Thickness of Intraretinal Layers in Patients with Type 2 Diabetes Mellitus Depending on a Concomitant Diabetic Neuropathy: Results of a Cross-Sectional Study Using Deviation Maps for OCT Data Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria for Patients and Controls
2.2. Spectral Domain OCT
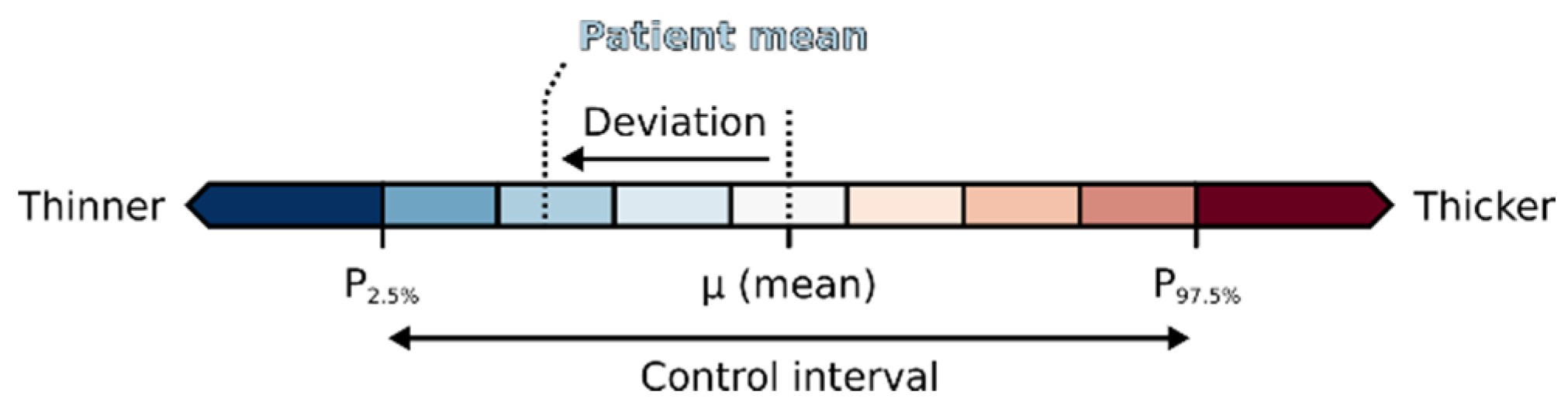
2.3. Visual Analysis
3. Results
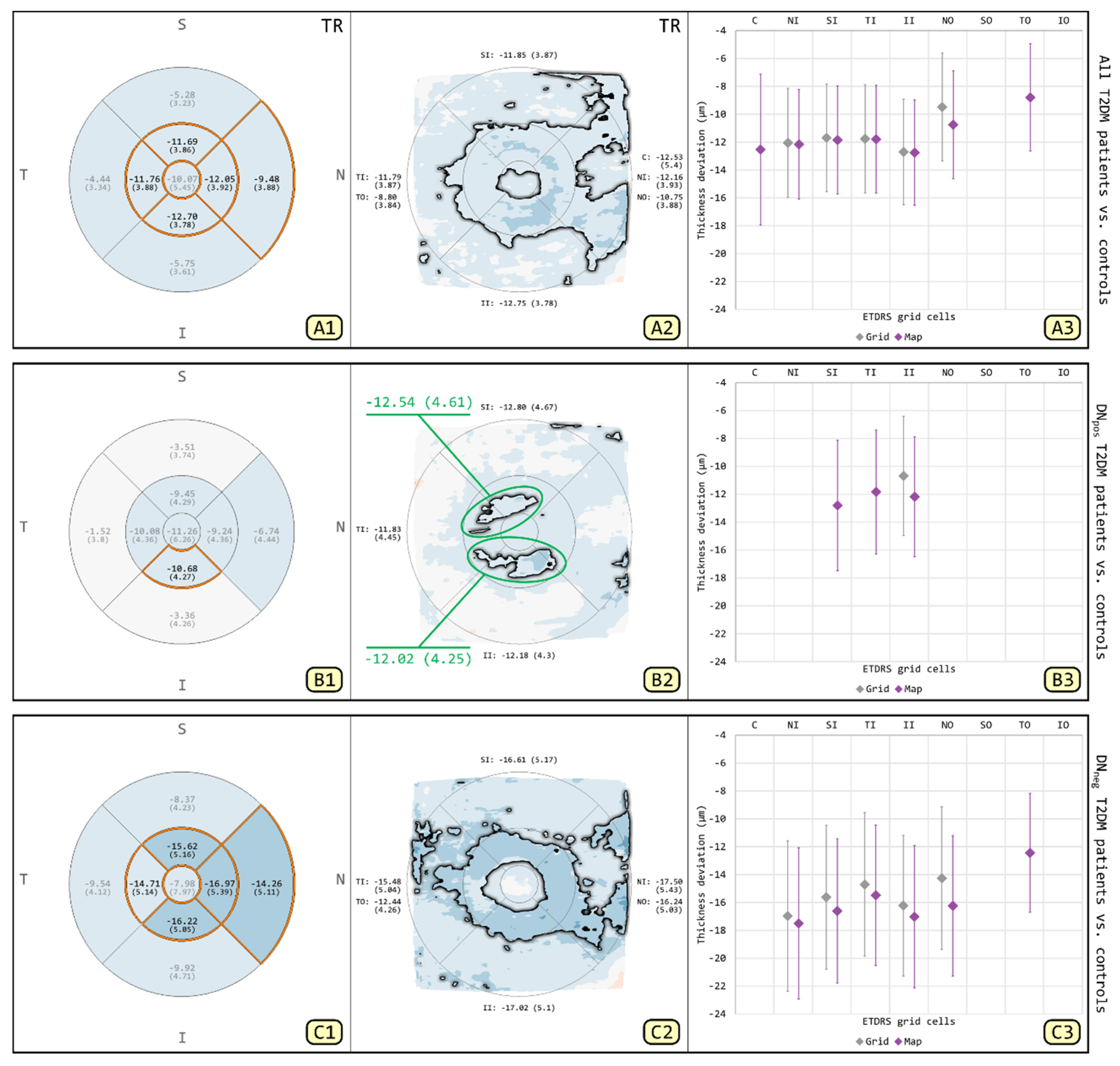
3.1. Comparison of T2DM Patients and Patient Subgroups to Controls for TR
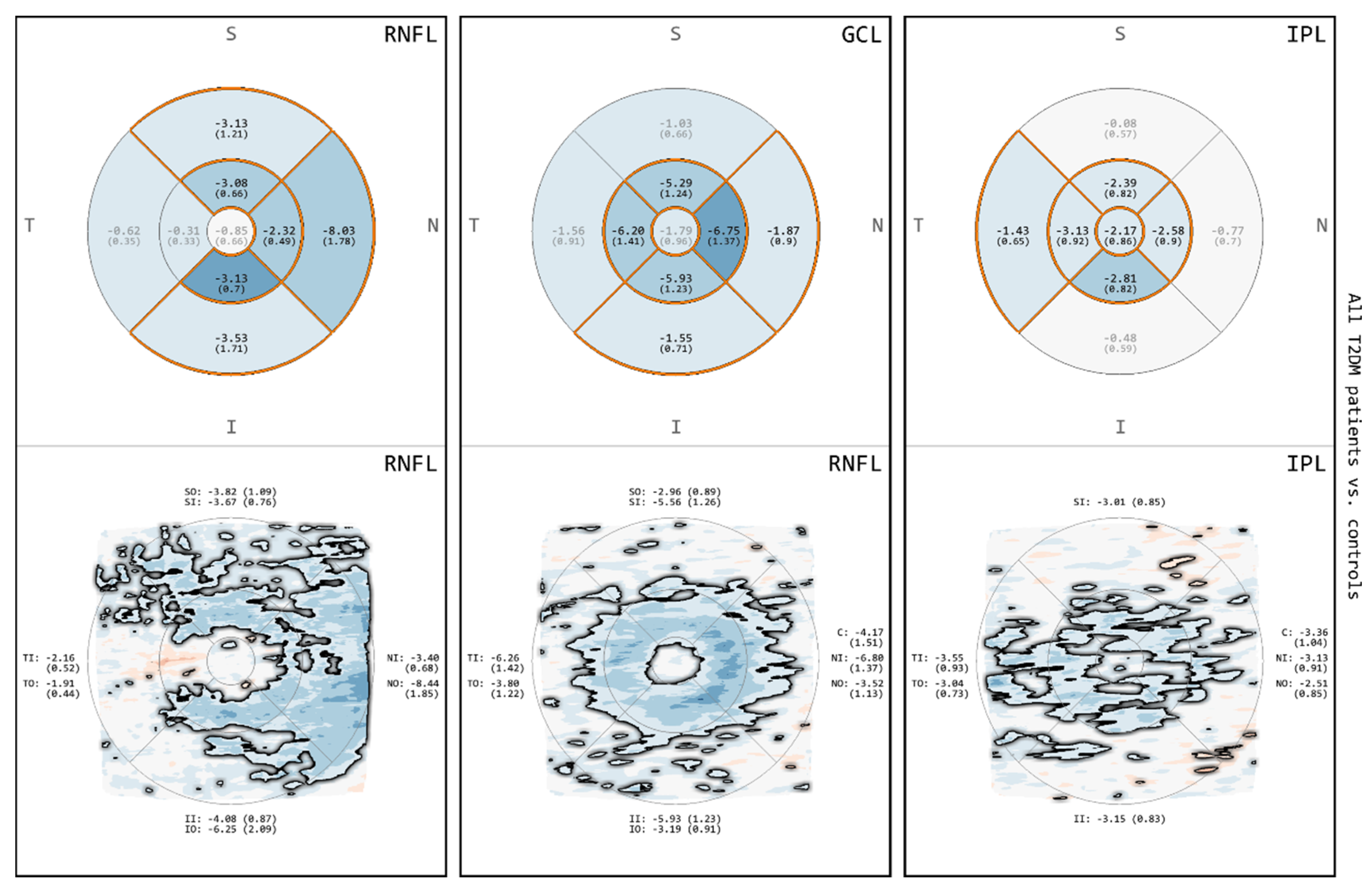
3.2. Comparison of All T2DM Patients to Controls for RNFL, GCL and IPL
3.3. Comparison between DNpos T2DM Patients and Controls for RNFL, GCL and IPL
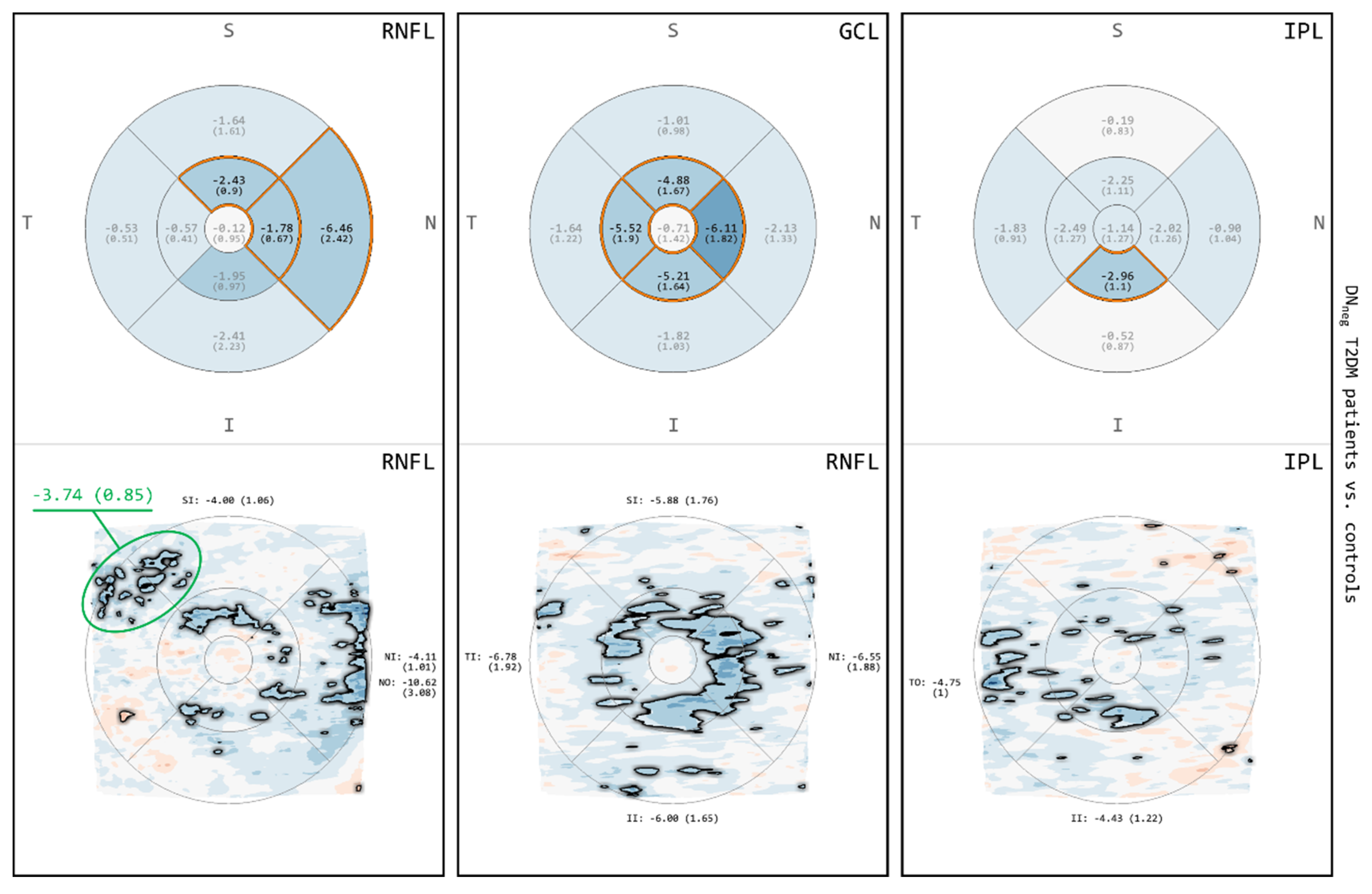
3.4. Comparison between DNneg T2DM Patients and Controls for RNFL, GCL and IPL
4. Discussion
4.1. Comparison of Grid-Based and Map-Based Analyses
4.2. Neurodegenerative Changes in Patients with T2DM
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OCT | Optical coherence tomography |
| VA | Visual Analytics |
| MA | Deviation map-based analysis |
| DevM | Deviation map |
| ETDRS | Early treatment diabetic retinopathy study |
| GA | ETDRS grid-based analysis |
| DM | Diabetes mellitus |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| DR | Diabetic retinopathy |
| DN | Diabetic peripheral neuropathy |
| DNneg | Patients without diabetic peripheral neuropathy |
| DNpos | Patients with diabetic peripheral neuropathy |
| FC | Foveal center |
| IMR | Inner macular ring |
| OMR | Outer macular ring |
| TR | Total thickness of the retina |
| RNFL | Retinal nerve fiver layer |
| GCL | Ganglion cell layer |
