Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells
Abstract
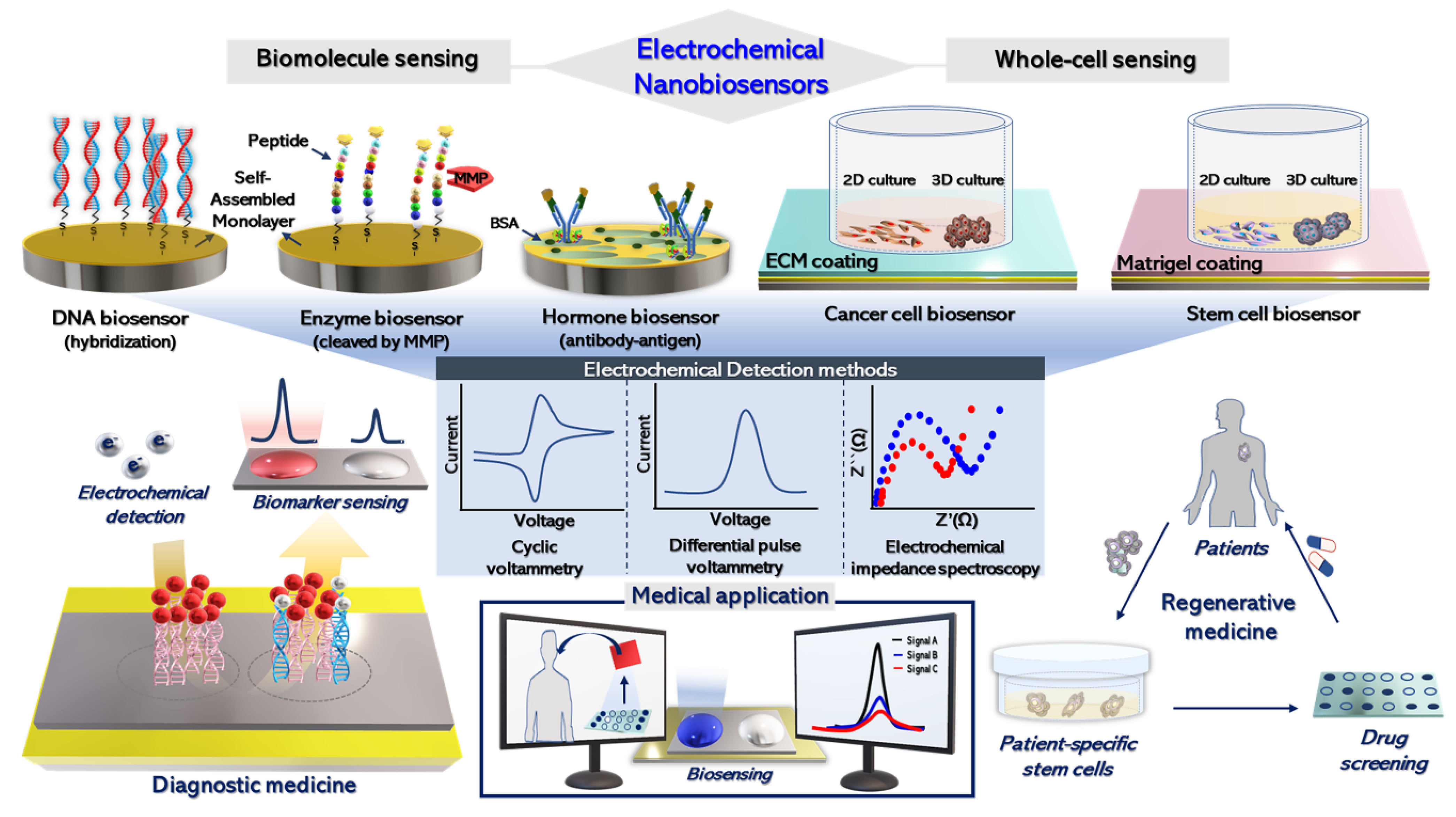
1. Introduction
2. Electrochemical Detection of Biomolecules
2.1. Electrochemical DNA Sensing Platforms
2.2. Electrochemical Biosensors for Enzyme Activity
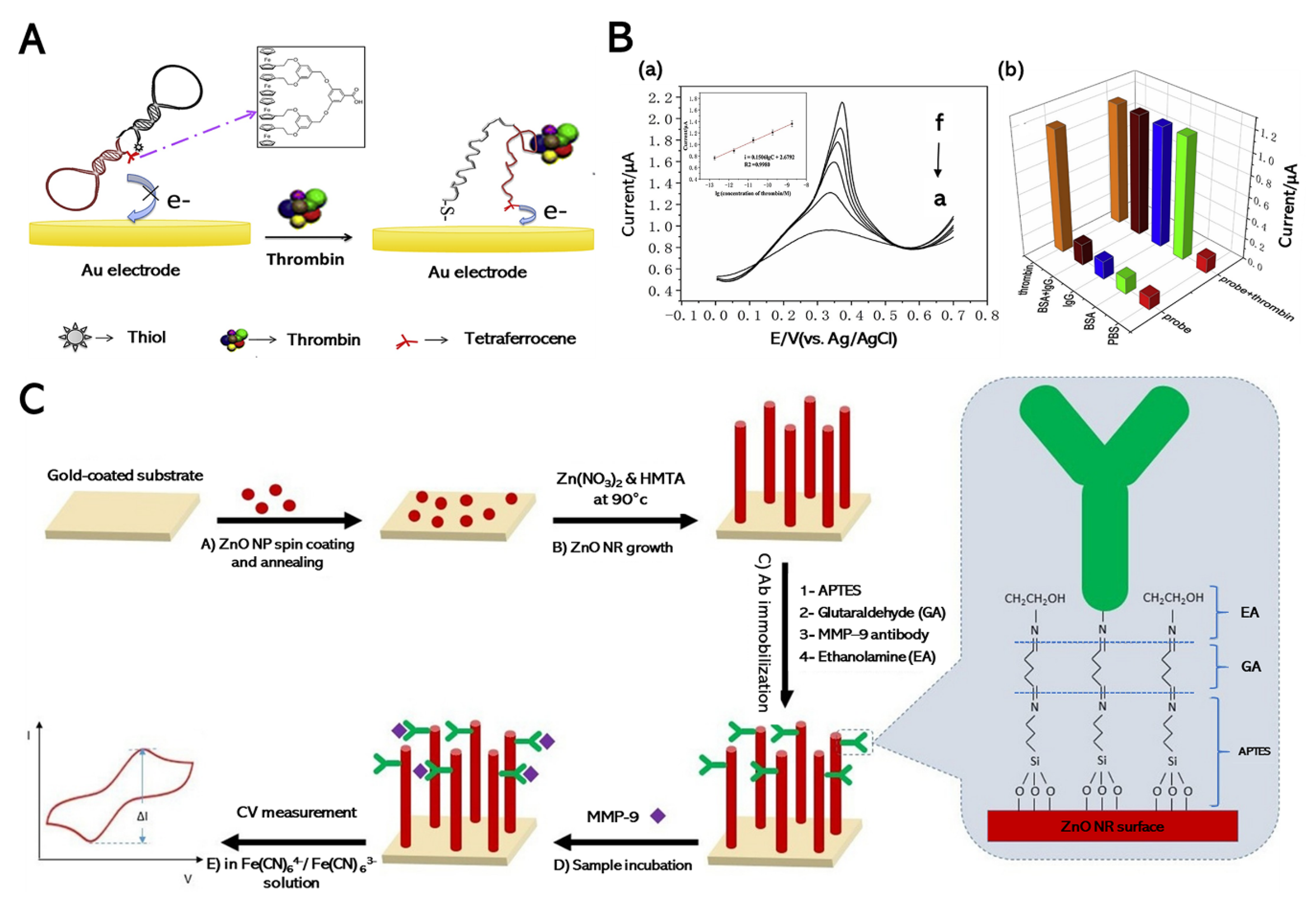
2.2.1. Electrochemical Detection of Thrombin
2.2.2. Electrochemical Detection of Matrix Metalloproteinase
3. Electrochemical Biosensors for Hormone Detection
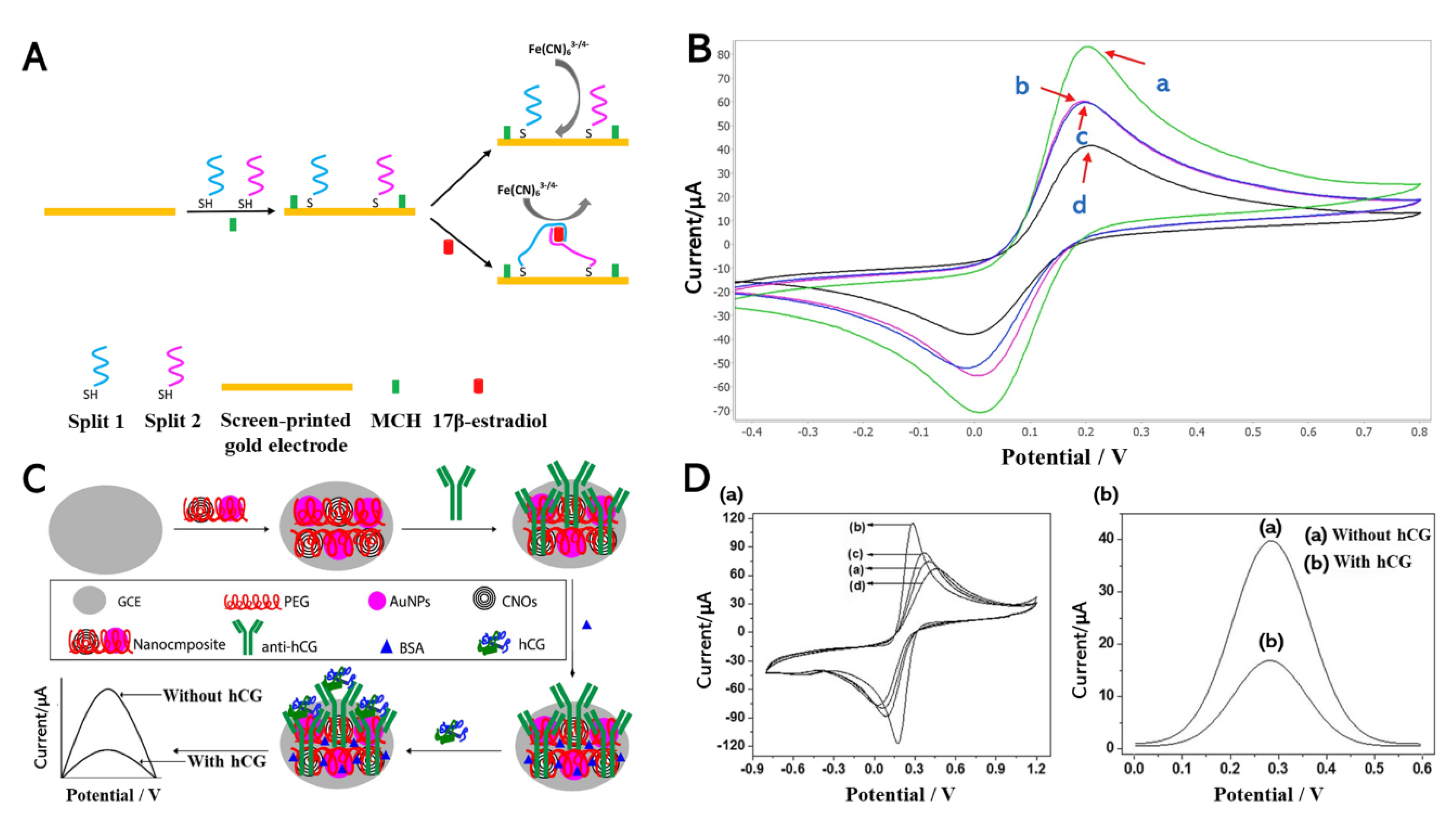
3.1. Electrochemical Detection of Estrogen Hormone
3.2. Electrochemical Detection of Human Chorionic Gonadotropin (hCG) Hormone
4. Electrochemical Biosensing for Highly Proliferative Cells
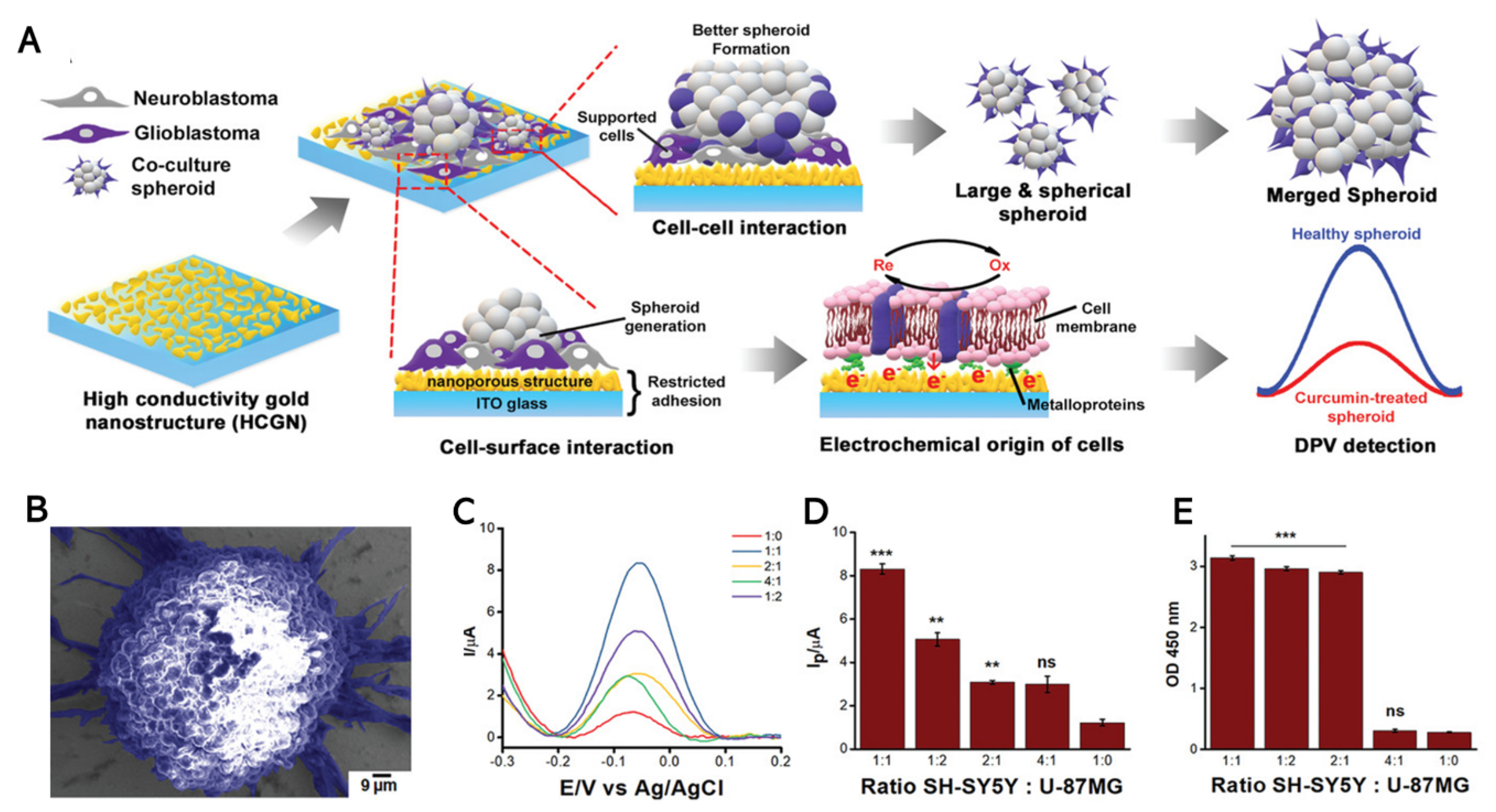
4.1. Electrochemical Detection of Cancer Cell Viability
4.2. Electrochemical Sensing of Stem Cell Pluripotency
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bruning, A.; Aatola, H.; Toivola, H.; Ikonen, N.; Savolainen-Kopra, C.; Blomqvist, S.; Pajkrt, D.; Wolthers, K.; Koskinen, J. Rapid detection and monitoring of human coronavirus infections. New Microbes New Infect. 2018, 24, 52–55. [Google Scholar] [CrossRef]
- French, D. Advances in bioanalytical techniques to measure steroid hormones in serum. Bioanalysis 2016, 8, 1203–1219. [Google Scholar] [CrossRef]
- Jackson, R.; Logue, B.A. A review of rapid and field-portable analytical techniques for the diagnosis of cyanide exposure. Anal. Chim. Acta 2017, 960, 18–39. [Google Scholar] [CrossRef]
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for chemists of terms used in biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kang, E.-S.; Baek, S.; Choo, S.-S.; Chung, Y.-H.; Lee, D.; Min, J.; Kim, T.-H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Nanostructures for nonlabeled and labeled electrochemical immunosensors: Simultaneous electrochemical detection of cancer markers: A review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef]
- Jung, I.Y.; Kim, J.S.; Choi, B.R.; Lee, K.; Lee, H. Hydrogel based biosensors for in vitro diagnostics of biochemicals, proteins, and genes. Adv. Healthc. Mater. 2017, 6, 1601475. [Google Scholar] [CrossRef]
- Laurila, T.; Sainio, S.; Caro, M.A. Hybrid carbon based nanomaterials for electrochemical detection of biomolecules. Prog. Mater. Sci. 2017, 88, 499–594. [Google Scholar] [CrossRef]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical detection of nucleic acids, proteins, small molecules and cells using a DNA-nanostructure-based universal biosensing platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Munge, B.S.; Stracensky, T.; Gamez, K.; DiBiase, D.; Rusling, J.F. Multiplex immunosensor arrays for electrochemical detection of cancer biomarker proteins. Electroanalysis 2016, 28, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Fu, R.; Wen, W.; Zhang, X.; Wang, S. Target-Driven Cascade-Amplified Release of Loads from DNA-Gated Metal–Organic Frameworks for Electrochemical Detection of Cancer Biomarker. ACS Appl. Mater. Interfaces 2019, 12, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Su, F.; Song, Y.; Hu, B.; Wang, M.; He, L.; Peng, D.; Zhang, Z. Aptamer-templated silver nanoclusters embedded in zirconium metal–organic framework for bifunctional electrochemical and SPR aptasensors toward carcinoembryonic antigen. ACS Appl. Mater. Interfaces 2017, 9, 41188–41199. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.-F.; Yang, C.-D.; Chen, C.-C.; Kuo, C.-T. Stepwise control of reduction of graphene oxide and quantitative real-time evaluation of residual oxygen content using EC-SPR for a label-free electrochemical immunosensor. Sens. Actuators B Chem. 2018, 258, 981–990. [Google Scholar] [CrossRef]
- Majd, S.M.; Salimi, A. Ultrasensitive flexible FET-type aptasensor for CA 125 cancer marker detection based on carboxylated multiwalled carbon nanotubes immobilized onto reduced graphene oxide film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef]
- Archana, R.; Sreeja, B.; Nagarajan, K.; Radha, S.; BalajiBhargav, P.; Balaji, C.; Padmalaya, G. Development of Highly Sensitive Ag NPs Decorated Graphene FET Sensor for Detection of Glucose Concentration. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3818–3825. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef]
- Xu, T.; Song, Y.; Gao, W.; Wu, T.; Xu, L.-P.; Zhang, X.; Wang, S. Superwettable electrochemical biosensor toward detection of cancer biomarkers. ACS Sens. 2018, 3, 72–78. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, Y.; Ye, C.; Sun, H.; Dai, D.; Wei, Q.; Lai, G.; Wu, T.; Yu, A.; Fu, L. Highly stable and regenerative graphene–diamond hybrid electrochemical biosensor for fouling target dopamine detection. Biosens. Bioelectron. 2018, 111, 117–123. [Google Scholar] [CrossRef]
- Törer, H.; Aydın, E.B.; Sezgintürk, M.K. A label-free electrochemical biosensor for direct detection of RACK 1 by using disposable, low-cost and reproducible ITO based electrode. Anal. Chim. Acta 2018, 1024, 65–72. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.B.; Park, J.S.; Min, N. Single cytosine-based electrochemical biosensor for low-cost detection of silver ions. Sens. Actuators B Chem. 2017, 245, 741–746. [Google Scholar] [CrossRef]
