Caterpillar Venom: A Health Hazard of the 21st Century
Abstract
1. Introduction
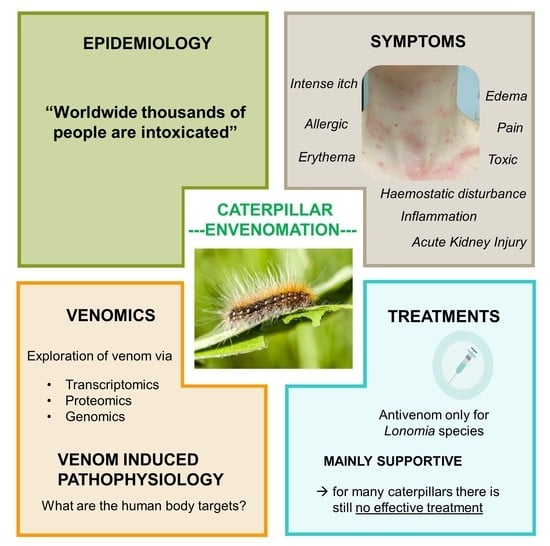
2. Evolving Global Epidemiology
3. Mechanism of Action
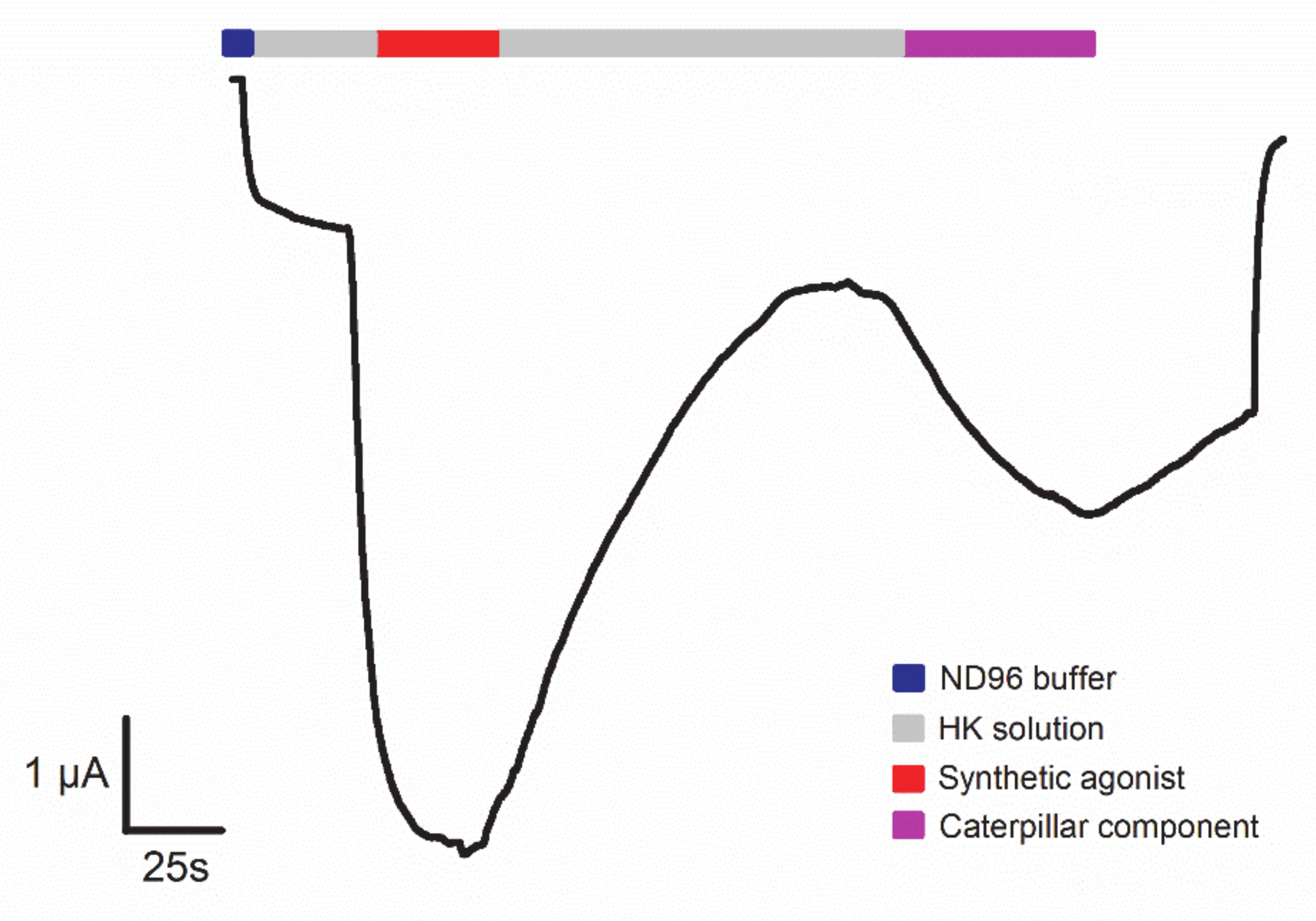
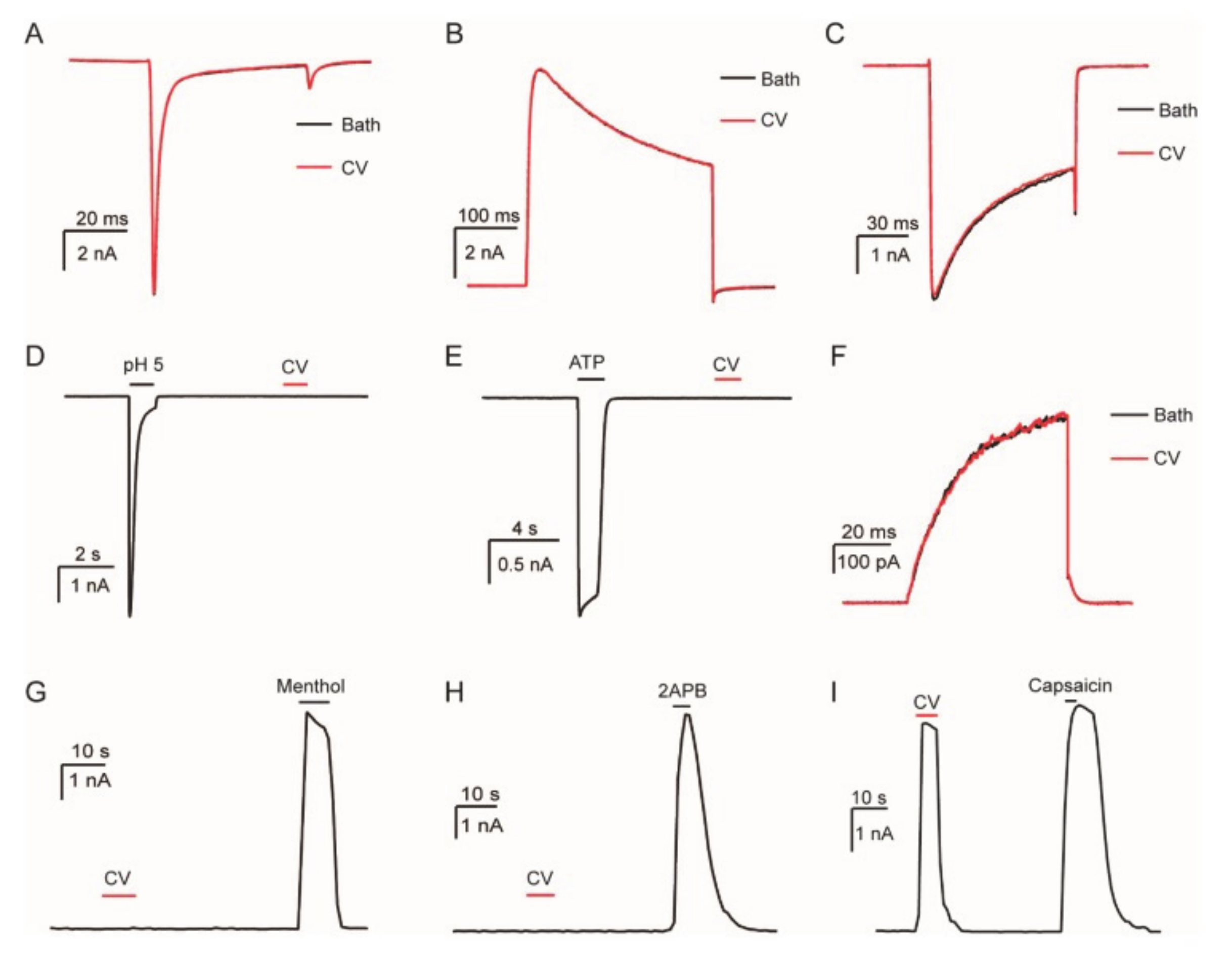
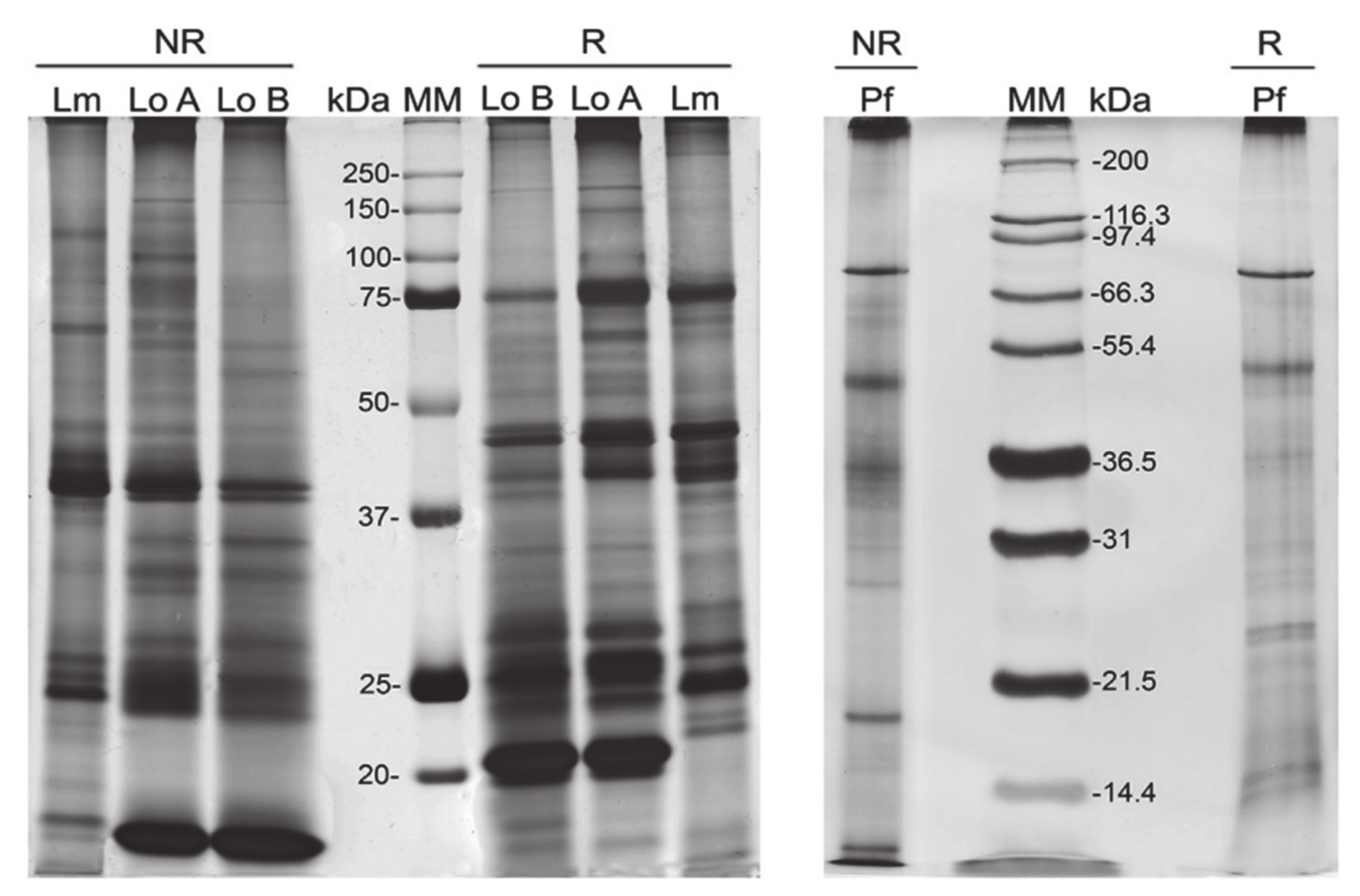
4. Technologies to Analyze Caterpillar Venom Composition and Function
5. Venom Induced Pathophysiology and Diagnosis
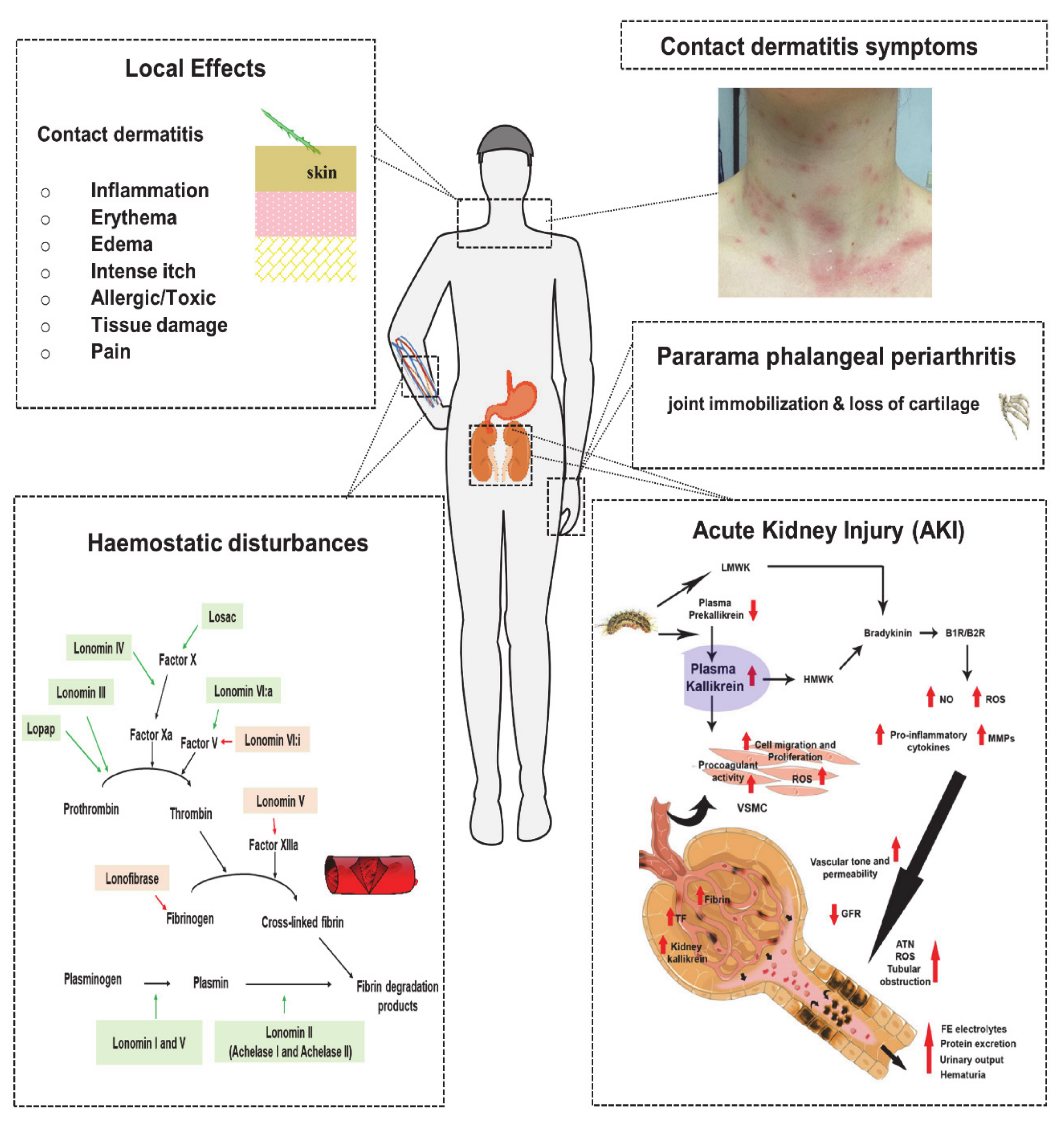
5.1. Local Effects
5.1.1. Contact Dermatitis
5.1.1.1. Allergic/Toxic Reaction
5.1.1.2. Edema and Erythema
5.1.1.3. Local Tissue Damage
5.1.1.4. Pain
5.2. Systemic Effect
5.2.1. Haemostatic Disturbances
5.2.2. Acute Kidney Injury
5.2.3. Pararama-Associated Phalangeal Periarthritis
6. Clinical Time Course
6.1. Immediate Effects
6.2. Short-Term Effects
6.3. Long-Term Effects
7. Treatments and Limitations
7.1. Antivenom for Management of Systemic Effects
7.2. Supportive Treatment for Management of Local Effects
8. Future Perspectives
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitter, C.; Davis, N.R.; Cummings, M.P. Phylogeny and Evolution of Lepidoptera. Annu. Rev. Èntomol. 2017, 62, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.P.; Scoble, M.J.; Karsholt, O. Lepidoptera phylogeny and systematics: The state of inventorying moth and butterfly diversity. Zootaxa 2007, 1668, 699–747. [Google Scholar] [CrossRef]
- Diaz, J.H. The Evolving Global Epidemiology, Syndromic Classification, Management, And Prevention of Caterpillar Envenoming. Am. J. Trop. Med. Hyg. 2005, 72, 347–57. [Google Scholar] [CrossRef] [PubMed]