| IPL | Inner plexiform layer |
| INL | Inner nuclear layer |
| MD ± SE | Mean deviation and standard error |
| NSS | Neuropathy symptom score |
| NDS | Neuropathy deficient score |
| cSLO | Confocal scanning laser ophthalmoscope |
| HSD | Honestly significant difference |
References
- Costa, R.A.; Skaf, M.; Melo, L.A.S.; Calucci, D.; Cardillo, J.A.; Castro, J.C.; Huang, D.; Wojtkowski, M. Retinal assessment using optical coherence tomography. Prog. Retin. Eye Res. 2006, 25, 325–353. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, R.K.; Röhlig, M.; Fischer, D.C.; Götze, A.; Jünemann, A.; Schumann, H.; Stachs, O. Deviation Maps for Understanding Thickness Changes of Inner Retinal Layers in Children with Type 1 Diabetes Mellitus. Curr. Eye Res. 2019, 44, 746–752. [Google Scholar] [CrossRef]
- DeBuc, D.C.; Somfai, G.M. Early detection of retinal thickness changes in diabetes using Optical Coherence Tomography. Med. Sci. Monit. 2010, 16, 15–21. [Google Scholar]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2015, 56, 6333. [Google Scholar] [CrossRef]
- Tekin, K.; Inanc, M.; Kurnaz, E.; Bayramoglu, E.; Aydemir, E.; Koc, M.; Kiziltoprak, H.; Aycan, Z. Quantitative evaluation of early retinal changes in children with type 1 diabetes mellitus without retinopathy. Clin. Exp. Optom. 2018, 101, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mendez, N.; Kommana, S.S.; Szirth, B.; Khouri, A.S. Structural Changes by Spectral Domain Optical Coherence Tomography in Patients With Type 1 Diabetes Mellitus. J. Diabetes Sci. Technol. 2016, 10, 271–276. [Google Scholar] [CrossRef]
