The Role of FAT10 in Alcoholic Hepatitis Pathogenesis
Abstract
1. Introduction
2. Role of FAT10 in Alcoholic Liver
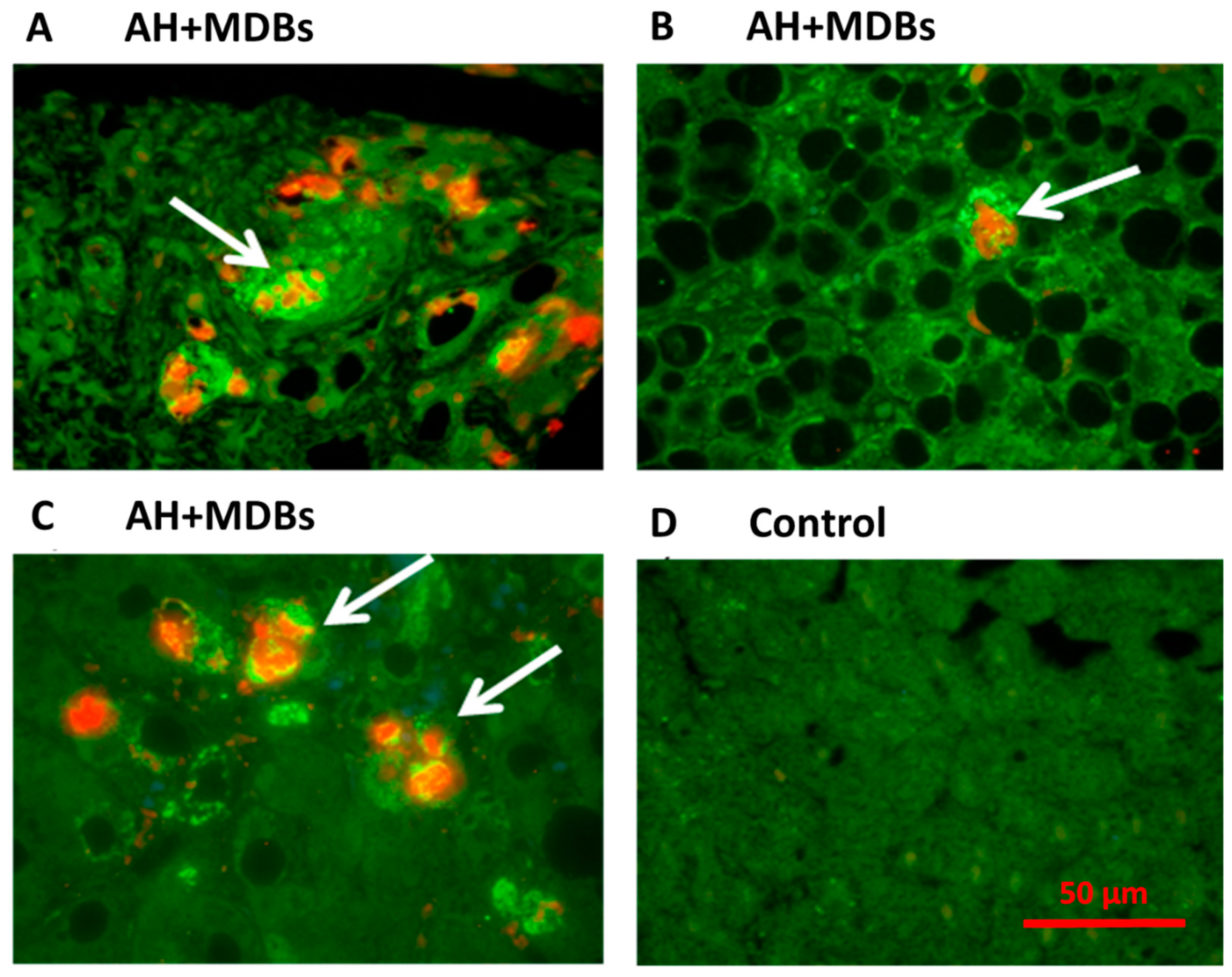
2.1. Role of MDB and Its Modulation in Animal Studies
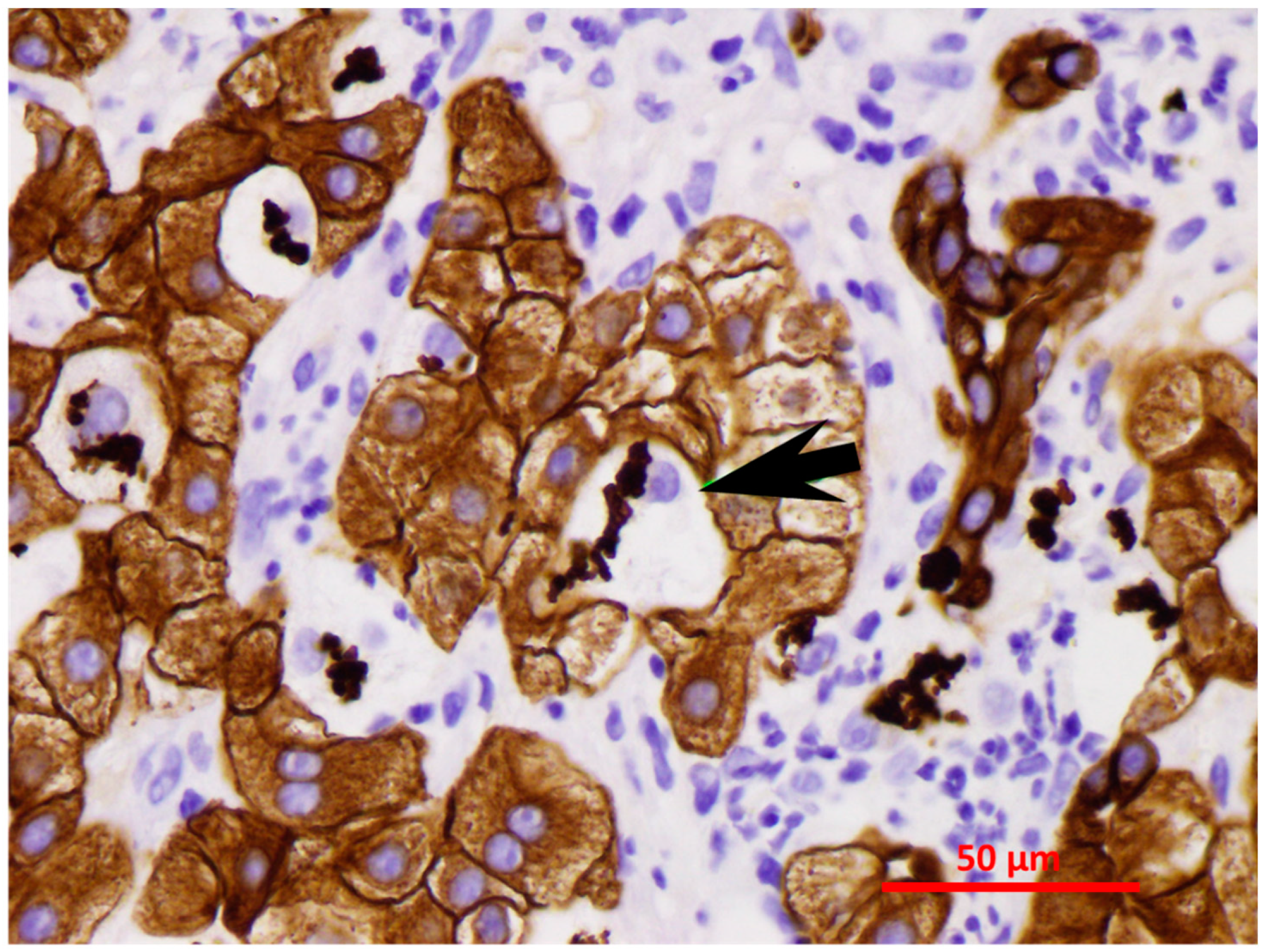
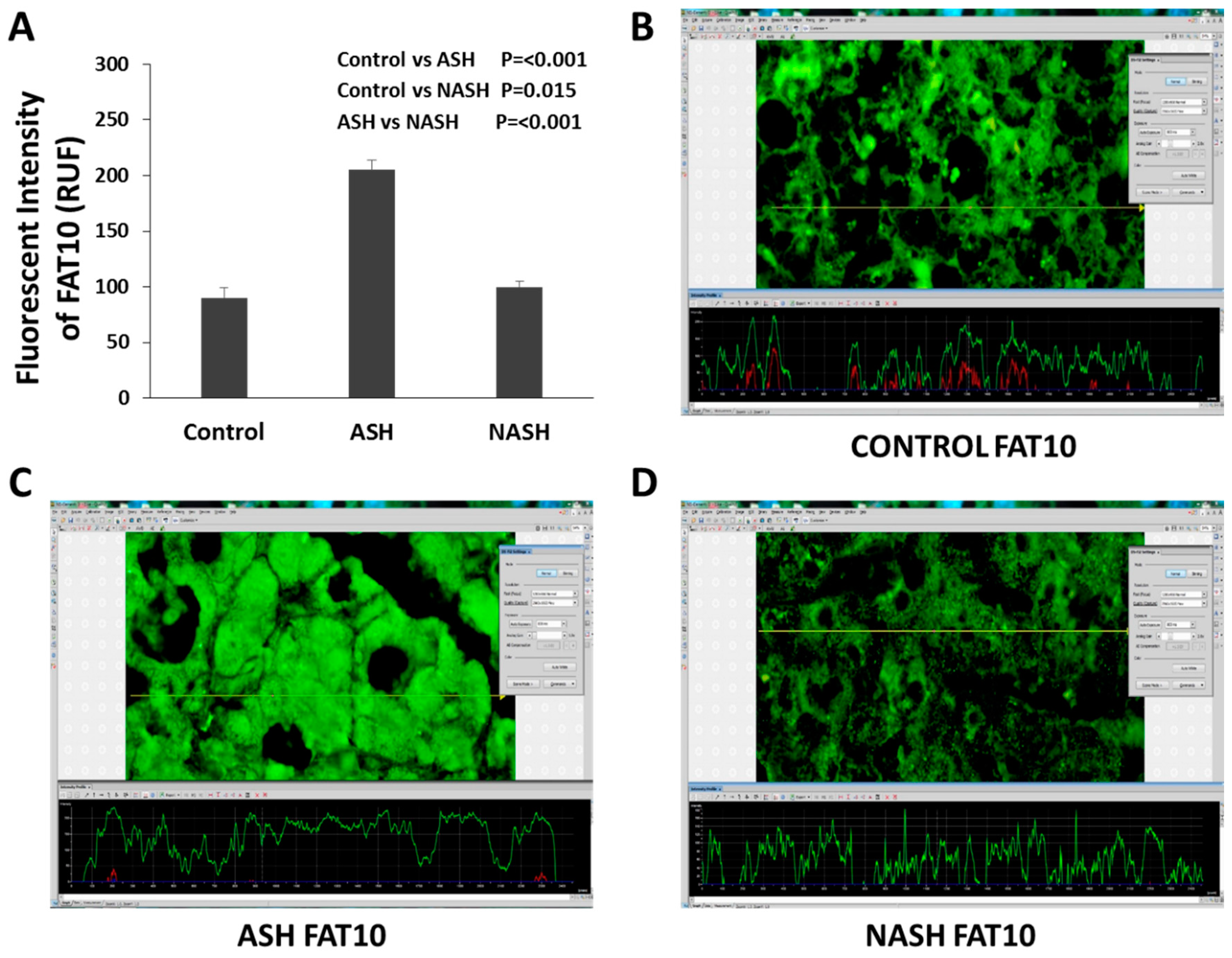
2.2. Role of FAT10 and MDB in Human Studies
2.3. Interaction of FAT10 and SUMO
2.4. Modulation of Proteasome by FAT10
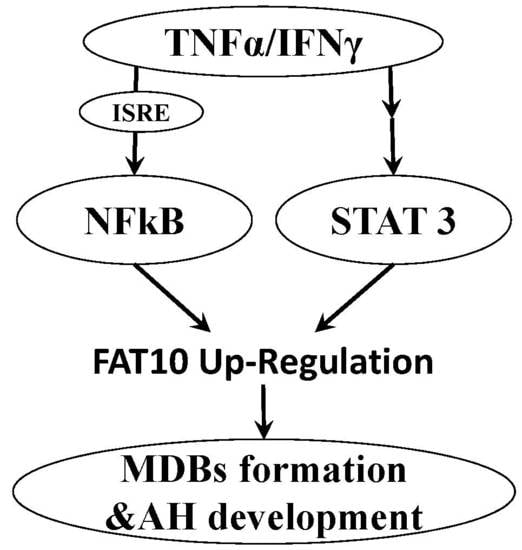
3. Molecular Pathways of FAT10 in Liver
3.1. Studies in FAT10 Knockout Animal
3.2. FAT10 and Ubiquitin-Proteasome System (UPS)
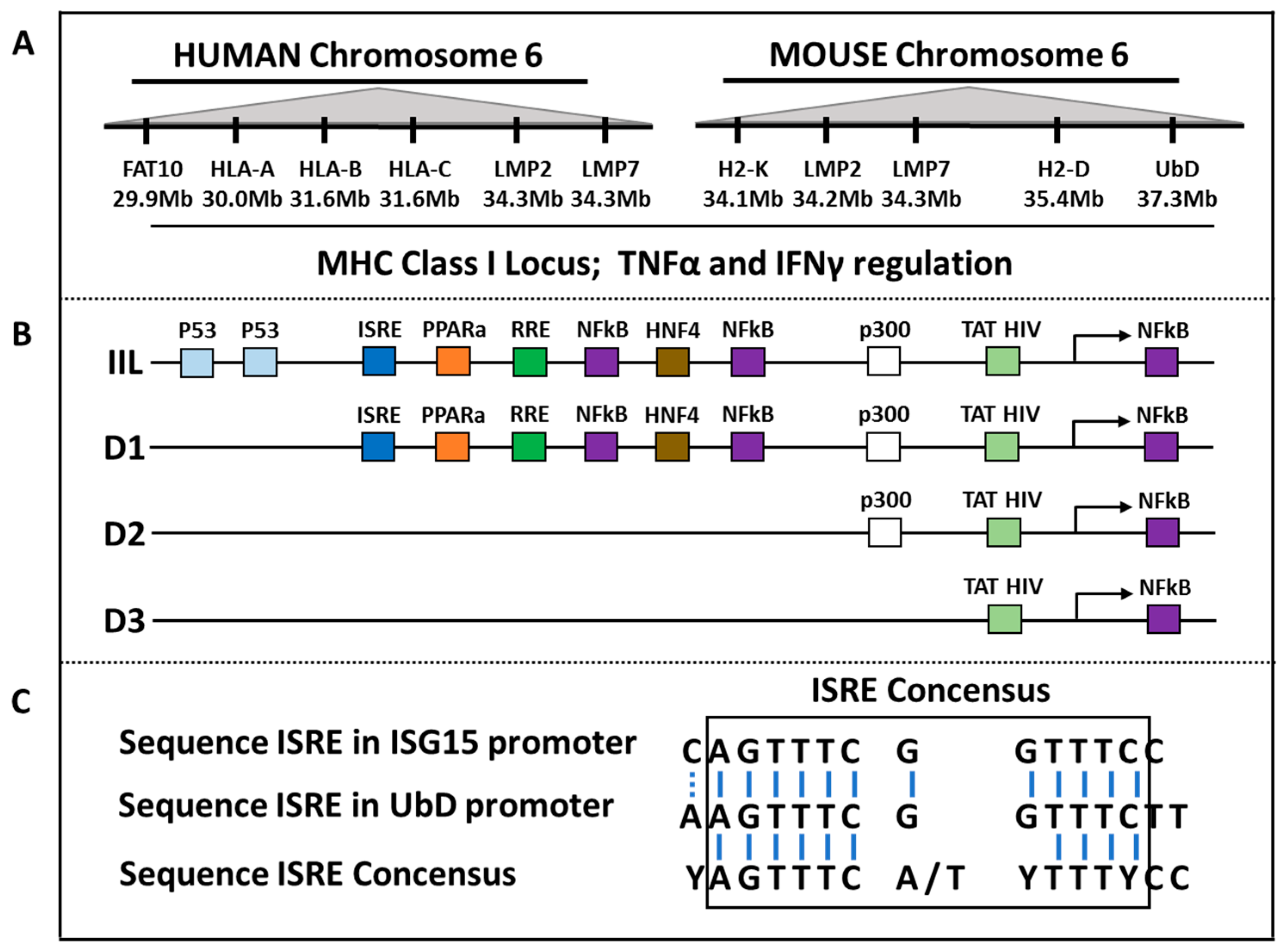
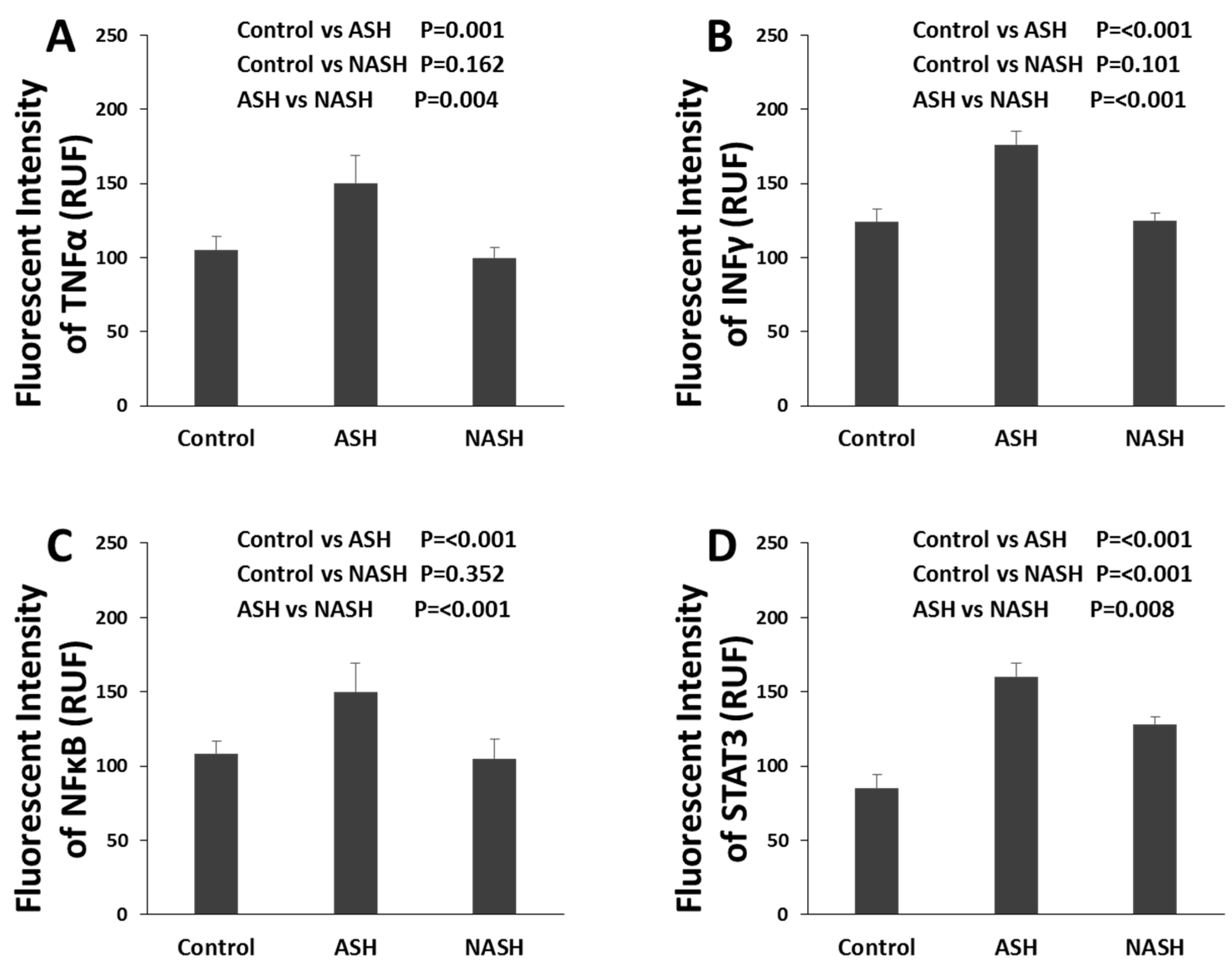
3.3. FAT10 and Cytokines
4. Treatment for Alcoholic Hepatitis
5. Conclusions
Funding
Conflicts of Interest
References
- Sheron, N. Alcohol and liver disease in Europe--Simple measures have the potential to prevent tens of thousands of premature deaths. J. Hepatol. 2016, 644, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, T.I. Alcohol and liver injury: Dose-related or permissive effect? Liver 1989, 9, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Groettrup, M.; Pelzer, C.; Schmidtke, G.; Hofmann, K. Activating the ubiquitin family: UBA6 challenges the field. Trends Biochem. Sci. 2008, 33, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Pan, J.; Zhang, C.; Fan, W.; Collinge, M.; Bender, J.R.; Weissman, S.M. A MHC-encoded ubiquitin-like protein (FAT10) binds noncovalently to the spindle assembly checkpoint protein MAD2. Proc. Natl. Acad. Sci. USA 1999, 96, 4313–4318. [Google Scholar] [CrossRef]
- Lee, C.; Ren, J.; Cheong, I.S.Y.; Ban, K.H.; Ooi, L.L.; Yong Tan, S.; Kan, A.; Nuchprayoon, I.; Jin, R.; Lee, K.H.; et al. Expression of the FAT10 gene is highly upregulated in hepatocellular carcinoma and other gastrointestinal and gynecological cancers. Oncogene 2003, 22, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Lukasiak, S.; Schiller, C.; Oehlschlaeger, P.; Schmidtke, G.; Krause, P.; Legler, D.F.; Autschbach, F.; Schirmacher, P.; Breuhahn, K.; Groettrup, M. Proinflammatory cytokines cause FAT10 upregulation in cancers of liver and colon. Oncogene 2008, 27, 6068–6074. [Google Scholar] [CrossRef]