- Pruna, R.; Palacio, F.; Baraket, A.; Zine, N.; Streklas, A.; Bausells, J.; Errachid, A.; López, M. A low-cost and miniaturized potentiostat for sensing of biomolecular species such as TNF-α by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2018, 100, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, M.-G. Advancements in DNA-assisted Immunosensors. BioChip J. 2020, 14, 18–31. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Huynh, K.-H.; Son, B.S.; Kim, H.-M.; Jun, B.-H. Sensitive Colorimetric Detection of Prostate Specific Antigen Using a Peroxidase-Mimicking Anti-PSA Antibody Coated Au Nanoparticle. BioChip J. 2020, 14, 158–168. [Google Scholar] [CrossRef]
- Haghparas, Z.; Kordrostami, Z.; Sorouri, M.; Rajabzadeh, M.; Khalifeh, R. Fabrication of Non-enzymatic Electrochemical Glucose Sensor Based on Nano-copper Oxide Micro Hollow-spheres. Biotechnol. Bioprocess Eng. 2020, 25, 528–535. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, F.; Hasebe, Y.; Jia, H.; Zhang, Z. A highly sensitive electrochemical biosensor for phenol derivatives using a graphene oxide-modified tyrosinase electrode. Bioelectrochemistry 2018, 122, 174–182. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M. Recent advances in design of electrochemical affinity biosensors for low level detection of cancer protein biomarkers using nanomaterial-assisted signal enhancement strategies. J. Pharm. Biomed. 2018, 147, 185–210. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: Recent progress, applications, and future perspective. Chem. Rev. 2018, 119, 120–194. [Google Scholar] [CrossRef]
- Pattadar, D.K.; Sharma, J.N.; Mainali, B.P.; Zamborini, F.P. Anodic stripping electrochemical analysis of metal nanoparticles. Curr. Opin. Electrochem. 2019, 13, 147–156. [Google Scholar] [CrossRef]
- Lu, L.; Lou, B.; Zou, S.; Kobayashi, H.; Liu, J.; Xiao, L.; Fan, J. Robust Removal of Ligands from Noble Metal Nanoparticles by Electrochemical Strategies. ACS Catal. 2018, 8, 8484–8492. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, J.; Sun, Y.; Wang, L.; Dong, X.; Ren, J.; He, W.; Xiao, F. Flexible nanohybrid microelectrode based on carbon fiber wrapped by gold nanoparticles decorated nitrogen doped carbon nanotube arrays: In situ electrochemical detection in live cancer cells. Biosens. Bioelectron. 2018, 100, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, X.; Nie, T.; Yin, L.; Wang, S.; Zhao, Y.; Wu, H.; Mei, H. A novel electrochemical sensing platform for detection of dopamine based on gold nanobipyramid/multi-walled carbon nanotube hybrids. Anal. Bioanal. Chem. 2020, 412, 2433–2441. [Google Scholar] [CrossRef]
- Dong, H.; Chen, H.; Jiang, J.; Zhang, H.; Cai, C.; Shen, Q. Highly sensitive electrochemical detection of tumor exosomes based on aptamer recognition-induced multi-DNA release and cyclic enzymatic amplification. Anal. Chem. 2018, 90, 4507–4513. [Google Scholar] [CrossRef]
- Park, H.-J.; Lee, W.-Y.; Chai, S.-Y.; Woo, J.-S.; Chung, H.-J.; Park, J.-K.; Song, H.; Hong, K. Expression of Insulin-like Growth Factor Binding Protein-3 and Regulation of the Insulin-like Growth Factor-I Axis in Pig Testis. Biotechnol. Bioprocess Eng. 2018, 23, 278–285. [Google Scholar] [CrossRef]
- Tao, H.; Chen, X.; Wei, A.; Song, X.; Wang, W.; Liang, L.; Zhao, Q.; Han, Z.; Han, Z.; Wang, X. Comparison of teratoma formation between embryonic stem cells and parthenogenetic embryonic stem cells by molecular imaging. Stem Cells Int. 2018, 2018, 7906531. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Schiffenbauer, Y.S.; Amouyal, N.; Ezov, N.; Steiner, M.; Izrael, M.; Lavon, N.; Hasson, A.; Revel, M.; Nyska, A. Compact MRI for the detection of teratoma development following intrathecal human embryonic stem cell injection in NOD-SCID mice. Neurotoxicology 2017, 59, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tang, Y.; Cao, Y.; Chen, T.; Chen, X.; Mao, X.; Yin, Y.; Chen, G. Amplified electrochemical detection of surface biomarker in breast cancer stem cell using self-assembled supramolecular nanocomposites. Electrochim. Acta 2018, 283, 1072–1078. [Google Scholar] [CrossRef]
- Poudineh, M.; Wang, Z.; Labib, M.; Ahmadi, M.; Zhang, L.; Das, J.; Ahmed, S.; Angers, S.; Kelley, S.O. Three-dimensional nanostructured architectures enable efficient neural differentiation of mesenchymal stem cells via mechanotransduction. Nano Lett. 2018, 18, 7188–7193. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Rahbarghazi, R.; Rashidi, M.-R. Label-free biosensors in the field of stem cell biology. Biosens. Bioelectron. 2018, 101, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Senel, M.; Sagir, T.; Isik, S. Highly sensitive detection of cancer cells with an electrochemical cytosensor based on boronic acid functional polythiophene. Biosens. Bioelectron. 2017, 90, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor nanoengineering: Design, operation, and implementation for biomolecular analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, P.; Pi, F.; Zhang, Y.; Li, Y.; Wang, J.; Sun, X. Development of a simple and convenient cell-based electrochemical biosensor for evaluating the individual and combined toxicity of DON, ZEN, and AFB1. Biosens. Bioelectron. 2017, 97, 345–351. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, Y.; Su, H.; Mao, L.; Chen, M. Sensitive detection of MCF-7 human breast cancer cells by using a novel DNA-labeled sandwich electrochemical biosensor. Biosens. Bioelectron. 2018, 122, 175–182. [Google Scholar] [CrossRef]
