Type 1 Diabetes Induces Hearing Loss: Functional and Histological Findings in An Akita Mouse Model
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Analysis of the Auditory Brainstem Response (ABR)
2.3. Metabolic Studies
2.4. Haematoxylin and Eosin (H&E) and Immunohistochemistry Staining
2.5. Electron Microscopy
2.6. SGN and Spiral Ligament (SL) Counts
2.7. Assessment of SV Thickness
2.8. CD31, Na+/K+-ATPase α1, Cleaved Caspase-3, and Intermediate Cell Intensity Quantitation
2.9. Statistical Analysis
2.10. Antibodies
3. Results
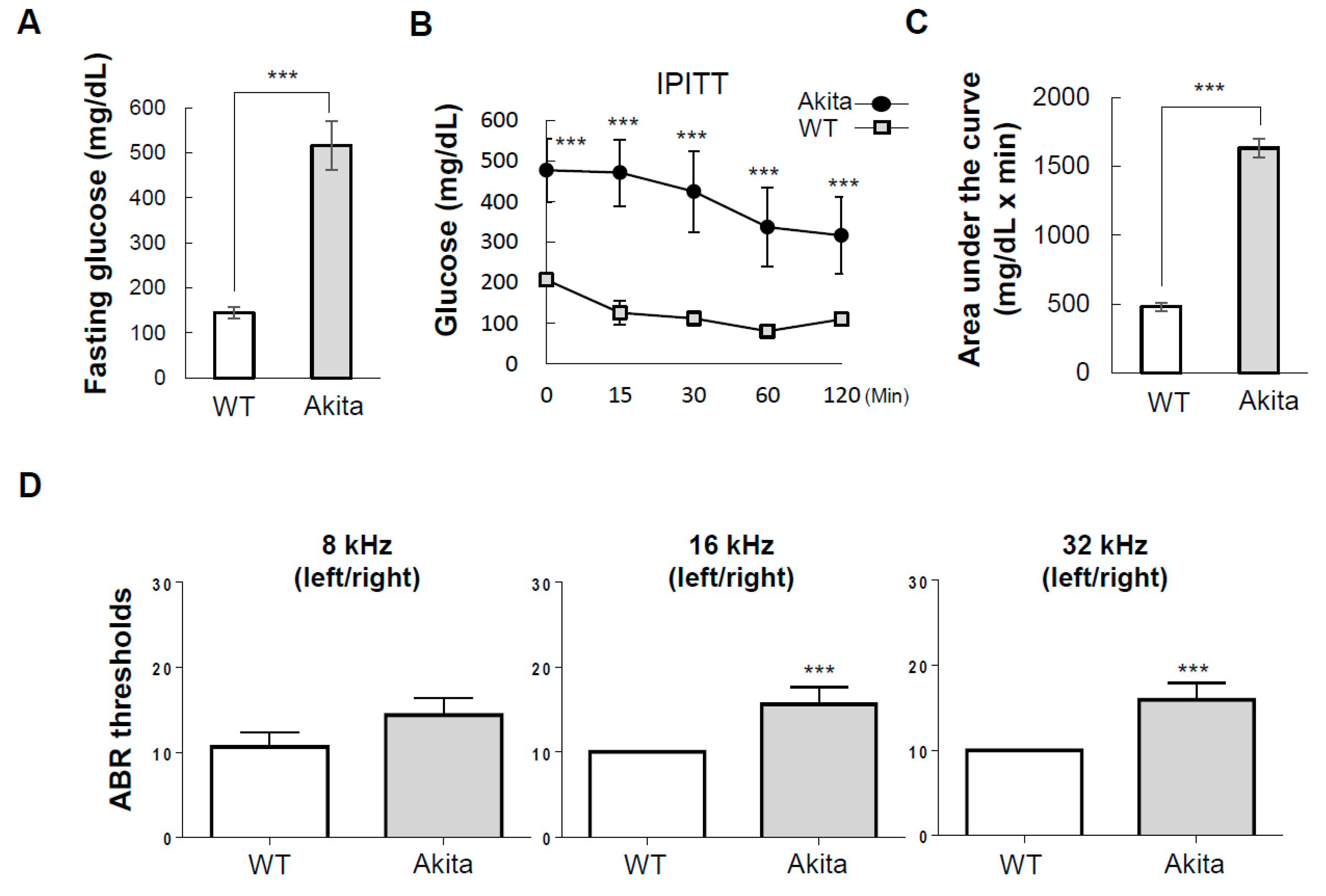
3.1. Akita Mice with Type 1 Diabetes Exhibit Hearing Loss
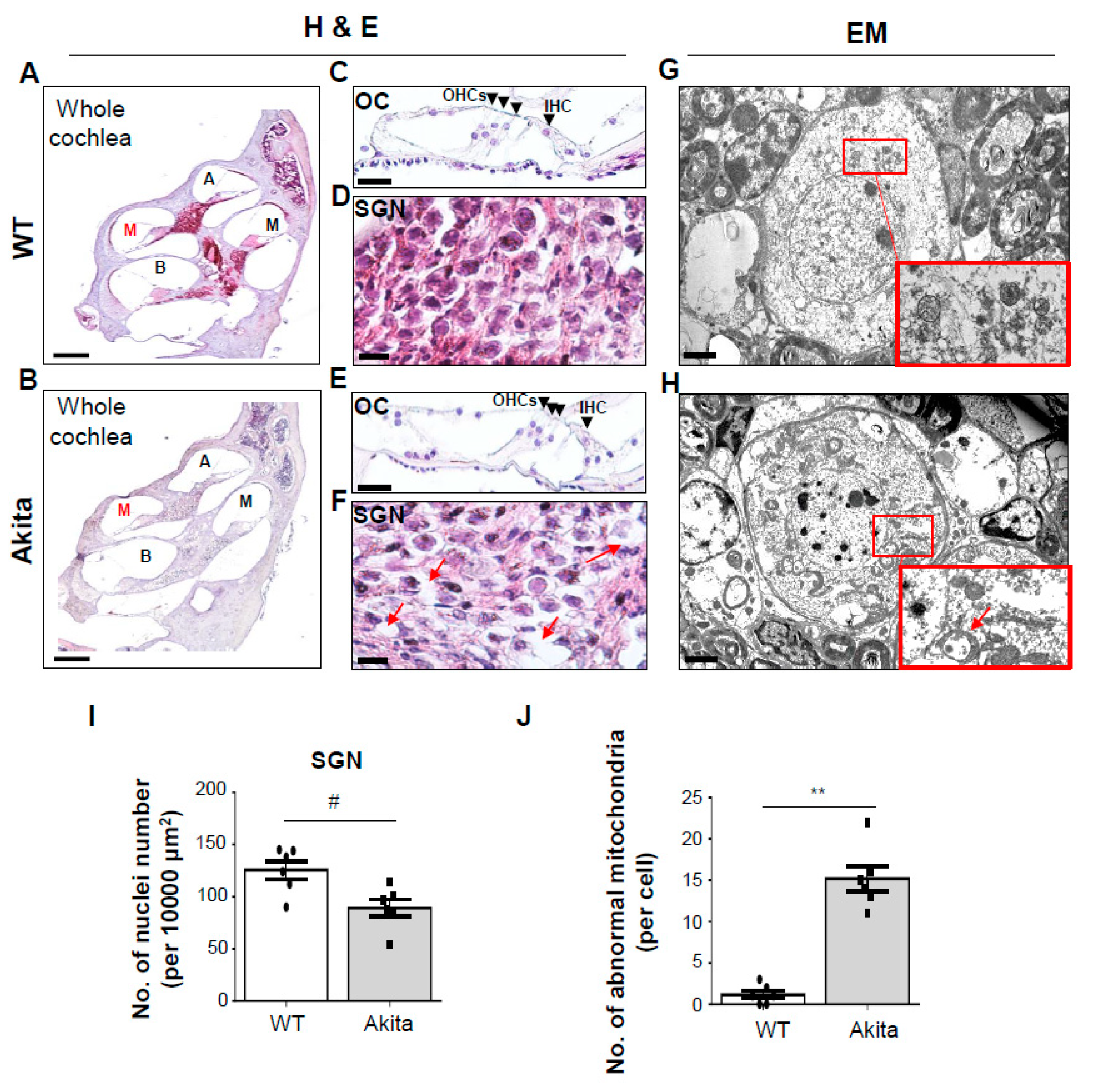
3.2. Type 1 Diabetes Induces Damage in the SGNs but Not the Organ of Corti (OC)
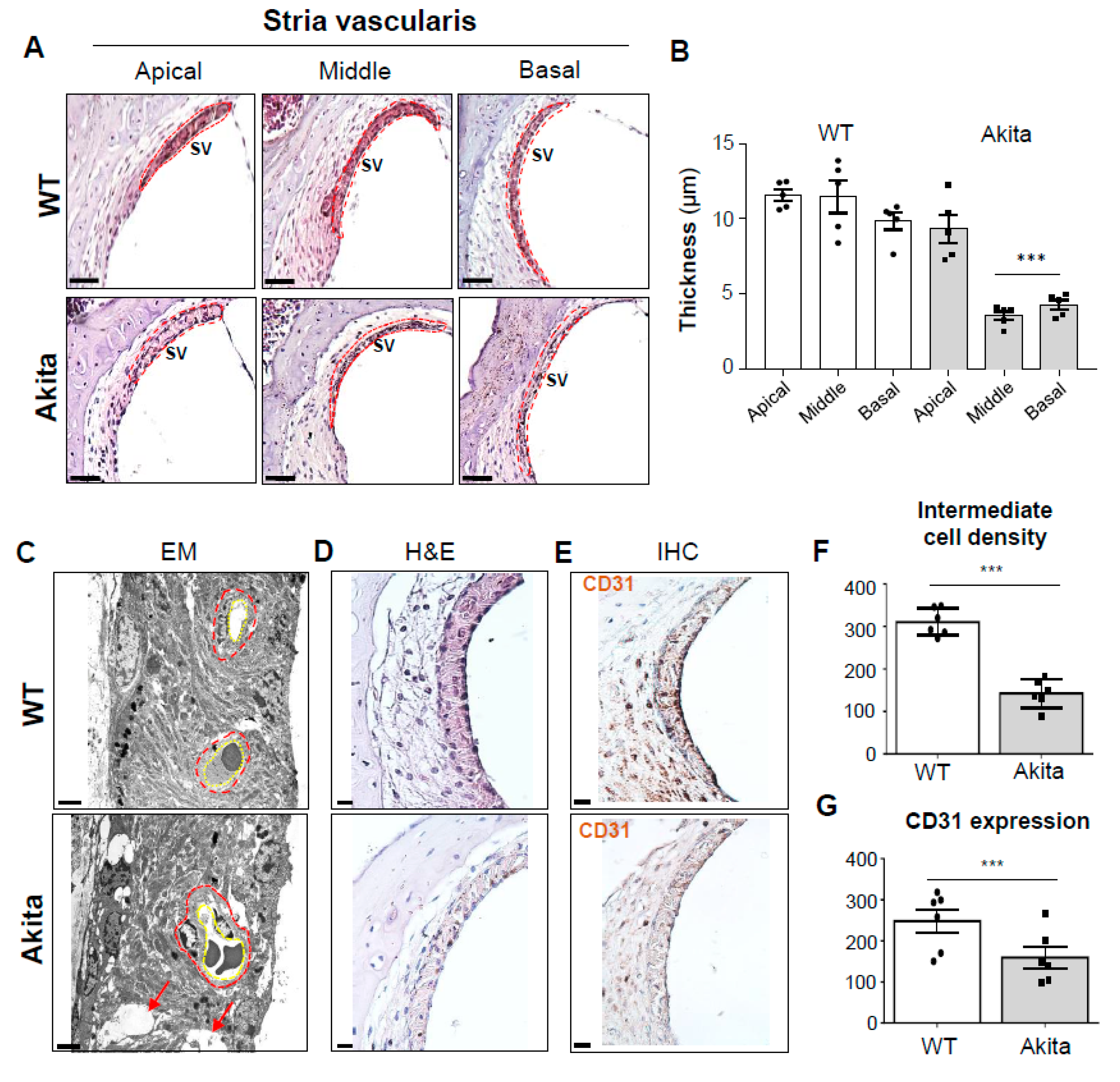
3.3. Type 1 Diabetes Induces Degeneration of the SV
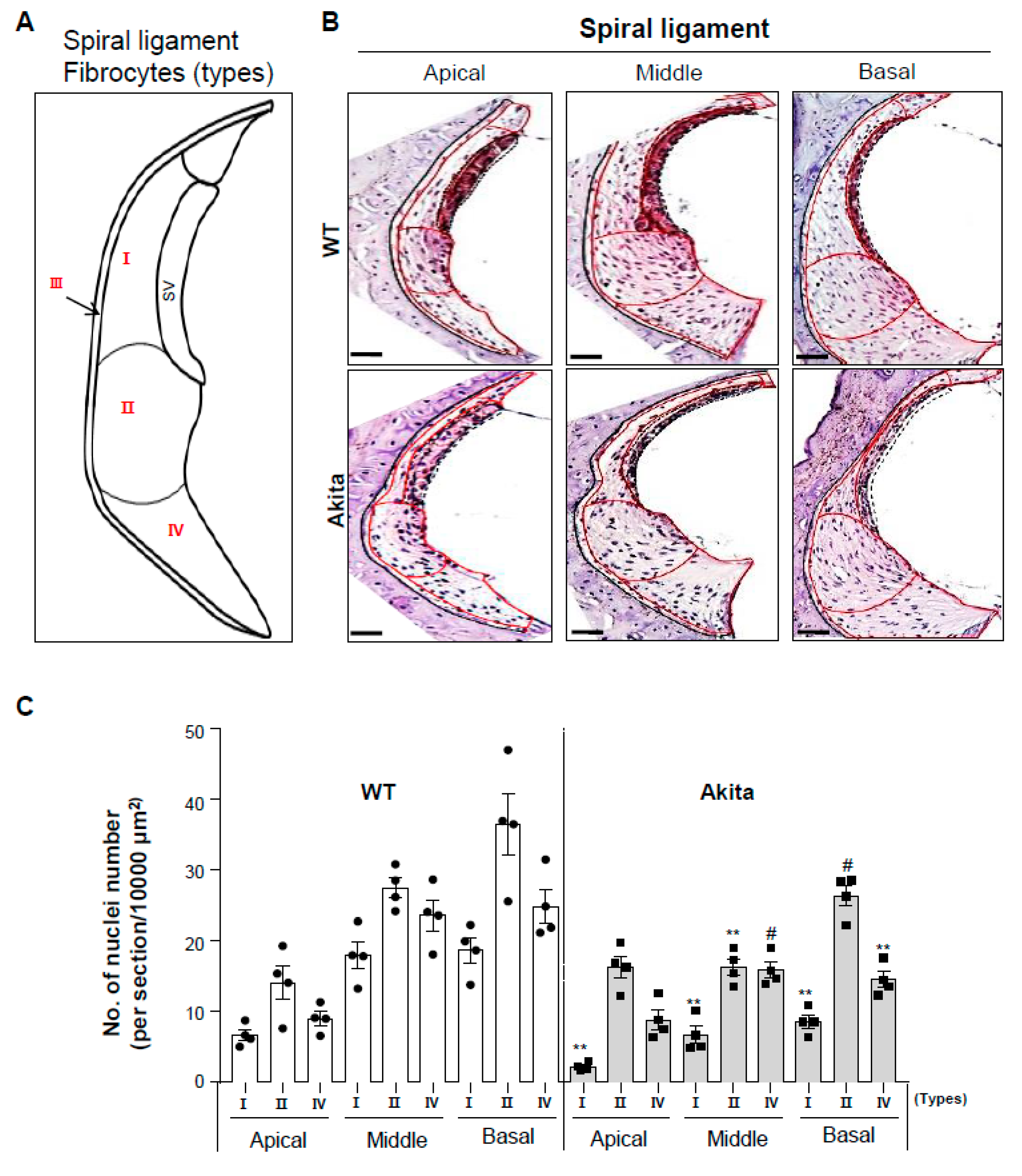
3.4. Type 1 Diabetes Induces a Decrease in Type I, II, and IV Fibrocytes in the Spiral Ligament
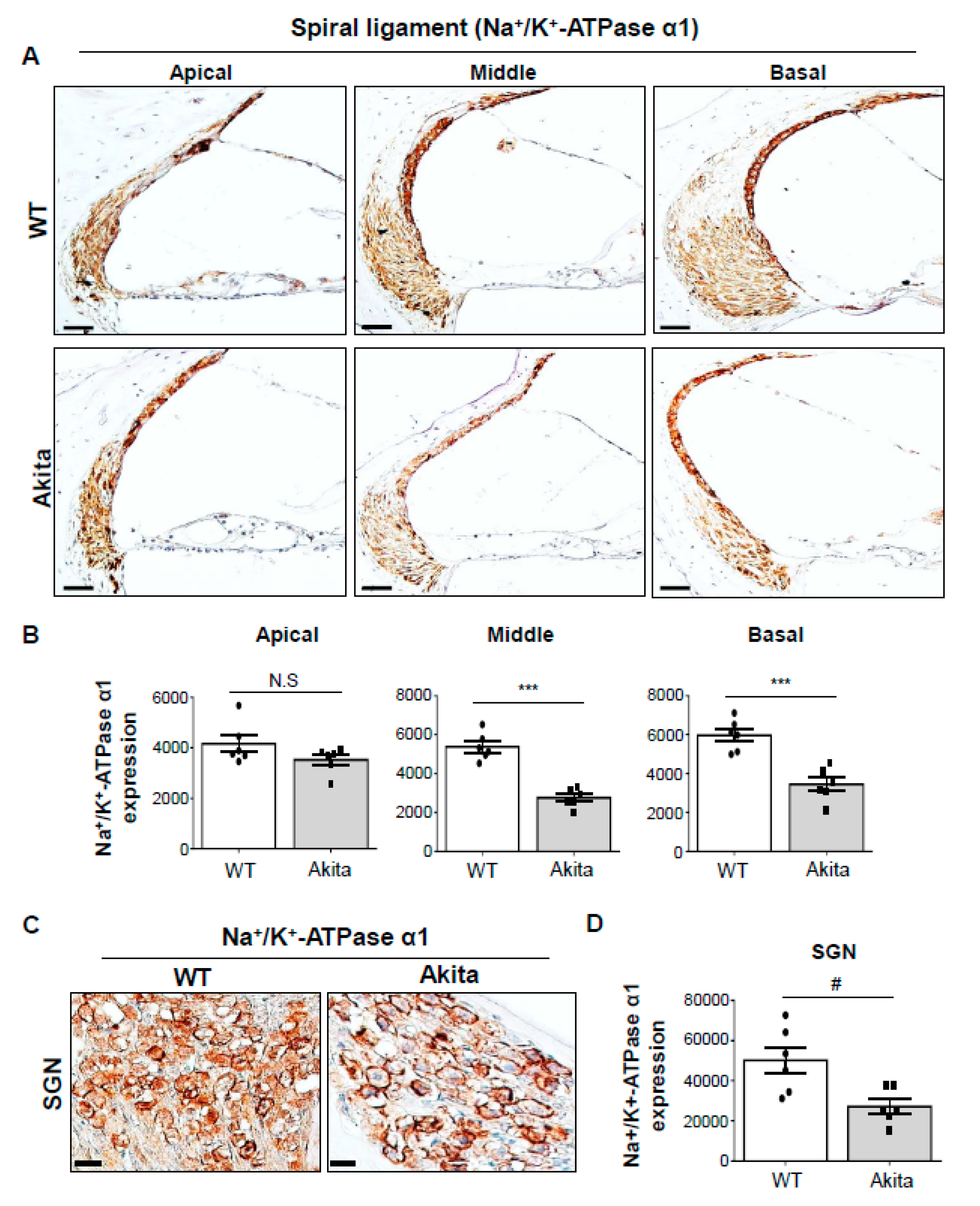
3.5. Type 1 Diabetes Induces a Decrease in Na+/K+-Atpase A1 Expression in the SL and SGNs
3.6. Activation of Caspase-3 is Associated with Degeneration of the SV and SL in the Type 1 Diabetes Cochlea
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABR | Auditory brainstem responses |
| SNHL | Sensorineural hearing loss |
| OC | Organ of Corti |
| SGN | Spiral ganglion neuron |
| LW | Lateral wall |
| SV | Stria vascularis |
| IHC | Inner hair cell |
| OHC | Outer hair cell |