- Raasi, S.; Schmidtke, G.; de Giuli, R.; Groettrup, M. A ubiquitin-like protein which is synergistically inducible by interferon-γ and tumor necrosis factor-α. Eur. J. Immunol. 1999, 29, 4030–4036. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.K.; Yoo, J.Y. NFκB and STAT3 synergistically activate the expression of FAT10, a gene counteracting the tumor suppressor p53. Mol. Oncol. 2014, 8, 642–655. [Google Scholar] [CrossRef]
- Oliva, J.; Bardag-Gorce, F.; Lin, A.; French, B.A.; French, S.W. The role of cytokines in UBD promoter regulation and Mallory-Denk body-like aggresomes. Exp. Mol. Pathol. 2010, 89, 1–8. [Google Scholar] [CrossRef]
- Aichem, A.; Groettrup, M. The ubiquitin-like modifier FAT10 in cancer development. Int. J. Biochem. Cell Biol. 2016, 79, 451–461. [Google Scholar] [CrossRef]
- Liu, S.; Jin, Y.; Zhang, D.; Wang, J.; Wang, G.; Lee, C.G.L. Investigating the promoter of FAT10 gene in HCC patients. Genes 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhan, B. UBA6 and its bispecific pathways for ubiquitin and FAT10. Int. J. Mol. Sci. 2019, 20, 2250. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xiong, H.; Chen, L.; Liu, X.; Zou, S.; Guan, J.; Wang, K. GRP78 promoter hepatocellular carcinoma proliferation by increasing FAT10 expression through the NFκB pathway. Exp. Cell Res. 2018, 365, 1–11. [Google Scholar] [CrossRef] [PubMed]
- French, S.W.; French, B.A.; Oliva, J.; Li, J.; Bardag-Gorce, F.; Tillman, B.; Canaan, A. FAT10 knock out mice livers fail to develop Mallory-Denk bodies in the DDC mouse model. Exp. Mol. Pathol. 2003, 93, 309–314. [Google Scholar] [CrossRef]
- Kurtovic, E.; Limaiem, F. Mallory Bodies. 30 Jul 2019. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Basaranoglu, M.; Turhan, N.; Sonsuz, A.; Basaranoglu, G. Mallory-Denk Bodies in chronic hepatitis. World J. Gastroenterol. 2011, 17, 2172–2177. [Google Scholar] [CrossRef]
- Zatloukal, K.; French, S.W.; Stumptner, C.; Strnad, P.; Harada, M.; Toivola, D.M.; Cadrin, M.; Omary, M.B. From Mallory to Mallory-Denk bodies: What, how and why? Exp. Cell Res. 2007, 313, 2033–2049. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Oliva, J.; Li, J.; French, B.A.; French, S.W. SAMe prevents the induction of the immunoproteasome and preserves the 26S proteasome in the DDC-induced MDB mouse model. Exp. Mol. Pathol. 2010, 88, 353–362. [Google Scholar] [CrossRef]