- Jo, J.; Yoon, J.; Lee, T.; Cho, H.-Y.; Lee, J.-Y.; Choi, J.-W. H2O2 biosensor consisted of hemoglobin-DNA conjugate on nanoporous gold thin film electrode with electrochemical signal enhancement. Nano Converg. 2019, 6, 1. [Google Scholar] [CrossRef]
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Singh, H.; Zhuang, S.; Misra, D.; Bhuyian, M.N.U.; Lee, E.S. Detection of cancer antigens (CA-125) using gold nano particles on interdigitated electrode-based microfluidic biosensor. Nano Converg. 2019, 6, 3. [Google Scholar] [CrossRef]
- Suhito, I.R.; Lee, W.; Baek, S.; Lee, D.; Min, J.; Kim, T.-H. Rapid and sensitive electrochemical detection of anticancer effects of curcumin on human glioblastoma cells. Sens. Actuators B Chem. 2019, 288, 527–534. [Google Scholar] [CrossRef]
- Flampouri, E.; Imar, S.; OConnell, K.; Singh, B. Spheroid-3D and monolayer-2D intestinal electrochemical biosensor for toxicity/viability testing: Applications in drug screening, food safety, and environmental pollutant analysis. ACS Sens. 2019, 4, 660–669. [Google Scholar] [CrossRef]
- Knutson, S.D.; Sanford, A.A.; Swenson, C.S.; Korn, M.M.; Manuel, B.A.; Heemstra, J.M. Thermoreversible Control of Nucleic Acid Structure and Function with Glyoxal Caging. J. Am. Chem. Soc. 2020, 142, 17766–17781. [Google Scholar] [CrossRef]
- Hannocks, M.-J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019, 75, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Flanagan, J.U.; Langley, R.J.; Hay, M.P.; Perry, J.K. Targeting growth hormone function: Strategies and therapeutic applications. Signal Transduct. Target. Ther. 2019, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoci, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, İ. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Wang, C.-F.; Sun, X.-Y.; Su, M.; Wang, Y.-P.; Lv, Y.-K. Electrochemical biosensors based on antibody, nucleic acid and enzyme functionalized graphene for the detection of disease-related biomolecules. Analyst 2020, 145, 1550–1562. [Google Scholar] [CrossRef]
- Asal, M.; Özen, Ö.; Şahinler, M.; Polatoğlu, İ. Recent developments in enzyme, DNA and immuno-based biosensors. Sensors 2018, 18, 1924. [Google Scholar] [CrossRef]
- Faria, H.A.M.; Zucolotto, V. Label-free electrochemical DNA biosensor for zika virus identification. Biosens. Bioelectron. 2019, 131, 149–155. [Google Scholar] [CrossRef]
- Salimian, R.; Shahrokhian, S.; Panahi, S. Enhanced electrochemical activity of a hollow carbon sphere/polyaniline-based electrochemical biosensor for HBV DNA marker detection. ACS Biomater. Sci. Eng. 2019, 5, 2587–2594. [Google Scholar] [CrossRef]
- Shabaninejad, Z.; Yousefi, F.; Movahedpour, A.; Ghasemi, Y.; Dokanehiifard, S.; Rezaei, S.; Aryan, R.; Savardashtaki, A.; Mirzaei, H. Electrochemical-based biosensors for microRNA detection: Nanotechnology comes into view. Anal. Chem. 2019, 581, 113349. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Molecularly imprinted polymers and surface imprinted polymers based electrochemical biosensor for infectious diseases. Sensors 2020, 20, 996. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Takemura, K.; Li, T.-C.; Suzuki, T.; Park, E.Y. Electrical pulse-induced electrochemical biosensor for hepatitis E virus detection. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Haque, M.H.; Yadav, S.; Islam, M.N.; Gopalan, V.; Nguyen, N.-T.; Lam, A.K.; Shiddiky, M.J. An electrochemical method for sensitive and rapid detection of FAM134B protein in colon cancer samples. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.K.; Barfidokht, A.; Tehrani, F.; Mishra, R.K. Food safety analysis using electrochemical biosensors. Foods 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G. Bionanomaterial-based electrochemical biosensing platforms for biomedical applications. Anal. Methods 2020, 12, 1688–1701. [Google Scholar] [CrossRef]
- Luong, J.H.; Narayan, T.; Solanki, S.; Malhotra, B.D. Recent Advances of Conducting Polymers and Their Composites for Electrochemical Biosensing Applications. J. Function. Biomater. 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Sedlackova, E.; Bytesnikova, Z.; Birgusova, E.; Svec, P.; Ashrafi, A.M.; Estrela, P.; Richtera, L. Label-Free DNA Biosensor Using Modified Reduced Graphene Oxide Platform as a DNA Methylation Assay. Materials 2020, 13, 4936. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, K.; Kong, Q.; Zhang, L.; Ge, S.; Yu, J. A self-powered origami paper analytical device with a pop-up structure for dual-mode electrochemical sensing of ATP assisted by glucose oxidase-triggered reaction. Biosens. Bioelectron. 2020, 148, 111839. [Google Scholar] [CrossRef]
