BAY 41-2272 Attenuates CTGF Expression via sGC/cGMP-Independent Pathway in TGFβ1-Activated Hepatic Stellate Cells
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Isolation of Mouse Primary HSCs
2.3. Cell Viability
2.4. Western Blotting
2.5. Immunofluorescence Staining
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Determination of Intracellular cGMP Levels
2.8. sGC Activity Assay
2.9. Statistical Analysis
3. Results
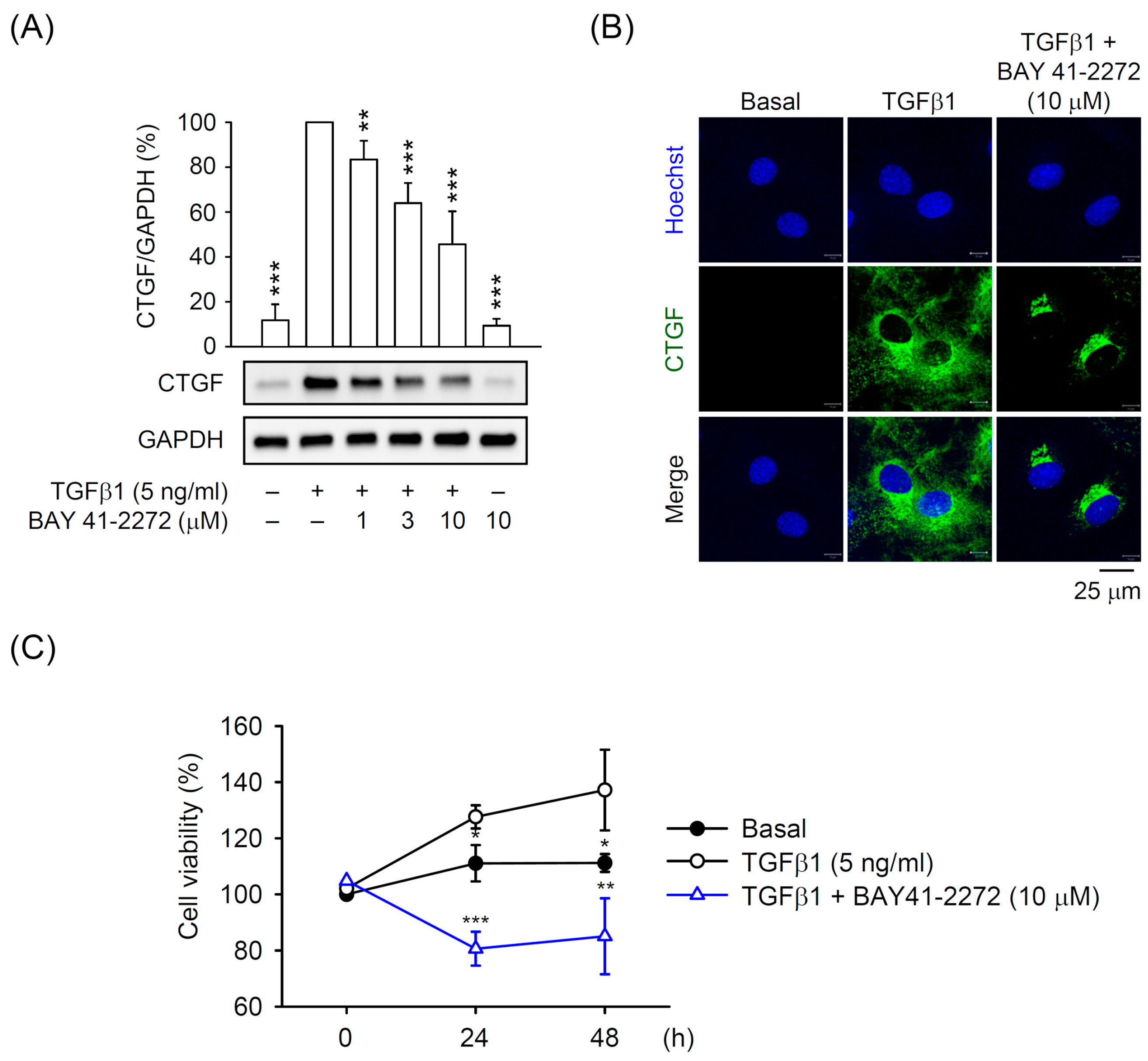
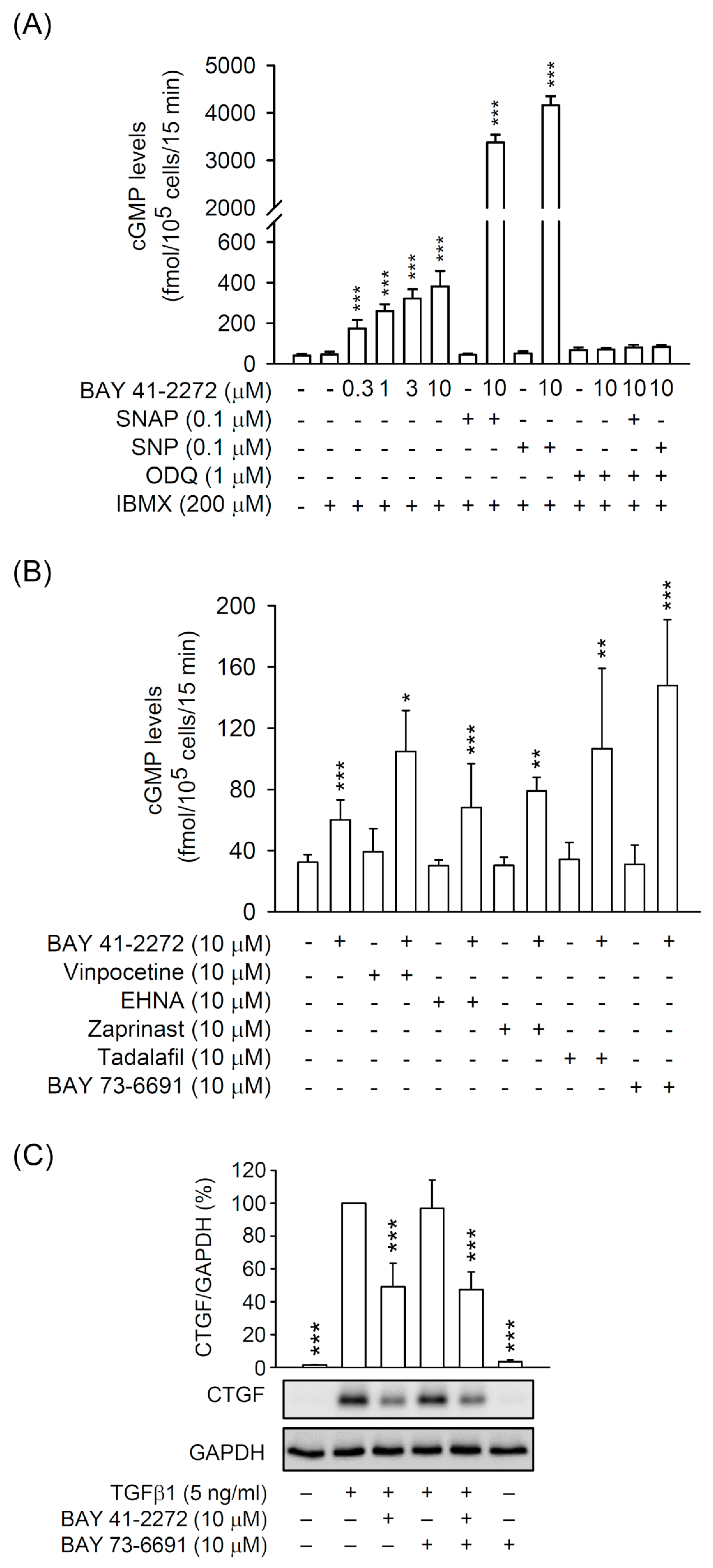
3.1. BAY 41-2272 sGC Stimulator Inhibited TGFβ1-Induced CTGF Expression and Cell Proliferation in Primary HSCs
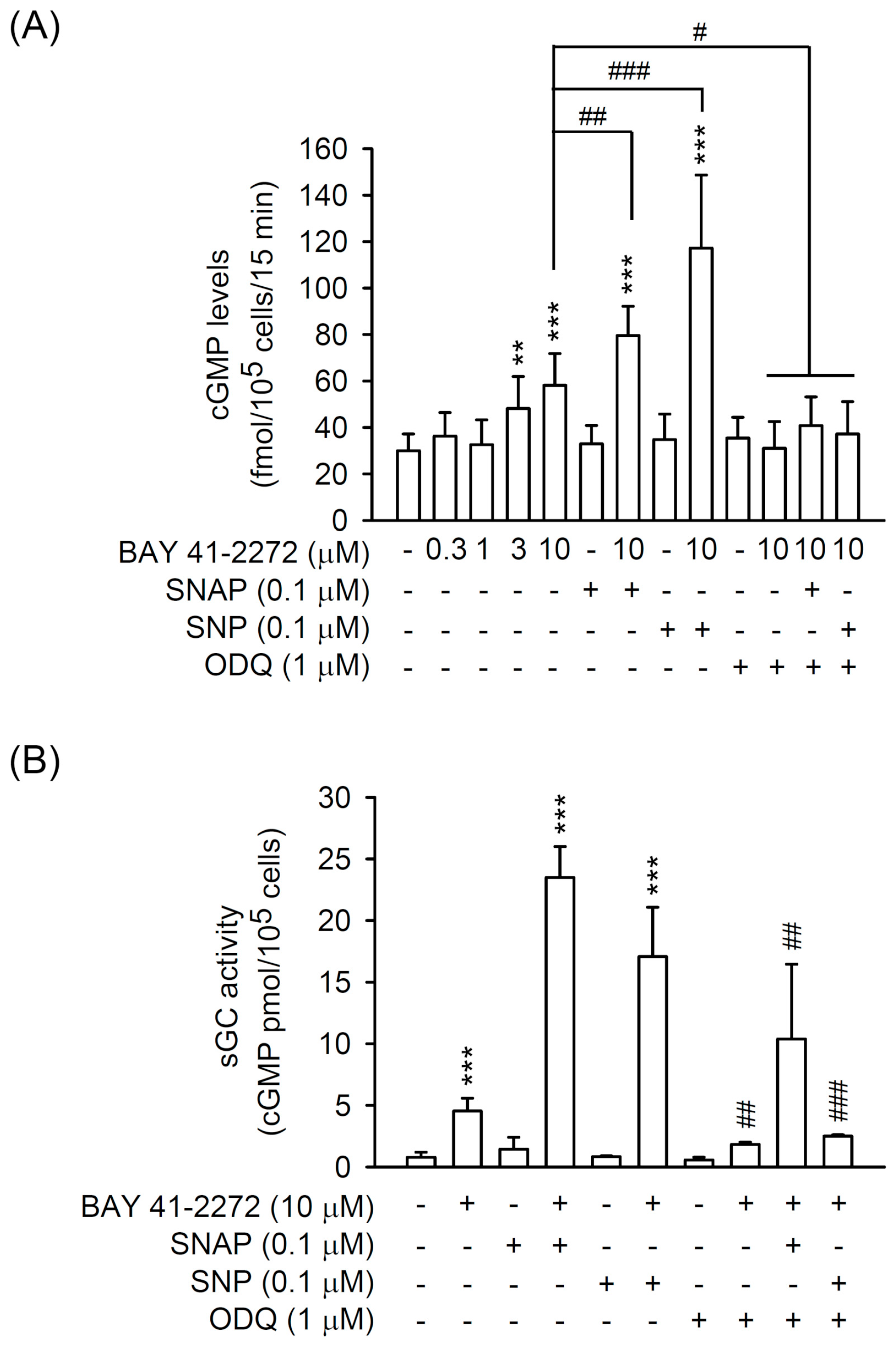
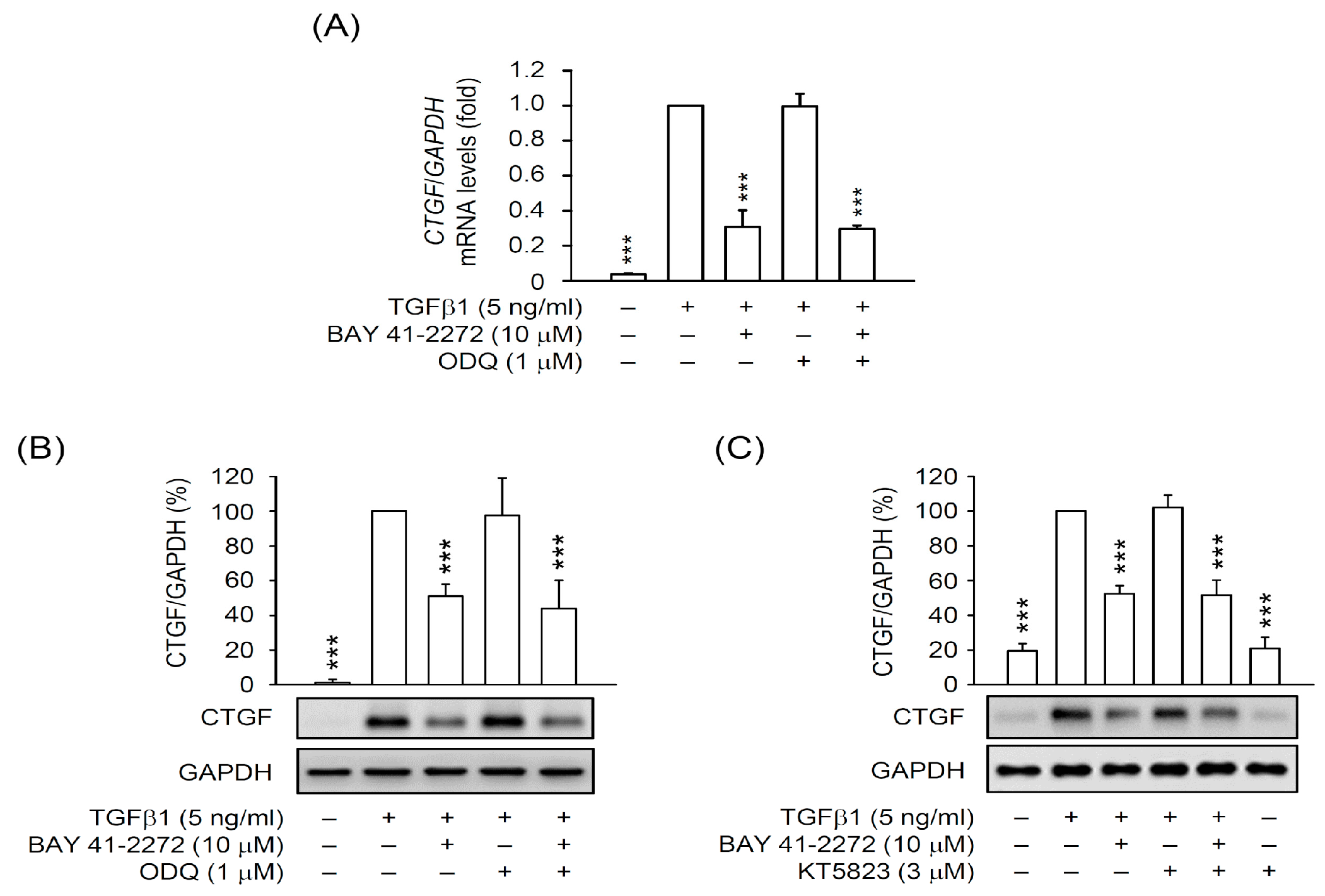
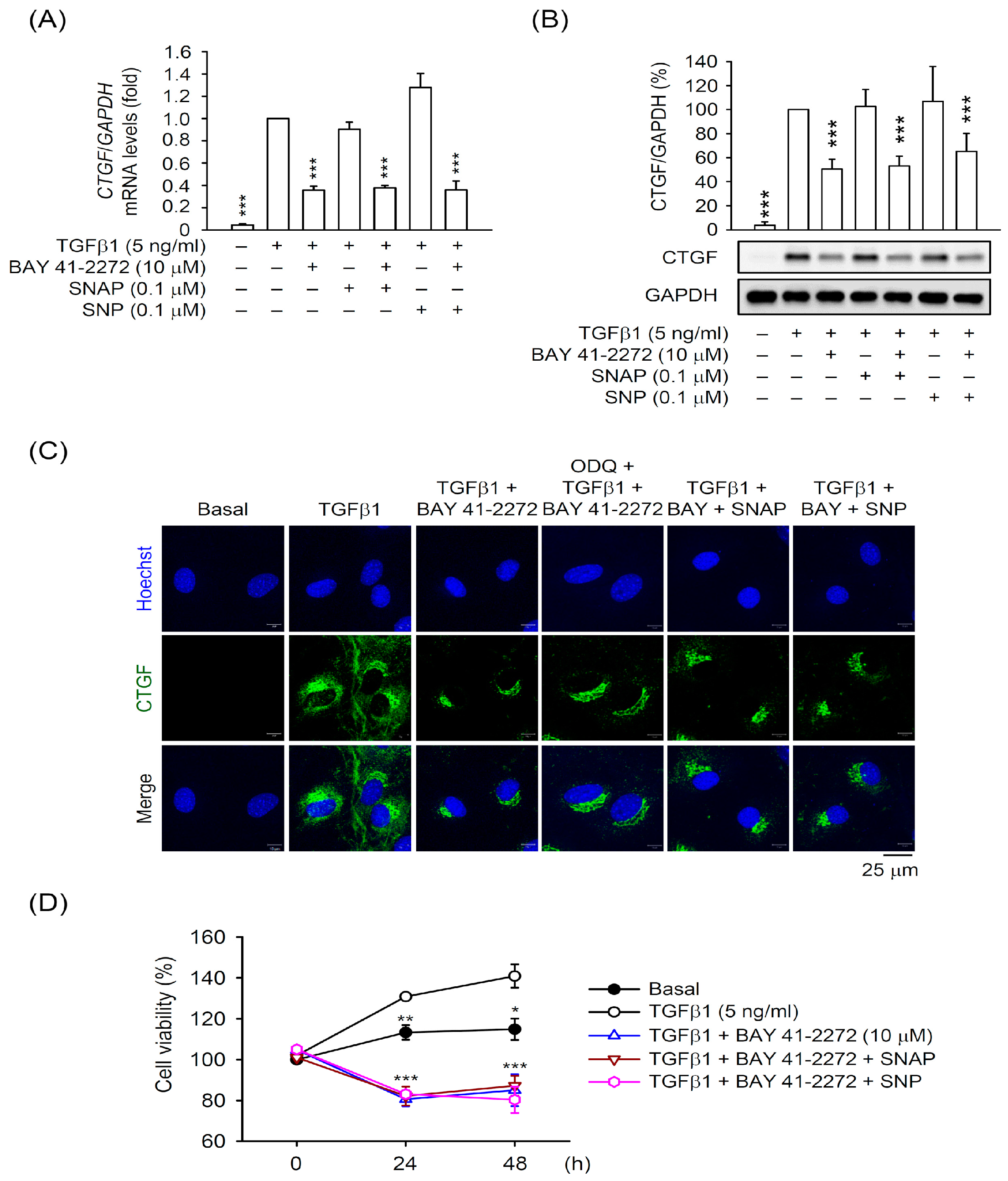
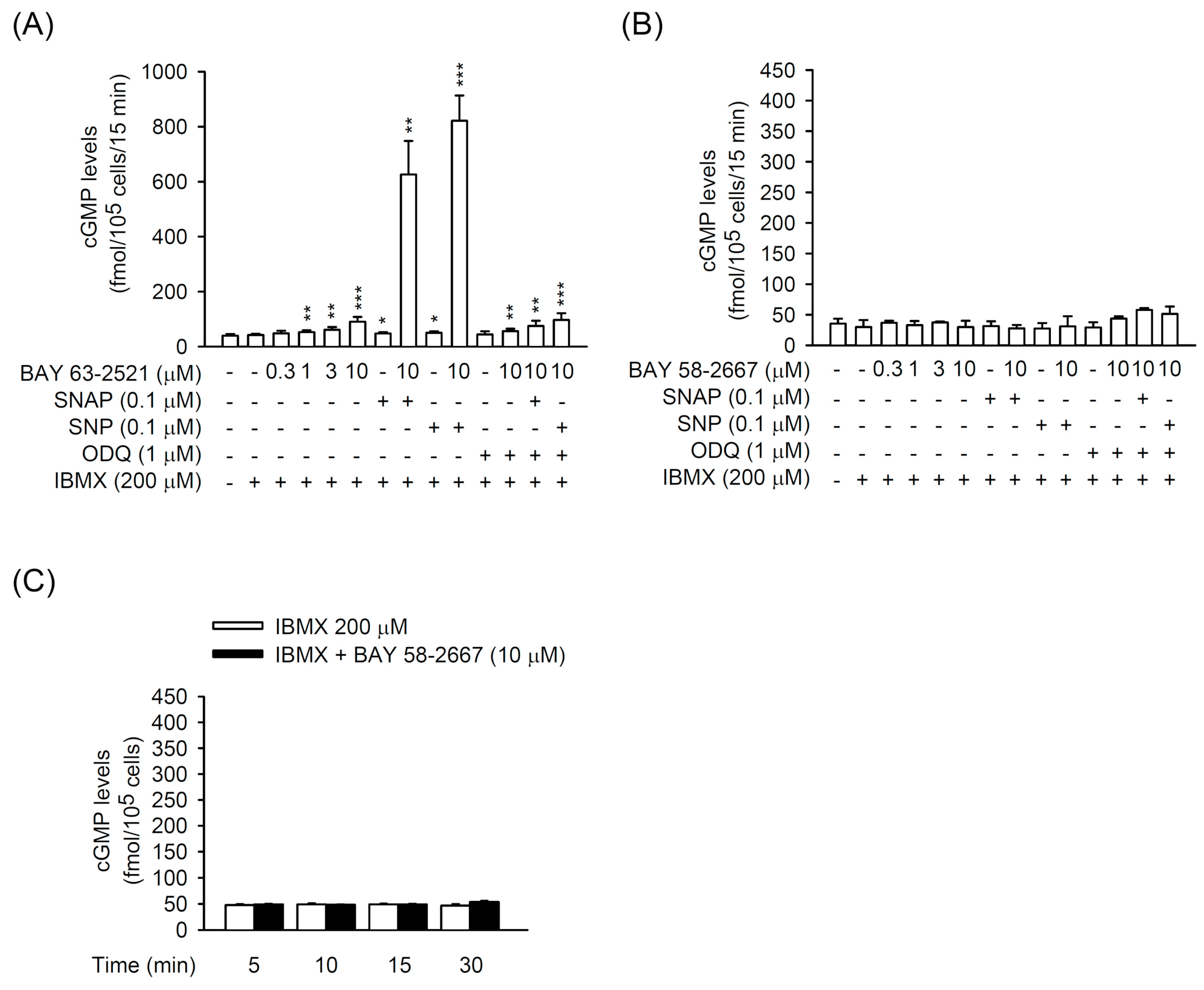
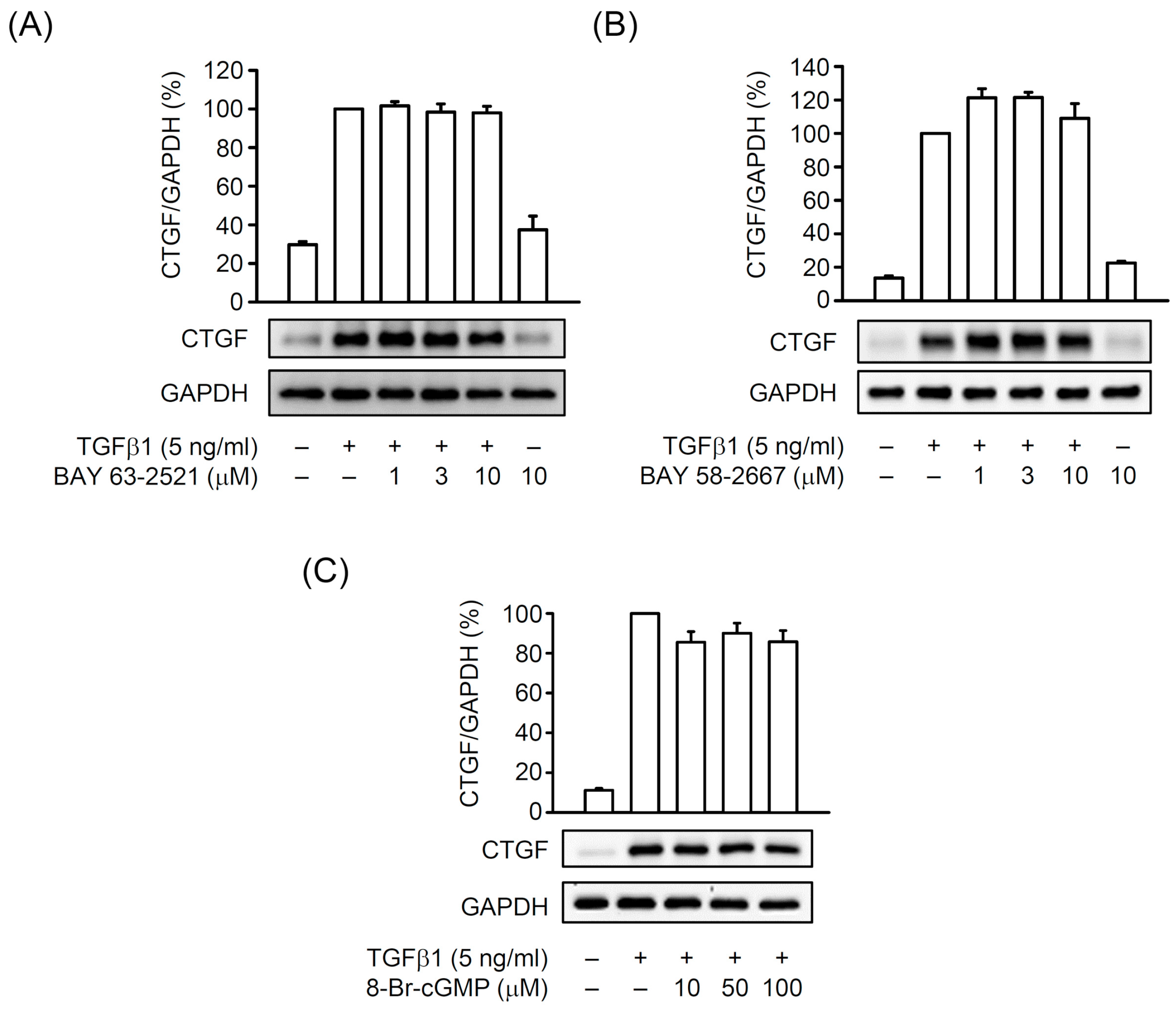
3.2. The BAY 41-2272-Inhibited CTGF Expression and Cell Proliferation Was not via sGC/cGMP Pathway in TGFβ1-Activated Primary HSCs
3.3. PDE9 Modulated the BAY 41-2272-Mediated sGC/cGMP Signaling But not CTGF Inhibition in Primary HSCs
3.4. The TGFβ1-Induced CTGF Expression is Independent of cGMP Formation in Primary HSCs
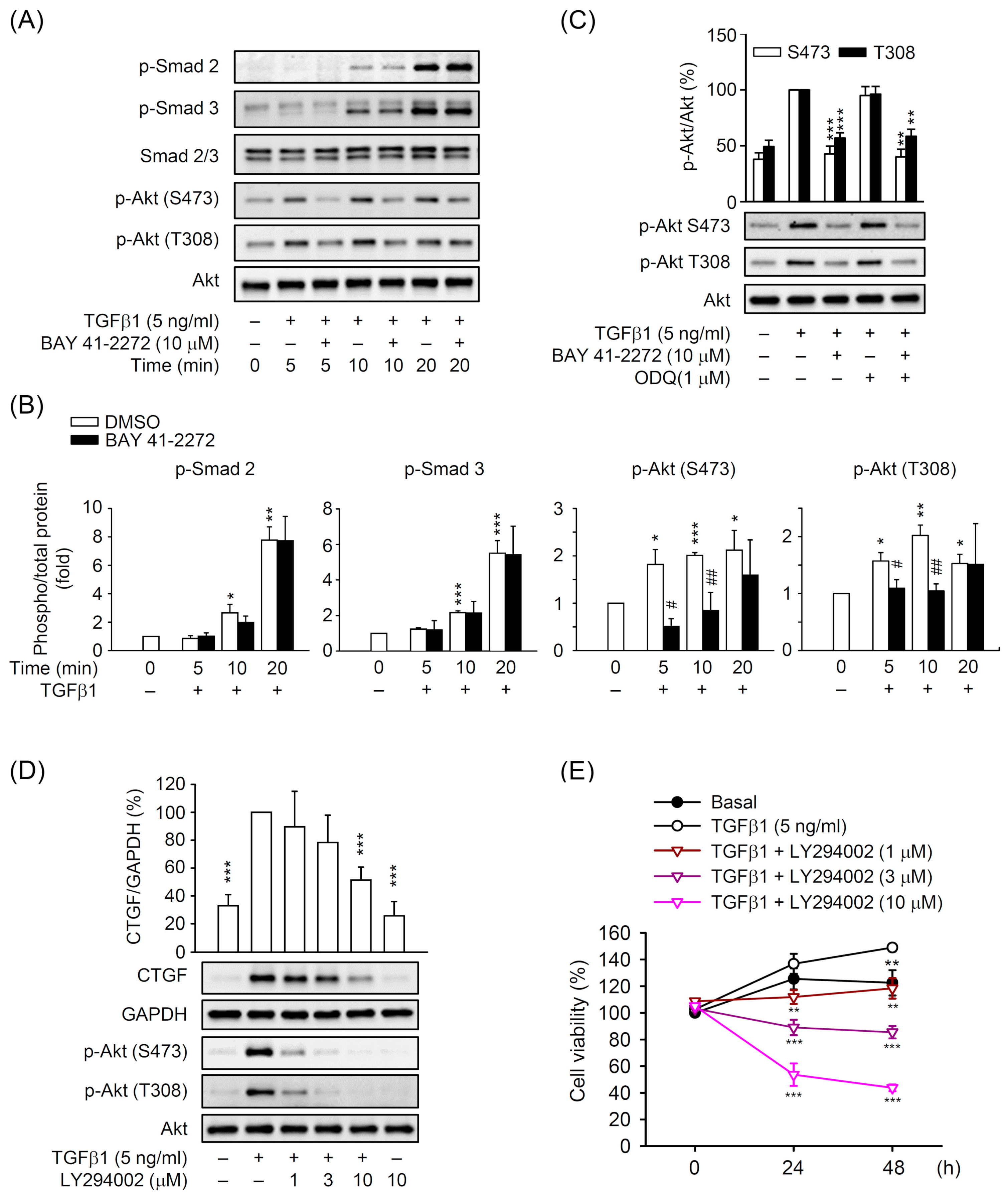
3.5. BAY 41-2272 Selectively Inhibited the TGFβ1-Induced Akt Activation in Primary HSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, J.; Wang, J.; Zhou, Q.; Yang, B.; He, Q.; Weng, Q. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: New insights into therapy. Pharmacol. Res. 2020, 155, 104720. [Google Scholar] [CrossRef] [PubMed]
- Khomich, O.; Ivanov, A.V.; Bartosch, B. Metabolic Hallmarks of Hepatic Stellate Cells in Liver Fibrosis. Cells 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, X.; Liang, J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp. Cell Res. 2017, 352, 420–426. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Kuo, L.M.; Chen, P.J.; Sung, P.J.; Chang, Y.C.; Ho, C.T.; Wu, Y.H.; Hwang, T.L. The Bioactive Extract of Pinnigorgia sp. Induces Apoptosis of Hepatic Stellate Cells via ROS-ERK/JNK-Caspase-3 Signaling. Mar. Drugs 2018, 16, 19. [Google Scholar] [CrossRef]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68–69, 435–451. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Hasko, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nature reviews. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef]
- Wobst, J.; Kessler, T.; Dang, T.A.; Erdmann, J.; Schunkert, H. Role of sGC-dependent NO signalling and myocardial infarction risk. J. Mol. Med. 2015, 93, 383–394. [Google Scholar] [CrossRef]
- Hollas, M.A.; Ben Aissa, M.; Lee, S.H.; Gordon-Blake, J.M.; Thatcher, G.R.J. Pharmacological manipulation of cGMP and NO/cGMP in CNS drug discovery. Nitric Oxide 2019, 82, 59–74. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Yi, R.; Yi, H.; Xiao, S.; Chen, Z.; Hu, G.; Li, Q. Soluble Guanylate Cyclase: A New Therapeutic Target for Fibrotic Diseases. Curr. Med. Chem. 2017, 24, 3203–3215. [Google Scholar] [CrossRef] [PubMed]
- Priviero, F.B.; Webb, R.C. Heme-dependent and independent soluble guanylate cyclase activators and vasodilation. J. Cardiovasc. Pharmacol. 2010, 56, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, Q.; Hu, L.; Yu, Z.; Yang, J.; Chang, Q.; Chen, Z.; Hu, G. Soluble Guanylate Cyclase Stimulators and Activators: Where are We and Where to Go? Mini Rev. Med. Chem. 2019, 19, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Perri, R.E.; Langer, D.A.; Chatterjee, S.; Gibbons, S.J.; Gadgil, J.; Cao, S.; Farrugia, G.; Shah, V.H. Defects in cGMP-PKG pathway contribute to impaired NO-dependent responses in hepatic stellate cells upon activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G535–G542. [Google Scholar] [CrossRef]