- French, S.W.; Mendoza, A.S.; Peng, Y. The mechanisms of Mallory-Denk body formation are similar to the formation of aggresomes in Alzheimer’s disease and other neurodegenerative disorders. Exp. Mol. Pathol. 2016, 100, 426–433. [Google Scholar] [CrossRef]
- Oliva, J.; Bardag-Gorce, F.; Li, J.; French, B.A.; Nguyen, S.K.; Lu, S.C.; French, S.W. Betaine prevents Mallory-Denk body formation in drug-primed mice by epigenetic mechanisms. Exp. Mol. Pathol. 2009, 86, 77–86. [Google Scholar] [CrossRef]
- Liu, H.; Gong, M.; French, B.A.; Li, J.; Tillman, B.; French, S.W. Mallory-Denk body (MDB) formation modulates Ufmylation expression epigenetically in alcoholic hepatitis (AH) and non-alcoholic Steatohepatitis. Exp. Mol. Pathol. 2014, 97, 477–483. [Google Scholar] [CrossRef]
- French, S.W.; Bardag-Gorce, F.; Li, J.; French, B.A.; Oliva, J. Mallory-Denk body pathogenesis revisited. World J. Hepatol. 2010, 2, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, K.; Moura, R.F.; Granvik, M.; Igoillo-Esteve, M.; Hohmeier, H.E.; Hendrickx, N.; Newgard, C.B.; Waelkens, E.; Cnop, M.; Schuit, F. Ubiquitin fold modifier 1 (UFM1) and its target UFBP1 protect pancreatic beta cells from ER stress-induced apoptosis. PLoS ONE 2011, 6, e18517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Wu, J.; Lei, G.; Li, H. Transcriptional regulation of the Ufm1 conjugation system in response to disturbance of the endoplasmic reticulum homeostasis and inhibition of vesicle trafficking. PLoS ONE 2012, 7, e48587. [Google Scholar] [CrossRef]
- Kwon, J.; Cho, H.J.; Han, S.H.; No, J.G.; Kwon, J.Y.; Kim, H. A novel LZAP-binding protein, NLBP, inhibits cell invasion. J. Biol. Chem. 2010, 285, 12232–12240. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, H.; Yoshimura, N.; Tamura, T.; Sone, M.; Ogishima, S.; Watase, K.; Tagawa, K.; Okazawa, H. Suppression of the novel ER protein Maxer by mutant ataxin-1 in Bergman glia contributes to non-cell-autonomous toxicity. EMBO J. 2010, 29, 2446–2460. [Google Scholar] [CrossRef]