| SC | Supporting cell; |
| SL | Spiral ligaments |
| Na+/K+-ATPase α1 | α1 subunit of Na+, K+-ATPase |
References
- Bagai, A.; Thavendiranathan, P.; Detsky, A.S. Does this patient have hearing impairment? JAMA 2006, 295, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Stachler, R.J.; Chandrasekhar, S.S.; Archer, S.M.; Rosenfeld, R.M.; Schwartz, S.R.; Barrs, D.M.; Brown, S.R.; Fife, T.D.; Ford, P.; Ganiats, T.G.; et al. Clinical practice guideline: Sudden hearing loss. Otolaryngol. Head Neck Surg. 2012, 146, S1–S35. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef]
- Chung, W.S.; Lin, C.L.; Kao, C.H. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism. A population-based cohort study. Thromb. Haemost. 2015, 114, 812–818. [Google Scholar] [CrossRef]
- Bainbridge, K.E.; Hoffman, H.J.; Cowie, C.C. Diabetes and hearing impairment in the United States: Audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann. Intern. Med. 2008, 149, 1–10. [Google Scholar] [CrossRef]
- Kim, M.B.; Zhang, Y.; Chang, Y.; Ryu, S.; Choi, Y.; Kwon, M.J.; Moon, I.J.; Deal, J.A.; Lin, F.R.; Guallar, E.; et al. Diabetes mellitus and the incidence of hearing loss: A cohort study. Int. J. Epidemiol. 2017, 46, 717–726. [Google Scholar] [CrossRef]
- Ashkezari, S.J.; Namiranian, N.; Rahmanian, M.; Atighechi, S.; Mohajeri-Tehrani, M.R.; Gholami, S. Is hearing impairment in diabetic patients correlated to other complications? J. Diabetes. Metab. Disord. 2018, 17, 173–179. [Google Scholar] [CrossRef]