- Sandner, P.; Stasch, J.P. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Respir. Med. 2017, 122 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-beta in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Diaz, D. Transforming Growth Factor-beta-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef]
- Fabregat, I.; Moreno-Caceres, J.; Sanchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P.; Consortium, I.-L. TGF-beta signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef]
- Carthy, J.M. TGFbeta signaling and the control of myofibroblast differentiation: Implications for chronic inflammatory disorders. J. Cell. Physiol. 2018, 233, 98–106. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Meyer, C.; Li, J.; Nadalin, S.; Konigsrainer, A.; Weng, H.; Dooley, S.; ten Dijke, P. Transforming growth factor-beta (TGF-beta)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J. Biol. Chem. 2013, 288, 30708–30719. [Google Scholar] [CrossRef]
- Colak, Y.; Senates, E.; Coskunpinar, E.; Oltulu, Y.M.; Zemheri, E.; Ozturk, O.; Doganay, L.; Mesci, B.; Yilmaz, Y.; Enc, F.Y.; et al. Concentrations of connective tissue growth factor in patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Dis. Markers 2012, 33, 77–83. [Google Scholar] [CrossRef]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018, 68–69, 44–66. [Google Scholar] [CrossRef]
- Huang, G.; Brigstock, D.R. Regulation of hepatic stellate cells by connective tissue growth factor. Front. Biosci. 2012, 17, 2495–2507. [Google Scholar]
- Wang, Y.; Kramer, S.; Loof, T.; Martini, S.; Kron, S.; Kawachi, H.; Shimizu, F.; Neumayer, H.H.; Peters, H. Enhancing cGMP in experimental progressive renal fibrosis: Soluble guanylate cyclase stimulation vs. phosphodiesterase inhibition. Am. J. Physiol. Ren. Physiol. 2006, 290, F167–F176. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Tsuruda, T.; Sekita, Y.; Hatakeyama, K.; Imamura, T.; Kato, J.; Asada, Y.; Stasch, J.P.; Kitamura, K. Pressure-independent effects of pharmacological stimulation of soluble guanylate cyclase on fibrosis in pressure-overloaded rat heart. Hypertens. Res. 2009, 32, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Zenzmaier, C.; Palumbo-Zerr, K.; Mancuso, R.; Distler, A.; Dees, C.; Zerr, P.; Huang, J.; Maier, C.; Pachowsky, M.L.; et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFbeta signalling. Ann. Rheum. Dis. 2015, 74, 1408–1416. [Google Scholar] [CrossRef]
- Kadoya, H.; Satoh, M.; Nagasu, H.; Sasaki, T.; Kashihara, N. Deficiency of endothelial nitric oxide signaling pathway exacerbates peritoneal fibrosis in mice. Clin. Exp. Nephrol. 2015, 19, 567–575. [Google Scholar] [CrossRef]
- Matei, A.E.; Beyer, C.; Gyorfi, A.H.; Soare, A.; Chen, C.W.; Dees, C.; Bergmann, C.; Ramming, A.; Friebe, A.; Hofmann, F.; et al. Protein kinases G are essential downstream mediators of the antifibrotic effects of sGC stimulators. Ann. Rheum. Dis. 2018, 77, 459. [Google Scholar] [CrossRef]
- Lambers, C.; Boehm, P.M.; Karabacak, Y.; Samaha, E.; Benazzo, A.; Jaksch, P.; Roth, M. Combined Activation of Guanylate Cyclase and Cyclic AMP in Lung Fibroblasts as a Novel Therapeutic Concept for Lung Fibrosis. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, L.M.; Chang, Y.; Wu, W.; Goldbach, C.; Ross, M.A.; Stolz, D.B.; Chen, L.; Fung, J.J.; Lu, L.; et al. In Vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology 2006, 44, 1171–1181. [Google Scholar] [CrossRef]
- Yu, M.C.; Chen, C.H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gaca, M.D.; Swenson, E.S.; Vellucci, V.F.; Reiss, M.; Wells, R.G. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J. Biol. Chem. 2003, 278, 11721–11728. [Google Scholar] [CrossRef] [PubMed]
- Lugnier, C.; Meyer, A.; Talha, S.; Geny, B. Cyclic nucleotide phosphodiesterases: New targets in the metabolic syndrome? Pharmacol. Ther. 2020, 208, 107475. [Google Scholar] [CrossRef] [PubMed]