- Cinti, S.; Proietti, E.; Casotto, F.; Moscone, D.; Arduini, F. Paper-based strips for the electrochemical detection of single and double stranded DNA. Anal. Chem. 2018, 90, 13680–13686. [Google Scholar] [CrossRef]
- Yang, J.; Gao, L.; Peng, C.; Zhang, W. Construction of self-signal DNA electrochemical biosensor employing WS 2 nanosheets combined with PIn6COOH. RSC Adv. 2019, 9, 9613–9619. [Google Scholar] [CrossRef]
- Dutta, S.; Chowdhury, A.D.; Biswas, S.; Park, E.Y.; Agnihotri, N.; De, A.; De, S. Development of an effective electrochemical platform for highly sensitive DNA detection using MoS2-polyaniline nanocomposites. Biochem. Eng. J. 2018, 140, 130–139. [Google Scholar] [CrossRef]
- Fan, T.; Du, Y.; Yao, Y.; Wu, J.; Meng, S.; Luo, J.; Zhang, X.; Yang, D.; Wang, C.; Qian, Y. Rolling circle amplification triggered poly adenine-gold nanoparticles production for label-free electrochemical detection of thrombin. Sens. Actuators B Chem. 2018, 266, 9–18. [Google Scholar] [CrossRef]
- Cheng, L.; Xu, C.; Cui, H.; Liao, F.; Hong, N.; Ma, G.; Xiong, J.; Fan, H. A sensitive homogenous aptasensor based on tetraferrocene labeling for thrombin detection. Anal. Chim. Acta 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. Ultrasensitive label-free homogeneous electrochemical aptasensor based on sandwich structure for thrombin detection. Sens. Actuators B Chem. 2018, 267, 412–418. [Google Scholar] [CrossRef]
- Chen, Y.; Song, X.; Li, L.; Tang, B. A High-Fidelity Electrochemical Platform Based on Au–Se Interface for Biological Detection. Anal. Chem. 2020, 92, 5855–5861. [Google Scholar] [CrossRef] [PubMed]
- Shabani, E.; Abdekhodaie, M.J.; Mousavi, S.A.; Taghipour, F. ZnO nanoparticle/nanorod-based label-free electrochemical immunoassay for rapid detection of MMP-9 biomarker. Biochem. Eng. J. 2020, 164, 107772. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.Y.; Lee, W.C.; Choi, S.; Lim, J.; Jeong, H.; Shin, D.-S.; Park, Y.J. A reference electrode-free electrochemical biosensor for detecting MMP-9 using a concentric electrode device. Sens. Actuators B Chem. 2017, 240, 735–741. [Google Scholar] [CrossRef]
- Ahirwar, R.; Dalal, A.; Sharma, J.G.; Yadav, B.K.; Nahar, P.; Kumar, A.; Kumar, S. An aptasensor for rapid and sensitive detection of estrogen receptor alpha in human breast cancer. Biotechnol. Bioeng. 2019, 116, 227–233. [Google Scholar] [CrossRef]
- Nameghi, M.A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An ultrasensitive electrochemical sensor for 17β-estradiol using split aptamers. Anal. Chim. Acta 2019, 1065, 107–112. [Google Scholar] [CrossRef]
- Liu, M.; Ke, H.; Sun, C.; Wang, G.; Wang, Y.; Zhao, G. A simple and highly selective electrochemical label-free aptasensor of 17β-estradiol based on signal amplification of bi-functional graphene. Talanta 2019, 194, 266–272. [Google Scholar] [CrossRef]
- Viet, N.X.; Hoan, N.X.; Takamura, Y. Development of highly sensitive electrochemical immunosensor based on single-walled carbon nanotube modified screen-printed carbon electrode. Mater. Chem. Phys. 2019, 227, 123–129. [Google Scholar] [CrossRef]
- Rizwan, M.; Hazmi, M.; Lim, S.A.; Ahmed, M.U. A highly sensitive electrochemical detection of human chorionic gonadotropin on a carbon nano-onions/gold nanoparticles/polyethylene glycol nanocomposite modified glassy carbon electrode. J. Electroanal. Chem. 2019, 833, 462–470. [Google Scholar] [CrossRef]
- Damiati, S.; Haslam, C.; Søpstad, S.; Peacock, M.; Whitley, T.; Davey, P.; Awan, S.A. Sensitivity comparison of macro-and micro-electrochemical biosensors for human chorionic gonadotropin (hCG) biomarker detection. IEEE Access 2019, 7, 94048–94058. [Google Scholar] [CrossRef]
- Kong, D.; Liao, F.; Lin, Y.; Cheng, L.; Peng, H.; Zhang, J.; Cui, H.; Hong, N.; Chen, C.; Wei, G. A homogenous electrochemical sensing DNA sensor by using bare Au electrode based on potential-assisted chemisorption technique. Sens. Actuators B Chem. 2018, 266, 288–293. [Google Scholar] [CrossRef]
- Dutta Chowdhury, A.; Agnihotri, N.; Doong, R.-a.; De, A. Label-free and nondestructive separation technique for isolation of targeted DNA from DNA–protein mixture using magnetic Au–Fe3O4 nanoprobes. Anal. Chem. 2017, 89, 12244–12251. [Google Scholar] [CrossRef]
- Abolhasan, R.; Mehdizadeh, A.; Rashidi, M.R.; Aghebati-Maleki, L.; Yousefi, M. Application of hairpin DNA-based biosensors with various signal amplification strategies in clinical diagnosis. Biosens. Bioelectron. 2019, 129, 164–174. [Google Scholar] [CrossRef]
- Momeneh, H.; Gholivand, M.B. Mycophenolate mofetil sensor based on molecularly imprinted polymer/multi-walled carbon nanotubes modified carbon paste electrode. Anal. Biochem. 2018, 557, 97–103. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic properties, catalytic mechanism, and applications. Angew. Chem. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable bioelectronics: Enzyme-based body-worn electronic devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef]