- Fukushima, H.; Cureoglu, S.; Schachern, P.A.; Kusunoki, T.; Oktay, M.F.; Fukushima, N.; Paparella, M.M.; Harada, T. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol. Head Neck Surg. 2005, 133, 100–106. [Google Scholar] [CrossRef]
- Gupta, S.; Eavey, R.D.; Wang, M.; Curhan, S.G.; Curhan, G.C. Type 2 diabetes and the risk of incident hearing loss. Diabetologia 2019, 62, 281–285. [Google Scholar] [CrossRef]
- Smith, T.L.; Raynor, E.; Prazma, J.; Buenting, J.E.; Pillsbury, H.C. Insulin-dependent diabetic microangiopathy in the inner ear. Laryngoscope 1995, 105, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Cureoglu, S.; Schachern, P.A.; Paparella, M.M.; Harada, T.; Oktay, M.F. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Kariya, S.; Cureoglu, S.; Fukushima, H.; Morita, N.; Baylan, M.Y.; Maeda, Y.; Nishizaki, K.; Paparella, M.M. Comparing the cochlear spiral modiolar artery in type-1 and type-2 diabetes mellitus:a human temporal bone study. Acta Med. Okayama 2010, 64, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Wackym, P.A.; Linthicum, F.H., Jr. Diabetes mellitus and hearing loss: Clinical and histopathologic relationships. Am. J. Otol. 1986, 7, 176–182. [Google Scholar]
- Ishikawa, T.; Naito, Y.; Taniguchi, K. Hearing impairment in WBN/Kob rats with spontaneous diabetes mellitus. Diabetologia 1995, 38, 649–655. [Google Scholar] [CrossRef]
- Nakae, S.; Tachibana, M. The cochlea of the spontaneously diabetic mouse. II. Electron microscopic observations of non-obese diabetic mice. Arch. Otorhinolaryngol. 1986, 243, 313–316. [Google Scholar] [CrossRef]