- Son, G.; Hines, I.N.; Lindquist, J.; Schrum, L.W.; Rippe, R.A. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology 2009, 50, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Son, M.K.; Ryu, Y.L.; Jung, K.H.; Lee, H.; Lee, H.S.; Yan, H.H.; Park, H.J.; Ryu, J.K.; Suh, J.K.; Hong, S.; et al. HS-173, a novel PI3K inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci. Rep. 2013, 3, 3470. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, F.; Guo, Q.; Li, M.; Wang, L.; Zhang, Z.; Jiang, S.; Jin, H.; Chen, A.; Tan, S.; et al. Curcumol induces RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling in hepatic stellate cells. Redox Biol. 2018, 19, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Gao, R.; Brigstock, D.R. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J. Biol. Chem. 2004, 279, 8848–8855. [Google Scholar] [CrossRef]
- Chen, L.; Charrier, A.L.; Leask, A.; French, S.W.; Brigstock, D.R. Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor. J. Hepatol. 2011, 55, 399–406. [Google Scholar] [CrossRef]
- Seo, H.Y.; Lee, S.H.; Lee, J.H.; Kang, Y.N.; Hwang, J.S.; Park, K.G.; Kim, M.K.; Jang, B.K. Src Inhibition Attenuates Liver Fibrosis by Preventing Hepatic Stellate Cell Activation and Decreasing Connetive Tissue Growth Factor. Cells 2020, 9, 558. [Google Scholar] [CrossRef]
- Chen, A.; Zheng, S. Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-kappaB and ERK signalling. Br. J. Pharmacol. 2008, 153, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Xie, Y.; Peng, M.; Ma, L.; Zhou, Y.; Zhang, Y.; Kang, W.; Wang, J.; Bai, X.; Wang, P.; et al. Inhibition of connective tissue growth factor suppresses hepatic stellate cell activation In Vitro and prevents liver fibrosis In Vivo. Clin. Exp. Med. 2014, 14, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Berger, P.; Zenzmaier, C. The Potential of sGC Modulators for the Treatment of Age-Related Fibrosis: A Mini-Review. Gerontology 2017, 63, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Kawada, N.; Kuroki, T.; Uoya, M.; Inoue, M.; Kobayashi, K. Smooth muscle alpha-actin expression in rat hepatic stellate cell is regulated by nitric oxide and cGMP production. Biochem. Biophys. Res. Commun. 