Simvastatin Impairs Glucose Homeostasis in Mice Depending on PGC-1α Skeletal Muscle Expression
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Intraperitoneal Glucose Tolerance Test (iGTT)
2.3. Sample Collection
2.4. In Vivo Skeletal Muscle Glucose Uptake
2.5. Quantification of Insulin Sensitivity
2.6. Muscle Glycogen Content
2.7. Quantitative Real-Time PCR
2.8. Western Blots
2.9. Statistical Analysis
3. Results
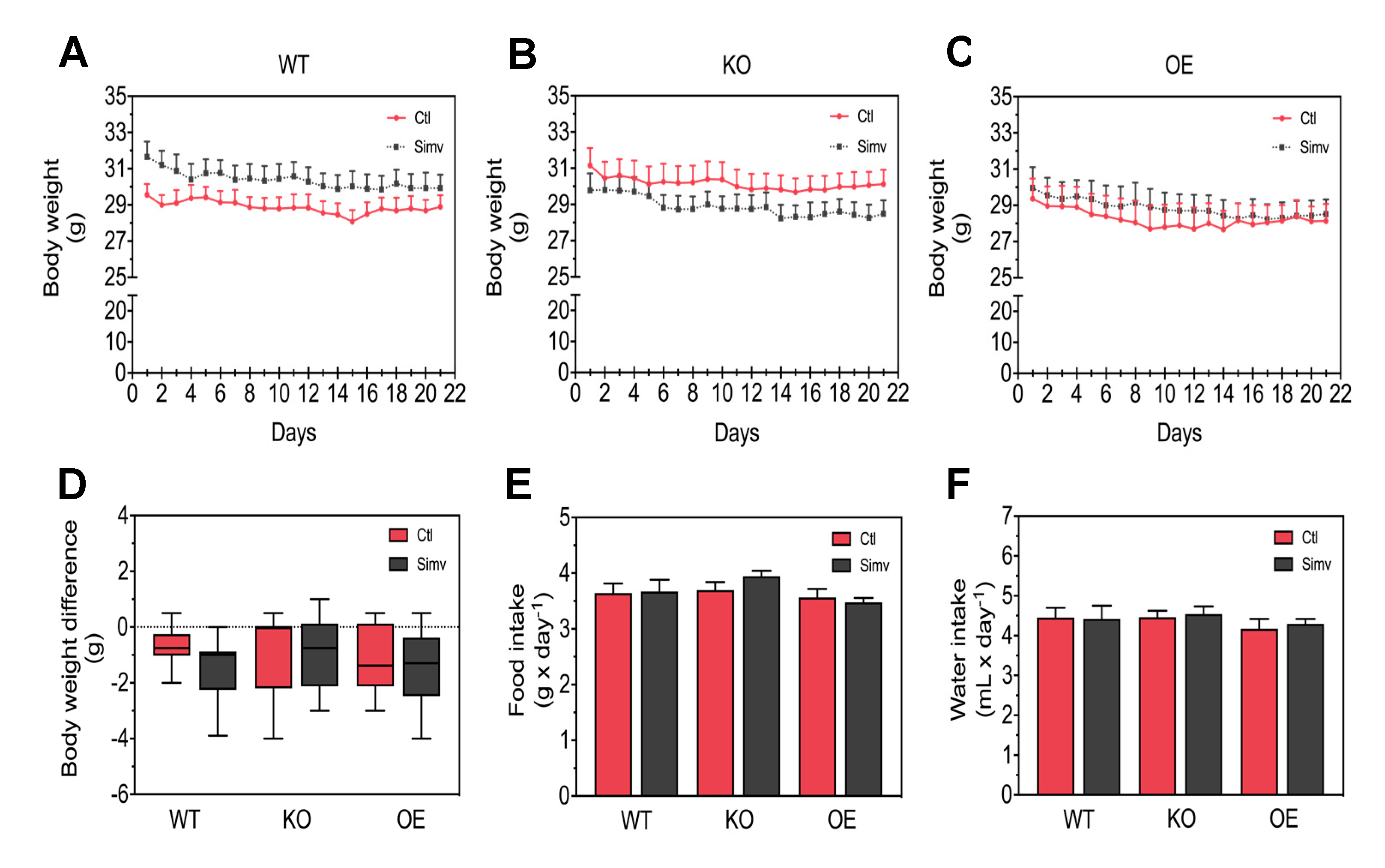
3.1. Physiological Characterization of the Mice
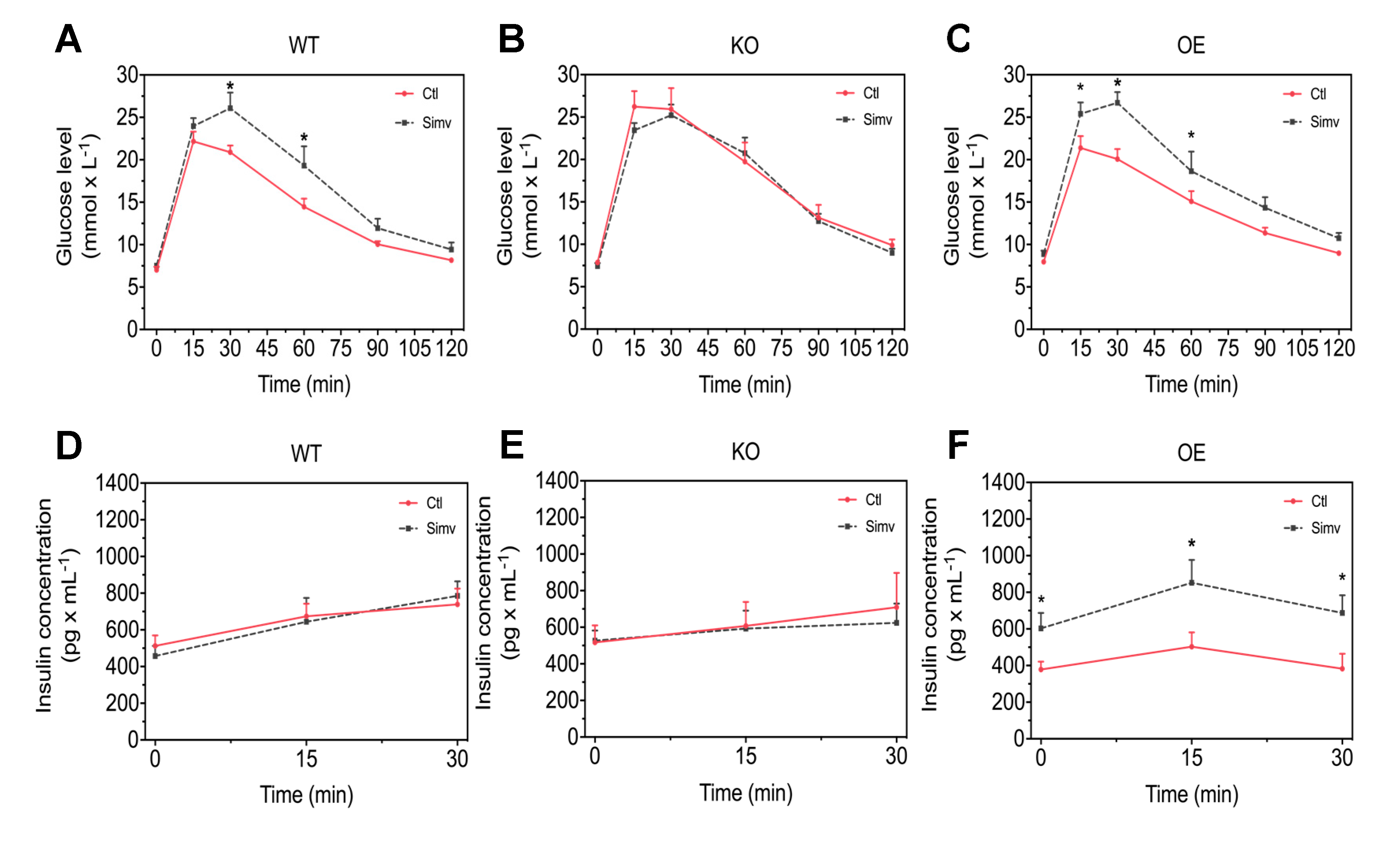
3.2. Simvastatin Impaired Glucose Homeostasis in WT and OE Mice Whereas KO Mice Showed Already Higher Blood Glucose Concentrations during the iGTT Without Simvastatin Treatment
3.3. Simvastatin Impaired Glucose Uptake and Glycogen Muscle Reserves in WT and KO Mice, But Not in OE Mice
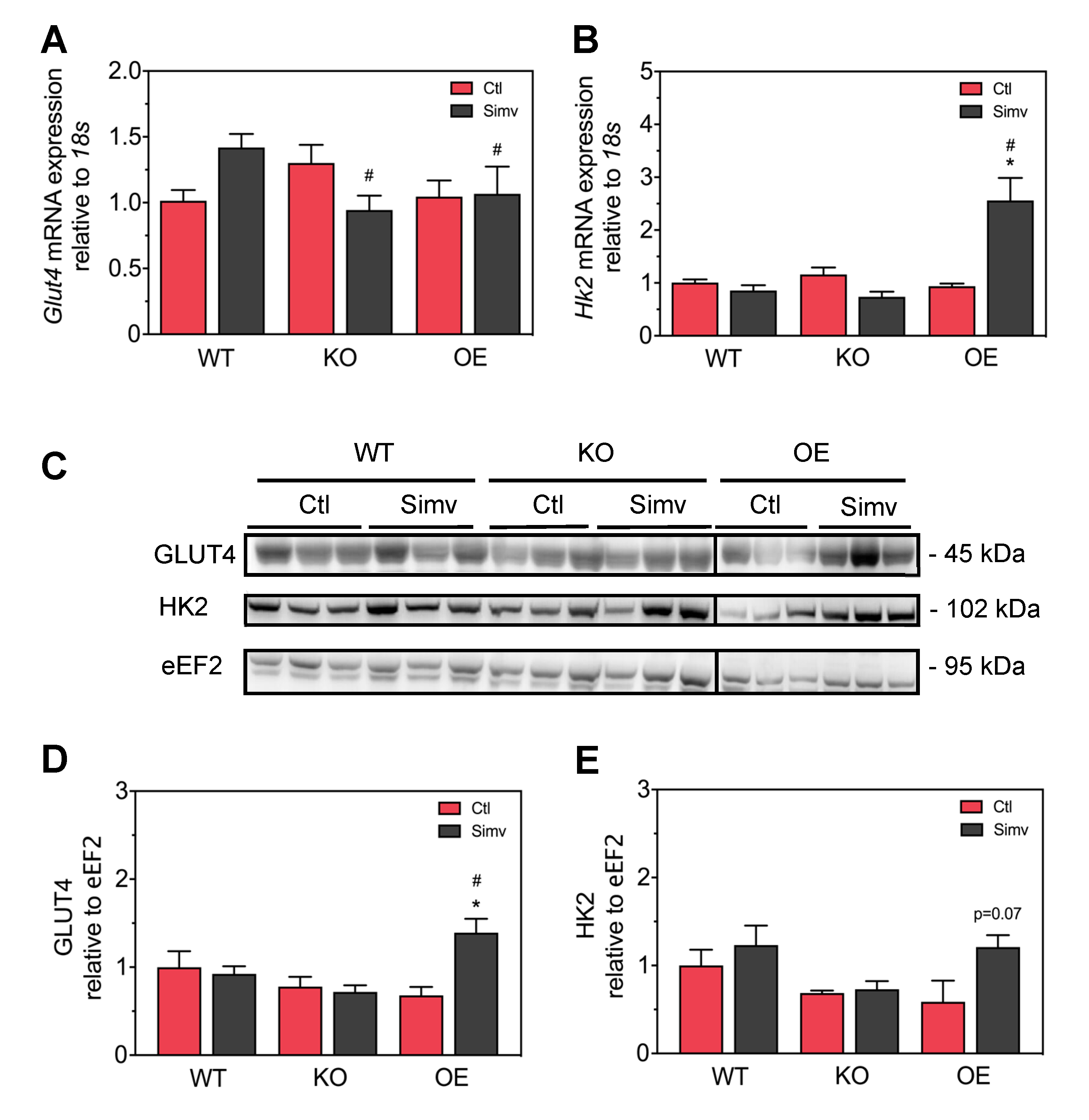
3.4. Simvastatin Had No Significant Impact on Skeletal Muscle GLUT4 and Hexokinase Protein Expression in WT and KO Mice But Increased the Expression of Both Proteins in OE Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L., 2nd; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.; Huffman, M.D.; Ebrahim, S. Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA 2013, 310, 2451. [Google Scholar] [CrossRef] [PubMed]
- Staffa, J.A.; Chang, J.; Green, L. Cerivastatin and Reports of Fatal Rhabdomyolysis. N. Engl. J. Med. 2002, 346, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Bitzur, R.; Cohen, H.; Kamari, Y.; Harats, D. Intolerance to Statins: Mechanisms and Management. Diabetes Care 2013, 36, S325–S330. [Google Scholar] [CrossRef]