- Boas, I.V.; Bonfá, G.; Tambourgi, D.V. Venomous caterpillars: From inoculation apparatus to venom composition and envenomation. Toxicon 2018, 153, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Flores, M.; Zannin, M.; Chudzinski-Tavassi, A. New Insight into the Mechanism of Lonomia obliqua Envenoming: Toxin Involvement and Molecular Approach. Pathophysiol. Haemost. Thromb. 2009, 37, 1–16. [Google Scholar] [CrossRef]
- Moraes, J.A.; Rodrigues, G.; Nascimento-Silva, V.; Renovato-Martins, M.; Berger, M.; Guimarães, J.A.; Barja-Fidalgo, C. Effects of Lonomia obliqua Venom on Vascular Smooth Muscle Cells: Contribution of NADPH Oxidase-Derived Reactive Oxygen Species. Toxins 2017, 9, 360. [Google Scholar] [CrossRef]
- Fagrell, B.; Jörneskog, G.; Larsson, S.; Holm, G.; Salomonsson, A.-C. Skin reactions induced by experimental exposure to setae from larvae of the northern pine processionary moth (Thaumetopoea pinivora). Contact Dermat. 2008, 59, 290–295. [Google Scholar] [CrossRef]
- Carrijo-Carvalho, L.C.; Chudzinski-Tavassi, A.M. The venom of the Lonomia caterpillar: An overview. Toxicon 2007, 49, 741–757. [Google Scholar] [CrossRef]
- Pentzold, S.; Zagrobelny, M.; Khakimov, B.; Engelsen, S.B.; Clausen, H.; Petersen, B.L.; Borch, J.; Møller, B.L.; Bak, S. Lepidopteran defence droplets—A composite physical and chemical weapon against potential predators. Sci. Rep. 2016, 6, 22407. [Google Scholar] [CrossRef]
