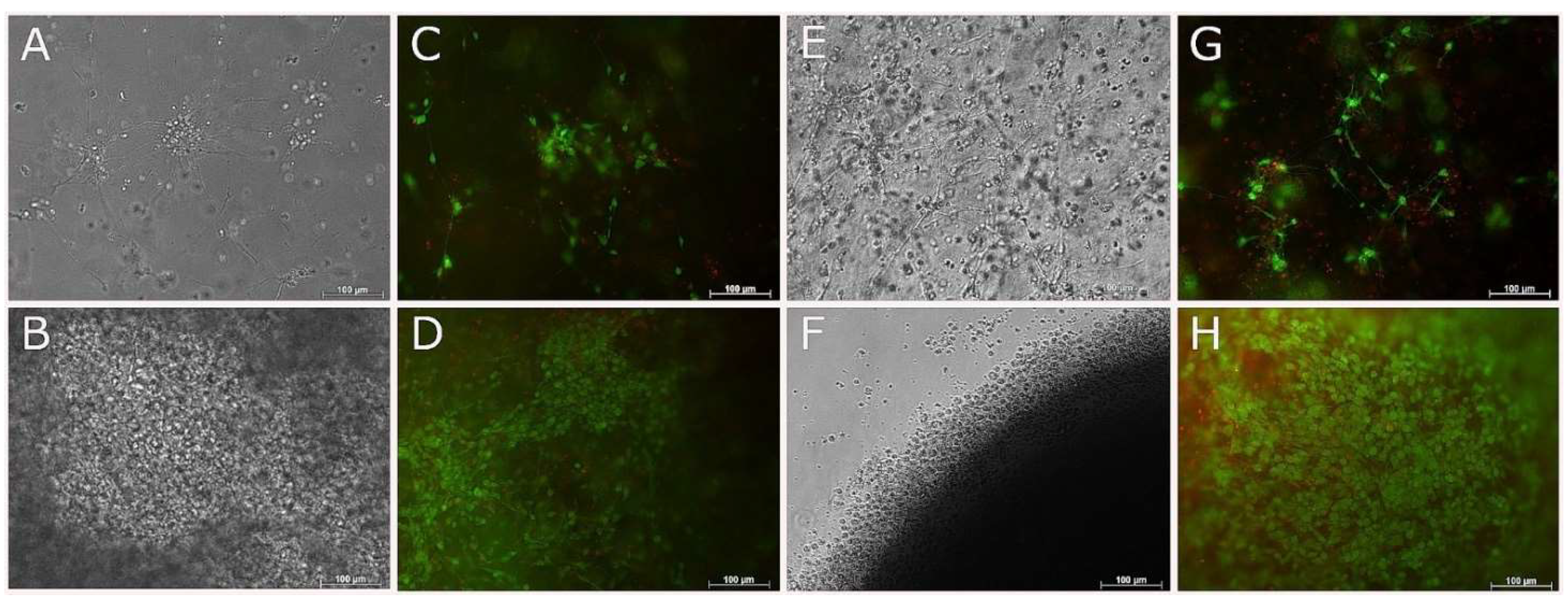
3.7. Compatibility of NSC-34 with UV-Cross-Linked Methacrylated Fish Collagen
3.8. 3D Coaxial Printing of Primary Myoblasts and NSC-34 Motor Neuron Cell Line
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Patino, M.G.; Neiders, M.E.; Andreana, S.; Noble, B.; Cohen, R.E. Collagen as an implantable material in medicine and dentistry. J. Oral Implantol. 2002, 28, 220–225. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R.; Stewart, E.M.; In Het Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Liu, X.; Carter, S.S.D.; Renes, M.J.; Kim, J.; Rojas-Canales, D.M.; Penko, D.; Angus, C.; Beirne, S.; Drogemuller, C.J.; Yue, Z.; et al. Development of a Coaxial 3D Printing Platform for Biofabrication of Implantable Islet-Containing Constructs. Adv. Healthc. Mater. 2019, 8, 1801181. [Google Scholar] [CrossRef]
- Otte, A. 3D Computer-Aided Design Reconstructions and 3D Multi-Material Polymer Replica Printings of the First “Iron Hand” of Franconian Knight Gottfried (Götz) von Berlichingen (1480–1562): An Overview. Prosthesis 2020, 2, 304–312. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Mechanical Properties of Laser-Sintered 3D-Printed Cobalt Chromium and Soft-Milled Cobalt Chromium. Prosthesis 2020, 2, 313–320. [Google Scholar] [CrossRef]
- Claudio-Rizoa, J.A.; Espíndola-Sernab, L.; Becerra-Rodriguezb, J.J.; Cano-Salazara, L.F.; Guíaa, T.E.F. Recent Advances in the Synthesis and Applications of Collagen Based Hydrogels: A Review. Mediterr. J. Basic Appl. Sci. 2019, 3, 54–98. [Google Scholar]
- Enea, D.; Gwynne, J.; Kew, S.; Arumugam, M.; Shepherd, J.; Brooks, R.; Ghose, S.; Best, S.; Cameron, R.; Rushton, N. Collagen fibre implant for tendon and ligament biological augmentation. In vivo study in an ovine model. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1783–1793. [Google Scholar] [CrossRef]
- Amri, M.; Firdaus, M.; Fauzi, M.; Chowdhury, S.R.; Fadilah, N.; Wan Hamirul, W.; Reusmaazran, M.; Aminuddin, B.; Ruszymah, B. Cytotoxic evaluation of biomechanically improved crosslinked ovine collagen on human dermal fibroblasts. Biomed. Mater. Eng. 2014, 24, 1715–1724. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Romero, A.; Guerrero, A. Influence of collagen concentration and glutaraldehyde on collagen-based scaffold properties. J. Biomed. Mater. Res. Part A 2016, 104, 1462–1468. [Google Scholar] [CrossRef]
- Harriger, M.D.; Supp, A.P.; Warden, G.D.; Boyce, S.T. Glutaraldehyde crosslinking of collagen substrates inhibits degradation in skin substitutes grafted to athymic mice. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1997, 35, 137–145. [Google Scholar] [CrossRef]