- Tachibana, M.; Nakae, S. The cochlea of the spontaneously diabetic mouse. I. Electron microscopic observation of KK mice. Arch. Otorhinolaryngol. 1986, 243, 238–241. [Google Scholar] [CrossRef]
- Rust, K.R.; Prazma, J.; Triana, R.J.; Michaelis, O.E.t.; Pillsbury, H.C. Inner ear damage secondary to diabetes mellitus. II. Changes in aging SHR/N-cp rats. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 397–400. [Google Scholar] [CrossRef]
- Raynor, E.M.; Carrasco, V.N.; Prazma, J.; Pillsbury, H.C. An assessment of cochlear hair-cell loss in insulin-dependent diabetes mellitus diabetic and noise-exposed rats. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 452–456. [Google Scholar] [CrossRef]
- Geering, K. Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 2008, 17, 526–532. [Google Scholar] [CrossRef]
- Skou, J.C. The identification of the sodium pump. Biosci. Rep. 2004, 24, 436–451. [Google Scholar] [CrossRef]
- Kerr, T.P.; Ross, M.D.; Ernst, S.A. Cellular localization of Na+,K+-ATPase in the mammalian cochlear duct: Significance for cochlear fluid balance. Am. J. Otolaryngol. 1982, 3, 332–338. [Google Scholar] [CrossRef]
- Gratton, M.A.; Smyth, B.J.; Schulte, B.A.; Vincent, D.A., Jr. Na,K-ATPase activity decreases in the cochlear lateral wall of quiet-aged gerbils. Hear. Res. 1995, 83, 43–50. [Google Scholar] [CrossRef]