- Mayence, C.; Mathien, C.; Sanna, A.; Houcke, S.; Tabard, P.; Roux, A.; Valentin, C.; Resiere, D.; Lemonnier, D.; Cho, F.N.; et al. Lonomia caterpillar envenoming in French Guiana reversed by the Brazilian antivenom: A successful case of international cooperation for a rare but deadly tropical hazard. Toxicon 2018, 151, 74–78. [Google Scholar] [CrossRef]
- Walker, A.A.; Robinson, S.D.; Yeates, D.K.; Jin, J.; Baumann, K.; Dobson, J.S.; Fry, B.G.; King, G.F. Entomo-venomics: The evolution, biology and biochemistry of insect venoms. Toxicon 2018, 154, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kuspis, D.A.; Rawlins, J.; Krenzelok, E.P. Human exposures to stinging caterpillar: Lophocampa caryae exposures. Am. J. Emerg. Med. 2001, 19, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Heinen, T.E.; Da Veiga, A.B.G. Arthropod venoms and cancer. Toxicon 2011, 57, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Favalesso, M.M.; Lorini, L.M.; Peichoto, M.; Guimarães, A.T.B. Potential distribution and ecological conditions of Lonomia obliqua Walker 1855 (Saturniidae: Hemileucinae) in Brazil. Acta Trop. 2019, 192, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Burdmann, E.; Jha, V. Acute kidney injury due to tropical infectious diseases and animal venoms: A tale of 2 continents. Kidney Int. 2017, 91, 1033–1046. [Google Scholar] [CrossRef]
- S. Brazil Ministry of Health. Accidents by Venomous Animals: What to Do and How to Avoid. Available online: https://saude.gov.br/saude-de-a-z/acidentes-por-animais-peconhentos (accessed on 7 April 2020).