- Chau, D.Y.; Collighan, R.J.; Verderio, E.A.; Addy, V.L.; Griffin, M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Silvipriya, K.; Kumar, K.K.; Bhat, A.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, C.; Maddern, G. Porcine and bovine surgical products: Jewish, Muslim, and Hindu perspectives. Arch. Surg. 2008, 143, 366–370. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.-H.; Kim, J.-H.; Do, J.T.; Song, H.; Bae, H. Cold water fish gelatin methacryloyl hydrogel for tissue engineering application. PLoS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef]
- Iswariya, S.; Bhanukeerthi, A.; Velswamy, P.; Uma, T.; Perumal, P.T. Design and development of a piscine collagen blended pullulan hydrogel for skin tissue engineering. RSC Adv. 2016, 6, 57863–57871. [Google Scholar]
- Tylingo, R.; Gorczyca, G.; Mania, S.; Szweda, P.; Milewski, S. Preparation and characterization of porous scaffolds from chitosan-collagen-gelatin composite. React. Funct. Polym. 2016, 103, 131–140. [Google Scholar] [CrossRef]
- Żelechowska, E.; Sadowska, M.; Turk, M. Isolation and some properties of collagen from the backbone of Baltic cod (Gadus morhua). Food Hydrocoll. 2010, 24, 325–329. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Chun, B.-S. Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus). Int. J. Biol. Macromol. 2019, 135, 668–676. [Google Scholar] [CrossRef]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, N.; Hayashi, A.; Yamanaka, K.; Sakiyama, A.; Nakano, A.; Shibata, M. Preparation and mechanical properties of photo-crosslinked fish gelatin/imogolite nanofiber composite hydrogel. Materials 2012, 5, 2573–2585. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Gad, A.; Abu-Hussein, A.E.-H.G.; Habib, S.I.; Badr, N.A.; Hashem, A.A. Chemical and biological evaluation of Egyptian Nile Tilapia (Oreochromis niloticas) fish scale collagen. Int. J. Biol. Macromol. 2015, 79, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Kuo, J.-M.; Wu, S.-J.; Tsai, H.-T. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion–hydro-extraction process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef]
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T.; Fujiki, K.; Nakao, M.; Yano, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chem. 2000, 70, 205–208. [Google Scholar] [CrossRef]
- Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of edible jellyfish exumbrella. J. Sci. Food Agric. 1999, 79, 855–858. [Google Scholar] [CrossRef]
- Shanmugam, V.; Ramasamy, P.; Subhapradha, N.; Sudharsan, S.; Seedevi, P.; Moovendhan, M.; Krishnamoorthy, J.; Shanmugam, A.; Srinivasan, A. Extraction, structural and physical characterization of type I collagen from the outer skin of Sepiella inermis (Orbigny, 1848). Afr. J. Biotechnol. 2012, 11, 14326–14337. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges Axinella cannabina and Suberites carnosus: Isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef]