- Chen, H.-J.; Chen, R.L.; Hsieh, B.-C.; Hsiao, H.-Y.; Kung, Y.; Hou, Y.-T.; Cheng, T.-J. Label-free and reagentless capacitive aptasensor for thrombin. Biosens. Bioelectron. 2019, 131, 53–59. [Google Scholar] [CrossRef]
- Reddel, C.J.; Tan, C.W.; Chen, V.M. Thrombin generation and cancer: Contributors and consequences. Cancers 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, G.; Zhang, M.; Xu, G.; Lin, J.; Luo, X. Aptamer based label free thrombin assay based on the use of silver nanoparticles incorporated into self-polymerized dopamine. Microchim. Acta 2018, 185, 253. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Prior, S.M.; Bembenek, J.P.; Niewada, M.; Broniatowska, E.; Członkowska, A.; Butenas, S.; Undas, A. Activation of blood coagulation and thrombin generation in acute ischemic stroke treated with rtPA. J. Thromb. Thrombolys. 2017, 44, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Xu, N.; Pan, J.; Chen, T.; Chen, Y.; Gao, W. Dual-signal amplification strategy for electrochemiluminescence sandwich biosensor for detection of thrombin. Sens. Actuators B Chem. 2017, 240, 742–748. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Z.; Xi, X.; Cao, T.; Wen, W.; Zhang, X.; Wang, S. An aptamer-based hook-effect-recognizable three-line lateral flow biosensor for rapid detection of thrombin. Biosens. Bioelectron. 2019, 133, 177–182. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Dukenbayev, K.; Shaimerdenova, M.; Bekniyazov, I.; Ayupova, T.; Sypabekova, M.; Molardi, C.; Tosi, D. Etched fiber Bragg grating biosensor functionalized with aptamers for detection of thrombin. Sensors 2018, 18, 4298. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Vanzetti, L.E.; Bossi, A.M.; Zeni, L. D-shaped plastic optical fibre aptasensor for fast thrombin detection in nanomolar range. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- De La Franier, B.; Thompson, M. Early stage detection and screening of ovarian cancer: A research opportunity and significant challenge for biosensor technology. Biosens. Bioelectron. 2019, 135, 71–81. [Google Scholar] [CrossRef]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Yin, J.; Guo, W.; Qin, X.; Zhao, J.; Pei, M.; Ding, F. A sensitive electrochemical aptasensor for highly specific detection of streptomycin based on the porous carbon nanorods and multifunctional graphene nanocomposites for signal amplification. Sens. Actuators B Chem. 2017, 241, 151–159. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, Z. Dual-reaction triggered sensitivity amplification for ultrasensitive peptide-cleavage based electrochemical detection of matrix metalloproteinase-7. Biosens. Bioelectron. 2018, 108, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vega, G.; García-Robaina, A.; Ismail, M.B.; Pasamar, H.; García-Berrocoso, T.; Montaner, J.; Zourob, M.; Othmane, A.; Del Campo, F.J.; Baldrich, E. Detection of plasma MMP-9 within minutes. Unveiling some of the clues to develop fast and simple electrochemical magneto-immunosensors. Biosens. Bioelectron. 2018, 115, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Lehr, D.; Schönbrodt, O.; Glaser, T.; Fechner, R.; Frost, F. Manufacturing of highly-dispersive, high-efficiency transmission gratings by laser interference lithography and dry etching. Microelectron. Eng. 2018, 191, 60–65. [Google Scholar] [CrossRef]
- Manjunatha, J. Electroanalysis of estriol hormone using electrochemical sensor. Sens. Bio-Sens. Res. 2017, 16, 79–84. [Google Scholar] [CrossRef]
- Govindasamy, M.; Subramanian, B.; Wang, S.-F.; Chinnapaiyan, S.; Ramalingam, R.J.; Al-Lohedan, H.A. Ultrasound-assisted synthesis of tungsten trioxide entrapped with graphene nanosheets for developing nanomolar electrochemical (hormone) sensor and enhanced sensitivity of the catalytic performance. Ultrason. Sonochem. 2019, 56, 134–142. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef]
- Cifrić, S.; Nuhić, J.; Osmanović, D.; Kišija, E. Review of Electrochemical Biosensors for Hormone Detection; Springer: Cham, Switzerland, 2020; pp. 173–177. [Google Scholar]
- Tieu, M.V.; Go, A.; Park, Y.J.; Nguyen, H.V.; Hwang, S.Y.; Lee, M. Highly Sensitive ELISA Using Membrane-Based Microwave-Mediated Electrochemical Immunoassay for Thyroid-Stimulating Hormone Detection. IEEE Sens. J. 2019, 19, 9826–9831. [Google Scholar] [CrossRef]
- Šišoláková, I.; Hovancová, J.; Oriňaková, R.; Oriňak, A.; Trnková, L.; Třísková, I.; Farka, Z.; Pastucha, M.; Radoňák, J. Electrochemical determination of insulin at CuNPs/chitosan-MWCNTs and CoNPs/chitosan-MWCNTs modified screen printed carbon electrodes. J. Electroanal. Chem. 2020, 860, 113881. [Google Scholar] [CrossRef]