- Kim, C.H.; Nam, H.S.; Lee, E.H.; Han, S.H.; Cho, H.J.; Chung, H.J.; Lee, N.S.; Choi, S.J.; Kim, H.; Ryu, J.S.; et al. Overexpression of a novel regulator of p120 catenin, NLBP, promotes lung adenocarcinoma proliferation. Cell Cycle 2013, 12, 2443–2453. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Morgan, T.R.; Mandayam, S.; Jamal, M.M. Alcohol and hepatocellular carcinoma. Gastroenterology 2004, 127, S87–S96. [Google Scholar] [CrossRef]
- Ramadori, P.; Cubero, F.J.; Liedtke, C.; Trautwein, C.; Nevzorova, Y.A. Alcohol and Hepatocellular Carcinoma: Adding Fuel to the Flame. Cancers 2017, 9, E130. [Google Scholar] [CrossRef]
- Testino, G.; Leone, S.; Borro, P. Alcohol and hepatocellular carcinoma: A review and a point of view. World. J. Gastroenterol. 2014, 20, 15943–15954. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Reinus, J.F. Prevalence and natural history of alcoholic liver disease. Clin. Liver Dis. 2012, 16, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Dam-Larsen, S.; Franzmann, M.; Andersen, I.B.; Christoffersen, P.; Jensen, L.B.; Sørensen, T.I.A.; Becker, U.; Bendtsen, F. Affiliations Long term prognosis of fatty liver: Risk of chronic liver disease and death. Gut 2004, 53, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Schuppan, D.; Hahn, E.G.; Seitz, H.K. Cocarcinogenic effects of alcohol in hepatocarcinogenesis. Gut 2002, 51, 132–139. [Google Scholar] [CrossRef]
- Stickel, F. Alcoholic cirrhosis and hepatocellular carcinoma. Adv. Exp. Med. Biol. 2015, 815, 113–130. [Google Scholar] [PubMed]
- Schmidtke, G.; Kalveram, B.; Groettrup, M. Degradation of FAT10 by the 26S proteasome is independent of ubiquitylation but relies on NUB1L. FEBS Lett. 2009, 583, 591–594. [Google Scholar] [CrossRef]
- Rani, N.; Aichem, A.; Schmidtke, G.; Kreft, S.G.; Groettrup, M. FAT10 and NUB1L bind to the VWA domain of Rpn10 and Rpn1 to enable proteasome-mediated proteolysis. Nat. Commun. 2012, 3, 749. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kalveram, B.; Raasi, S.; Groettrup, M.; Schmidtke, G. FAT10, a ubiquitin-independent signal for proteasomal degradation. Mol. Cell Biol. 2005, 25, 3483–3491. [Google Scholar] [CrossRef]