- Ciminera, M.; Auger-Rozenberg, M.-A.; Caron, H.; Herrera, M.; Scotti-Saintagne, C.; Scotti, I.; Tysklind, N.; Roques, A. Genetic Variation and Differentiation of Hylesia metabus (Lepidoptera: Saturniidae): Moths of Public Health Importance in French Guiana and in Venezuela. J. Med. Èntomol. 2018, 56, 137–148. [Google Scholar] [CrossRef]
- Quintana, M.; Sciani, J.M.; Auada, A.V.V.; Martínez, M.M.; Sánchez, M.N.; Santoro, M.L.; Fan, H.W.; Peichoto, M. Stinging caterpillars from the genera Podalia, Leucanella and Lonomia in Misiones, Argentina: A preliminary comparative approach to understand their toxicity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 202, 55–62. [Google Scholar] [CrossRef]
- McMillan, C.W.; Purcell, W.R. The Puss Caterpillar, Alias Woolly Slug. N. Engl. J. Med. 1964, 271, 147–149. [Google Scholar] [CrossRef]
- Forrester, M.B. Megalopyge opercularis Caterpillar Stings Reported to Texas Poison Centers. Wilderness Environ. Med. 2018, 29, 215–220. [Google Scholar] [CrossRef]
- Battisti, A.; Larsson, S.; Roques, A. Processionary Moths and Associated Urtication Risk: Global Change–Driven Effects. Annu. Rev. Èntomol. 2017, 62, 323–342. [Google Scholar] [CrossRef]
- Berardi, L.; Pivato, M.; Arrigoni, G.; Mitali, E.; Trentin, A.R.; Olivieri, M.; Kerdelhué, C.; Dorkeld, F.; Nidelet, S.; Dubois, E.; et al. Proteome Analysis of Urticating Setae From Thaumetopoea pityocampa (Lepidoptera: Notodontidae). J. Med. Èntomol. 2017, 54, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Mindlin, M.J.; Waroux, O.L.P.D.; Case, S.; Walsh, B. The arrival of oak processionary moth, a novel cause of itchy dermatitis, in the UK: Experience, lessons and recommendations. Public Health 2012, 126, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Boas, I.V.; Gonçalves-De-Andrade, R.M.; Pidde-Queiroz, G.; Assaf, S.L.M.R.; Portaro, F.C.V.; Sant’Anna, O.A.; Berg, C.W.V.D.; Tambourgi, D.V. Premolis semirufa (Walker, 1856) Envenomation, Disease Affecting Rubber Tappers of the Amazon: Searching for Caterpillar-Bristles Toxic Components. PLoS Negl. Trop. Dis. 2012, 6, e1531. [Google Scholar] [CrossRef]
- E Perkins, L.; Cribb, B.W.; Pagendam, E.D.; Zalucki, M.P. Variation in Morphology and Airborne Dispersal of the Urticating Apparatus of Ochrogaster lunifer (Lepidoptera: Notodontidae), an Australian Processionary Caterpillar, and Implications for Livestock and Humans. J. Insect Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Vega, J.; Moneo, I. Skin Reactions on Exposure to the Pine Processionary Caterpillar (Thaumetopoea pityocampa). Actas Dermo-Sifiliográficas (English Edition) 2011, 102, 658–667. [Google Scholar] [CrossRef]
- Fuentes, V.; Zapatero, R.L.; Martínez, M.M.; Alonso, L.E.; Beitia, Z.B.; Mazuecos, J.M.B. Allergy to the pine processionary caterpillar (Thaumetopoea pityocampa). Allergol. Immunopathol. 1999, 29, 1418–1423. [Google Scholar]
- Laustsen, A.H.; Gutiérrez, J.M.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef]
- Greeney, H.F.; Dyer, L.A.; Smilanich, A.M. Feeding by lepidopteran larvae is dangerous: A review of caterpillars’ chemical, physiological, morphological, and behavioral defenses against natural enemies. Invertebr. Surviv. J. 2012, 9, 7–34. [Google Scholar]
- Cabrera, G.; Salazar, V.; Montesino, R.; Tambara, Y.; Struwe, W.B.; Leon, E.; Harvey, D.J.; Lesur, A.; Rincon, M.; Domon, B.; et al. Structural characterization and biological implications of sulfated N-glycans in a serine protease from the neotropical moth Hylesia metabus (Cramer 1775) (Lepidoptera: Saturniidae). Glycobiology 2015, 26, cwv096. [Google Scholar] [CrossRef]
- Jourdain, F.; Girod, R.; Vassal, J.; Chandre, F.; Lagneau, C.; Fouque, F.; Guiral, D.; Raude, J.; Robert, V. The moth Hylesia metabus and French Guiana lepidopterism: Centenary of a public health concern. Parasite 2012, 19, 117–128. [Google Scholar] [CrossRef]