- Uliana, C.V.; Peverari, C.R.; Afonso, A.S.; Cominetti, M.R.; Faria, R.C. Fully disposable microfluidic electrochemical device for detection of estrogen receptor alpha breast cancer biomarker. Biosens. Bioelectron. 2018, 99, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, G.; Wang, B.; Shi, L.; Gao, T.; Li, G. Fabrication of reusable electrochemical biosensor and its application for the assay of α-glucosidase activity. Anal. Chim. Acta 2018, 1026, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, X.; Lu, J.; Mao, X.; Xiang, Y.; Shu, Y.; Li, G. Design of DNA nanostructure-based interfacial probes for the electrochemical detection of nucleic acids directly in whole blood. Chem. Sci. 2018, 9, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Chen, Z.; Liu, Y.; Ren, H.; Liu, L. Peptide aptamer-based biosensor for the detection of human chorionic gonadotropin by converting silver nanoparticles-based colorimetric assay into sensitive electrochemical analysis. Sens. Actuators B Chem. 2017, 243, 784–791. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Dong, C.; Li, Y.; Kou, Q.; Yan, J.; Zhang, L. Ultrasensitive ELISA for the detection of hCG based on assembled gold nanoparticles induced by functional polyamidoamine dendrimers. Anal. Chim. Acta 2018, 1042, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Pourmoghadam, Z.; Soltani-Zangbar, M.S.; Sheikhansari, G.; Azizi, R.; Eghbal-Fard, S.; Mohammadi, H.; Siahmansouri, H.; Aghebati-Maleki, L.; Danaii, S.; Mehdizadeh, A. Intrauterine administration of autologous hCG-activated peripheral blood mononuclear cells improves pregnancy outcomes in patients with recurrent implantation failure; A double-blind, randomized control trial study. J. Reprod. Immunol. 2020, 142, 103182. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Hallaj, R.; Fathi, F.; Soleimani, F. CuO/WO3 nanoparticles decorated graphene oxide nanosheets with enhanced peroxidase-like activity for electrochemical cancer cell detection and targeted therapeutics. Mater. Sci. Eng. C 2019, 99, 1374–1383. [Google Scholar] [CrossRef]
- Ruiyi, L.; Tinling, P.; Hongxia, C.; Jinsong, S.; Zaijun, L. Electrochemical detection of cancer cells in human blood using folic acid and glutamic acid-functionalized graphene quantum dot-palladium@ gold as redox probe with excellent electrocatalytic activity and target recognition. Sens. Actuators B Chem. 2020, 309, 127709. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Naghib, S.M.; Majidzadeh-A, K.; Zargartalebi, H.; Sanati-Nezhad, A. Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens. Bioelectron. 2018, 117, 104–111. [Google Scholar] [CrossRef]
- Kaushik, A.; Tiwari, S.; Jayant, R.D.; Vashist, A.; Nikkhah-Moshaie, R.; El-Hage, N.; Nair, M. Electrochemical biosensors for early stage Zika diagnostics. Trends Biotechnol. 2017, 35, 308–317. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Q.; Xiao, F.; Duan, H. 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Chandra, P. Clinical implications and electrochemical biosensing of monoamine neurotransmitters in body fluids, in vitro, in vivo, and ex vivo models. Biosens. Bioelectron. 2018, 121, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. NAt. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Fournel, L.; Wu, Z.; Alifano, M.; Lincet, H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem. Sci. 2019, 44, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Nasr, B.; Chatterton, R.; Yong, J.H.M.; Jamshidi, P.; D’Abaco, G.M.; Bjorksten, A.R.; Kavehei, O.; Chana, G.; Dottori, M.; Skafidas, E. Self-organized nanostructure modified microelectrode for sensitive electrochemical glutamate detection in stem cells-derived brain organoids. Biosensors 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Jarolimova, P.; Voltrova, B.; Blahnova, V.; Sovkova, V.; Pruchova, E.; Hybasek, V.; Fojt, J.; Filova, E. Mesenchymal stem cell interaction with Ti 6 Al 4 V alloy pre-exposed to simulated body fluid. RSC Adv. 2020, 10, 6858–6872. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, J.-H.; Choi, J.-W. Applications of Bionano Sensor for Extracellular Vesicles Analysis. Materials 2020, 13, 3677. [Google Scholar] [CrossRef]
- Chen, K.G.; Mallon, B.S.; Park, K.; Robey, P.G.; McKay, R.D.; Gottesman, M.M.; Zheng, W. Pluripotent stem cell platforms for drug discovery. Trends Mol. Med. 2018, 24, 805–820. [Google Scholar] [CrossRef]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically conductive materials: Opportunities and challenges in tissue engineering. Biomolecules 2019, 9, 448. [Google Scholar] [CrossRef]
- Elkhenany, H.; AlOkda, A.; El-Badawy, A.; El-Badri, N. Tissue regeneration: Impact of sleep on stem cell regenerative capacity. Life Sci. 2018, 214, 51–61. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Q.; Zou, K.; Wang, H.; Zhang, X.; Yang, Z.; Lee, W.Y.W.; Wei, B.; Gu, W.; Yang, Y.P. Administration of allogeneic mesenchymal stem cells in lengthening phase accelerates early bone consolidation in rat distraction osteogenesis model. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar]