- Zeng, S.-K.; Zhang, C.-H.; Lin, H.; Yang, P.; Hong, P.-Z.; Jiang, Z. Isolation and characterisation of acid-solubilised collagen from the skin of Nile tilapia (Oreochromis niloticus). Food Chem. 2009, 116, 879–883. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Isolation and characterization of collagen from bigeye snapper (Priacanthus macracanthus) skin. J. Sci. Food Agric. 2005, 85, 1203–1210. [Google Scholar] [CrossRef]
- Woo, J.-W.; Yu, S.-J.; Cho, S.-M.; Lee, Y.-B.; Kim, S.-B. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocoll. 2008, 22, 879–887. [Google Scholar] [CrossRef]
- Okazaki, E.; Osako, K. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014, 149, 264–270. [Google Scholar]
- Muralidharan, N.; Shakila, R.J.; Sukumar, D.; Jeyasekaran, G. Skin, bone and muscle collagen extraction from the trash fish, leather jacket (Odonus niger) and their characterization. J. Food Sci. Technol. 2013, 50, 1106–1113. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Wang, H.; Zhang, H.; Wang, M.; Liu, L. Physical-chemical properties of collagens from skin, scale, and bone of grass carp (Ctenopharyngodon idellus). J. Aquat. Food Prod. Technol. 2014, 23, 264–277. [Google Scholar] [CrossRef]
- Bao, Z.; Gao, M.; Fan, X.; Cui, Y.; Yang, J.; Peng, X.; Xian, M.; Sun, Y.; Nian, R. Development and characterization of a photo-cross-linked functionalized type-I collagen (Oreochromis niloticus) and polyethylene glycol diacrylate hydrogel. Int. J. Biol. Macromol. 2020, 155, 163–173. [Google Scholar] [CrossRef]
- Wang, J.K.; Yeo, K.P.; Chun, Y.Y.; Tan, T.T.Y.; Tan, N.S.; Angeli, V.; Choong, C. Fish scale-derived collagen patch promotes growth of blood and lymphatic vessels in vivo. Acta Biomater. 2017, 63, 246–260. [Google Scholar] [CrossRef]
- Caruso, G. Fishery wastes and by-products: A resource to be valorised. J. Fish. Sci. 2015, 9, 80–83. [Google Scholar]
- Yuan, N.; Tian, W.; Sun, L.; Yuan, R.; Tao, J.; Chen, D. Neural stem cell transplantation in a double-layer collagen membrane with unequal pore sizes for spinal cord injury repair. Neural Regen. Res. 2014, 9, 1014. [Google Scholar]
- Egawa, E.Y.; Kato, K.; Hiraoka, M.; Nakaji-Hirabayashi, T.; Iwata, H. Enhanced proliferation of neural stem cells in a collagen hydrogel incorporating engineered epidermal growth factor. Biomaterials 2011, 32, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.M.; Stenger, D.A.; Shaffer, K.M.; Maric, D.; Barker, J.L.; Ma, W. Primary neural precursor cell expansion, differentiation and cytosolic Ca2+ response in three-dimensional collagen gel. J. Neurosci. Methods 2000, 102, 187–195. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakamura, M.; Okano, H.; Toyama, Y. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen Type-1 Gel. Restor. Neurol. Neurosci. 2007, 25, 109–117. [Google Scholar] [PubMed]