- Bagci, A.M.; Shahidi, M.; Ansari, R.; Blair, M.; Blair, N.P.; Zelkha, R. Thickness Profiles of Retinal Layers by Optical Coherence Tomography Image Segmentation. Am. J. Ophthalmol. 2008, 146, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification. Ophthalmology 1991, 98, 786–806. [CrossRef]
- Goebel, W.; Kretzchmar-Gross, T. Retinal Thickness in Diabetic Retinopathy: A Study Using Optical Coherence Tomography (OCT). Retina 2002, 22, 759–767. [Google Scholar] [CrossRef]
- Röhlig, M.; Schmidt, C.; Prakasam, R.K.; Rosenthal, P.; Schumann, H.; Stachs, O. Visual analysis of retinal changes with optical coherence tomography. Vis. Comput. 2018, 34, 1209–1224. [Google Scholar] [CrossRef]
- Röhlig, M.; Prakasam, R.K.; Stüwe, J.; Schmidt, C.; Stachs, O.; Schumann, H. Enhanced Grid-Based Visual Analysis of Retinal Layer Thickness with Optical Coherence Tomography. Information 2019, 10, 266. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Yan, Y.; Shen, X. Diabetic macular morphology changes may occur in the early stage of diabetes. BMC Ophthalmol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Frydkjaer-Olsen, U.; Hansen, R.S.; Peto, T.; Grauslund, J. Structural neurodegeneration correlates with early diabetic retinopathy. Int. Ophthalmol. 2018, 38, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, M.; Yuan, Y.; Huang, S.; Zhu, D.; Ma, Q.; Ye, X.; Lu, F. Macular Thickness Profiles of Intraretinal Layers in Myopia Evaluated by Ultrahigh-Resolution Optical Coherence Tomography. Am. J. Ophthalmol. 2015, 160, 53–61. [Google Scholar] [CrossRef]