- Oliva, J.; Bardag-Gorce, F.; French, B.A.; Li, J.; McPhaul, L.; Amidi, F.; Dedes, J.; Habibi, A.; Nguyen, S.; French, S.W. Fat10 is an epigenetic marker for liver preneoplasia in a drug-primed mouse model of tumorigenesis. Exp. Mol. Pathol. 2008, 84, 102–112. [Google Scholar] [CrossRef]
- French, S.W.; Lee, J.; Zhong, J.; Morgan, T.R.; Buslon, V.; Lungo, W.; French, B.A. Alcoholic liver disease-Hepatocellular carcinoma transformation. J. Gastrointest. Oncol. 2012, 3, 174–181. [Google Scholar]
- Jia, Y.; French, B.; Tillman, B.; French, S. Different roles of FAT10, FOXO1, and ADRA2A in hepatocellular carcinoma tumorigenesis in patients with alcoholic steatohepatitis (ASH) vs non-alcoholic steatohepatitis (NASH). Exp. Mol. Pathol. 2018, 105, 144–149. [Google Scholar] [CrossRef]
- Seeler, J.S.; Dejean, A. SUMO and the robustness of cancer. Nat. Rev. Cancer. 2017, 17, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Zhi-Jian, H.; Yan-Hu, F.; Bao-Hong, G.; Yu-Min, L.; Hao, C. The Post-Translational Modification, SUMOylation, and Cancer (Review). Int. J. Oncol. 2018, 52, 1081–1094. [Google Scholar] [CrossRef]
- Tomasi, M.L.; Ramani, K.; Ryoo, M.; Cossu, C.; Floris, A.; Murray, B.J.; Iglesias-Ara, A.; Spissu, Y.; Mavila, N. SUMOylation regulates cytochrome P450 2E1 expression and activity in alcoholic liver disease. FASEB J. 2018, 32, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Annette, A.; Sailer, C.; Ryu, S.; Catone, N.; Stankovic-Valentin, N.; Schmidtke, G.; Melchior, F.; Stengel, F.; Groettrup, M. The ubiquitin-like modifier FAT10 interferes with SUMO activation. Nat. Commun. 2019, 10, 1–17. [Google Scholar]
- Ganesan, M.; Hindman, J.; Tillman, B.; Jaramillo, L.; Poluektova, L.I.; French, B.A.; Kharbanda, K.K.; French, S.W.; Osna, N.A. FAT10 suppression stabilizes oxidized proteins in liver cells: Effects of HCV and ethanol. Exp. Molec. Pathol. 2015, 99, 506–516. [Google Scholar] [CrossRef]
- French, B.A.; Oliva, J.; Bardag-Gorce, F.; French, S.W. The immunoproteasome in steatohepatitis: Its role in Mallory-Denk body formation. Exp. Mol. Pathol. 2011, 90, 252–256. [Google Scholar] [CrossRef]