- Paniz-Mondolfi, A.E.; Pérez-Alvarez, A.M.; Lundberg, U.; Fornés, L.; Reyes-Jaimes, O.; Hernández-Pérez, M.; Hossler, E. Tropical medicine rounds Cutaneous lepidopterism : Dermatitis from contact with moths of Hylesia metabus (Cramer 1775) (Lepidoptera : Saturniidae), the causative agent of caripito itch. Int. J. Dermatol. 2011, 50, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Sano-Martins, I.S.; Duarte, A.C.; Guerrero, B.; Moraes, R.H.P.; Barros, E.J.G.; Arocha-Piñango, C.L. Hemostatic disorders induced by skin contact with Lonomia obliqua (Lepidoptera, Saturniidae) caterpillars. Revista do Instituto de Medicina Tropical de São Paulo 2017, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boas, I.V.; Gonçalves-De-Andrade, R.M.; Squaiella-Baptistão, C.C.; Sant’Anna, O.A.; Tambourgi, D.V. Characterization of Phenotypes of Immune Cells and Cytokines Associated with Chronic Exposure to Premolis semirufa Caterpillar Bristles Extract. PLoS ONE 2013, 8, e71938. [Google Scholar] [CrossRef]
- Bastos, L.D.C.; Da Veiga, A.B.G.; Guimaraes, J.A.; Tonussi, C.R. Nociceptive and edematogenic responses elicited by a crude bristle extract of Lonomia obliqua caterpillars. Toxicon 2004, 43, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.I.D.C.; Da Silveira, R.B.; Nader, H.B.; Dietrich, C.P.; Gremski, W.; Veiga, S. Identification and partial characterisation of hyaluronidases in Lonomia obliqua venom. Toxicon 2005, 45, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.C.F.; Wiezel, G.A.; Amorim, F.G.; Arantes, E.C. Arthropod venom Hyaluronidases: Biochemical properties and potential applications in medicine and biotechnology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Bala, E.; Hazarika, R.; Singh, P.; Yasir, M.; Shrivastava, R. A biological overview of Hyaluronidase: A venom enzyme and its inhibition with plants materials. Mater. Today Proc. 2018, 5, 6406–6412. [Google Scholar] [CrossRef]
- Boas, I.V.; Pidde-Queiroz, G.; Magnoli, F.C.; Gonçalves-De-Andrade, R.M.; Berg, C.W.V.D.; Tambourgi, D.V. A Serine Protease Isolated from the Bristles of the Amazonic Caterpillar, Premolis semirufa, Is a Potent Complement System Activator. PLoS ONE 2015, 10, e0118615. [Google Scholar] [CrossRef]
- Berardi, L.; Battisti, A.; Negrisolo, E. The allergenic protein Tha p 2 of processionary moths of the genus Thaumetopoea (Thaumetopoeinae, Notodontidae, Lepidoptera): Characterization and evolution. Gene 2015, 574, 317–324. [Google Scholar] [CrossRef]
- Sánchez, M.N.; Sciani, J.M.; Quintana, M.A.; Martínez, M.M.; Tavares, F.L.; Gritti, M.A.; Fan, H.W.; Teibler, G.P.; Peichoto, M. Understanding toxicological implications of accidents with caterpillars Megalopyge lanata and Podalia orsilochus (Lepidoptera: Megalopygidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 216, 110–119. [Google Scholar] [CrossRef]
- Lamdin, J.; Howell, D.; Kocan, K.; Murphey, D.; Arnold, D.; Fenton, A.; Odell, G.; Ownby, C. The venomous hair structure, venom and life cycle of Lagoa crispata, a puss caterpillar of Oklahoma. Toxicon 2000, 38, 1163–1189. [Google Scholar] [CrossRef]
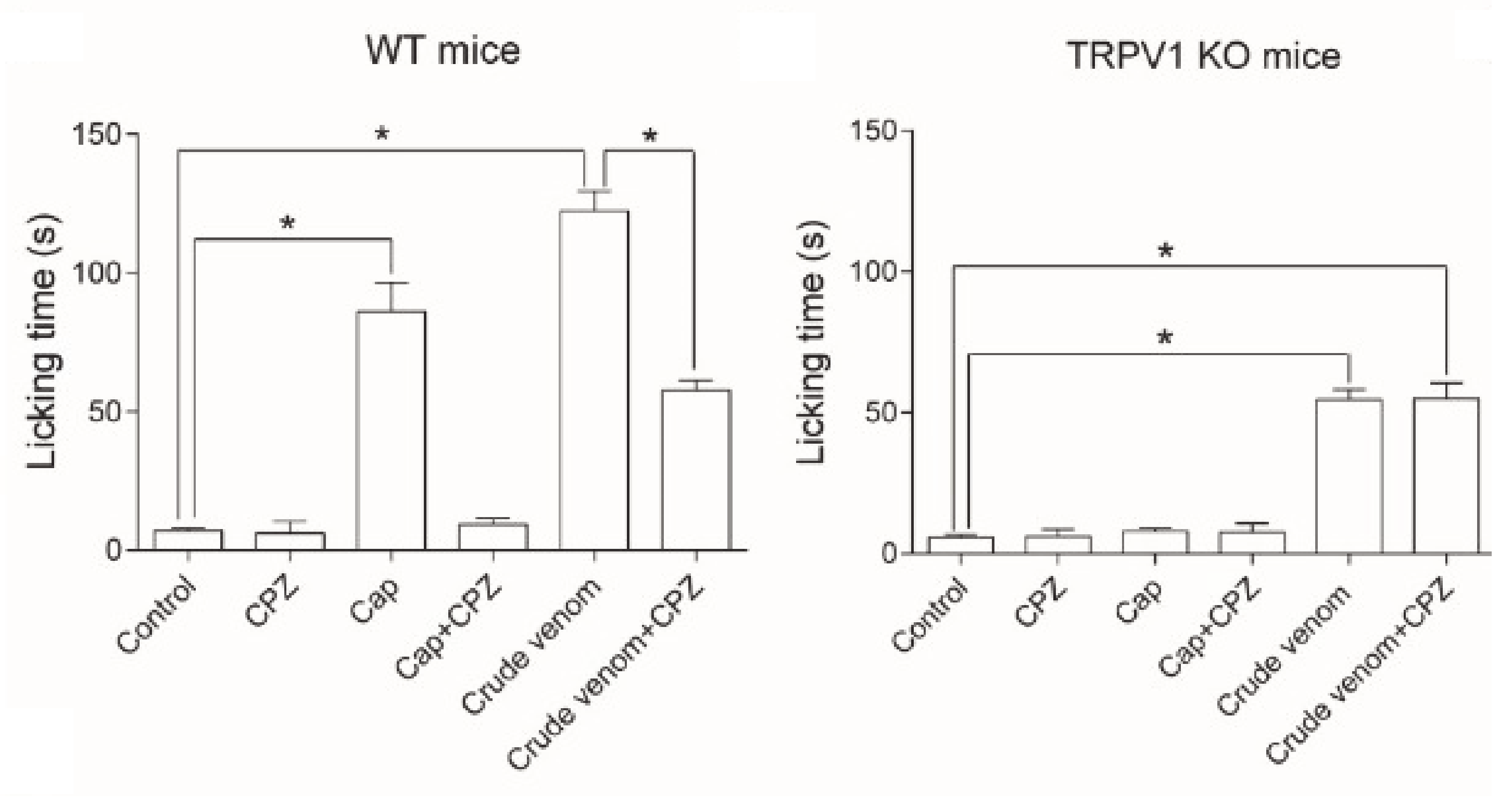
- Yao, Z.; Kamau, P.; Han, Y.; Hu, J.; Luo, A.; Luo, L.; Zheng, J.; Tian, Y.; Lai, R. The Latoia consocia Caterpillar Induces Pain by Targeting Nociceptive Ion Channel TRPV1. Toxins 2019, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Holm, G.; Fagrell, B.; Larsson, S. Urticating Hairs in Arthropods: Their Nature and Medical Significance. Annu. Rev. Èntomol. 2011, 56, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Da Veiga, A.B.G.; Blochtein, B.; Guimaraes, J.A. Structures involved in production, secretion and injection of the venom produced by the caterpillar Lonomia obliqua (Lepidoptera, Saturniidae). Toxicon 2001, 39, 1343–1351. [Google Scholar] [CrossRef]