- Choung, Y.H.; Kim, S.W.; Tian, C.; Min, J.Y.; Lee, H.K.; Park, S.N.; Lee, J.B.; Park, K. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope 2011, 121, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Kayo, T.; Ikeda, T.; Koizumi, A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997, 46, 887–894. [Google Scholar] [CrossRef]
- Fujita, T.; Yamashita, D.; Katsunuma, S.; Hasegawa, S.; Tanimoto, H.; Nibu, K. Increased inner ear susceptibility to noise injury in mice with streptozotocin-induced diabetes. Diabetes 2012, 61, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.S.; Schulte, B.A. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear. Res. 1991, 56, 53–64. [Google Scholar] [CrossRef]
- Spicer, S.S.; Schulte, B.A. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear. Res. 1996, 100, 80–100. [Google Scholar] [CrossRef]
- Yakovlev, A.G.; Faden, A.I. Caspase-dependent apoptotic pathways in CNS injury. Mol. Neurobiol. 2001, 24, 131–144. [Google Scholar] [CrossRef]
- Nicotera, T.M.; Hu, B.H.; Henderson, D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J. Assoc. Res. Otolaryngol. 2003, 4, 466–477. [Google Scholar] [CrossRef]
- Tadros, S.F.; D’Souza, M.; Zhu, X.; Frisina, R.D. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis 2008, 13, 1303–1321. [Google Scholar] [CrossRef]
- Haryuna, T.S.; Purba, A.H.; Farhat, F.; Alviandi, W. The Antiapoptotic Effect of Curcumin in the Fibroblast of the Cochlea in an Ototoxic Rat Model. Iran. J. Otorhinolaryngol. 2018, 30, 247–253. [Google Scholar] [PubMed]