- Iwashita, M.; Ohta, H.; Fujisawa, T.; Cho, M.; Ikeya, M.; Kidoaki, S.; Kosodo, Y. Brain-stiffness-mimicking tilapia collagen gel promotes the induction of dorsal cortical neurons from human pluripotent stem cells. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Fischbach, G.D.; Dichter, M.A. Electrophysiologic and morphologic properties of neurons in dissociated chick spinal cord cell cultures. Dev. Biol. 1974, 37, 100–116. [Google Scholar] [CrossRef]
- Cashman, N.R.; Durham, H.D.; Blusztajn, J.K.; Oda, K.; Tabira, T.; Shaw, I.T.; Dahrouge, S.; Antel, J.P. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. 1992, 194, 209–221. [Google Scholar] [CrossRef]
- Hunter, D.D.; Cashman, N.; Morris-Valero, R.; Bulock, J.W.; Adams, S.P.; Sanes, J.R. An LRE (leucine-arginine-glutamate)-dependent mechanism for adhesion of neurons to S-laminin. J. Neurosci. 1991, 11, 3960–3971. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Di Bella, C.; Thompson, F.; Augustine, C.; Beirne, S.; Cornock, R.; Richards, C.J.; Chung, J.; Gambhir, S.; Yue, Z.; et al. Development of the Biopen: A handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 2016, 8, 015019. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform IR spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Surewicz, W.K.; Mantsch, H.H. New Insight into Protein Secondary Structure from Resolution-Enhanced Infrared-Spectra. Biochim. Biophys. Acta 1988, 952, 115–130. [Google Scholar] [CrossRef]
- Chadefaux, C.; Le Hô, A.S.; Bellot-Gurlet, L.; Reiche, I. Curve-fitting Micro-ATR-FTIR studies of the amide I and II bands of type I collagen in archaeological bone materials. E-Preserv. Sci. 2009, 6, 129–137. [Google Scholar]
- Anand, S.; Kamath, S.; Chuang, L.; Kasapis, S.; Lopata, A.L. Biochemical and thermo-mechanical analysis of collagen from the skin of Asian Sea bass (Lates calcarifer) and Australasian Snapper (Pagrus auratus), an alternative for mammalian collagen. Eur. Food Res. Technol. 2013, 236, 873–882. [Google Scholar] [CrossRef]
- Sylvester, M.F.; Yannas, I.V.; Salzman, E.W.; Forbes, M.J. Collagen banded fibril structure and the collagen-platelet reaction. Thromb. Res. 1989, 55, 135–148. [Google Scholar] [CrossRef]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maity, P.P.; Moulik, D.; Dhara, S. Accelerating full thickness wound healing using collagen sponge of mrigal fish (Cirrhinus cirrhosus) scale origin. Int. J. Biol. Macromol. 2016, 93, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, W.T.; Nagapudi, K.; Thomas, B.S.; Chaikof, E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Biomatrix, A. Analysis of Type 1 Collagen Products. 2019. Available online: https://advancedbiomatrix.com/sds-banding.html (accessed on 25 December 2020).