- El-Fayoumi, D.; Badr Eldine, N.M.; Esmael, A.F.; Ghalwash, D.; Soliman, H.M. Retinal Nerve Fiber Layer and Ganglion Cell Complex Thicknesses Are Reduced in Children With Type 1 Diabetes With No Evidence of Vascular Retinopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 5355. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Kim, H.W.; Kwon, J.W.; Shim, Y.; Jee, D.H.; Yun, J.S.; Ahn, Y.B.; Park, C.K.; Ko, S.H. Early inner retinal thinning and cardiovascular autonomic dysfunction in type 2 diabetes. PLoS ONE 2017, 12, e0174377. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, R.; Sah, S.; Gupta, N. Study of Retinal Nerve Fibre Layer Thickness in Patients with Diabetes Mellitus Using Fourier Domain Optical Coherence Tomography. J. Clin. Diagn. Res. 2016, 10. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Du, X.; Wu, Q. Comparison of spectral-domain optical coherence tomography for intra-retinal layers thickness measurements between healthy and diabetic eyes among Chinese adults. PLoS ONE 2017, 12, e0177515. [Google Scholar] [CrossRef]
- Oshitari, T.; Hanawa, K.; Adachi-Usami, E. Changes of macular and RNFL thicknesses measured by Stratus OCT in patients with early stage diabetes. Eye 2009, 23, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Thakku, S.G.; Sabanayagam, C.; Tan, G.; Agrawal, R.; Cheung, C.M.G.; Lamoureux, E.L.; Wong, T.Y.; Cheng, C.Y. Characterisation of choroidal morphological and vascular features in diabetes and diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 1038–1044. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Li, W.; Hua, Y.J.; Wu, Q. The thickness and volume of the choroid, outer retinal layers and retinal pigment epithelium layer changes in patients with diabetic retinopathy. Int. J. Ophthalmol. 