- Makishima, K.; Tanaka, K. Pathological changes of the inner ear and central auditory pathway in diabetics. Ann. Otol. Rhinol. Laryngol. 1971, 80, 218–228. [Google Scholar] [CrossRef]
- Kovar, M. The inner ear in diabetes mellitus. ORL J. Otorhinolaryngol. Relat. Spec. 1973, 35, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.P.; Biurrun, O.; Lorente, J.; Conget, J.I.; de Espana, R.; Esmatjes, E.; Gomis, R. Auditory function in young patients with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 1991, 11, 17–22. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Kruse, C.; Juhn, S.K. Cochlear vascular changes in streptozotocin diabetes in chinchillas. Trans. Sect. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 1977, 84, 443–451. [Google Scholar]
- Rubini, R.; Biasiolo, F.; Fogarolo, F.; Magnavita, V.; Martini, A.; Fiori, M.G. Brainstem auditory evoked potentials in rats with streptozotocin-induced diabetes. Diabetes Res. Clin. Pract. 1992, 16, 19–25. [Google Scholar] [CrossRef]
- Vasilyeva, O.N.; Frisina, S.T.; Zhu, X.; Walton, J.P.; Frisina, R.D. Interactions of hearing loss and diabetes mellitus in the middle age CBA/CaJ mouse model of presbycusis. Hear. Res. 2009, 249, 44–53. [Google Scholar] [CrossRef]
- Teng, Z.P.; Tian, R.; Xing, F.L.; Tang, H.; Xu, J.J.; Zhang, B.W.; Qi, J.W. An association of type 1 diabetes mellitus with auditory dysfunction: A systematic review and meta-analysis. Laryngoscope 2017, 127, 1689–1697. [Google Scholar] [CrossRef]