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modelling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Smith, V.; Jackson, M.; Tran, M.; Thomas, M.; Patel, A.; Lorusso, E.; Nimbalkar, S.; Cai, Y.; McAleer, C.W.; et al. A Human-Based Functional NMJ System for Personalized ALS Modeling and Drug Testing. Adv. Ther. 2020, 3, 2000133. [Google Scholar] [CrossRef]
- Natarajan, A.; Sethumadhavan, A.; Krishnan, U.M. Toward Building the Neuromuscular Junction: In Vitro Models To Study Synaptogenesis and Neurodegeneration. ACS Omega 2019, 4, 12969–12977. [Google Scholar] [CrossRef]
- 3D Bioprinting and Differentiation of Primary Skeletal Muscle Progenitor Cells; Catherine Ngan, A.Q., O’Connell, C., Agdalena KIta, M., Bourke, J., Wallace, G.G., Choong, P., Robert, M., Kapsa, I., Eds.; Springer: New York, NY, USA, 2020; Volume 2140, pp. 229–242. [Google Scholar]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Swieszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef]
- Schuh, C.M.; Day, A.G.; Redl, H.; Phillips, J. An optimized collagen-fibrin blend engineered neural tissue promotes peripheral nerve repair. Tissue Eng. Part A 2018, 24, 1332–1340. [Google Scholar] [CrossRef]
- Kotch, F.W.; Raines, R.T. Self-assembly of synthetic collagen triple helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Novak, U.; Kaye, A.H. Extracellular matrix and the brain: Components and function. J. Clin. Neurosci. 2000, 7, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Burden, S.J. MuSK controls where motor axons grow and form synapses. Nat. Neurosci. 2008, 11, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Maj, M.; Bezakova, G.; Magyar, J.P.; Brenner, H.R.; Ruegg, M.A. Muscle-wide secretion of a miniaturized form of neural agrin rescues focal neuromuscular innervation in agrin mutant mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11406–11411. [Google Scholar] [CrossRef]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