2018, 11, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Pritchard, N.; Vagenas, D.; Edwards, K.; Sampson, G.P.; Russell, A.W.; Malik, R.A.; Efron, N. Retinal Tissue Thickness is Reduced in Diabetic Peripheral Neuropathy. Curr. Eye Res. 2016, 41, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Pritchard, N.; Sampson, G.P.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Diagnostic capability of retinal thickness measures in diabetic peripheral neuropathy. J. Optom. 2017, 10, 215–225. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All T2DM Patients (n = 33) | DNpos T2DM Patients (n = 21) | DNneg T2DM Patients (n = 12) |
|---|---|---|---|
| Age (yrs) | 60.4 ± 12.0 | 64.0 ± 9.0 | 54.0 ± 14.0 |
| Gender (male/female) | 21/12 | 15/6 | 6/6 |
| Height (cm) | 174 ± 10 | 176 ± 10 | 171 ± 9 |
| Weight (kg) | 99.3 ± 21.4 | 101.2 ± 21.4 | 95.9 ± 21.9 |
| BMI (kg/m2) | 32.7 ± 6.3 | 32.5 ± 5.2 | 33.1 ± 8.0 |
| Systolic blood pressure (mmHg) | 133 ± 16 | 134 ± 18 | 130 ± 10 |
| Diastolic blood pressure (mmHg) | 79 ± 11 | 79 ± 12 | 77 ± 6 |
| Duration of DM (yrs) | 13.3 ± 8.8 | 16.0± 9.6 | 8.7 ± 4.5 |
| Mean daily insulin dosage (IU/kg) | 0.515 ± 0.323 (n = 30) | 0.516 ± 0.335 (n = 19) | 0.512 ± 0.317 (n = 11) |
| HbA1c (%) | 8.2 ± 0.8 | 8.2 ± 0.8 | 8.2 ± 0.9 |
| Neurologic disability score, NDS (pts) | 1.7 ± 1.9 | 2.7 ± 1.8 | 0 |
| Neurologic symptom score, NSS (pts) | 2.7 ± 1.9 | 3.9 ± 0.9 | 0.5 ± 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakasam, R.K.; Matuszewska-Iwanicka, A.; Fischer, D.-C.; Schumann, H.; Tschöpe, D.; Stratmann, B.; Hettlich, H.