- Moneo, I.; Vega, J.; Caballero, M.L.; Vega, J.; Alday, E. Isolation and characterization of Tha p 1, a major allergen from the pine processionary caterpillar Thaumetopoea pityocampa. Allergy 2003, 58, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Marisa, A.; Paola, M.; Carrijo-Carvalho, L.C.; Esther, M. Toxins from Lonomia obliqua—Recombinant Production and Molecular Approach. In An Integrated View of the Molecular Recognition and Toxinology—From Analytical Procedures to Biomedical Applications; IntechOpen: London, UK, 2013. [Google Scholar]
- Rodríguez-Mahillo, A.I.; Gonzalez-Muñoz, M.; Vega, J.M.; Lopez, J.A.; Yart, A.; Kerdelhué, C.; Camafeita, E.; Ortiz, J.C.G.; Vogel, H.; Toffolo, E.P.; et al. Setae from the pine processionary moth (Thaumetopoea pityocampa) contain several relevant allergens. Contact Dermat. 2012, 67, 367–374. [Google Scholar] [CrossRef]
- Triant, D.A.; Cinel, S.D.; Kawahara, A.Y. Lepidoptera genomes: Current knowledge, gaps and future directions. Curr. Opin. Insect Sci. 2018, 25, 99–105. [Google Scholar] [CrossRef]
- Berger, M.; De Moraes, J.A.; Beys-Da-Silva, W.O.; Santi, L.; Terraciano, P.B.; Driemeier, D.; Cirne-Lima, E.O.; Passos, E.P.; Vieira, M.A.R.; Barja-Fidalgo, T.C.; et al. Renal and vascular effects of kallikrein inhibition in a model of Lonomia obliqua venom-induced acute kidney injury. PLoS Negl. Trop. Dis. 2019, 13, e0007197. [Google Scholar] [CrossRef]
- Munitz, A.; Piliponsky, A.M.; Levi-Schaffer, F. IgE-Independent Activation of Human Mast Cells Indicates their Role in the Late Phase Reaction of Allergic Inflammation. Cell Tissue Bank. 2003, 4, 25–28. [Google Scholar] [CrossRef]
- Shaik-Dasthagirisaheb, Y.; Varvara, G.; Murmura, G.; Saggini, A.; Potalivo, G.; Caraffa, A.; Antinolfi, P.; Tetè, S.; Tripodi, D.; Conti, F.; et al. Vascular Endothelial Growth Factor (VEGF), Mast Cells and Inflammation. Int. J. Immunopathol. Pharmacol. 2013, 26, 327–335. [Google Scholar] [CrossRef]
- Galicia-Curiel, M.F.; Quintanar, J.L.; Jiménez, M.; Salinas, E. Mast cells respond to urticating extract from lepidoptera larva Morpheis ehrenbergii in the rat. Toxicon 2014, 77, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, E.; Yanagihara, A.; Karlsson, E.; Tytgat, J. Jellyfish and other cnidarian envenomations cause pain by affecting TRPV1 channels. FEBS Lett. 2006, 580, 5728–5732. [Google Scholar] [CrossRef] [PubMed]
- Lamy, M.; Vincendeau, P.; Ducombs, G.; Pastureaud, M.H. Irritating substance extracted from the Thaumetopoea pityocampa caterpillar; mechanism of action. Cell. Mol. Life Sci. 1983, 39, 299. [Google Scholar] [CrossRef] [PubMed]
- Gottschling, S.; Meyer, S.; Dill-Mueller, D.; Wurm, D.; Gortner, L. Outbreak Report of Airborne Caterpillar Dermatitis in a Kindergarten. Dermatology 2007, 215, 5–9. [Google Scholar] [CrossRef]
- Faninger, A. Dermatitis erucarum (Euproctis chrysorrhea). Med. Glas. 1972, 26. [Google Scholar]
- Lamy, M.; Pastureaud, M.-H.; Novak, F.; Ducombs, G.; Vincedeau, P.; Maleville, J.; Texier, L. Thaumetopoein: An urticating protein from the hairs and integument of the pine processionary caterpillar (Thaumetopoea pityocampa schiff., Lepidoptera, Thaumetopoeidae). Toxicon 1986, 24, 347–356. [Google Scholar] [CrossRef]
- Kalender, Y.; Kalender, S.; Uzunhisarcikli, M.; Ogutcu, A.; Açikgoz, F. Effects of Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) larvae on the degranulation of dermal mast cells in mice; an electron microscopic study. Folia Boil. 2004, 52, 13–17. [Google Scholar]
- Werno, J.; Lamy, M.; Vincendeau, P. Caterpillar hairs as allergens. Lancet 1993, 342, 936–937. [Google Scholar] [CrossRef]
- Lamy, M. Contact dermatitis (erucism) produced by processionary caterpillars (Genus Thaumetopoea). J. Appl. Èntomol. 1990, 110, 425–437. [Google Scholar] [CrossRef]
- S.n. WHO/IUIS Allergen Nomenclature Sub-Committee. “Allergen Tha p 1,” Allergen Nomenclature. 2006. Available online: http://www.allergen.org/viewallergen.php?aid=614 (accessed on 18 April 2020).