- Krähenbühl, S.; Pavik-Mezzour, I.; Von Eckardstein, A. Unmet Needs in LDL-C Lowering: When Statins Won’t Do! Drugs 2016, 76, 1175–1190. [Google Scholar] [CrossRef]
- Bouitbir, J.; Sanvee, G.M.; Panajatovic, M.V.; Singh, F.; Krähenbühl, S. Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol. Res. 2020, 154, 104201. [Google Scholar] [CrossRef]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef]
- Bays, H. Statin Safety: An Overview and Assessment of the Data—2005. Am. J. Cardiol. 2006, 97, 6C–26C. [Google Scholar] [CrossRef]
- Law, M.; Rudnicka, A.R. Statin Safety: A Systematic Review. Am. J. Cardiol. 2006, 97, S52–S60. [Google Scholar] [CrossRef]
- Bouitbir, J.; Charles, A.-L.; Echaniz-Laguna, A.; Kindo, M.; Daussin, F.; Auwerx, J.; Piquard, F.; Geny, B.; Zoll, J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: A ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur. Hear. J. 2012, 33, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Bouitbir, J.; Singh, F.; Charles, A.-L.; Schlagowski, A.-I.; Bonifacio, A.; Echaniz-Laguna, A.; Geny, B.; Krähenbühl, S.; Zoll, J. Statins Trigger Mitochondrial Reactive Oxygen Species-Induced Apoptosis in Glycolytic Skeletal Muscle. Antioxid. Redox Signal. 2016, 24, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.; Zoll, J.; Duthaler, U.; Charles, A.L.; Panajatovic, M.V.; Laverny, G.; McWilliams, T.G.; Metzger, D.; Geny, B.; Krahenbuhl, S.; et al. PGC-1β modulates statin-associated myotoxicity in mice. Arch. Toxicol. 2019, 93, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Panajatovic, M.V.; Singh, F.; Roos, N.J.; Duthaler, U.; Handschin, C.; Krähenbühl, S.; Bouitbir, J. PGC-1α plays a pivotal role in simvastatin-induced exercise impairment in mice. Acta Physiol. 2019, 228, e13402. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; De Craen, A.J.; Seshasai, S.R.K.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Sukhija, R.; Prayaga, S.; Marashdeh, M.; Bursac, Z.; Kakar, P.; Bansal, D.; Sachdeva, R.; Kesan, S.H.; Mehta, J. Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J. Investig. Med. 2009, 57, 495–499. [Google Scholar] [CrossRef]
- Cederberg, H.; Stancáková, A.; Yaluri, N.; Modi, S.R.; Kuusisto, J.; Laakso, M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: A 6 year follow-up study of the METSIM cohort. Diabetologia 2015, 58, 1109–1117. [Google Scholar] [CrossRef]
- Olotu, B.; Shepherd, M.; Novak, S.; Lawson, K.; Wilson, J.; Richards, K.; Rasu, R. Use of statins and the risk of incident diabetes: A retrospective cohort study. Am. J. Cardiovasc. Drugs 2016, 16, 377–390. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef]
- Crandall, J.P.; Mather, K.; Rajpathak, S.N.; Goldberg, R.B.; Watson, K.; Foo, S.; Ratner, R.; Barrett-Connor, E.; Temprosa, M. Statin use and risk of developing diabetes: Results from the Diabetes Prevention Program. BMJ Open Diabetes Res. Care 2017, 5, e000438. [Google Scholar] [CrossRef]
- Sanvee, G.M.; Bouitbir, J.; Krähenbühl, S. Insulin prevents and reverts simvastatin-induced toxicity in C2C12 skeletal muscle cells. Sci. Rep. 2019, 9, 7409. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Łukaszuk, B.; Kurek, K.; Mikłosz, A.; Żendzian-Piotrowska, M.; Chabowski, A. The Role of PGC-1α in the Development of Insulin Resistance in Skeletal Muscle—Revisited. Cell. Physiol. Biochem. 2015, 37, 2288–2296. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Handschin, C.; Choi, C.S.; Chin, S.; Kim, S.; Kawamori, D.; Kurpad, A.J.; Neubauer, N.; Hu, J.; Mootha, V.K.; Kim, Y.B.; et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J. Clin. Invest. 2007, 117, 3463–3474. [Google Scholar] [CrossRef]