- Madhurantakam, S.; Babu, K.J.; Rayappan, J.B.B.; Krishnan, U.M. Fabrication of mediator-free hybrid nano-interfaced electrochemical biosensor for monitoring cancer cell proliferation. Biosens. Bioelectron. 2017, 87, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, T.; Yu, G.; Wu, S.; Sun, J.; Jia, H.; Wang, H.; Yang, X.; Zhang, Y. A label-free electrochemical biosensor for ultra-sensitively detecting telomerase activity based on the enhanced catalytic currents of acetaminophen catalyzed by Au nanorods. Biosens. Bioelectron. 2019, 124, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Cuzzani, O.; Binette, F.; Sternberg, H.; West, M.D.; Nasonkin, I.O. Pluripotent stem cells for retinal tissue engineering: Current status and future prospects. Stem Cell Rev. Rep. 2018, 14, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Jossen, V.; van den Bos, C.; Eibl, R.; Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl. Microbiol. Biotechnol. 2018, 102, 3981–3994. [Google Scholar] [CrossRef]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human pluripotent stem cell culture: Current status, challenges, and advancement. Stem Cells Int. 2018, 2018, 7396905. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Lin, H.-C.; Lai, C.-L.; Chen, P.-Y.; Lai, C.-H. Mannosyl electrochemical impedance cytosensor for label-free MDA-MB-231 cancer cell detection. Biosens. Bioelectron. 2018, 116, 100–107. [Google Scholar] [CrossRef]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly (glutamic acid)/MWNT nanocomposite. Microchim. Acta 2018, 185, 405. [Google Scholar] [CrossRef]
- Sun, D.; Lu, J.; Chen, D.; Jiang, Y.; Wang, Z.; Qin, W.; Yu, Y.; Chen, Z.; Zhang, Y. Label-free electrochemical detection of HepG2 tumor cells with a self-assembled DNA nanostructure-based aptasensor. Sens. Actuators B Chem. 2018, 268, 359–367. [Google Scholar] [CrossRef]
- Sun, D.; Lu, J.; Zhong, Y.; Yu, Y.; Wang, Y.; Zhang, B.; Chen, Z. Sensitive electrochemical aptamer cytosensor for highly specific detection of cancer cells based on the hybrid nanoelectrocatalysts and enzyme for signal amplification. Biosens. Bioelectron. 2016, 75, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, S.; Demir, B.; Eyrilmez, G.O.; Kesici, S.; Saylam, A.; Demirkol, D.O.; Özçubukçu, S.; Timur, S.; Toppare, L. Comparative cell adhesion properties of cysteine extended peptide architectures. RSC Adv. 2016, 6, 2695–2702. [Google Scholar] [CrossRef]
- Angeline, N.; Suhito, I.R.; Kim, C.H.; Hong, G.P.; Park, C.G.; Bhang, S.H.; Luo, Z.; Kim, T.H. A fibronectin-coated gold nanostructure composite for electrochemical detection of effects of curcumin-carrying nanoliposomes on human stomach cancer cells. Analyst 2020, 145, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Suhito, I.R.; Angeline, N.; Lee, K.H.; Kim, H.; Park, C.G.; Luo, Z.; Kim, T.H. A Spheroid-Forming Hybrid Gold Nanostructure Platform That Electrochemically Detects Anticancer Effects of Curcumin in a Multicellular Brain Cancer Model. Small 2020, 2002436. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Kim, S.U.; Park, M.-K.; Choi, J.W. Electrochemical Detection of Human Mesenchymal Stem Cell Differentiation on Fabricated Gold Nano-Dot Cell Chips. J. Nanosci. Nanotechnol. 2015, 15, 7929–7934. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-C.; Choo, S.-S.; Kim, K.-T.; Hong, K.-S.; Moon, S.-H.; Cha, H.-J.; Kim, T.-H. Conductive hybrid matrigel layer to enhance electrochemical signals of human embryonic stem cells. Sens. Actuators B Chem. 2017, 242, 224–230. [Google Scholar] [CrossRef]
- Suhito, I.R.; Kang, E.-S.; Kim, D.-S.; Baek, S.; Park, S.-J.; Moon, S.-H.; Luo, Z.; Lee, D.; Min, J.; Kim, T.-H. High density gold nanostructure composites for precise electrochemical detection of human embryonic stem cells in cell mixture. Colloids Surf. B Biointerfaces 2019, 180, 384–392. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.; Naumov, A. Graphene oxide as a multifunctional platform for intracellular delivery, imaging, and cancer sensing. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Suhito, I.R.; Han, Y.; Min, J.; Son, H.; Kim, T.-H. In situ label-free monitoring of human adipose-derived mesenchymal stem cell differentiation into multiple lineages. Biomaterials 2018, 154, 223–233. [Google Scholar] [CrossRef]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Choo, S.-S.; Kim, S.-J.; Song, I.; Kim, T.-H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef]
- Kim, B.-S.; Cho, C.-S. Injectable Hydrogels for Regenerative Medicine. Tissue Eng. Regen. Med 2018, 15, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kim, R.Y.; Park, B.-W.; Lee, S.; Choi, S.W.; Park, J.-H.; Choi, J.J.; Kim, S.-W.; Jang, J.; Cho, D.-W. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-W.; Wang, P.-H.; Tsui, K.-H. Mesenchymal stem cell in wound healing and regeneration. J. Chin. Med. Assoc. 2018, 81, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, M.; Liu, W.; Li, Y.; Li, M. Stem Cell-Based Therapies for Liver Diseases: An Overview and Update. Tissue Eng. Regen. Med. 2019, 16, 107–118. [Google Scholar] [CrossRef]
- Angelova, P.R.; Barilani, M.; Lovejoy, C.; Dossena, M.; Viganò, M.; Seresini, A.; Piga, D.; Gandhi, S.; Pezzoli, G.; Abramov, A.Y. Mitochondrial dysfunction in Parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol. 2018, 14, 474–484. [Google Scholar] [CrossRef]