- Gschloessl, B.; Dorkeld, F.; Berges, H.; Beydon, G.; Bouchez, O.; Branco, M.; Bretaudeau, A.; Burban, C.; Dubois, E.; Gauthier, P.; et al. Draft genome and reference transcriptomic resources for the urticating pine defoliator Thaumetopoea pityocampa (Lepidoptera: Notodontidae). Mol. Ecol. Resour. 2018, 18, 602–619. [Google Scholar] [CrossRef]
- Ziprkowski, L.; Rolant, F.; Ziprkowski, F.R.L. Study of the Toxin from Poison Hairs of Thaumetopoea Wilkinsoni Caterpillars. J. Investig. Dermatol. 1972, 58, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, C.; Junior, J.R.; Fernandes, D.; Sordi, R.; Guimaraes, J.A.; Assreuy, J.; Termignoni, C. Kallikrein–kinin system activation by Lonomia obliqua caterpillar bristles: Involvement in edema and hypotension responses to envenomation. Toxicon 2007, 49, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Medeiros, R.; Fernandes, E.S.; Ferreira, J.; Cabrini, A.D.; Campos, M.M. Kinin B1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br. J. Pharmacol. 2004, 143, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, G.; Lundberg, U.; Rodríguez, L.A.E.; Herrera, M.; Machado, W.; Portela, M.; Palomares, S.; Espinosa, L.A.; Ramos, Y.; Durán, R.; et al. Protein content of the Hylesia metabus egg nest setae (Cramer 1775) (Lepidoptera: Saturniidae) and its association with the parental investment for the reproductive success and lepidopterism. J. Proteom. 2017, 150, 183–200. [Google Scholar] [CrossRef]
- Clémençon, B.; Kuhn-Nentwig, L.; Langenegger, N.; Kopp, L.; Peigneur, S.; Tytgat, J.; Nentwig, W.; Lüscher, B.P. Neurotoxin Merging: A Strategy Deployed by the Venom of the Spider Cupiennius salei to Potentiate Toxicity on Insects. Toxins 2020, 12, 250. [Google Scholar] [CrossRef]
- Berger, M.; Beys-da-Silva, W.O.; Santi, L.; de Oliveira, I.M.; Jorge, P.M.; Henriques, J.A.P.; Driemeier, D.; Vieira, M.A.R.; Guimarães, J.A. Acute Lonomia obliqua caterpillar envenomation-induced physiopathological alterations in rats: Evidence of new toxic venom activities and the efficacy of serum therapy to counteract systemic tissue damage. Toxicon 2013, 74, 179–192. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef]
- Maíra, M.P.B.; Carla, F.B.-F.; Camila, C.P.; Taís, F.G.; Marcus, T.S.; Eduardo, M.D.C.; Stephen, H.; Fábio, B. Management of severe pain after dermal contact with caterpillars (erucism): A prospective case series. Clin. Toxicol. 2019, 57, 338–342. [Google Scholar]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–84. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical Pharmacology of Indomethacin in Preterm Infants: Implications in Patent Ductus Arteriosus Closure. Pediatr. Drugs 2013, 15, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Seibert, C.S.; Tanaka-Azevedo, A.M.; Santoro, M.L.; Mackessy, S.P.; Torquato, R.J.S.; Lebrun, I.; Tanaka, A.S.; Sano-Martins, I.S. Purification of a phospholipase A2 from Lonomia obliqua caterpillar bristle extract. Biochem. Biophys. Res. Commun. 2016, 342, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Priel, A.; Zhou, S.; King, D.; Siemens, J.; Julius, D. A Bivalent Tarantula Toxin Activates the Capsaicin Receptor, TRPV1, by Targeting the Outer Pore Domain. Cell 2010, 141, 834–45. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Silva, M.E.; Valente, R.H.; León, I.R.; Tambourgi, D.V.; Ramos, O.H.P.; Perales, J.; Chudzinski-Tavassi, A.M. Immunochemical and proteomic technologies as tools for unravelling toxins involved in envenoming by accidental contact with Lonomia obliqua caterpillars. Toxicon 2008, 51, 1017–1028. [Google Scholar] [CrossRef]
- Chudzinski-Tavassi, A.; Carrijo-Carvalho, L.C. Biochemical and biological properties of Lonomia obliqua bristle extract. J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 156–171. [Google Scholar] [CrossRef]
- Alvarez-Flores, M.; Furlin, D.; Ramos, O.H.P.; Balan, A.; Konno, K.; Chudzinski-Tavassi, A. Losac, the First Hemolin that Exhibits Procogulant Activity through Selective Factor X Proteolytic Activation. J. Boil. Chem. 2010, 286, 6918–6928. [Google Scholar] [CrossRef]
- Alvarez-Flores, M.; Fritzen, M.; Reis, C.V.; Chudzinski-Tavassi, A.M. Losac, a factor X activator from Lonomia obliqua bristle extract: Its role in the pathophysiological mechanisms and cell survival. Biochem. Biophys. Res. Commun. 2006, 343, 1216–1223. [Google Scholar] [CrossRef]
- Bernardi, L.; Pinto, A.F.M.; Mendes, E.; Yates, J.; Lamers, M.L. Lonomia obliqua bristle extract modulates Rac1 activation, membrane dynamics and cell adhesion properties. Toxicon 2019, 162, 32–39. [Google Scholar] [CrossRef]
- Chan, K.; Lee, A.; Onell, R.; Etches, W.; Nahirniak, S.; Bagshaw, S.M.; Larratt, L.M. Caterpillar-induced bleeding syndrome in a returning traveller. Can. Med Assoc. J. 2008, 179, 158–161. [Google Scholar] [CrossRef][Green Version]
- Rosing, J.; Govers-Riemslag, J.W.; Yukelson, L.; Tans, G. Factor V Activation and Inactivation by Venom Proteases. Pathophysiol. Haemost. Thromb. 2001, 31, 241–246. [Google Scholar] [CrossRef]
- Fernandes-Pedrosa, M.F.; Félix-Silva, J.; Menezes, Y.A. Toxins from Venomous Animals: Gene Cloning, Protein Expression and Biotechnological Applications. In An Integrated View of the Molecular Recognition and Toxinology: From Analytical Procedures to Biomedical Applications; IntechOpen: London, UK, 2012. [Google Scholar]
- Brusco, I.; Justino, A.B.; Silva, C.R.; Fischer, S.; Cunha, T.M.; Scussel, R.; Machado-de-Ávila, R.A.; Ferreira, J.; Oliveira, S.M. Kinins and their B 1 and B 2 receptors are involved in fibromyalgia-like pain symptoms in mice. Biochem. Pharmacol. 2019, 168, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Samartin, L.G.; Luchini, G.; Pidde, C.C. Squaiella-baptistão, and D. V Tambourgi, Complement System Inhibition Modulates the Pro-Inflammatory Effects of a Snake Venom Metalloproteinase. Front. Immunol. 2019, 10, 1–11. [Google Scholar]
- Haddad, V.; Cardoso, J.L.C.; Lupi, O.; Tyring, S.K. Tropical dermatology: Venomous arthropods and human skin: Part, I. Insecta. J. Am. Acad. Dermatol. 2012, 67, 331-e1. [Google Scholar] [CrossRef]