- Handschin, C.; Spiegelman, B.M. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006, 27, 728–735. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Bonifacio, A.; Sanvee, G.M.; Bouitbir, J.; Krähenbühl, S. The AKT/mTOR signaling pathway plays a key role in statin-induced myotoxicity. Biochim. Biophys. Acta. 2015, 1853, 1841–1849. [Google Scholar] [CrossRef]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P.; NIH Mouse Metabolic Phenotyping Center Consortium. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- Kleinert, M.; Sylow, L.; Fazakerley, D.J.; Krycer, J.R.; Thomas, K.C.; Oxbøll, A.-J.; Jordy, A.B.; Jensen, T.E.; Yang, G.; Schjerling, P.; et al. Acute mTOR inhibition induces insulin resistance and alters substrate utilization in vivo. Mol. Metab. 2014, 3, 630–641. [Google Scholar] [CrossRef]
- Maarbjerg, S.J.; Jørgensen, S.B.; Rose, A.J.; Jeppesen, J.; Jensen, T.E.; Treebak, J.T.; Birk, J.B.; Schjerling, P.; Wojtaszewski, J.F.; Richter, E.A. Genetic impairment of AMPKα2 signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am. J. Physiol. Metab. 2009, 297, E924–E934. [Google Scholar] [CrossRef] [PubMed]
- Fueger, P.T.; Hess, H.S.; Bracy, D.P.; Pencek, R.R.; Posey, K.A.; Charron, M.J.; Wasserman, D.H. Regulation of Insulin-Stimulated Muscle Glucose Uptake in the Conscious Mouse: Role of Glucose Transport Is Dependent on Glucose Phosphorylation Capacity. Endocrinology 2004, 145, 4912–4916. [Google Scholar] [CrossRef][Green Version]