-J.; Guthoff, R.F.; Stachs, O.; Röhlig, M. Thickness of Intraretinal Layers in Patients with Type 2 Diabetes Mellitus Depending on a Concomitant Diabetic Neuropathy: Results of a Cross-Sectional Study Using Deviation Maps for OCT Data Analysis. Biomedicines 2020, 8, 190. https://doi.org/10.3390/biomedicines8070190
Prakasam RK, Matuszewska-Iwanicka A, Fischer D-C, Schumann H, Tschöpe D, Stratmann B, Hettlich H-J, Guthoff RF, Stachs O, Röhlig M. Thickness of Intraretinal Layers in Patients with Type 2 Diabetes Mellitus Depending on a Concomitant Diabetic Neuropathy: Results of a Cross-Sectional Study Using Deviation Maps for OCT Data Analysis. Biomedicines. 2020; 8(7):190. https://doi.org/10.3390/biomedicines8070190
Chicago/Turabian StylePrakasam, Ruby Kala, Aleksandra Matuszewska-Iwanicka, Dagmar-Christiane Fischer, Heidrun Schumann, Diethelm Tschöpe, Bernd Stratmann, Hans-Joachim Hettlich, Rudolf F. Guthoff, Oliver Stachs, and Martin Röhlig. 2020. "Thickness of Intraretinal Layers in Patients with Type 2 Diabetes Mellitus Depending on a Concomitant Diabetic Neuropathy: Results of a Cross-Sectional Study Using Deviation Maps for OCT Data Analysis" Biomedicines 8, no. 7: 190. https://doi.org/10.3390/biomedicines8070190
APA StylePrakasam, R. K., Matuszewska-Iwanicka, A., Fischer, D.-C., Schumann, H., Tschöpe, D., Stratmann, B., Hettlich, H.-J., Guthoff, R. F., Stachs, O., & Röhlig, M. (2020). Thickness of Intraretinal Layers in Patients with Type 2 Diabetes Mellitus Depending on a Concomitant Diabetic Neuropathy: Results of a Cross-Sectional Study Using Deviation Maps for OCT Data Analysis. Biomedicines, 8(7), 190. https://doi.org/10.3390/biomedicines8070190