- Gao, Y.; Theng, S.S.; Zhuo, J.; Teo, W.B.; Ren, J.; Lee, C.G. FAT10, an Ubiquitin-like Protein, Confers Malignant Properties in Non-tumorigenic and Tumorigenic Cells. Carcinogenesis 2013, 35, 923–934. [Google Scholar] [CrossRef]
- Qing, X.; French, B.A.; Oliva, J.; French, S.W. Increased expression of FAT10 in colon benign, premalignant and malignant epithelial neoplasms. Exp. Mol. Pathol. 2011, 90, 51–54. [Google Scholar] [CrossRef]
- Yan, D.W.; Li, D.W.; Yang, Y.X.; Xia, J.; Wang, X.L.; Zhou, C.Z.; Fan, J.W.; Wen, Y.G.; Sun, H.C.; Wang, Q.; et al. Ubiquitin D is correlated with colon cancer progression and predicts recurrence for stage II-III disease after curative surgery. Br. J. Cancer 2010, 103, 961–969. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Li, J.; Tillman, B.; Morgan, T.R.; French, B.A.; French, S.W. TLR3/4 signaling is mediated via the NFκB-CXCR4/7 pathway in human alcoholic hepatitis and non-alcoholic steatohepatitis which formed Mallory-Denk bodies. Exp. Mol. Pathol. 2014, 97, 234–240. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Tillman, B.; French, B.A.; French, S.W. Ufmylation and FATylation pathways are downregulated in human alcoholic and nonalcoholic steatohepatitis, and mice fed DDC, where Mallory-Denk bodies (MDBs) form. Exp. Mol. Pathol. 2014, 97, 81–88. [Google Scholar] [CrossRef]
- Theng, S.S.; Wang, W.; Mah, W.C.; Chan, C.; Zhuo, J.; Gao, Y.; Qin, H.; Lim, L.; Chong, S.S.; Song, J.; et al. Disruption of FAT10-MAD2 binding inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2014, 111, E5282–E5291. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, G.; Aichem, A.; Groettrup, M. FAT10ylation as a signal for proteasomal degradation. Biochim. Biophys. Acta 2014, 1843, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Thrower, J.S.; Hoffman, L.; Rechsteiner, M.; Pickart, C.M. Recognition of thepolyubiquitin proteolytic signal. EMBO J. 2000, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, S.; Bercovich, B.; Ciechanover, A. FAT10 is a proteasomal degradation signal that is itself regulated by ubiquitination. Mol. Biol. Cell 2012, 23, 225–232. [Google Scholar] [CrossRef]
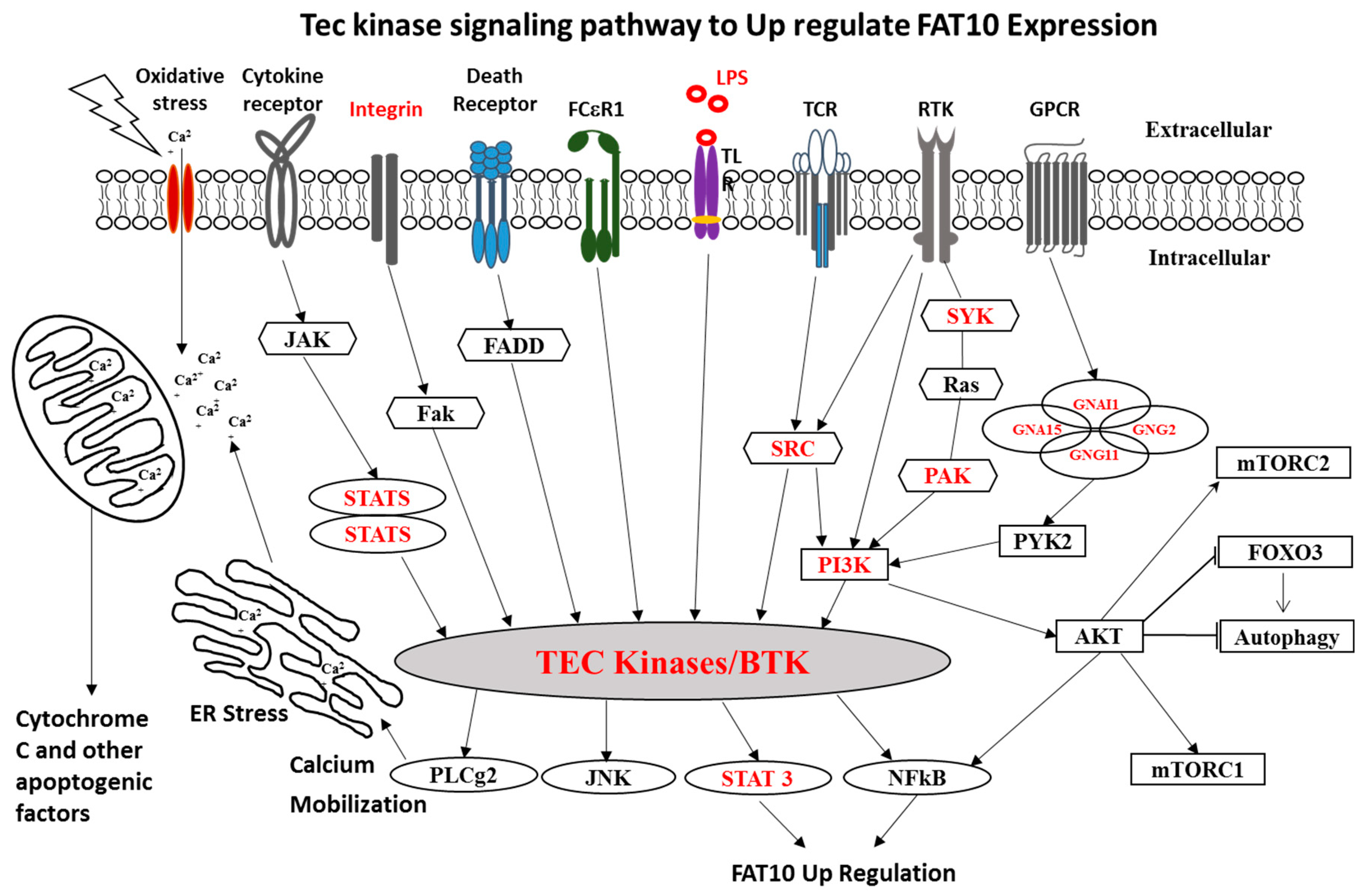
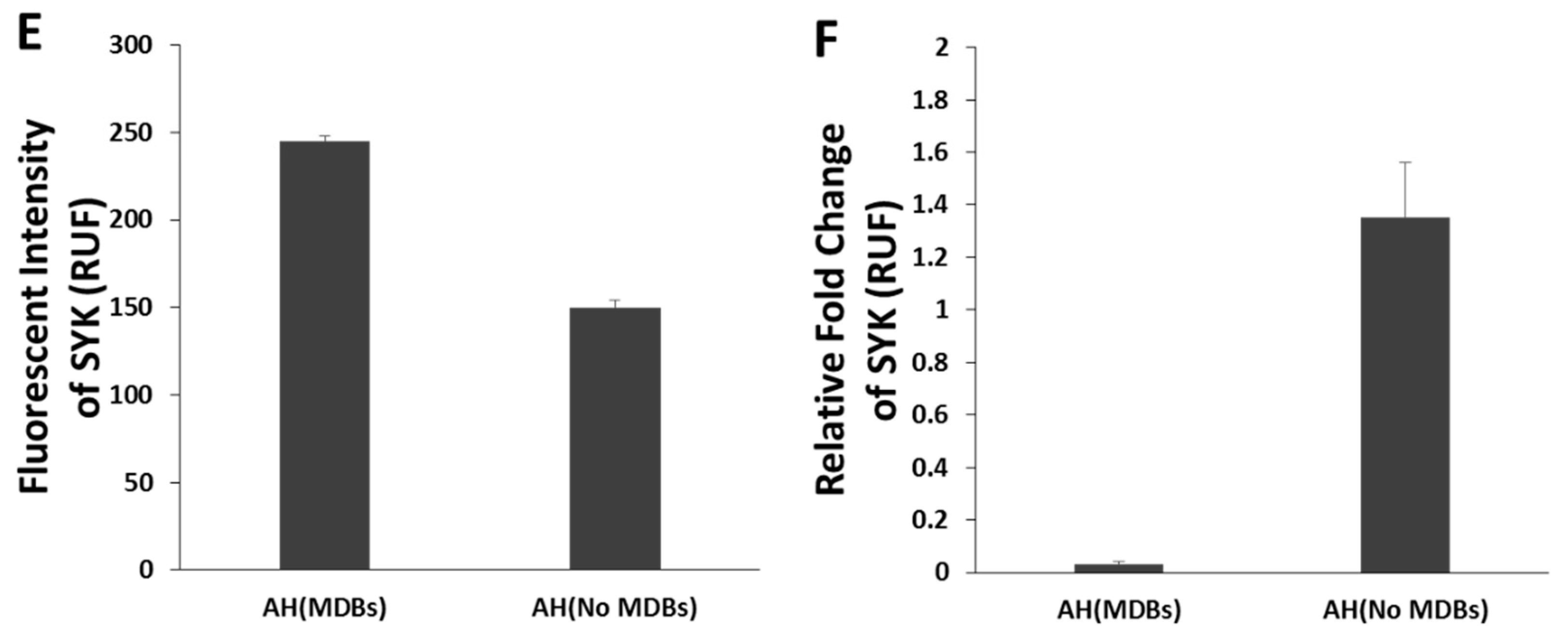