- Robertson, D. Effects of acoustic trauma on stereocilia structure and spiral ganglion cell tuning properties in the guinea pig cochlea. Hear. Res. 1982, 7, 55–74. [Google Scholar] [CrossRef]
- Liberman, M.C.; Dodds, L.W. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984, 16, 55–74. [Google Scholar] [CrossRef]
- Puel, J.L.; Ruel, J.; Gervais d’Aldin, C.; Pujol, R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport 1998, 9, 2109–2114. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, K.R.; Chung, W.H.; Cho, Y.S.; Hong, S.H. Early sensorineural hearing loss in ob/ob mouse, an animal model of type 2 diabetes. Clin. Exp. Otorhinolaryngol. 2008, 1, 211–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keithley, E.M.; Feldman, M.L. Spiral ganglion cell counts in an age-graded series of rat cochleas. J. Comp. Neurol. 1979, 188, 429–442. [Google Scholar] [CrossRef]
- White, J.A.; Burgess, B.J.; Hall, R.D.; Nadol, J.B. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear. Res. 2000, 141, 12–18. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Raynor, E.; Robison, W.G.; Garrett, C.G.; McGuirt, W.T.; Pillsbury, H.C.; Prazma, J. Consumption of a high-galactose diet induces diabetic-like changes in the inner ear. Otolaryngol. Head Neck Surg. 1995, 113, 748–754. [Google Scholar] [CrossRef]
- Gao, Y.; Yechikov, S.; Vazquez, A.E.; Chen, D.; Nie, L. Impaired surface expression and conductance of the KCNQ4 channel lead to sensorineural hearing loss. J. Cell Mol. Med. 2013, 17, 889–900. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, H.B. The role of an inwardly rectifying K(+) channel (Kir4.1) in the inner ear and hearing loss. Neuroscience 2014, 265, 137–146. [Google Scholar] [CrossRef]