1996, 229, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Failli, P.; De, F.R.; Caligiuri, A.; Gentilini, A.; Romanelli, R.G.; Marra, F.; Batignani, G.; Guerra, C.T.; Laffi, G.; Gentilini, P.; et al. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology 2000, 119, 479–492. [Google Scholar] [CrossRef]
- Thirunavukkarasu, C.; Watkins, S.C.; Gandhi, C.R. Mechanisms of endotoxin-induced NO, IL-6, and TNF-alpha production in activated rat hepatic stellate cells: Role of p38 MAPK. Hepatology 2006, 44, 389–398. [Google Scholar] [CrossRef]
- Uemura, T.; Gandhi, C.R. Inhibition of DNA synthesis in cultured hepatocytes by endotoxin-conditioned medium of activated stellate cells is transforming growth factor-beta and nitric oxide-independent. Br. J. Pharmacol. 2001, 133, 1125–1133. [Google Scholar] [CrossRef]
- Urtasun, R.; Cubero, F.J.; Vera, M.; Nieto, N. Reactive nitrogen species switch on early extracellular matrix remodeling via induction of MMP1 and TNFalpha. Gastroenterology 2009, 136, 1410–1422. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Hall, K.C.; Bernier, S.G.; Jacobson, S.; Liu, G.; Zhang, P.Y.; Sarno, R.; Catanzano, V.; Currie, M.G.; Masferrer, J.L. sGC stimulator praliciguat suppresses stellate cell fibrotic transformation and inhibits fibrosis and inflammation in models of NASH. Proc. Natl. Acad. Sci. USA 2019, 116, 11057–11062. [Google Scholar] [CrossRef]