- Afifiyan, N.; Tillman, B.; French, B.A.; Sweeny, O.; Masouminia, M.; Samadzadeh, S.; French, S.W. The role of Tec Kinase signalling pathways in the development of Mallory-Denk bodies in balloon cells in alcoholic hepatitis. Exp. Mol. Pathol. 2017, 103, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Afifiyan, N.; Tillman, B.; French, B.A.; Masouminia, M.; Samadzadeh, S.; French, S.W. Over expression of proteins that alter the intracellular signaling pathways in the cytoplasm of the liver cells forming Mallory-Denk bodies. Exp. Mol. Pathol. 2017, 102, 106–114. [Google Scholar] [CrossRef][Green Version]
- Qu, C.; Zheng, D.; Li, S.; Lidofsky, A.; Holmes, J.A.; Chen, J.; He, L.; Wei, L.; Liao, Y.; Yuan, H.; et al. Tyrosine kinase SYK is a potential therapeutic target for liver fibrosis. Hepatology 2018, 68, 1125–1139. [Google Scholar] [CrossRef]
- Gong, P.; Canaan, A.; Wang, B.; Leventhal, J.; Snyder, A.; Nair, V.; Cohen, C.D.; Kretzler, M.; D’Agati, V.; Weissman, S.; et al. The Ubiquitin-Like Protein FAT10 Mediates NF-κB Activation. J. Am. Soc. Nephrol. 2010, 21, 316–326. [Google Scholar] [CrossRef]
- Kandel-Kfir, M.; Garcia-Milan, R.; Gueta, I.; Lubitz, I.; Ben-Zvi, I.; Shaish, A.; Shir, L.; Harats, D.; Mahajan, M.; Canaan, A.; et al. IFNγ potentiates TNFα/TNFR1 signaling to induce FAT10 expression in macrophages. Mol. Immunol. 2020, 117, 101–109. [Google Scholar] [CrossRef]
- Allon, C.; Xiaofeng, Y.; Carmen, J.B.; Jin, L.; Isaac, L.; Serwa, L.G.; Katrina, C.; Naohiko, K.; Yasuhiro, N.; Yuan-Ching, L.; et al. FAT10/diubiquitin-like Protein-Deficient Mice Exhibit Minimal Phenotypic Differences. Mol. Cell Biol. 2006, 26, 5180–5189. [Google Scholar] [CrossRef]
- Polachi, N.; Bai, G.; Li, T.; Chu, Y.; Wang, X.; Li, S.; Gu, N.; Wu, J.; Li, W.; Zhang, Y.; et al. Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer-A comprehensive review. Eur. J. Med. Chem. 2016, 123, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Theng, S.S.; Mah, W.C.; Lee, C.G. Silibinin down-regulates FAT10 and modulate TNFα/IFNγ-induced chromosomal instability and apoptosis sensitivity. Co. Biol. 2015, 1–9. [Google Scholar] [CrossRef]

| # | Fat Macro | Fat Micro | PMN | Lymp | Other Findings | Fibrosis Stage | Duct Metaplasia | MDBs |
|---|---|---|---|---|---|---|---|---|