- Rahlenbeck, S.I.; Utikal, J. The oak processionary moth: A new health hazard? Br. J. Gen. Pr. 2015, 65, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Brownstein, S.; Kapasi, M.; O’Connor, M.; Blanco, P. Ophthalmia nodosa secondary to caterpillar-hair-induced conjunctivitis in a child. Can. J. Ophthalmol. 2020, 55, e56–e59. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.D.; Campos, C.M.; Gonçalves, L.R.; Sousa-E-Silva, M.C.; Higashi, H.G.; Yamagushi, I.K.; Kelen, E.M. Development of an antivenom against toxins of Lonomia obliqua caterpillars. Toxicon 1996, 34, 1045–1049. [Google Scholar] [CrossRef]
- Rocha-Campos, A.C.; Sousa-E-Silva, M.C.; Oliveira, J.E.; Da Silva, W.D.; Ribela, M.T.; Yamagushi, I.K.; Fernandes, I.; Gonçalves, L.R.; Higashi, H.G. Specific heterologous F(ab’)2 antibodies revert blood incoagulability resulting from envenoming by Lonomia obliqua caterpillars. Am. J. Trop. Med. Hyg. 2001, 64, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Sano-Martins, I.S.; González, C.; Anjos, I.V.; Diaz, J.; Gonçalves, L.R.C. Effectiveness of Lonomia antivenom in recovery from the coagulopathy induced by Lonomia orientoandensis and Lonomia casanarensis caterpillars in rats. PLoS Negl. Trop. Dis. 2018, 12, e0006721. [Google Scholar] [CrossRef]
- Gonçalves, L.R.C.; Sousa-E-Silva, M.C.C.; Tomy, S.C.; Sano-Martins, I.S. Efficacy of serum therapy on the treatment of rats experimentally envenomed by bristle extract of the caterpillar Lonomia obliqua: Comparison with epsilon-aminocaproic acid therapy. Toxicon 2007, 50, 349–356. [Google Scholar] [CrossRef]
- Junior, V.H.; Lastória, J.C. Envenomation by caterpillars (erucism): Proposal for simple pain relief treatment. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 21. [Google Scholar] [CrossRef]
- Tsai, M.K.; Yang, D.H. Caterpillar-Induced Protracted Anaphylaxis. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjI6YeH4eDpAhXqy6YKHVdNB3AQFjAAegQIARAB&url=http%3A%2F%2Faustinpublishinggroup.com%2Fallergy%2Fdownload.php%3Ffile%3Dfulltext%2Faja-v5-id1033.pdf&usg=AOvVaw393wKfvuACAlXDld1qf3HL (accessed on 30 May 2020).
- Boas, I.V.; Alvarez-Flores, M.; Chudzinski-Tavassi, A.; Tambourgi, D.V. Envenomation by Caterpillars. In Toxinology; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 429–449. [Google Scholar]


| Superfamily | Family | Species | Occurrence | Cases | Venom Effects | References | |
|---|---|---|---|---|---|---|---|
| Year | Number | ||||||
| Bombycoidea | Saturniidae | Hylesia metabus | Venezuela, French, Guiana | - | Epidemic outbreaks Number not defined | Pruriginous dermatitis Edema and Erythema Local tissue damage Pain | [17,30,31,32] |
| Leucanella memusae | Argentina | - | Not defined | Local tissue damage Pain | [18] | ||
| Lonomia achelous | Argentina | - | Not defined | Edema and Erythema Haemostatic disturbances Acute kidney injury Pain | [8,33,34] | ||
| Lonomia obliqua | Southern Brazil | 2000–2018 | 60.588 cases 33 mortalities incidence rate of 3,2 envenomations per 100,000 inhabitants | Edema and Erythema Local tissue damage Pain Haemostatic disturbances Acute kidney injury | [8,13,14,15] [16,18,35,36] | ||
| Lasiocampoidea | Lasiocampidae | Dendrolimus pini | Eastern Europe, Central Asia, Northern Asia, Northern Africa | - | Not defined | Dendrolimiasis: Urticating dermatitis Edema and Erythema Osteoarthritis Pain | [3,4] |
| Noctuoidea | Erebidae | Euproctis chrysorrhea | Japan, China | - | Hundred thousand persons Exact number not defined | Contact dermatitis Edema and Erythema Conjunctivitis Allergic reactions Pain | [4,21] |
| Erebidae Subfamily: Arctiidae | Premolis semirufa | Brazil | - | Not defined | Local tissue damage Edema and Erythema Pain Joint immobilization Loss of cartilage | [24,34,37,38,39] | |
| Superfamily | Family | Species | Occurrence | Cases | Venom effects | References | |
|---|---|---|---|---|---|---|---|
| Year | Number | ||||||
| Noctuoidea | Notodontidae | Ochrogaster lunifer | Australia | - | Endemic to Australia Number not defined | Equine Amnionitis Fetal Loss of horses Contact dermatitis Ophthalmia Severe allergic reaction Pain | [22,25] |
| Notodontidae | Thaumetopoea pityocampa | Europe: France,Italy, | - | 18% of humans infected in outbreak areas 60% of veterinary practitioners in France experience symptoms | Contact dermatitis Edema and Erythema Severe allergic reaction Burning pain Conjunctivitis | [3,21,22,40] | |
| Subfamily Thaumetopoeinae | Thaumetopoea processionea | Belgium, Netherlands, United Kingdom, Germany | Epidemic outbreaks Number not defined | [3,23,40] | |||
| Papilionoidea | Nymphalidae | Morpheis ehrenbergii | Mexico | - | Not defined | Contact dermatitis Pain | [53] |
| Zygaenoidea | Megalopygidae | Megalopyge lanata | Argentina | - | Not defined | Edema and Erythema Pain | [41] |
| Megalopyge opercularis | Virginia, Texas | 2000–2016 | 3484 cases | Contact dermatitis Edema and Erythema Local tissue damage Pain | [20] | ||
| Lagoa crispate | Oklahoma | - | Not defined | Edema and Erythema Pain | [42] | ||
| Podalia ca. fuscescens | Argentina | - | Not defined | Edema and Erythema Pain | [18] | ||
| Podalia orsilochus | Argentina | - | Not defined | Edema and Erythema Pain | [18] | ||
| Limacodidae | Latoia consocia | China, Taiwan | - | Not defined | Pain | [43] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seldeslachts, A.; Peigneur, S.; Tytgat, J. Caterpillar Venom: A Health Hazard of the 21st Century. Biomedicines 2020, 8, 143. https://doi.org/10.3390/biomedicines8060143
Seldeslachts A, Peigneur S, Tytgat J. Caterpillar Venom: A Health Hazard of the 21st Century. Biomedicines. 2020; 8(6):143. https://doi.org/10.3390/biomedicines8060143
Chicago/Turabian StyleSeldeslachts, Andrea, Steve Peigneur, and Jan Tytgat. 2020. "Caterpillar Venom: A Health Hazard of the 21st Century" Biomedicines 8, no. 6: 143. https://doi.org/10.3390/biomedicines8060143
APA StyleSeldeslachts, A., Peigneur, S., & Tytgat, J. (2020). Caterpillar Venom: A Health Hazard of the 21st Century. Biomedicines, 8(6), 143. https://doi.org/10.3390/biomedicines8060143