- Wangemann, P. Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 2006, 576, 11–21. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kimura, R.S.; Paul, D.L.; Takasaka, T.; Adams, J.C. Gap junction systems in the mammalian cochlea. Brain Res. Brain Res. Rev. 2000, 32, 163–166. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.Y.; Kim, Y.J.; Gil, E.S.; Kim, H.; Jang, J.H.; Choung, Y.-H. Type 1 Diabetes Induces Hearing Loss: Functional and Histological Findings in An Akita Mouse Model. Biomedicines 2020, 8, 343. https://doi.org/10.3390/biomedicines8090343
Lee YY, Kim YJ, Gil ES, Kim H, Jang JH, Choung Y-H. Type 1 Diabetes Induces Hearing Loss: Functional and Histological Findings in An Akita Mouse Model. Biomedicines. 2020; 8(9):343. https://doi.org/10.3390/biomedicines8090343
Chicago/Turabian StyleLee, Yun Yeong, Yeon Ju Kim, Eun Sol Gil, Hantai Kim, Jeong Hun Jang, and Yun-Hoon Choung. 2020. "Type 1 Diabetes Induces Hearing Loss: Functional and Histological Findings in An Akita Mouse Model" Biomedicines 8, no. 9: 343. https://doi.org/10.3390/biomedicines8090343
APA StyleLee, Y. Y., Kim, Y. J., Gil, E. S., Kim, H., Jang, J. H., & Choung, Y.-H. (2020). Type 1 Diabetes Induces Hearing Loss: Functional and Histological Findings in An Akita Mouse Model. Biomedicines, 8(9), 343. https://doi.org/10.3390/biomedicines8090343