- Sarafidis, P.A.; Lasaridis, A.N.; Nilsson, P.M.; Pikilidou, M.I.; Stafilas, P.C.; Kanaki, A.; Kazakos, K.; Yovos, J.; Bakris, G.L. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley’s indices in patients with hypertension and type II diabetes. J. Hum. Hypertens. 2007, 21, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Pacini, G.; Omar, B.; Ahrén, B. Methods and Models for Metabolic Assessment in Mice. J. Diabetes Res. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Soonthornpun, S.; Setasuban, W.; Thamprasit, A.; Chayanunnukul, W.; Rattarasarn, C.; Geater, A. Novel Insulin Sensitivity Index Derived from Oral Glucose Tolerance Test. J. Clin. Endocrinol. Metab. 2003, 88, 1019–1023. [Google Scholar] [CrossRef]
- Harris, R.C.; Hultman, E.; Nordesjö, L.O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand. J. Clin. Lab. Investig. 1974, 33, 109–120. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Larsen, S.; Stride, N.; Hey-Mogensen, M.; Hansen, C.N.; Bang, L.E.; Bundgaard, H.; Nielsen, L.B.; Helge, J.W.; Dela, F. Simvastatin Effects on Skeletal Muscle. J. Am. Coll. Cardiol. 2013, 61, 44–53. [Google Scholar] [CrossRef]
- Schrauwen, P.; Saris, W.H.M.; Hesselink, M.K.C. An alternative function for human uncoupling protein 3: Protection of mitochondria against accumulation of nonesterified fatty acids inside the mitochondrial matrix. FASEB J. 2001, 15, 2497–2502. [Google Scholar] [CrossRef]
- Moro, C.; Bajpeyi, S.; Smith, S.R. Determinants of intramyocellular triglyceride turnover: Implications for insulin sensitivity. Am. J. Physiol. Metab. 2008, 294, E203–E213. [Google Scholar] [CrossRef] [PubMed]