- Hwang, T.L.; Tang, M.C.; Kuo, L.M.; Chang, W.D.; Chung, P.J.; Chang, Y.W.; Fang, Y.C. YC-1 potentiates cAMP-induced CREB activation and nitric oxide production in alveolar macrophages. Toxicol. Appl. Pharmacol. 2012, 260, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Breitenstein, S.; Roessig, L.; Sandner, P.; Lewis, K.S. Novel sGC Stimulators and sGC Activators for the Treatment of Heart Failure. Handb. Exp. Pharmacol. 2017, 243, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.P. Soluble Guanylate Cyclase Stimulators and Activators. Handb. Exp. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Abdelaziz, N.; Colombo, F.; Mercier, I.; Calderone, A. Nitric oxide attenuates the expression of transforming growth factor-beta(3) mRNA in rat cardiac fibroblasts via destabilization. Hypertension 2001, 38, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Martic, M.; Darby, I.A.; Kelynack, K.J.; Bisucci, T.; Tait, M.G.; Becker, G.J. Intracellular cyclic nucleotide analogues inhibit in vitro mitogenesis and activation of fibroblasts derived from obstructed rat kidneys. Nephron. Exp. Nephrol. 2004, 96, e59–e66. [Google Scholar] [CrossRef]
- Frey, R.; Becker, C.; Saleh, S.; Unger, S.; van der Mey, D.; Muck, W. Clinical Pharmacokinetic and Pharmacodynamic Profile of Riociguat. Clin. Pharmacokinet. 2018, 57, 647–661. [Google Scholar] [CrossRef]
- Schwabl, P.; Brusilovskaya, K.; Supper, P.; Bauer, D.; Konigshofer, P.; Riedl, F.; Hayden, H.; Fuchs, C.D.; Stift, J.; Oberhuber, G.; et al. The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci. Rep. 2018, 8, 9372. [Google Scholar] [CrossRef]
- Flores-Costa, R.; Alcaraz-Quiles, J.; Titos, E.; Lopez-Vicario, C.; Casulleras, M.; Duran-Guell, M.; Rius, B.; Diaz, A.; Hall, K.; Shea, C.; et al. The soluble guanylate cyclase stimulator IW-1973 prevents inflammation and fibrosis in experimental non-alcoholic steatohepatitis. Br. J. Pharmacol. 2018, 175, 953–967. [Google Scholar] [CrossRef]