| 01-003 | 2+ | 1+ | 0 | 4+ | 0 | 4+ | positive | 2+ |
| 01-011 | 1+ | 0 | 0 | 0 | 0 | 4+ | positive | 1+ |
| 01-015 | 3+ | 1+ | 1+ | 1+ | 0 | 4+ | positive | 3+ |
| 01-016 | 4+ | 0 | 3+ | 1+ | bile thrombi, EM | 4+ | positive | 4+ |
| 01-017 | 2+ | 0 | 2+ | 4+ | PMN satellitosis | 4+ | positive | 4+ |
| 01-019 | 4+ | 0 | 0 | 1+ | 0 | 4+ | positive | 3+ |
| 01-021 | 0 | 0 | 1+ | 4+ | EM apoptosis | 4+ | positive | 1+ |
| 03-001 | 1+ | 0 | 3+ | 4+ | bile thrombi, EM | 4+ | positive | 4+ |
| 03-005 | 2+ | 0 | 0 | 4+ | EM | 4+ | positive | 1+ |
| 03-006 | 1+ | 0 | 4+ | 1+ | satellitosis, EM | 4+ | positive | 4+ |
| 03-007 | 1+ | 0 | 0 | 2+ | 0 | 4+ | positive | 1+ |
| 03-012 | 2+ | 0 | 3+ | 3+ | best satellitosis, EM | 4+ | positive | 4+ |
| 03-014 | 2+ | 0 | 2+ | 0 | most MDBs | 4+ | positive | 4+ |
| 03-015 | 4+ | 0 | 1+ | 0 | 0 | 4+ | positive | 2+ |
| 03-017 | 3+ | 0 | 2+ | 3+ | EM | 3+ | positive | 1+ |
| 03-018 | 4+ | 0 | 4+ | 2+ | satellitosis | 4+ | positive | 3+ |
| 03-019 | 3+ | 0 | 3+ | 0 | satellitosis, EM | 4+ | positive | 4+ |
| 03-020 | 1+ | 0 | 3+ | 0 | satellitosis | 4+ | positive | 1+ |
| 03-022 | 3+ | 1+ | 0 | 2+ | autophagy, EM | 4+ | positive | 3+ |
| 03-023 | 1+ | 0 | 4+ satellitosis | 1+ | autophagy of MDBs, EM | 4+ | positive | 4+ |
| 03-024 | 4+ | 1+ | 3+ | 1+ | PMN lymphocytes, EM | 4+ | positive | 4+ |
| 03-025 | 4+ | 1+ | 1+ | 1+ | autophagy, EM | 3+ | positive | 3+ |
| 03-027 | 1+ | 0 | 4+ | 4+ | PMN satellitosis | 4+ | positive | 4+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Ji, P.; French, S.W. The Role of FAT10 in Alcoholic Hepatitis Pathogenesis. Biomedicines 2020, 8, 189. https://doi.org/10.3390/biomedicines8070189
Jia Y, Ji P, French SW. The Role of FAT10 in Alcoholic Hepatitis Pathogenesis. Biomedicines. 2020; 8(7):189. https://doi.org/10.3390/biomedicines8070189
Chicago/Turabian StyleJia, Yue, Ping Ji, and Samuel W. French. 2020. "The Role of FAT10 in Alcoholic Hepatitis Pathogenesis" Biomedicines 8, no. 7: 189. https://doi.org/10.3390/biomedicines8070189
APA StyleJia, Y., Ji, P., & French, S. W. (2020). The Role of FAT10 in Alcoholic Hepatitis Pathogenesis. Biomedicines, 8(7), 189. https://doi.org/10.3390/biomedicines8070189