- Sanvee, G.M.; Panajatovic, M.V.; Bouitbir, J.; Krähenbühl, S. Mechanisms of insulin resistance by simvastatin in C2C12 myotubes and in mouse skeletal muscle. Biochem. Pharmacol. 2019, 164, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Befroy, D.E.; Codella, R.; Kim, S.; Reznick, R.M.; Hwang, Y.-J.; Liu, Z.-X.; Lee, H.-Y.; Distefano, A.; Samuel, V.T.; et al. Paradoxical effects of increased expression of PGC-1 on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl. Acad. Sci. USA 2008, 105, 19926–19931. [Google Scholar] [CrossRef] [PubMed]
- Summermatter, S.; Shui, G.; Maag, D.; Santos, G.; Wenk, M.R.; Handschin, C. PGC-1α Improves Glucose Homeostasis in Skeletal Muscle in an Activity-Dependent Manner. Diabetes 2012, 62, 85–95. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panajatovic, M.V.; Singh, F.; Krähenbühl, S.; Bouitbir, J. Simvastatin Impairs Glucose Homeostasis in Mice Depending on PGC-1α Skeletal Muscle Expression. Biomedicines 2020, 8, 351. https://doi.org/10.3390/biomedicines8090351
Panajatovic MV, Singh F, Krähenbühl S, Bouitbir J. Simvastatin Impairs Glucose Homeostasis in Mice Depending on PGC-1α Skeletal Muscle Expression. Biomedicines. 2020; 8(9):351. https://doi.org/10.3390/biomedicines8090351
Chicago/Turabian StylePanajatovic, Miljenko Valentin, François Singh, Stephan Krähenbühl, and Jamal Bouitbir. 2020. "Simvastatin Impairs Glucose Homeostasis in Mice Depending on PGC-1α Skeletal Muscle Expression" Biomedicines 8, no. 9: 351. https://doi.org/10.3390/biomedicines8090351
APA StylePanajatovic, M. V., Singh, F., Krähenbühl, S., & Bouitbir, J. (2020). Simvastatin Impairs Glucose Homeostasis in Mice Depending on PGC-1α Skeletal Muscle Expression. Biomedicines, 8(9), 351. https://doi.org/10.3390/biomedicines8090351