- Suhito, I.R.; Angeline, N.; Choo, S.-S.; Woo, H.Y.; Paik, T.; Lee, T.; Kim, T.-H. Nanobiosensing Platforms for Real-Time and Non-Invasive Monitoring of Stem Cell Pluripotency and Differentiation. Sensors 2018, 18, 2755. [Google Scholar] [CrossRef]
- Ino, K.; Nashimoto, Y.; Taira, N.; Azcon, J.R.; Shiku, H. Intracellular electrochemical sensing. Electroanalysis 2018, 30, 2195–2209. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, H.K.; Yang, L.; Chueng, S.T.D.; Choi, J.W.; Lee, K.B. Nondestructive Real-Time Monitoring of Enhanced Stem Cell Differentiation Using a Graphene-Au Hybrid Nanoelectrode Array. Adv. Mater. 2018, 30, 1802762. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, J.-H.; Chueng, S.-T.D.; Pongkulapa, T.; Yang, L.; Cho, H.-Y.; Choi, J.-W.; Lee, K.-B. Nondestructive characterization of stem cell neurogenesis by a magneto-plasmonic nanomaterial-based exosomal mirna detection. ACS Nano 2019, 13, 8793–8803. [Google Scholar] [CrossRef]
- Ma, W.; Shao, X.; Zhao, D.; Li, Q.; Liu, M.; Zhou, T.; Xie, X.; Mao, C.; Zhang, Y.; Lin, Y. Self-assembled tetrahedral DNA nanostructures promote neural stem cell proliferation and neuronal differentiation. ACS Appl. Mater. Interfaces 2018, 10, 7892–7900. [Google Scholar] [CrossRef]


| Target | Substrate | Immobilization Strategies | Detection Methods | Ref. |
|---|---|---|---|---|
| DNA | Screen-printed electrode | Au nanoparticles/TFO probe/Methylene Blue/Target DNA (ssDNA or ds DNA) | CV/SWV | [68] |
| DNA | Carbon paste electrode | WS2/PIn6COOH/ssDNA | CV/EIS | [69] |
| DNA | Platinum electrode | MoS2-polyaniline/ssDNA/Methylene Blue (MB) | CV/DPV | [70] |
| Thrombin | Au electrode | Poly-adenine/aptamer1/thrombin/aptamer2/padlock | CV/DPV/EIS | [71] |
| Thrombin | Au electrode | Thiol-group/aptamer/tetra-ferrocene | DPV/EIS | [72] |
| Thrombin | Glassy-carbon electrode | Graphene oxide/MNP-TBA1 (Magnetic nanoparticle-thrombin binding aptamer)/HAP-TBA2 (Hydroxyapatite-TBA2) | CV/SWV | [73] |
| MMP-2 | Au electrode | Selenium/peptide/Na2MoO4/ssDNA | CV/EIS | [74] |
| MMP-9 | ZnO nanoparticle electrode | Gold-coated glass/ZnO-NP/APTES/Glutaraldehyde/MMP-9 Antibody | CV/EIS | [75] |
| MMP-9 | Au electrode | L-cysteine/EDC/NHS/Peptide/MB | CV | [76] |
| Estrogen (ER alpha) | Screen-printed electrode | 5′-thiol-modified DNA aptamer/Tris-(2-carboxyethyl) phosphine hydrochloride | DPV | [77] |
| Estrogen (17-β Estradiol) | Au electrode | Split aptamer 1/E2/Split aptamer 2 | CV/DPV | [78] |
| Estrogen (17-β Estradiol) | Au electrode | 6-mercapto-1-hexanol (MCH)/Aptamer-Graphene | DPV/EIS | [79] |
| Human chorionic gonadotrophin (hCG) | Screen-printed carbon electrode | Carbon-nanotube/Antibody 1/hCG/Au-Antibody 2 | CV/DPV | [80] |
| Human chorionic gonadotrophin (hCG) | Glassy-carbon electrode | Carbon nano-onions (CNOs)/gold nanoparticles (AuNPs)/Polyethylene glycol (PEG) | CV/SWV | [81] |
| Human chorionic gonadotrophin (hCG) | Screen-printed carbon electrode | PANHS/Anti-hCG antibody/BSA/hCG | CV/SWV | [82] |
| Target | Substrate | Immobilization Strategies | Detection Methods | Ref. |
|---|---|---|---|---|
| MDA-MB-231 cells | Glassy-carbon electrode | Mannose-C2NH2/Con A or BSA Mannose-C2NH2/Cell mixture‘ | CV/EIS | [139] |
| MCF-7 cells | Glassy-carbon electrode | MWCNT/PGA composite/MUC-1 aptamer/Glutathione/Apt-AgNPs | CV/DPV/EIS | [140] |
| HepG2 cells | Screen-printed gold electrode | DNA nanotetrahedron/TLS11a aptamer probe/Pd-Pt nanocage (labeled with cDNA) | DPV | [141] |
| HepG2 cells | Glassy-carbon electrode | Fe3O4/MnO2/Au-Pd/HRP–aptamer/Hemin/G-quadruplex (nano probe) | CV/DPV | [142] |
| U-87 MG cells | ITO glass electrode | Gold layer/L-cysteine/TAT and RGD-C-peptide | CV/EIS | [143] |
| HER2 cells | Fluorine doped tin oxide (FTO) glass | Nitrogen-doped graphene/AgNP/Poly aniline/Anti-HER2 | DPV | [120] |
| MKN-28 cells | ITO glass electrode | HAuCl4/Fibronectin and Collagen-solution | DPV/EIS | [144] |
| SH-SY5Y/U-87 MG cells | ITO glass electrode | Gold nanostructure | DPV | [145] |
| hMSCs | ITO glass electrode | Nano-porous Alumina Mask/Au dot/L-cysteine/RGD-peptide composite | CV | [146] |
| hESCs | ITO glass electrode | Matrigel/GNPs/RGD peptide/Gold layer | DPV | [147] |
| hESCs | ITO glass electrode | Matrigel/High density gold nanostructure | DPV | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhito, I.R.; Koo, K.-M.; Kim, T.-H. Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines 2021, 9, 15. https://doi.org/10.3390/biomedicines9010015
Suhito IR, Koo K-M, Kim T-H. Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines. 2021; 9(1):15. https://doi.org/10.3390/biomedicines9010015
Chicago/Turabian StyleSuhito, Intan Rosalina, Kyeong-Mo Koo, and Tae-Hyung Kim. 2021. "Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells" Biomedicines 9, no. 1: 15. https://doi.org/10.3390/biomedicines9010015
APA StyleSuhito, I. R., Koo, K.-M., & Kim, T.-H. (2021). Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines, 9(1), 15. https://doi.org/10.3390/biomedicines9010015