- Knorr, A.; Hirth-Dietrich, C.; Alonso-Alija, C.; Harter, M.; Hahn, M.; Keim, Y.; Wunder, F.; Stasch, J.P. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60-2770 in experimental liver fibrosis. Arzneimittel-Forschung 2008, 58, 71–80. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Q.L.; Shen, L.; Tao, Y.Y.; Liu, C.H. MicroRNA-101 suppresses liver fibrosis by downregulating PI3K/Akt/mTOR signaling pathway. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 575–584. [Google Scholar] [CrossRef]
- Lao, Y.; Li, Y.; Zhang, P.; Shao, Q.; Lin, W.; Qiu, B.; Lv, Y.; Tang, L.; Su, S.; Zhang, H.; et al. Targeting Endothelial Erk1/2-Akt Axis as a Regeneration Strategy to Bypass Fibrosis during Chronic Liver Injury in Mice. Mol. Ther. 2018, 26, 2779–2797. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Bai, R.; Wang, L.; Gao, J.; Zhang, H. Artesunate may inhibit liver fibrosis via the FAK/Akt/beta-catenin pathway in LX-2 cells. BMC Pharmacol. Toxicol. 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-J.; Kuo, L.-M.; Wu, Y.-H.; Chang, Y.-C.; Lai, K.-H.; Hwang, T.-L. BAY 41-2272 Attenuates CTGF Expression via sGC/cGMP-Independent Pathway in TGFβ1-Activated Hepatic Stellate Cells. Biomedicines 2020, 8, 330. https://doi.org/10.3390/biomedicines8090330
Chen P-J, Kuo L-M, Wu Y-H, Chang Y-C, Lai K-H, Hwang T-L. BAY 41-2272 Attenuates CTGF Expression via sGC/cGMP-Independent Pathway in TGFβ1-Activated Hepatic Stellate Cells. Biomedicines. 2020; 8(9):330. https://doi.org/10.3390/biomedicines8090330
Chicago/Turabian StyleChen, Po-Jen, Liang-Mou Kuo, Yi-Hsiu Wu, Yu-Chia Chang, Kuei-Hung Lai, and Tsong-Long Hwang. 2020. "BAY 41-2272 Attenuates CTGF Expression via sGC/cGMP-Independent Pathway in TGFβ1-Activated Hepatic Stellate Cells" Biomedicines 8, no. 9: 330. https://doi.org/10.3390/biomedicines8090330
APA StyleChen, P.-J., Kuo, L.-M., Wu, Y.-H., Chang, Y.-C., Lai, K.-H., & Hwang, T.-L. (2020). BAY 41-2272 Attenuates CTGF Expression via sGC/cGMP-Independent Pathway in TGFβ1-Activated Hepatic Stellate Cells. Biomedicines, 8(9), 330. https://doi.org/10.3390/biomedicines8090330