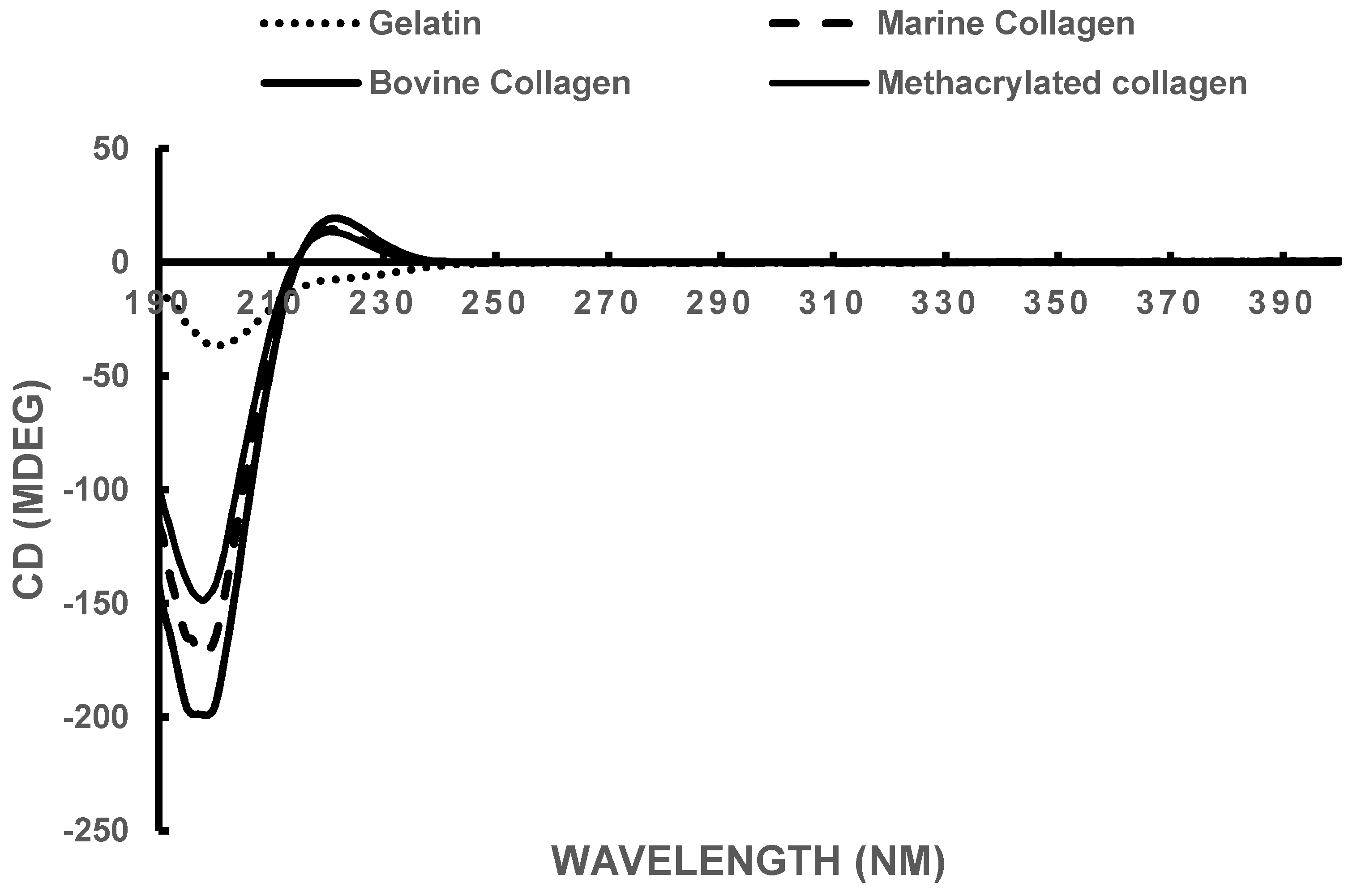

| Sample | Rotatory Minimum (nm) | Rotatory Maximum (nm) | Crossover Point (nm) |
|---|---|---|---|
| Bovine Collagen | 198 | 222 | 215 |
| Marine Collagen | 198 | 220 | 214 |
| Methacrylated Marine Collagen | 198 | 220 | 214 |
| Gelatin | 199 | - | - |
| Theoretical Collagen [64] | 205 | 221 | 212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, B.; Albillos Sanchez, A.; Tangey, B.; Gilmore, K.; Yue, Z.; Liu, X.; Wallace, G. Light Cross-Linkable Marine Collagen for Coaxial Printing of a 3D Model of Neuromuscular Junction Formation. Biomedicines 2021, 9, 16. https://doi.org/10.3390/biomedicines9010016
Sanz B, Albillos Sanchez A, Tangey B, Gilmore K, Yue Z, Liu X, Wallace G. Light Cross-Linkable Marine Collagen for Coaxial Printing of a 3D Model of Neuromuscular Junction Formation. Biomedicines. 2021; 9(1):16. https://doi.org/10.3390/biomedicines9010016
Chicago/Turabian StyleSanz, Borja, Ane Albillos Sanchez, Bonnie Tangey, Kerry Gilmore, Zhilian Yue, Xiao Liu, and Gordon Wallace. 2021. "Light Cross-Linkable Marine Collagen for Coaxial Printing of a 3D Model of Neuromuscular Junction Formation" Biomedicines 9, no. 1: 16. https://doi.org/10.3390/biomedicines9010016
APA StyleSanz, B., Albillos Sanchez, A., Tangey, B., Gilmore, K., Yue, Z., Liu, X., & Wallace, G. (2021). Light Cross-Linkable Marine Collagen for Coaxial Printing of a 3D Model of Neuromuscular Junction Formation. Biomedicines, 9(1), 16. https://doi.org/10.3390/biomedicines9010016





